Abstract
Platensimycin (PTM) is a recently discovered broad-spectrum antibiotic produced by Streptomyces platensis. It acts by selectively inhibiting the elongation-condensing enzyme FabF of the fatty acid biosynthesis pathway in bacteria. We report here that PTM is also a potent and highly selective inhibitor of mammalian fatty acid synthase. In contrast to two agents, C75 and cerulenin, that are widely used as inhibitors of mammalian fatty acid synthase, platensimycin specifically inhibits fatty acid synthesis but not sterol synthesis in rat primary hepatocytes. PTM preferentially concentrates in liver when administered orally to mice and potently inhibits hepatic de novo lipogenesis, reduces fatty acid oxidation, and increases glucose oxidation. Chronic administration of platensimycin led to a net reduction in liver triglyceride levels and improved insulin sensitivity in db/+ mice fed a high-fructose diet. PTM also reduced ambient glucose levels in db/db mice. These results provide pharmacological proof of concept of inhibiting fatty acid synthase for the treatment of diabetes and related metabolic disorders in animal models.
Diabetes affects nearly 24 million people in the United States, which is an increase of ∼3 million in 2 y; nearly 8% of the US population is diabetic. Currently approved therapies for the treatment of diabetes suffer from inadequate efficacy and/or liabilities, including hypoglycemia, weight gain, edema, fractures, lactic acidosis, and gastrointestinal intolerance (1). Cumulative data from the National Health and Nutrition Examination Survey during the period 1999–2006 showed significant improvement in the proportion of people with diagnosed diabetes achieving glycemic and cholesterol target levels; however, only one in eight diagnosed diabetics was able to achieve the recommended glycemic, blood pressure, and LDL cholesterol target levels at the same time (2), underscoring the need to develop novel modalities for the treatment of diabetes.
Platensimycin (PTM) is a novel broad-spectrum Gram-positive antibiotic produced by Streptomyces platensis. It was discovered by a target-based whole-cell screening strategy using an antisense differential sensitivity assay (3). Platensimycin inhibits bacterial growth by selectively inhibiting the elongation-condensing enzyme FabF of the fatty acid biosynthesis pathway (3–5). Bacterial fatty acid synthesis (FAS II) differs from mammalian fatty acid synthesis (FAS I) in that the former is catalyzed by a multienzyme complex whereas the latter is catalyzed by a single large protein (6).
Fatty acid synthase (FAS) catalyzes the synthesis of long-chain fatty acids (primarily palmitate) by condensation of acetyl-CoA and malonyl-CoA (6). The mammalian enzyme is a homodimer, with each polypeptide containing seven catalytic domains that act in concert to catalyze the reactions leading to palmitate synthesis. FAS activity is believed to be a determinant of the maximal capacity of liver to synthesize fatty acids by de novo lipogenesis (DNL). Similar to global knockout of acetyl-CoA carboxylase 1 (ACC1), which generates malonyl-CoA for fatty acid synthesis, deletion of the gene coding for FAS results in embryonic lethality (7). By contrast, liver-specific FAS knockout in mice does not lead to an overt phenotype when the animals are fed a normal chow diet (8); however, when animals are fed a zero-fat diet, the lack of FAS does not protect them from the development of fatty liver but rather exacerbates it (8). This phenotype could be attributed to at least two consequences of FAS deficiency: a decrease in DNL that is a direct consequence of FAS deficiency, and a reduction in β-oxidation that is an indirect consequence of FAS deficiency. The latter is evidenced by a threefold increase in hepatic malonyl-CoA concentrations and a significant decrease in blood ketone body levels in liver-specific FAS KO mice (8). The increase in hepatic malonyl-CoA levels is believed to lead to the inhibition of carnitine palmitoyltransferase 1 (CPT-1), impairing entry of fatty acids into the mitochondria for oxidation (9). It was also hypothesized that “new fat” from the diet or synthesized via FAS activity specifically activates a pool of nuclear receptors (e.g., PPARα) that in turn leads to enhanced β-oxidation (8). One such candidate requiring FAS activity for synthesis is 1-palmitoyl-2-oleoyl-sn-glycerol-3-phosphocholine, a putative endogenous ligand of PPARα (10).
FAS is mainly regulated at the transcriptional level by nutrients and hormones. In particular, glucose (via ChREBP), insulin (via SREBP-1c), and the thyroid hormone triiodothyronine increase FAS activity, whereas glucagon and saturated and polyunsaturated fatty acids decrease it (11). Increased fatty acid synthase (FASN) gene expression in adipose tissue is linked to visceral fat accumulation, impaired insulin sensitivity, and increased circulating fasting insulin, IL-6, leptin, and RBP4 (12) in humans.
In this report, we evaluated the effect of the antibiotic platensimycin, a FAS inhibitor, on free fatty acid (FFA) and sterol synthesis in comparison with C75 and cerulenin. We demonstrate that platensimycin is an inhibitor of mammalian FAS that potently and selectively inhibits hepatocyte fatty acid synthesis and fatty acid oxidation, without affecting sterol synthesis. We also demonstrate that platensimycin is liver-preferring in vivo and that the net effect of chronic treatment with this agent is to lower liver triglyceride (TG) levels. Chronic administration of platensimycin led to improved insulin sensitivity in db/+ mice fed a high-fructose diet and reduced ambient glucose levels in db/db mice, providing pharmacological proof of concept of inhibiting fatty acid synthase for the treatment of diabetes and related metabolic disorders.
Results
Platensimycin Is a Potent Inhibitor of Mammalian FAS.
Given its high degree of amino acid sequence conservation, we evaluated the activity of platensimycin against FAS of higher species. Using an established in vitro assay of FAS activity (13), we observed that platensimycin inhibited purified rat and human FAS, with IC50 values of 0.18 and 0.30 μM, respectively.
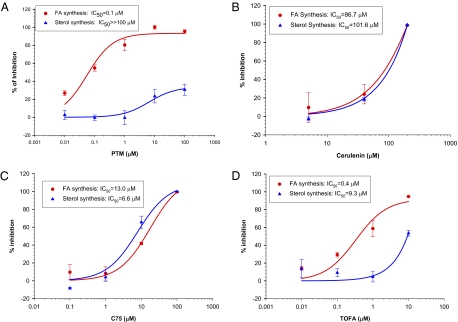
Because liver is a primary site of fatty acid synthesis, we examined whether platensimycin inhibits FAS of rat primary hepatocytes, using a de novo lipogenesis assay. We compared the effects of platensimycin, platencin, cerulenin, C75, as well as TOFA [5-(tetradecyloxy)-2-furoic acid] under the same conditions. As shown in Fig. 1 and Table 1, platensimycin inhibited FFA synthesis in rat primary hepatocytes, with an IC50 of 0.063 μM. As expected of a specific FAS inhibitor, platensimycin did not inhibit sterol synthesis. Platencin [a balanced FAS II FabH/FabF inhibitor (14, 15)] showed a similar activity profile but considerably reduced potency. By contrast, two widely used and structurally distinct FAS inhibitors, C75 and cerulenin, inhibited FFA as well as sterol synthesis, with similar IC50 values. TOFA, an ACC inhibitor, preferentially inhibited FFA synthesis as expected, but also inhibited sterol synthesis, albeit with a more than 20-fold reduced potency (Table 1 and Fig. 1).
Fig. 1.
Effect of platensimycin (A), two widely used FAS inhibitors (cerulenin and C75, B and C), and the ACC inhibitor TOFA (D) on fatty acid and sterol synthesis in rat primary hepatocytes.
Table 1.
Platensimycin is a highly selective mammalian FAS inhibitor
| Platensimycin | Platencin | C75 | TOFA | Cerulenin | |
| Purified enzyme | |||||
| Human FAS (IC50, μM) | 0.3 | 6.3 | >100 | >100 | ND |
| Rat FAS (IC50, μM) | 0.2 | 2.5 | >100 | >100 | ND |
| Rat hepatocytes | |||||
| Fatty acid synthesis (IC50, μM) | 0.1 | 2.0 | 13 | 0.4 | 86.7 |
| Sterol synthesis (IC50, μM) | >>100 | >>100 | 6.6 | 9.3 | 101.6 |
| Selectivity (FA/sterol) | >>1075 | >>50 | 0.6 | 13.4 | 1.0 |
| Fatty acid oxidation (% of control) | 59.7 at 10 μM | 66 at 10 μM | 156 at 40 μM | 245 at 10 μM | 81 at 40 μM |
| LDH assay (% of cell death) | 0 at 100 μM | 0 at 100 μM | 13.3 at 40 μM | 0 at 100 μM | 8.5 at 40 μM |
IC50 values are the average of triplicate determinations, except for the IC50 of platencin for rat FAS, which was n = 1. All IC50 values in the FAS assay were obtained without preincubation with the inhibitors. ND, not determined.
Platensimycin Is Preferentially Concentrated in Liver Following Oral Administration.
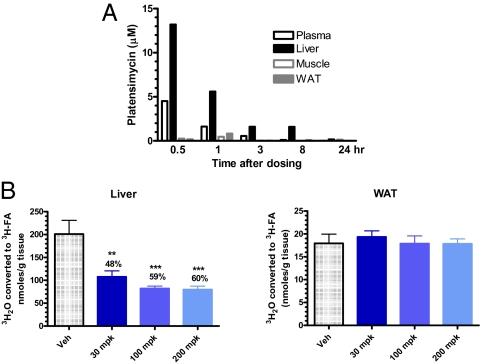
PTM is rapidly cleared from the circulation, where it is needed to exert its antibiotic function, leading to poor systemic exposure and preventing its development as an antibiotic. PTM exhibits a high volume of distribution (Vdss ∼12 L/kg) in mice, which suggested high tissue distribution. We therefore wondered whether platensimycin is quickly distributed or concentrated in tissues from blood. To investigate this, we determined the tissue distribution of platensimycin following oral dosing of mice. As shown in Fig. 2A, exposure of platensimycin in the liver is 2.9×, 3.5×, 2.9×, and 15.3× higher than in blood at 0.5, 1, 3, and 8 h after an oral dose of 100 mg/kg (mpk), respectively. Levels of PTM were detectable only in liver at 24 h after such a single dose. Exposure in the brain is low: The plasma-to-brain ratios of PTM were 19.4×, 31.2×, and 4.7× at 0.5, 1, and 3 h, respectively. Brain exposure was below the detection limit at 8 and 24 h postdosing. PTM levels in muscle and adipose were also significantly below the plasma levels at all time points. Additional studies revealed that platensimycin is excreted primarily in the bile. The enrichment of platensimycin in the liver is also supported by the observation that at doses of up to 200 mpk, PTM inhibited fatty acid synthesis in liver only but not in extrahepatic tissue such as adipose (Fig. 2B).
Fig. 2.
Platensimycin preferentially distributes to the liver following oral dosing (n = 3) (A) and inhibits fatty acid synthesis in the liver, but not in adipose tissue (WAT) (n = 5) (B). db/db mice were used. For A, PTM was dosed orally at 100 mpk; for B, PTM was administered via i.p. injection at the doses indicated. Lower limits of detection were 0.0045, 0.045, and 0.009 μM for plasma, brain, and liver. For fatty acid synthesis measurements, the [3H]H20 incorporation method was used.
Platensimycin Potently Inhibits Liver Fatty Acid Synthesis in Vivo.
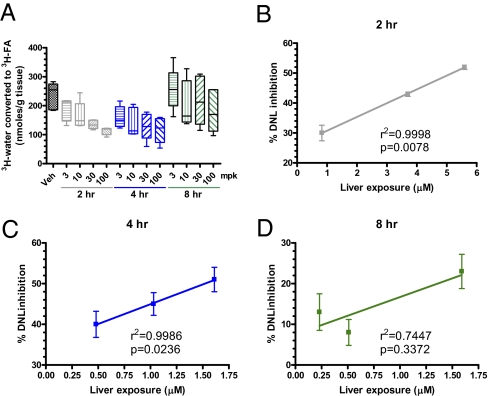
The potent and selective inhibitory activity of platensimycin toward purified mammalian FAS enzyme and in rat primary hepatocytes, coupled with its oral bioavailability, led us to investigate whether platensimycin impacted lipid metabolism in rodents. PTM significantly inhibited hepatic FFA synthesis in a dose-dependent manner (Fig. 2B). By contrast, doses of PTM up to 200 mpk did not inhibit fatty acid synthesis in adipose (Fig. 2B). These data are fully consistent with the tissue distribution data and support the argument that PTM is a liver-preferring compound that has little-to-no efficacy in extrahepatic tissues. We next administered PTM to mice orally at 3, 10, 30, and 100 mpk and monitored its effect on hepatic fatty acid synthesis at different time points (Fig. 3). At 2 h postdose, inhibition of fatty acid synthesis is linearly correlated with liver exposure of PTM (Fig. 3B). The inhibition of liver fatty acid synthesis is also dose-proportional up to 4 h postdosing (Fig. 3C), demonstrating that FAS inhibition by platensimycin persists for at least 4 h in a dose-dependent manner, and the effect lasts up to 8 h (Fig. 3D).
Fig. 3.
Platensimycin levels in liver are positively correlated with DNL inhibition in db/db mice. (A) rate of hepatic lipogenesis as a function of time and PTM dosages; (B–D) correlation of liver PTM levels and lipogenesis inhibition at 2, 4, and 8 h. Veh, vehicle.
Platensimycin Inhibits Fatty Acid Oxidation and Increases Glucose Oxidation.
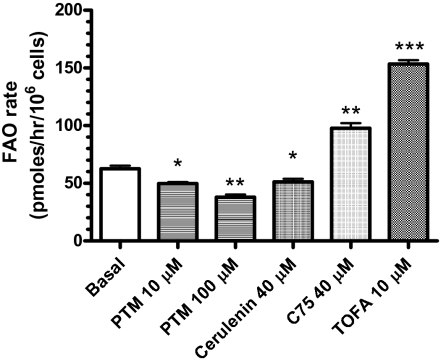
FAS inhibition by platensimycin is anticipated to result in elevated hepatic malonyl-CoA levels. This in turn should lead to inhibition of liver CPT-1, which is required for entry of long-chain fatty acids into mitochondria. Accordingly, platensimycin treatment should also inhibit hepatic fatty acid oxidation (FAO). We tested this hypothesis using both primary rat hepatocytes in vitro as well as db/db mice in vivo. We first evaluated the effect of platensimycin, cerulenin, C75, and TOFA on FAO in primary rat hepatocytes. As shown in Fig. 4, FAO in rat primary hepatocytes was inhibited by 21% and 39% when incubated with 10 or 100 μM PTM, respectively. The ACC inhibitor TOFA increased FAO by 145%, as expected. Surprisingly, C75 increased FAO by 56% whereas cerulenin inhibited FAO by 18%, as expected for a FAS inhibitor (Table 1). The increase in FAO induced by C75 is likely caused by actions other than its inhibition of FAS, consistent with the off-target activities of C75 that have been reported (16–18).
Fig. 4.
Platensimycin inhibits fatty acid oxidation in primary rat hepatocytes. n = 3 wells. Data are expressed as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 versus vehicle.
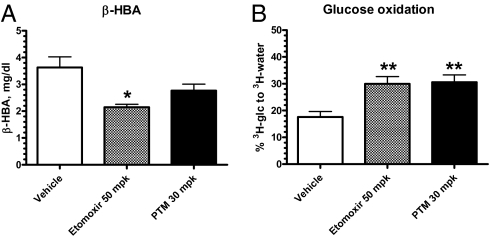
To determine the effects of platensimycin on hepatic FAO in vivo, we measured its effects on the plasma level of the ketone body d-β-hydroxybutyrate (β-HBA) in treated db/db mice. Etomoxir, an inhibitor of CPT-1, was included as a positive control. As expected, etomoxir significantly decreased plasma β-HBA levels (Fig. 5A). Platensimycin at 30 mpk also caused a trend toward a decrease of β-HBA, consistent with its inhibition of FAO in rat primary hepatocytes (Fig. 4). Levels of plasma TG, FFA, and glucose were not changed under these conditions.
Fig. 5.
Platensimycin reduces fatty acid oxidation (A) and increases whole-body glucose oxidation (B) in vivo. db/db mice of 14.5 wk of age were used (n = 7 mice per group). *P < 0.05, **P < 0.01 versus vehicle.
In a parallel experiment, the effect of etomoxir and platensimycin on glucose oxidation was evaluated. Consistent with the inhibitory effects on FAO by both compounds, etomoxir at 50 mpk and platensimycin at 30 mpk increased whole-body glucose oxidation (Fig. 5B). The liver-preferring tissue distribution of PTM (Fig. 2A) makes it likely that the liver is responsible for the increase in glucose oxidation measured in these studies. Unfortunately, direct assessment of the effect of PTM on the rate of glucose oxidation by isolated primary hepatocytes was not possible, due to the low rates of glucose oxidation observed in the cultured hepatocytes.
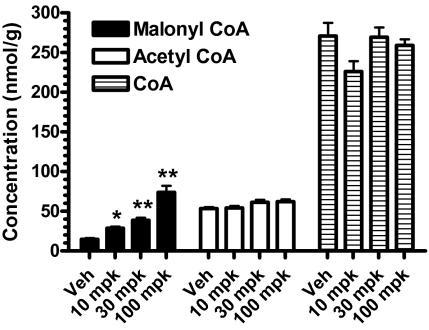
We also determined the levels of hepatic malonyl-CoA, acetyl-CoA, and CoA following platensimycin treatment to assess the correlation between changes in these metabolites and liver FAO. Levels of malonyl-CoA were increased in the livers of db/db mice dosed with PTM in a dose-dependent manner (Fig. 6). The highest values for malonyl-CoA were obtained 1 h postdose, with levels returning to near baseline at 4 and 8 h. This is in contrast to the effect of platensimycin on DNL, where even at 8 h postdosing, inhibition of fatty acid synthesis still persisted (Fig. 3), despite the return of malonyl-CoA levels to normal. Levels of acetyl-CoA and free CoA were not affected (Fig. 6), further demonstrating the specificity of platensimycin's inhibitory effect on FAS.
Fig. 6.
Platensimycin increases malonyl-CoA but not acetyl-CoA or CoA levels in the livers of db/db mice at 1 h postdosing. Mice received an oral dose of platensimycin as indicated at 9:00 AM and food was removed. Livers were collected and quickly frozen at 1, 4, and 8 h postdose. n = 5 per group. *P < 0.05, **P < 0.01 versus vehicle.
Platensimycin Causes a Net Reduction of Liver Triglyceride Levels and Increases Insulin Sensitivity.
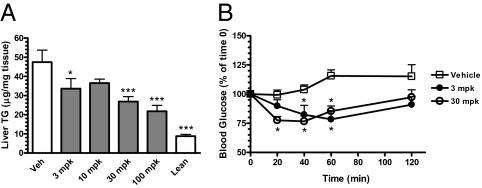
Because platensimycin inhibits both fatty acid synthesis as well as FAO (the latter being an indirect result of malonyl-CoA accumulation upon FAS inhibition), we examined the effect of chronic platensimycin treatment and FAS inhibition on liver TG levels. To increase the magnitude of in vivo de novo lipogenesis, we performed chronic studies in db/+ mice fed a high-fructose diet. Platensimycin was administered to db/+ mice in drinking water for 4 wk. At the end of the study, db/+ mice treated with platensimycin exhibited a dose-dependent decrease in liver TG levels (Fig. 7A). These data demonstrate that the net effect of FAS inhibition by PTM on liver TG levels is in favor of a reduction. These results are in agreement with the observation that PTM caused sustained inhibition of fatty acid synthesis, but only a transient increase in malonyl-CoA levels and consequently inhibition of FAO (Figs. 3 and 6). When an insulin tolerance test was performed on db/+ mice at the end of the study, mice that received PTM at 3 or 30 mpk both exhibited enhanced glucose lowering versus vehicle-treated animals (Fig. 7B), demonstrating increased insulin sensitivity.
Fig. 7.
Chronic platensimycin treatment leads to a reduction of liver triglyceride levels (A) and increased insulin sensitivity as determined by an insulin tolerance test (B). Dosed animals were db/+ mice fed a high-fructose diet (n = 8 mice per group). *P < 0.05, ***P < 0.001 versus vehicle.
Platensimycin Ameliorates Hyperglycemia in db/db Mice.
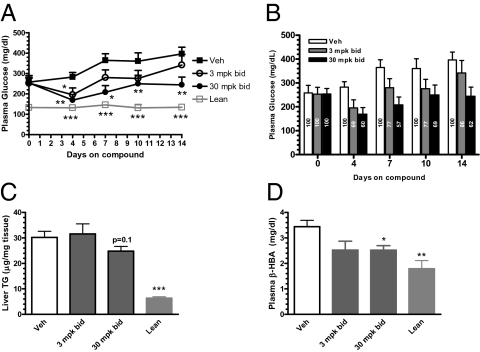
Because hepatic triglyceride has been reported to be a significant independent predictor of fasting endogenous glucose production (19, 20) and platensimycin reduced liver TG levels after chronic dosing, we next examined the effect of PTM treatment on plasma glucose levels of overtly diabetic db/db mice. Mice were orally gavaged with platensimycin twice daily for 2 wk at doses of 3 or 30 mpk. Plasma glucose levels were monitored on days 0, 4, 7, 10, and 14 of treatment. In the mice receiving vehicle alone, plasma glucose levels increased with time, reflecting the natural progression toward more severe diabetes in the db/db mouse model. By contrast, blood glucose levels in the mice that received platensimycin at both 3 and 30 mpk were decreased on day 4 of treatment. The 30-mpk PTM-treated group maintained statistically significant decreased blood glucose levels throughout the 2 wk of treatment (Fig. 8A). On day 14 of PTM treatment, dosing at 3 and 30 mpk decreased plasma glucose levels by 10% and 38%, respectively, compared with vehicle-treated control mice (Fig. 8A). Lower levels of liver TG (Fig. 8B) and circulating β-HBA (Fig. 8C) were also observed in the treated animals. The glucose-lowering effect was not caused by changes in food intake or levels of plasma insulin, as these values were not affected by PTM treatment. Taken together, these data demonstrate that inhibition of hepatic fatty acid synthesis by platensimycin is sufficient to lower blood glucose levels in diabetic db/db mice, and that this is accompanied by a decrease in liver TG levels as well as circulating β-HBA.
Fig. 8.
Chronic treatment of db/db mice with platensimycin lowers plasma glucose and plasma ketone bodies, and leads to a trend toward liver TG reduction. (A) Time course of plasma glucose changes. (B) Change in glucose as a percentage of vehicle as a function of treatment duration. Vehicle glucose was set as 100%, and glucose levels in the treated groups were normalized to the respective vehicle groups. (C) Terminal liver TG levels. (D) Terminal plasma β-HBA level (n = 7–8 mice per group). *P < 0.05, **P < 0.01, ***P < 0.001 versus vehicle.
To further investigate the mechanism(s) of glucose lowering by PTM in db/db mice, we performed ex vivo studies to evaluate the effects of PTM on hepatic gluconeogenesis and glucose utilization by NMR. We also investigated the effect of PTM treatment on glucose uptake by skeletal muscle in vivo. Hepatic glucose production was suppressed by PTM treatment, whereas glucose uptake by skeletal muscle was not affected (Figs. S1 and S2). These data are consistent with the liver-preferring distribution of PTM and provide insight into the mechanism of liver-mediated blood glucose lowering in db/db mice (Fig. 2A).
Discussion
Fatty acid synthesis and oxidation are two important metabolic functions of the liver; both of these processes are regulated by nutritional status. Inhibition of FAS is the only known mechanism by which dual inhibition of both fatty acid synthesis and fatty acid oxidation can occur. Using platensimycin, a liver-targeted natural product isolated from S. platensis, we have demonstrated that the net consequence of FAS inhibition is a reduction in hepatic TG levels. Interestingly, this reduction is associated with increased insulin sensitivity. We further present pharmacological evidence that inhibition of FAS by platensimycin is sufficient to ameliorate diabetes in db/db mice.
C75 and cerulenin are two electrophilic molecules that are frequently used as inhibitors of mammalian FAS. C75, in particular, is a relatively nonspecific inhibitor of FAS: (i) The plasma concentration of C75 present at a dose that completely blocks food intake is only ∼1% of the level needed for FAS inhibition in vitro (17); (ii) C75 is a nonspecific neuronal activator of pro-opiomelanocortin, neuropeptide Y, and Purkinje neurons, whereas cerulenin is not (18); and (iii) C75 increases FAO in primary rat hepatocytes, in contrast to what would be expected of a FAS inhibitor (Fig. 4). Both C75 and cerulenin inhibit cholesterol synthesis. By contrast, as expected for a specific FAS inhibitor, platensimycin does not appreciably inhibit sterol synthesis (Fig. 1). This observation supports the assertion that platensimycin is a highly specific inhibitor of FAS and that it is significantly more selective than either C75 or cerulenin.
Agents that can decrease liver steatosis offer promise as a new treatment modality for diabetes and the metabolic syndrome. As an illustration of this point, the effect of pioglitazone treatment on tissue lipid levels in type 2 diabetic patients has been measured using noninvasive approaches such as 1H magnetic resonance spectroscopy. Pioglitazone treatment of type 2 diabetics resulted in a more than 50% decrease in both hepatic and muscle triglyceride content, despite a significant body weight gain of about 6 kg on average. Using stepwise regression analysis, hepatic triglyceride was found to be a significant independent predictor of fasting endogenous glucose production (EGP), accounting for 53% of its variation (P < 0.001). Similarly, hepatic triglyceride content accounted for 36% of the variation seen in the percent suppression of postprandial EGP observed at 150 min (P = 0.03). In contrast, whereas muscle triglyceride content decreased with pioglitazone treatment, it did not correlate with EGP. There was no relationship between visceral fat content and EGP (19). An earlier diet-intervention study also revealed that moderate weight loss normalizes fasting hyperglycemia in patients with poorly controlled type 2 diabetes; this is accomplished by mobilizing a relatively small pool of intrahepatic lipid, which reverses hepatic insulin resistance and normalizes rates of basal glucose production, independently of changes in insulin-stimulated peripheral glucose metabolism (20). These and other studies underscore the potential benefit of decreasing hepatic TG content by platensimycin pharmacotherapy, or other means, for the treatment of type 2 diabetes as well as related metabolic disorders.
The role of FAS in vivo has been studied by generation of conditional knockout models, because global KO of FAS causes embryonic lethality (7). The role of FAS in the hypothalamus was examined in a series of studies using conditional FAS knockout mice (21, 22). High-fat feeding of conditional FAS knockout mice in which inactivation of FAS was limited to the hypothalamus and pancreatic β-cells demonstrated that these mice are protected from diet-induced obesity and exhibit increased insulin sensitivity in the liver but not in the periphery (22). The hyperinsulinemic-euglycemic clamp data obtained in that study correlated well with biochemical data which demonstrated increased phosphorylation of Akt, GSK-3β, and FOXO1 in the liver but not in the gastrocnemius muscle of the conditional FAS KO mice. Liver mRNA levels of PGC-1α, PEPCK, and G6Pase were decreased, and mRNA of glucokinase was increased in the KO mice (22). These studies also support the hypothesis that FAS functions as a nutritional sensor within the hypothalamus.
On the other hand, pharmacological approaches to FAS inhibition using existing tool compounds suggest that AMP-activated protein kinase (AMPK) may function as the true physiological energy sensor in the hypothalamus. Both systemic and intracerebroventricular treatment of mice with nonselective FAS inhibitors (cerulenin and C75) led to inhibition of feeding and dramatic weight loss. These effects of C75 could be mediated by at least two known actions of C75: It inhibits expression of neuropeptide Y in the hypothalamus, and it acts in a leptin-independent manner that appears to be mediated by malonyl-CoA (23). C75 treatment also results in elevated hypothalamic neuronal ATP levels and rapidly reduces the levels of phosphorylated AMPKα, which may contribute to the reduction of food intake induced by C75 (24). Because the effect of FAS inhibition by C75 can be reversed by aminoimidazole carboxamide ribonucleotide, an AMPK activator (24), AMPK may be functioning as an energy sensor in the hypothalamus. However, the specificity of C75 on FAS was again brought into question because the effective concentration of C75 needed to inhibit the enzyme activity of rat fatty acid synthase in vitro is up to 100 times higher than that achieved in the hypothalamus at a dose that causes complete blockade of food intake. This discrepancy suggests that the anorectic effect of C75 could be independent of its inhibition of FAS in the hypothalamus (17).
Mice deficient in hepatic FAS are devoid of phenotypes when fed a chow diet. When fed a zero-fat diet, hepatosteatosis was observed. Recent studies suggest that FAS activity in liver is required to generate 1-palmitoyl-2-oleoyl-sn-glycerol-3-phosphocholine, a putative endogenous ligand of PPARα (10). Thus, reduced PPARα activity secondary to FAS deficiency might directly contribute to liver steatosis under zero-fat diet conditions. Zero-fat diet-induced liver steatosis may not be relevant to the human condition, where obesity and liver steatosis result from overconsumption of high-fat-content foods. Thus, increasing FAS activity to maintain PPARα activation as a potential therapeutic strategy in human is likely to have very limited utility (25).
When FAS is deleted in a subset of neurons and in the islet β-cells, driven by Cre recombinase excision under the control of rat insulin promoter, mice become resistant to diet-induced obesity. Such mice are also resistant to diet-induced insulin resistance. This observation is consistent with a role of malonyl-CoA in brain sensing of nutrients, because malonyl-CoA should have accumulated secondary to FAS deficiency. Because platensimycin does not readily cross the blood–brain barrier, we do not know whether platensimycin will cause similar metabolic changes when dosed centrally.
Inhibition of FAS elicits cell-cycle arrest and apoptosis; accordingly, FAS is also considered a potential drug target for oncology. Knockdown of FAS, but not two enzymes upstream of it, ATP-citrate lyase and acetyl-CoA carboxylase, leads to caspase-8 activation and cell death. One potential explanation for the specific role of FAS in caspase-8 activation is suggested by the observation that FAS inhibition leads to up-regulation of DNA damage-inducible transcript 4, a stress-response gene that negatively regulates the mammalian target of rapamycin (mTOR) pathway (26). The net consequence of FAS inhibition is thus down-regulation of the mTOR pathway. However, FAS inhibition, using C75 and cerulenin as tools, has also been reported to increase mTOR signaling in the hypothalamus, as determined by increased phosphorylation of S6K1 and S6, two downstream targets of mTOR (27). These observations suggest that additional studies are needed to understand the benefit of FAS inhibition in tumor therapy.
Finally, PTM may also be effective as an anti-infective therapy. When considering FAS as a target for antibiotic development, the potential of exogenous fatty acids present in circulation to bypass inhibition of the de novo lipogenesis pathway needs further investigation (28, 29).
In summary, we have demonstrated that platensimycin, initially isolated as a novel antibiotic inhibiting FAS II machinery of Gram-positive bacteria, is also a potent and selective inhibitor of mammalian FAS. Platensimycin treatment led to improved liver steatosis of high-fructose-diet-fed db/+ mice and ameliorated the diabetes of db/db mice. The superior potency and selectivity of platensimycin over currently available FAS inhibitors cerulenin and C75, coupled with its liver-selective action in vivo, demonstrates its utility as an agent suitable for further investigations of the effects of hepatic FAS inhibition in mammals and potentially in the clinic. Most promisingly, our studies provide an example of preclinical proof of concept for FAS inhibition as a unique modality for the treatment of diabetes, liver steatosis, and related metabolic disorders.
Materials and Methods
Detailed descriptions of procedures are provided in SI Materials and Methods.
Supply of Inhibitors.
PTM was isolated from S. platensis as described earlier (4, 5). Platensimycin was converted to the monosodium salt, and the aqueous solution of the lyophilized form was used for all experiments described herein. Cerulenin, C75, and TOFA were obtained from Sigma-Aldrich.
FAS Enzymatic Activity Assay.
Human and rat FAS were purified as described previously (13). Human enzyme was purified from SKBr3 cells and rat FAS was purified from rat liver. FAS activity was determined by the formation of [3H]palmitate from substrate [3H]acetyl-CoA as previously described (13) using phospholipid-coated Flash Plates (PerkinElmer) (30). IC50 values are the average of triplicate determinations, except for the IC50 of platencin for rat FAS, which was n = 1. The reaction was initiated by the addition of enzyme and the inhibitors were not preincubated with the enzyme.
Supplementary Material
Acknowledgments
We thank Manu Chakravarthy for a critical review of the manuscript.
Footnotes
Conflict of interest statement: All authors are or were employed by Merck & Company, Inc.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1002588108/-/DCSupplemental.
References
- 1.Nathan DM, et al. American Diabetes Association European Association for the Study of Diabetes Medical management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy: A consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheung BMY, et al. Diabetes prevalence and therapeutic target achievement in the United States, 1999 to 2006. Am J Med. 2009;122:443–453. doi: 10.1016/j.amjmed.2008.09.047. [DOI] [PubMed] [Google Scholar]
- 3.Singh SB, Phillips JW, Wang J. Highly sensitive target-based whole-cell antibacterial discovery strategy by antisense RNA silencing. Curr Opin Drug Discov Devel. 2007;10:160–166. [PubMed] [Google Scholar]
- 4.Singh SB, et al. Isolation, structure, and absolute stereochemistry of platensimycin, a broad spectrum antibiotic discovered using an antisense differential sensitivity strategy. J Am Chem Soc. 2006;128:11916–11920. doi: 10.1021/ja062232p. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, et al. Platensimycin is a selective FabF inhibitor with potent antibiotic properties. Nature. 2006;441:358–361. doi: 10.1038/nature04784. [DOI] [PubMed] [Google Scholar]
- 6.White SW, Zheng J, Zhang YM, Rock CO. The structural biology of type II fatty acid biosynthesis. Annu Rev Biochem. 2005;74:791–831. doi: 10.1146/annurev.biochem.74.082803.133524. [DOI] [PubMed] [Google Scholar]
- 7.Chirala SS, et al. Fatty acid synthesis is essential in embryonic development: Fatty acid synthase null mutants and most of the heterozygotes die in utero. Proc Natl Acad Sci USA. 2003;100:6358–6363. doi: 10.1073/pnas.0931394100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakravarthy MV, et al. “New” hepatic fat activates PPARα to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab. 2005;1:309–322. doi: 10.1016/j.cmet.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Foster DW. The role of the carnitine system in human metabolism. Ann N Y Acad Sci. 2004;1033:1–16. doi: 10.1196/annals.1320.001. [DOI] [PubMed] [Google Scholar]
- 10.Chakravarthy MV, et al. Identification of a physiologically relevant endogenous ligand for PPARα in liver. Cell. 2009;138:476–488. doi: 10.1016/j.cell.2009.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radenne A, et al. Hepatic regulation of fatty acid synthase by insulin and T3: Evidence for T3 genomic and nongenomic actions. Am J Physiol Endocrinol Metab. 2008;295:E884–E894. doi: 10.1152/ajpendo.90438.2008. [DOI] [PubMed] [Google Scholar]
- 12.Berndt J, et al. Fatty acid synthase gene expression in human adipose tissue: Association with obesity and type 2 diabetes. Diabetologia. 2007;50:1472–1480. doi: 10.1007/s00125-007-0689-x. [DOI] [PubMed] [Google Scholar]
- 13.Chung CC, et al. A fluorescence-based thiol quantification assay for ultra-high-throughput screening for inhibitors of coenzyme A production. Assay Drug Dev Technol. 2008;6:361–374. doi: 10.1089/adt.2007.105. [DOI] [PubMed] [Google Scholar]
- 14.Jayasuriya H, et al. Isolation and structure of platencin: A FabH and FabF dual inhibitor with potent broad-spectrum antibiotic activity. Angew Chem Int Ed Engl. 2007;46:4684–4688. doi: 10.1002/anie.200701058. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, et al. Discovery of platencin, a dual FabF and FabH inhibitor with in vivo antibiotic properties. Proc Natl Acad Sci USA. 2007;104:7612–7616. doi: 10.1073/pnas.0700746104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rendina AR, Cheng D. Characterization of the inactivation of rat fatty acid synthase by C75: Inhibition of partial reactions and protection by substrates. Biochem J. 2005;388:895–903. doi: 10.1042/BJ20041963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rohrbach KW, et al. Disconnection between the early onset anorectic effects by C75 and hypothalamic fatty acid synthase inhibition in rodents. Eur J Pharmacol. 2005;511:31–41. doi: 10.1016/j.ejphar.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi KA, Smart JL, Liu H, Cone RD. The anorexigenic fatty acid synthase inhibitor, C75, is a nonspecific neuronal activator. Endocrinology. 2004;145:184–193. doi: 10.1210/en.2003-0337. [DOI] [PubMed] [Google Scholar]
- 19.Ravikumar B, et al. Pioglitazone decreases fasting and postprandial endogenous glucose production in proportion to decrease in hepatic triglyceride content. Diabetes. 2008;57:2288–2295. doi: 10.2337/db07-1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen KF, et al. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54:603–608. doi: 10.2337/diabetes.54.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chakravarthy MV, et al. Brain fatty acid synthase activates PPARα to maintain energy homeostasis. J Clin Invest. 2007;117:2539–2552. doi: 10.1172/JCI31183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chakravarthy MV, et al. Inactivation of hypothalamic FAS protects mice from diet-induced obesity and inflammation. J Lipid Res. 2009;50:630–640. doi: 10.1194/jlr.M800379-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loftus TM, et al. Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science. 2000;288:2379–2381. doi: 10.1126/science.288.5475.2379. [DOI] [PubMed] [Google Scholar]
- 24.Kim EK, et al. C75, a fatty acid synthase inhibitor, reduces food intake via hypothalamic AMP-activated protein kinase. J Biol Chem. 2004;279:19970–19976. doi: 10.1074/jbc.M402165200. [DOI] [PubMed] [Google Scholar]
- 25.Rotman N, Wahli W. Fatty acid synthesis and PPARα hand in hand. Chem Biol. 2009;16:801–802. doi: 10.1016/j.chembiol.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Knowles LM, Yang C, Osterman A, Smith JW. Inhibition of fatty-acid synthase induces caspase-8-mediated tumor cell apoptosis by up-regulating DDIT4. J Biol Chem. 2008;283:31378–31384. doi: 10.1074/jbc.M803384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Proulx K, Cota D, Woods SC, Seeley RJ. Fatty acid synthase inhibitors modulate energy balance via mammalian target of rapamycin complex 1 signaling in the central nervous system. Diabetes. 2008;57:3231–3238. doi: 10.2337/db07-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brinster S, et al. Type II fatty acid synthesis is not a suitable antibiotic target for Gram-positive pathogens. Nature. 2009;458:83–86. doi: 10.1038/nature07772. [DOI] [PubMed] [Google Scholar]
- 29.Balemans W, et al. Essentiality of FASII pathway for Staphylococcus aureus. Nature. 2010;463:E3. doi: 10.1038/nature08667. discussion E4. [DOI] [PubMed] [Google Scholar]
- 30.Weiss DR, Glickman JF. Characterization of fatty acid synthase activity using scintillation proximity. Assay Drug Dev Technol. 2003;1:161–166. doi: 10.1089/154065803321537872. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.