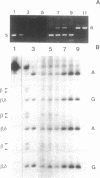
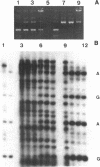
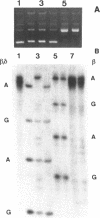
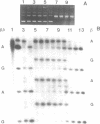
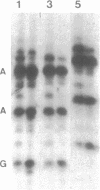
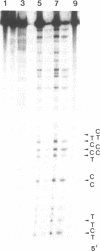
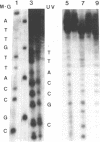
Abstract
Hot piperidine is often used to cleave abasic and UV-irradiated DNA at the sites of damage. It can inflict non-specific damage on DNA, probably because it is a strong base and creates significant concentrations of hydroxyl ions which can attack purines and pyrimidines. We show that several other amines can cleave abasic DNA at or near neutral pH without non-specific damage. One diamine, N,N'-dimethylethylenediamine, efficiently cleaves abasic DNA at pH 7.4 by either beta- or beta,delta-elimination, depending on temperature. Using end-labelled oligonucleotides we show that cleavage depends mainly on elimination reactions, but that 4',5'-cyclization is also significant. This reagent also cleaves at photoproducts induced by UVC and UVB, producing the same overall pattern as piperidine, but with no non-specific damage. It should prove valuable in locating low levels of photoproducts in DNA, such as those induced by natural sunlight.
Full text
PDF
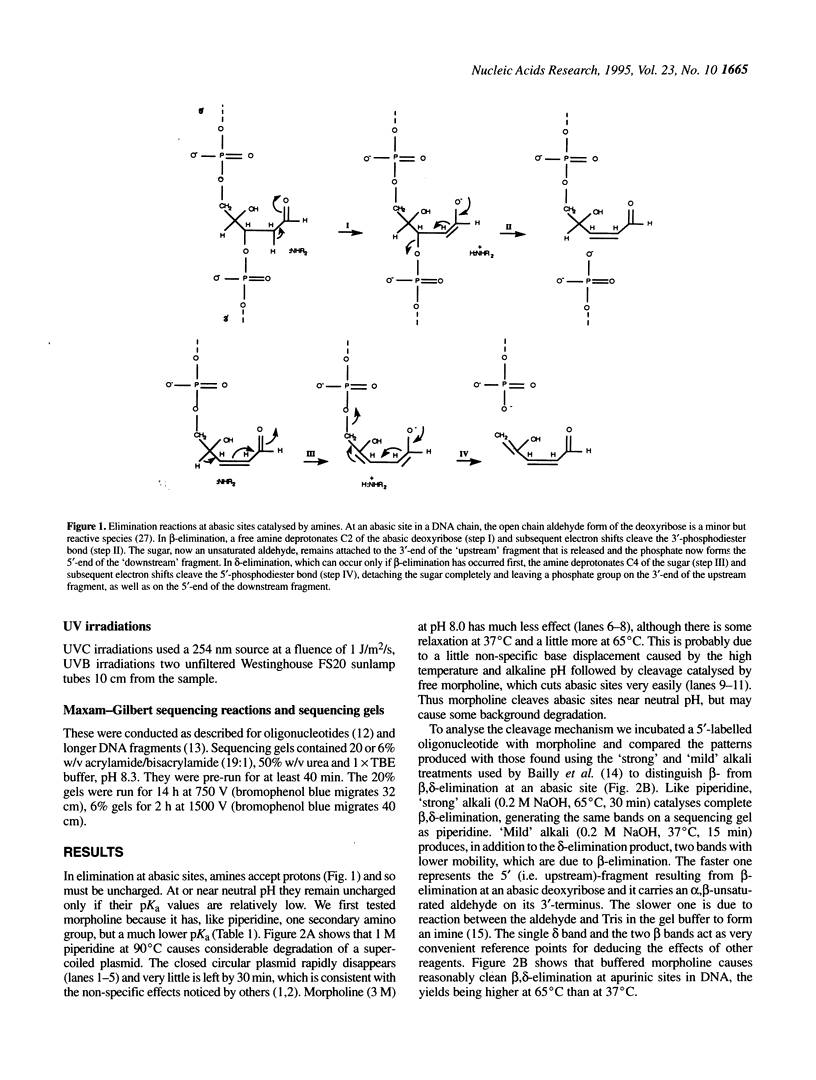




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailly V., Derydt M., Verly W. G. Delta-elimination in the repair of AP (apurinic/apyrimidinic) sites in DNA. Biochem J. 1989 Aug 1;261(3):707–713. doi: 10.1042/bj2610707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly V., Sente B., Verly W. G. Bacteriophage-T4 and Micrococcus luteus UV endonucleases are not endonucleases but beta-elimination and sometimes beta delta-elimination catalysts. Biochem J. 1989 May 1;259(3):751–759. doi: 10.1042/bj2590751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly V., Verly W. G. Possible roles of beta-elimination and delta-elimination reactions in the repair of DNA containing AP (apurinic/apyrimidinic) sites in mammalian cells. Biochem J. 1988 Jul 15;253(2):553–559. doi: 10.1042/bj2530553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banaszuk A. M., Deugau K. V., Sherwood J., Michalak M., Glick B. R. An efficient method for the sequence analysis of oligodeoxyribonucleotides. Anal Biochem. 1983 Feb 1;128(2):281–286. doi: 10.1016/0003-2697(83)90376-7. [DOI] [PubMed] [Google Scholar]
- Behmoaras T., Toulmé J. J., Hélène C. A tryptophan-containing peptide recognizes and cleaves DNA at apurinic sites. Nature. 1981 Aug 27;292(5826):858–859. doi: 10.1038/292858a0. [DOI] [PubMed] [Google Scholar]
- Brun F., Toulmé J. J., Hélène C. Interactions of aromatic residues of proteins with nucleic acids. Fluorescence studies of the binding of oligopeptides containing tryptophan and tyrosine residues to polynucleotides. Biochemistry. 1975 Feb 11;14(3):558–563. doi: 10.1021/bi00674a015. [DOI] [PubMed] [Google Scholar]
- Doetsch P. W., Cunningham R. P. The enzymology of apurinic/apyrimidinic endonucleases. Mutat Res. 1990 Sep-Nov;236(2-3):173–201. doi: 10.1016/0921-8777(90)90004-o. [DOI] [PubMed] [Google Scholar]
- Douki T., Cadet J. Far-UV photochemistry and photosensitization of 2'-deoxycytidylyl-(3'-5')-thymidine: isolation and characterization of the main photoproducts. J Photochem Photobiol B. 1992 Aug 31;15(3):199–213. doi: 10.1016/1011-1344(92)85124-d. [DOI] [PubMed] [Google Scholar]
- Drew H. R. Structural specificities of five commonly used DNA nucleases. J Mol Biol. 1984 Jul 15;176(4):535–557. doi: 10.1016/0022-2836(84)90176-1. [DOI] [PubMed] [Google Scholar]
- FELSENFELD G., HUANG S. The interaction of polynucleotides with metal ions, amino acids, and polyamines. Biochim Biophys Acta. 1960 Jan 29;37:425–433. doi: 10.1016/0006-3002(60)90498-4. [DOI] [PubMed] [Google Scholar]
- Haukanes B. I., Helland D. E., Kleppe K. Action of a mammalian AP-endonuclease on DNAs of defined sequences. Nucleic Acids Res. 1989 Feb 25;17(4):1493–1509. doi: 10.1093/nar/17.4.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejmadi V., Stevenson C., Kumar S., Davies R. J. Alkali-labile photolesions mapping to purine sites in ultraviolet-irradiated DNA. Photochem Photobiol. 1994 Feb;59(2):197–203. doi: 10.1111/j.1751-1097.1994.tb05022.x. [DOI] [PubMed] [Google Scholar]
- Kan L. S., Voituriez L., Cadet J. The Dewar valence isomer of the (6-4) photoadduct of thymidylyl-(3'-5')-thymidine monophosphate: formation, alkaline lability and conformational properties. J Photochem Photobiol B. 1992 Mar 13;12(4):339–357. doi: 10.1016/1011-1344(92)85040-2. [DOI] [PubMed] [Google Scholar]
- Knowland J., McKenzie E. A., McHugh P. J., Cridland N. A. Sunlight-induced mutagenicity of a common sunscreen ingredient. FEBS Lett. 1993 Jun 21;324(3):309–313. doi: 10.1016/0014-5793(93)80141-g. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Andersson A. Rate of chain breakage at apurinic sites in double-stranded deoxyribonucleic acid. Biochemistry. 1972 Sep 12;11(19):3618–3623. doi: 10.1021/bi00769a019. [DOI] [PubMed] [Google Scholar]
- Lippke J. A., Gordon L. K., Brash D. E., Haseltine W. A. Distribution of UV light-induced damage in a defined sequence of human DNA: detection of alkaline-sensitive lesions at pyrimidine nucleoside-cytidine sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3388–3392. doi: 10.1073/pnas.78.6.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattes W. B., Hartley J. A., Kohn K. W. Mechanism of DNA strand breakage by piperidine at sites of N7-alkylguanines. Biochim Biophys Acta. 1986 Oct 16;868(1):71–76. doi: 10.1016/0167-4781(86)90088-6. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumder A., Gerlt J. A., Absalon M. J., Stubbe J., Cunningham R. P., Withka J., Bolton P. H. Stereochemical studies of the beta-elimination reactions at aldehydic abasic sites in DNA: endonuclease III from Escherichia coli, sodium hydroxide, and Lys-Trp-Lys. Biochemistry. 1991 Jan 29;30(4):1119–1126. doi: 10.1021/bi00218a033. [DOI] [PubMed] [Google Scholar]
- Pfeifer G. P., Drouin R., Riggs A. D., Holmquist G. P. In vivo mapping of a DNA adduct at nucleotide resolution: detection of pyrimidine (6-4) pyrimidone photoproducts by ligation-mediated polymerase chain reaction. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1374–1378. doi: 10.1073/pnas.88.4.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre J., Laval J. Specific nicking of DNA at apurinic sites by peptides containing aromatic residues. J Biol Chem. 1981 Oct 25;256(20):10217–10220. [PubMed] [Google Scholar]
- Smith C. A., Taylor J. S. Preparation and characterization of a set of deoxyoligonucleotide 49-mers containing site-specific cis-syn, trans-syn-I, (6-4), and Dewar photoproducts of thymidylyl(3'-->5')-thymidine. J Biol Chem. 1993 May 25;268(15):11143–11151. [PubMed] [Google Scholar]
- Treiber D. K., Chen Z., Essigmann J. M. An ultraviolet light-damaged DNA recognition protein absent in xeroderma pigmentosum group E cells binds selectively to pyrimidine (6-4) pyrimidone photoproducts. Nucleic Acids Res. 1992 Nov 11;20(21):5805–5810. doi: 10.1093/nar/20.21.5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu C. P., Wu R. Sequence analysis of short DNA fragments. Methods Enzymol. 1980;65(1):620–638. [PubMed] [Google Scholar]
- Zhao X., Kao J. L., Taylor J. S. Preparation and characterization of a deoxyoligonucleotide 49-mer containing a site-specific thymidylyl-(3',5')-deoxyadenosine photoproduct. Biochemistry. 1995 Jan 31;34(4):1386–1392. doi: 10.1021/bi00004a033. [DOI] [PubMed] [Google Scholar]