Abstract
TNF plays a crucial role in the pathogenesis of Crohn disease. Dysregulated TNF production in mice that bear the genetic deletion of the TNF AU-rich regulatory elements (ARE) (TnfΔARE/+ mice) results in TNF receptor I (TNFRI)-dependent spontaneous Crohn-like pathology. Current concepts consider intestinal epithelial cell (IEC) responses to TNF to be critical for intestinal pathology, but the potential contribution of IEC-derived TNF in disease pathogenesis has not been addressed. In this study we examined whether IEC are sufficient as cellular targets or sources of TNF in the development of intestinal pathology. Using IEC-specific reactivation of a hypomorphic TnfΔAREneo allele in mice, we show that selective chronic overproduction of TNF by IEC suffices to cause full development of Crohn-like pathology. Epithelial TNF overexpression leads to early activation of the underlying intestinal myofibroblast, a cell type previously identified as a sufficient target of TNF for disease development in the TnfΔARE model. By contrast, restricted TNFRI expression on IEC although sufficient to confer IEC apoptosis after acute exogenous TNF administration, fails to induce pathology following chronic specific targeting of IEC by endogenous TNF in TnfΔARE/+ mice. Our results argue against IEC being early and sufficient responders to chronic TNF-mediated pathogenic signals and suggest that proinflammatory aberrations leading to chronic TNF production by IEC may initiate pathology in Crohn disease.
Keywords: inflammatory bowel disease, ileitis, mucosal, mesenchymal
Inflammatory bowel disease (IBD) is a chronic inflammatory disorder of the gastrointestinal tract resulting from inappropriate and sustained activation of the mucosal immune system against the bacterial microflora of the gut (1). IBD is represented by two major forms: ulcerative colitis (UC), which is manifested in the colon, and Crohn disease (CD), which is manifested primarily in the ileum but also in the colon (2). Genome-wide association studies in patients with CD indicate a putative role for genes of the innate and adaptive immunity system and an emerging role for genes implicated in autophagy (3). Animal models of disease suggest that adaptive immune responses are required for disease manifestation and that the gut microflora appear to drive inflammation (4). However, the exact mechanisms underlying IBD pathogenesis and especially the early pathogenic events remain largely obscure.
TNF is critically implicated in IBD pathophysiology, highlighted by the successful use of anti-TNF therapies for the treatment of patients who have CD (5). TNF is shown to exert pathogenic activities in several animal models of IBD (6), whereas protective roles have been described also (7, 8). The crucial pathogenic role of TNF in CD has been verified experimentally in mice that bear the genetic deletion of the TNF AU-rich regulatory elements (ARE) that leads to defective posttranscriptional regulation of tnf expression and chronic TNF overproduction (9). TnfΔARE/+ mice chronically overproduce TNF and spontaneously develop CD8+ T lymphocyte-dependent Crohn-like IBD pathology in the ileum (9–11). Restricting TNF overexpression in myeloid cells or T lymphocytes suffices for the induction of intestinal pathology in this model, indicating the pathogenic potential of TNF derived from innate or adaptive effectors (10). The dominant role of TNF receptor I (TNFRI) in mediating TNF pathogenic signals leading to intestinal pathology has been established previously in this model (9). Most importantly, by restricting TNFRI expression in mesenchymal cells, the intestinal myofibroblast (IMF) was identified as a primary and sufficient cellular target of TNF for intestinal pathology in the TnfΔARE model (12).
An important cell type prominently linked to IBD pathogenesis is the intestinal epithelial cell (IEC), which lies at the interphase between the huge luminal bacterial microflora and the gut-associated lymphoid tissues (13). IEC participate in luminal microbiota recognition by the innate immune system and the clearance of bacterial pathogens, either through the production of antimicrobial peptides or through autophagy (14–16). Importantly, mutations associated with CD pathology in genes such as nucleotide-binding oligomerization domain-containing 2 (NOD2), autophagy-related 16-like 1 (ATG16L1), and immunity-related GTPase family M (IRGM) have been associated with defective bacterial clearance by epithelial cells (14, 17, 18), and the NOD2 and ATG16L1 mutations have been associated with inflammatory activation of macrophages in mice (19, 20). Although human IEC have been reported to overexpress TNF in patients who have CD (21), whether IEC have a role in initiating inflammatory responses in the gut was unknown. In turn, a prominent role for the IEC as a target of TNF has been reported, associating TNF with either the alteration of the epithelial junctions (22) or the induction of IEC apoptosis (23). In this context we aimed to identify the role of IEC as a potential source and target of TNF in a model of TNF-driven Crohn-like IBD. Here we show that TNF overexpression specifically by IEC leads to the early activation of the underlying mesenchymal cells and is sufficient for the full induction of Crohn-like IBD pathology in the mouse. By contrast, although IEC-restricted TNFRI expression is sufficient for the induction of IEC apoptosis in response to exogenous TNF administration, chronic targeting of IEC by endogenous TNF is not sufficient to induce IBD pathology. These findings provide insight into the mechanisms of CD pathogenesis by showing that IEC are potential TNF producers and that IEC and mesenchymal cells can form a cellular axis of TNF function in the gut that is sufficient to cause the full spectrum of pathology seen in human disease.
Results
IEC-Specific TNF Overexpression Is Sufficient for the Induction of Crohn-Like IBD.
IEC, and in particular Paneth cells, from patients who have CD have been reported to overexpress TNF mRNA (21). Therefore, we examined whether IEC may constitute an additional source of TNF overproduction in the TnfΔARE model. By performing RT-PCR in primary ileal IEC, we established that TnfΔARE/+ IEC significantly overexpress TNF as compared with WT IEC (Fig. S1). To estimate the impact of IEC as an early local cellular source of TNF as compared with the other cell types in the ileum, we directly compared TNF expression in IEC and in nonepithelial tissue in WT and TnfΔARE/+ mice at age 4 wk, a stage of disease development at which no inflammatory infiltrates are observed. Although IEC in WT mice appear to express less TNF than do the other intestinal cell types, IEC in TnfΔARE/+ mice express significantly more TNF than does the rest of the tissue and indeed constitute the major source of TNF (Fig. 1A). These observations show that in TnfΔARE/+ mice IEC are the major local source of TNF overproduction before the recruitment of immune effector cells.
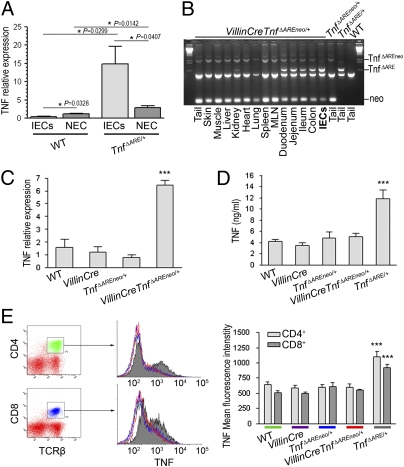
Fig. 1.
IEC are the major early source of TNF in the ileum of TnfΔARE/+ mice. VillinCreTnfΔAREneo/+ mice overproduce TNF specifically by IEC. (A) Quantitative RT-PCR analysis comparing TNF expression in primary ileal IEC and the respective nonepithelial compartment (NEC) in individual mice. Littermate 4-wk-old WT (n = 5) and TnfΔARE/+ (n = 7) mice were used. P values were calculated with two-tailed t test, unpaired for comparison of WT vs.TnfΔARE/+ and paired for comparison of IEC vs. NEC. (B) PCR analysis for detection of the activated TnfΔARE mutation in tissues and primary isolated IEC from VillinCreTnfΔAREneo/+ mice. MLN, mesenteric lymph node. (C) Quantitative RT-PCR analysis for TNF expression in primary IEC isolated from 2-mo-old WT (n = 3), VillinCre (n = 3), TnfΔAREneo/+ (n = 3), and VillinCreTnfΔAREneo/+ (n = 4) littermates. (***P < 0.001; one-way ANOVA.) (D) TNF production determined by ELISA in culture supernatants from LPS-stimulated bone marrow-derived macrophages from 2-mo-old mice (n = 3 per genotype). (***P < 0.001; one-way ANOVA.) (E) TNF expression determined by flow cytometry following intracellular staining for TNF in TCRβ+-gated splenic T lymphocytes from 2-mo-old mice (n = 3–5 per genotype). (***P < 0.001; one-way ANOVA.) In B–E, data shown are representative of two independent experiments. In A–E, mice were analyzed individually. Error bars show SEM.
To investigate the potential role of IEC in introducing inflammatory signals in the intestinal mucosa, we generated mice overproducing TNF specifically by IEC. For this purpose, we crossed the IEC-specific VillinCre transgenic mouse (24) with TnfΔAREneo/+ mice bearing a hypomorphic TnfΔAREneo allele in which a LoxP-flanked neomycin (neo) cassette is inserted next to the tnfΔARE mutation but can be activated upon cre-mediated deletion of the neo cassette (9, 10). In accordance with the reported pattern of expression of the Villin-Cre transgene, we observed in VillinCreTnfΔAREneo/+ mice the activation of the TnfΔARE allele in isolated IEC from the ileum and in all parts of the small intestine and colon but not in other tissues examined (Fig. 1B). Performing quantitative RT-PCR analysis in primary IEC isolated from the ileum, we verified that IEC of VillinCreTnfΔAREneo/+ mice express significantly higher levels of TNF than do IEC from WT, VillinCre, and TnfΔAREneo/+ controls (Fig. 1C). To confirm the specificity of TNF overexpression in IEC, we examined TNF production from macrophages and T-cell receptor β–positive (TCRβ+) splenic T lymphocytes and observed that, unlike control TnfΔARE/+ mice, VillinCreTnfΔAREneo/+ mice did not display increased TNF levels in these cell types (Fig. 1 D and E).
Interestingly, IEC-specific TNF overproduction in VillinCreTnfΔAREneo/+ mice was sufficient to induce spontaneous intestinal pathology in the terminal ileum (Fig. 2 A–C and G). Histological examination of the ileum of VillinCreTnfΔAREneo/+ mice revealed mild inflammatory changes consistent with a Crohn-like IBD phenotype starting at age 2–3 mo (Fig. 2A) and progressing at age 4 mo to severe pathology characterized by broadened and blunted intestinal villi and the infiltration of acute and chronic inflammatory cells in the mucosa, often extending to the submucosa (Fig. 2B). In some cases transmural inflammation was observed in 8-mo-old VillinCreTnfΔAREneo/+ mice (Fig. 2C). By contrast none of the WT, VillinCre, or TnfΔAREneo/+ controls exhibited signs of intestinal inflammation up to age 8 mo (Fig. 2 D–G). FACS analysis of the lamina propria cell populations in the ileum of diseased VillinCreTnfΔAREneo/+ mice revealed a profile similar to that in TnfΔARE/+ mice, characterized by massive accumulation of granulocytes, macrophages, CD4+ and IFNγ-producing CD8+ T lymphocytes, and increased Th-17 responses (Fig. S2). Inflammation was not observed in the duodenum, jejunum, or colon of the VillinCreTnfΔAREneo/+ mice (Fig. S3). The histopathological findings in the intestinal pathology developing in VillinCreTnfΔAREneo/+ mice are similar to the findings in TnfΔARE/+mice, in which TNF is systemically overproduced (9). Interestingly, the course of disease is comparatively delayed, indicating possible synergies with pathogenic TNF overproduction by additional cell types (Fig. S4). These results show that chronic TNF overexpression by IEC is a condition sufficient for the induction of Crohn-like IBD and highlight the pathogenic potential of aberrant IEC proinflammatory responses involving chronic dysregulated TNF overproduction.
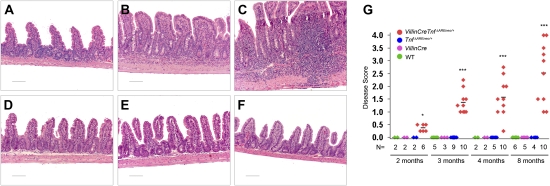
Fig. 2.
Intestinal epithelial TNF overexpression leads to Crohn-like IBD pathology. Histological examination of the ileum of VillinCreTnfΔAREneo/+ mice (A–C) and littermate controls (D–F). (A) Two-month-old VillinCreTnfΔAREneo/+ mice appear normal with no signs of inflammatory infiltration. (B) Severe pathology in a 4-mo-old VillinCreTnfΔAREneo/+ mouse characterized by massive inflammatory infiltration in the intestinal mucosa and submucosa. Intestinal villi are broadened and blunted. (C) Extensive submucosal and transmural inflammation in an 8-mo-old VillinCreTnfΔAREneo/+ mouse. (D) WT, (E) VillinCre, and (F) TnfΔAREneo/+ littermate control mice are normal with no signs of intestinal inflammation at age 8 mo. Paraffin sections were stained with H&E. (Scale bars: 100 μm.) (G) Disease score distribution in the ileum of VillinCreTnfΔAREneo/+ and littermate mice at age 2 mo (*P < 0.05), 3 mo (***P < 0.001), 4 mo (***P < 0.001), and 8 mo (***P < 0.001).
Epithelial Cell-Derived TNF Leads to Mesenchymal Cell Activation Before the Onset of Inflammatory Infiltrations.
The sufficiency of IEC-derived TNF overproduction for the development of full-blown Crohn-like IBD pathology in VillinCreTnfΔAREneo/+ mice suggests that epithelial TNF may lead to an early activation of tissue-damaging processes in the intestine. Matrix metalloproteinases (MMP) are critically implicated in such processes in CD (25), so we used MMP9 as a relevant activation marker, because it is abundantly expressed in the inflamed bowel of patients who have CD (26). By performing gelatin zymography in ileal samples from 2-mo-old VillinCreTnfΔAREneo/+ mice, an age at which no signs of inflammatory infiltration are detected, we observed strong increase of pro-MMP9 gelatinolytic activity (Fig. 3A) indicating an early activation of tissue-damaging processes. The activity of MMP2, which participates in wound-healing processes (25), was not altered (Fig. 3A).
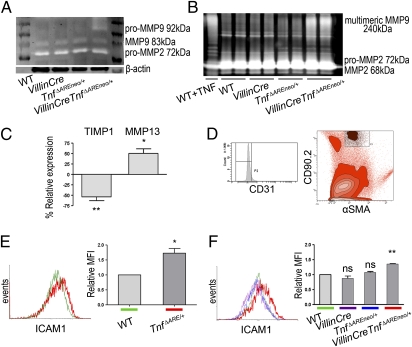
Fig. 3.
Early activation of IMF in the ileum of VillinCreTnfΔAREneo/+ mice. (A) Gelatin zymogram showing MMP9 and MMP2 activity in whole-tissue extracts from the ileum of VillinCreTnfΔAREneo/+ and littermate control mice at age 2 mo. Lines represent individual mice. Data shown are representative of three independent experiments. (B) Gelatin zymogram showing MMP9 and MMP2 secretion from IMF isolated from VillinCreTnfΔAREneo/+ mice (n = 4) and littermates (n = 4 each) at age 2 mo. Lines represent duplicates of separate samples within a single experiment. WT IMF treated with TNF were used as positive control. Data are representative of two independent experiments. (C) Quantitative RT-PCR analysis for TIMP1 (**P < 0.01) and MMP13 (*P < 0.05) expression in IMF isolated from 2-mo-old VillinCreTnfΔAREneo/+ mice and the respective controls in three independent experiments. One sample t test was performed for statistical significance. Error bars show SD. (D) Discrimination of IMF (CD90.2+αSMA+CD31− cells) among intestinal cell populations by FACS analysis. (E and F) ICAM1 expression measured on gated freshly isolated IMF. Analysis was performed in pooled tissues from WT (n = 3) and TnfΔARE/+ (n = 3) 4-wk-old mice in three independent experiments (*P < 0.05) (E) and WT (n = 3), VillinCre (n = 3), TnfΔAREneo/+ (n = 3), and VillinCreTnfΔAREneo/+ (n = 3) 2-mo-old mice in three independent experiments (**P < 0.01) (F). One sample t test was performed for statistical significance. Error bars show SD. MFI, mean fluorescence intensity.
In TnfΔARE/+ mice, the mesenchymal cells underlying the epithelial monolayer, the subepithelial IMF, become activated early and constitute sufficient targets of TNF for the induction of Crohn-like IBD (12). Because IEC are an adequate cellular source of TNF for the development of IBD pathology, we tested whether epithelial TNF leads to an early activation of the underlying IMF promoting pathogenic processes. Gelatin zymography in culture supernatants of isolated IMF from 2-mo-old VillinCreTnfΔAREneo/+ and littermate control mice exhibited severely increased spontaneous MMP9 multimers secretion compared with controls (Fig. 3B). In addition, by performing RT-PCR analysis, we observed that 2-mo-old VillinCreTnfΔAREneo/+ IMF show decreased tissue inhibitor of metalloproteinases-1 (TIMP1) and increased MMP13 expression levels (Fig. 3C). The deregulation of these markers indicates the activation of tissue-remodeling processes (25). To examine further the activation status of IMF in vivo, we performed FACS analysis in freshly isolated total intestinal populations identifying IMF as CD90.2+αSMA+CD31− cells (Fig. 3D), as previously described for human specimens (27). We observed significant early overexpression of the activation marker intercellular adhesion molecule-1 (ICAM1) in both TnfΔARE/+ and VillinCreTnfΔAREneo/+ IMF as compared with littermate controls (Fig. 3 E and F), and this observation also was validated in cultured IMF (Fig. S5). The misbalanced MMP–TIMP expression and the up-regulation of the immunoactivating adhesion molecule ICAM1 (28) demonstrate that IEC-derived TNF leads to an early pathogenic activation of IMF. Because the targeting of mesenchymal cells by TNF and the overexpression of TNF by IEC are conditions independently sufficient for IBD development, these results suggest that IEC and mesenchymal cells in the gut may form a cellular axis of epithelial TNF production and IMF targeting that may be sufficient for the development of CD pathology.
Chronic Targeting of IEC by Endogenous TNF Is Not Sufficient for the Induction of IBD Pathogenesis.
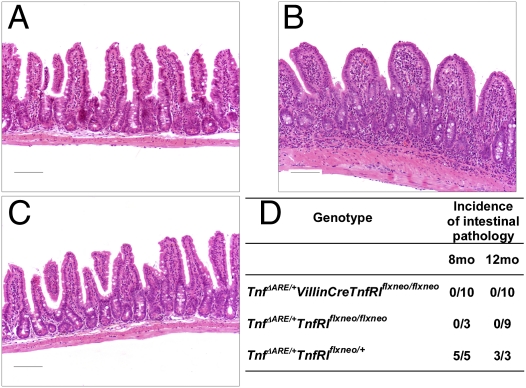
It currently is thought that TNF acts on the intestinal epithelium in ways that lead to IBD pathogenesis. It has been established that acute administration of exogenous TNF leads to IEC apoptosis in a TNFRI-dependent manner (29), although it remains unknown whether in this setting apoptosis is caused directly by TNF targeting the IEC or is a secondary event. To study the in vivo effects of TNF specifically on IEC, we generated mice expressing functional TNFRI selectively in this cell type. We used previously generated mice bearing a conditional gain-of-function allele for TNFRI (TnfRIflxneo/flxneo mice) in which TNFRI expression is inhibited by the presence of a floxed neo cassette but is restored upon Cre-mediated excision of the cassette (30). By crossing these mice with the IEC-specific VillinCre transgenic mice (24), we generated VillinCreTnfRIflxneo/flxneo mice bearing a reactivated TnfRI allele selectively in IEC (Fig. S6). To test whether acute exogenous TNF exerts its apoptotic effect on IEC via epithelial TNFRI, we injected i.v. 12 μg of murine recombinant TNF in VillinCreTnfRIflxneo/flxneo, TnfRIflxneo/flxneo, TnfRI−/− (31), and WT control mice and performed histological and TUNEL analysis in tissues obtained 1 h after injections. Although IEC apoptosis was not detected in the absence of TNFRI expression in control TnfRI−/− and TnfRIflxneo/flxneo mice, we observed extensive IEC apoptosis at the villi tips and detachment of apoptotic IEC from the lumen in VillinCreTnfRIflxneo/flxneo mice expressing TNFRI selectively in IEC, as also occurs in WT controls (Fig. 4 A and B). We verified that the apoptotic cells observed were IEC by performing E-cadherin immunostaining and TUNEL analysis in serial tissue sections (Fig. 4 C and D). Furthermore, we quantified the apoptotic effect of TNF on IEC by collecting the cells detached by lavage (Fig. 4E). FACS analysis for E-cadherin and CD45 along with DNA content analysis upon propidium iodide staining further showed that the vast majority of the cells collected were apoptotic IEC (Fig. 4 F–H). These results show that acute exogenous TNF administration leads directly to extensive IEC apoptosis via epithelial TNFRI. Possibly, then, chronic TNF overproduction in TnfΔARE/+ mice may involve a direct effect of TNF on the IEC through the induction of IEC apoptosis. Therefore, using TUNEL assays, we examined IEC apoptosis in TnfΔARE/+ mice before disease onset (at age 5 wk) and in advanced disease (at age 4 mo). We detected no significant differences in the apoptotic ratios measured in TnfΔARE/+ and WT mice (Fig. S7). Therefore it is possible either that TNF-induced IEC apoptosis occurs at a much lower frequency and may not be readily assessed in a setting of chronic as opposed to acute TNF overexpression or that IEC apoptosis is not a mechanism fundamentally implicated in the pathogenesis of the TnfΔARE disease. To address these alternatives and additional possible effects of TNF in IEC function, we examined whether intact IEC-specific TNFRI expression would suffice for the induction of IBD pathology caused by chronic TNF overproduction in the TnfΔARE model. For this purpose we crossed VillinCreTnfRIflxneo/flxneo mice, in which IEC are the only available cellular target for TNF, with TnfΔARE/+ mice and assessed the development of intestinal pathology. Interestingly, in TnfΔARE/+VillinCreTnfRIflxneo/flxneo mice expressing TNFRI selectively in IEC, signs of intestinal inflammation were not observed even at age 12 mo (Fig. 5 A and D). At age 8 mo, control TnfΔARE/+TnfRIflxneo/+ mice, which bear a functional TnfRI allele, developed severe IBD pathology (Fig. 5 B and D), but signs of inflammation were not observed at the same age in TnfΔARE/+TnfRIflxneo/flxneo mice, which do not express TNFRI (Fig. 5 C and D). These results indicate that the chronic targeting of IEC by endogenous TNF in TnfΔARE/+ mice is not sufficient for the development of inflammation and IBD pathology. Thus, despite the detrimental effects of exogenous TNF administration in the epithelium, chronic spontaneous overproduction of TNF targeting the IEC seems not to be critically involved in initiating TNF-mediated Crohn-like IBD pathology.
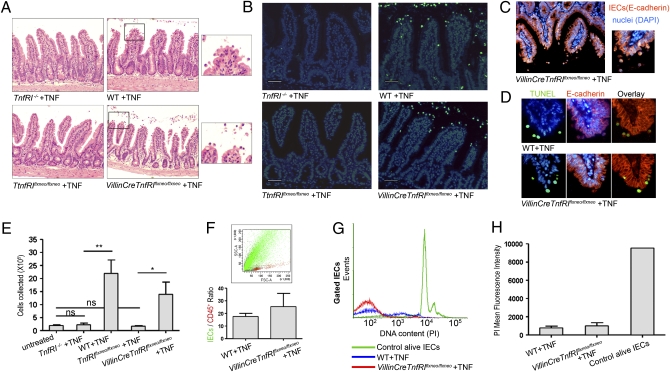
Fig. 4.
Acute exogenous TNF administration leads to IEC apoptosis in a direct manner via epithelial TNFRI. (A–D) Ileal sections from TnfRI−/− (n = 4), WT (n = 5), TnfRIflxneo/flxneo (n = 5), and VillinCreTnfRIflxneo/flxneo (n = 3) mice injected i.v. with 12 μg of murine recombinant TNF. Data represent two independent experiments. (A) Histological examination of H&E-stained sections. (B) TUNEL assay. (Scale bars: 50 μm.) (C) Representative E-cadherin immunostaining. (D) Colocalization of TUNEL and E-cadherin staining in serial paraffin sections. (E–H) Quantitation of TNF-induced IEC apoptosis. Data represent two independent experiments. (E) Number of cells collected from the small intestine of TNF-injected mice. P values (**P < 0.01, *P < 0.05) were calculated with two-tailed t test. Error bars show SEM. (F) Representative FACS analysis for the detection of the markers E-cadherin (epithelial) and CD45 (hemopoietic) on the cells collected. Mean ratio of E-cadherin+/CD45+ cells. Error bars show SEM. (G) DNA content analysis with propidium iodide (PI) staining in gated E-cadherin+/CD45− cells: representative histograms. (H) Quantitation of PI staining data. Error bars show SD.
Fig. 5.
Chronic selective targeting of IEC by endogenous TNF in TnfΔARE/+ mice is not sufficient for the development of intestinal pathology. (A–C) Histological examination of the ileum of 8-mo-old TnfΔARE/+VillinCreTnfRIflxneo/flxneo mice and littermate controls. (A) TnfΔARE/+VillinCreTnfRIflxneo/flxneo mice that express TNFRI selectively in IEC show normal tissue structure without signs of inflammation or tissue damage. (B) Severe intestinal pathology characterized by extensive mucosal and submucosal inflammation in positive-control TnfΔARE/+TnfRIflxneo/+ mice. (C) Negative-control TnfΔARE/+TnfRIflxneo/flxneo mice that do not express TNFRI appear normal. Paraffin sections were stained with H&E. (Scale bars: 100 μm.) (D) Incidence of IBD pathology in the ileum of TnfΔARE/+VillinCreTnfRIflxneo/flxneo mice and littermates at age 8 and 12 mo.
Discussion
In this study we found a proinflammatory role for IEC in triggering IBD pathogenesis. We show that TNF overexpression specifically by IEC leads to the early activation of the underlying mesenchymal cells and is sufficient for the full induction of IBD pathology specifically in the ileum. Notably, the ileal localization of pathology in the TnfΔARE model is a pivotal characteristic of human CD and distinguishes this model from the majority of mouse models of IBD in which disease is colonic. Although the mechanisms underlying this localization remain unclear, possible explanations could be the involvement of divergent molecular and cellular pathways and differential microbiota contributions as well as potential differences in the local mucosal immune system structure and function. Along with this role of IEC as a cellular source of TNF, we demonstrated aspects of the role of IEC as a TNF target. We show that selective TNFRI expression on IEC, although sufficient to confer apoptosis after acute exogenous TNF administration, fails to induce pathology after chronic specific targeting of IEC by endogenous TNF.
TNF has been implicated in disease development in mouse models of colon inflammation associated with loss of the integrity of the intestinal barrier, such as the epithelial-specific NF-κB essential modulator (NEMO)-deficient mice (32) and the epithelial-specific TGF-β–activated kinase 1 (TAK1)-deficient mice (33), in which the epithelium undergoes TNF-mediated apoptosis (32, 33). These models show that impairment of NF-κB signaling sensitizes IEC to the apoptotic effect of endogenous TNF, resulting in colon inflammation (32, 33). Furthermore, in the SAMP/YitFc ileitis model, in which intrinsic defects in nonhemopoietic cells have been reported to underlie ileitis susceptibility, the beneficial effect of anti-TNF administration has been associated with suppressed epithelial apoptosis (34, 35). By contrast, in the TnfΔARE model, in which the primary pathogenic trigger is dysregulated chronic TNF overproduction, the IEC response to endogenous TNF does not appear sufficient to cause pathology. Although we cannot exclude a potential requirement for TNF-mediated effects on IEC contributing to the IBD pathology, our data argue against epithelial TNF targeting as a primary cause of pathology in TNF-driven Crohn-like IBD.
Previous studies have indicated that several mutations associated with CD affect genes mediating IEC functions related to autophagy or bacterial recognition and clearance, suggesting a possible proinflammatory role of IEC in the development of pathology. Indeed, in patients who have CD the Paneth cell, an epithelial cell type involved in bacterial clearance, strongly overexpresses TNF (21). Importantly also, mice bearing IEC-specific deletion of the endoplasmic reticulum stress response-related transcription factor X-box-binding protein 1 (XBP1) show impaired epithelial antimicrobial function and spontaneously develop IBD pathology in the small intestine (36). Hypomorphic variants of XBP1 have been found to be associated with both CD and UC (36), and XBP1-deficient IEC overproduce TNF in vivo (36), indicating that a genetic alteration associated with human IBD can lead directly to epithelial overproduction of TNF. Relevant to these observations, our results provide evidence for aspects of the role of IEC as a cellular source of TNF with regard to IBD pathogenesis. We originally show that IEC are able to introduce early inflammatory signals in the intestine sufficient to lead to the development of intestinal pathology in the ileum similar to that observed in patients who have CD. Because IEC-derived TNF can be an early trigger of CD bearing full pathogenic potential, it would be interesting to examine whether genetic alterations found to be associated with IBD in humans lead to epithelial TNF overexpression. However, mice genetically engineered to carry most of the CD-associated mutations in genes like Nod2 and Atg16l1 did not manifest spontaneous phenotypes of inflammation in the small intestine (14, 17, 37). Therefore, for pathology to develop, additional factors, both genetic and environmental, must act synergistically with any particular mutation. TNF overproduction can be a point of convergence of such pathways (37).
The dynamic cross-talk between the intestinal microflora and the epithelium is the major environmental parameter that may lead to a proinflammatory function of IEC, because they respond differently to commensal and pathogenic bacteria (38). Indeed, human primary IEC, although unresponsive to noninvasive bacterial strains, overproduce TNF in response to bacterial invasion ex vivo (39), highlighting the effect of microflora composition on the tolerant or inflammatory character of IEC. On the other hand, important differences are observed in the composition of the intestinal microflora in patients who have CD or UC and healthy controls (40), and infections are considered to be implicated in both disease onset and relapse in humans (41). Interestingly, it has been shown that Escherichia coli bacterial isolates from inflamed tissues of patients who have CD trigger NF-κB activation and TNF secretion by IEC (42). Furthermore, the hypoxia generated under conditions of infection, tissue damage, and inflammation can be an additional trigger or positive-feedback mechanism for epithelial TNF production, because IEC respond to hypoxia by releasing TNF (43). The pathogenic potential of epithelial TNF production shown in the present study may indicate that in humans conditions leading to local TNF production, such as genetic mutation or bacterial invasion, independently or in combination, may lead to chronic TNF overproduction, breaking the delicate homeostatic balance of the intestine and resulting in pathology.
An immediate cellular target of epithelial TNF that is capable of mediating development of disease is the adjacent subepithelial IMF. Indeed, activated IMF can express cytokines, chemokines, adhesion molecules, and prostaglandins promoting the inflammatory response as well as growth factors and MMP degrading the extracellular matrix and reorganizing the tissue structure (44, 45). Notably, our group previously identified IMF as early and sufficient targets of TNF in Crohn-like IBD pathology in the TnfΔARE model (12). In this study we show that epithelial-derived TNF is able to trigger activation of the underlying IMF even before the appearance of pathological signs or recruitment of immune effector cells in the lamina propria. We show that epithelial TNF leads to misbalanced MMP and TIMP1 expression by IMF and to ICAM1 up-regulation. Therefore, the interaction of IEC and IMF via TNF seems to bear full pathogenic capacity in this model. Treatment of IMF isolated from patients who have active CD with anti-TNF antibodies used currently for CD therapy, such as infliximab, leads to their functional modulation, enhancing the expression of TIMP-1 and downregulating MMP (46). These observations together with our present results suggest that in future studies it will be important to examine genetic and/or environmental parameters that lead to epithelial TNF overproduction in the human intestine. Such studies should provide essential information on the early events leading to CD pathology in humans and may lead to novel applications in disease prevention and therapy.
Materials and Methods
Mice.
The TnfΔARE/+, TnfΔAREneo/+ (9), TnfRIflxneo/flxneo (30), and TnfRI−/− (31) mice have been described previously. VillinCre transgenic mice (24) were provided by D. L. Gumucio (Department of Cell and Developmental Biology, University of Michigan Medical School, Ann Arbor, MI). All mice were bred and maintained on a C57BL/6J or on a mixed C57BL/6J×129S6 genetic background in the animal facilities of the Biomedical Sciences Research Center (BSRC) Alexander Fleming under specific pathogen-free conditions. All mice were used in accordance with the guidance of the Institutional Animal Care and Use Committee of BSRC Alexander Fleming.
Histological Assessment of Intestinal Pathology.
Histological analysis was performed in the terminal ileum and parts of the colon, duodenum, and jejunum. The tissue was embedded in paraffin and stained with H&E. Semiquantitative assessment of intestinal pathology was performed in a blinded fashion. The following scoring system was used: 0 = normal; 1 = villi blunting and mucosal inflammation; 2 = villi blunting, extensive mucosal inflammation, and submucosal inflammation; 3 = extensive submucosal inflammation; 4 = transmural inflammation.
Statistical Analysis.
Statistical analysis was performed using GraphPad Prism software with P values <0.05 regarded as statistically significant.
Supplementary Material
Acknowledgments
We thank D. L. Gumucio for providing the VillinCre transgenic mice; T. Fotsis and A. S. Politou for advice; Ksanthi Kranidioti for discussions and technical advice; Spiros Lalos for excellent technical assistance in histopathology; and Panos Athanasakis, Nikos Giannakas, and Peggy Andriopoulou for technical assistance. This work was supported by Grant LSHG-CT-2005–005203 from the European Commission Integrated Functional Genomics in Mutant Mouse Models as Tools to Investigate the Complexity of Human Immunological Disease (MUGEN) program, Grant 223151 from the European Commission program Inflammation and Cancer Research in Europe (INFLACARE), and by Grant GSRT-PENED-03EΔ770 from the Hellenic Ministry for Development.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. F.C. is a guest editor invited by the Editorial Board.
1Deceased December 10, 2010.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1007811108/-/DCSupplemental.
References
- 1.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 2.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 3.Cho JH. The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol. 2008;8:458–466. doi: 10.1038/nri2340. [DOI] [PubMed] [Google Scholar]
- 4.Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- 5.Targan SR, et al. Crohn's Disease cA2 Study Group. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. N Engl J Med. 1997;337:1029–1035. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- 6.Apostolaki M, Armaka M, Victoratos P, Kollias G. Cellular mechanisms of TNF function in models of inflammation and autoimmunity. Curr Dir Autoimmun. 2010;11:1–26. doi: 10.1159/000289195. [DOI] [PubMed] [Google Scholar]
- 7.Noti M, Corazza N, Mueller C, Berger B, Brunner T. TNF suppresses acute intestinal inflammation by inducing local glucocorticoid synthesis. J Exp Med. 2010;207:1057–1066. doi: 10.1084/jem.20090849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pagnini C, et al. Probiotics promote gut health through stimulation of epithelial innate immunity. Proc Natl Acad Sci USA. 2010;107:454–459. doi: 10.1073/pnas.0910307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F, Kollias G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: Implications for joint and gut-associated immunopathologies. Immunity. 1999;10:387–398. doi: 10.1016/s1074-7613(00)80038-2. [DOI] [PubMed] [Google Scholar]
- 10.Kontoyiannis D, et al. Genetic dissection of the cellular pathways and signaling mechanisms in modeled tumor necrosis factor-induced Crohn's-like inflammatory bowel disease. J Exp Med. 2002;196:1563–1574. doi: 10.1084/jem.20020281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Apostolaki M, et al. Role of beta7 integrin and the chemokine/chemokine receptor pair CCL25/CCR9 in modeled TNF-dependent Crohn's disease. Gastroenterology. 2008;134:2025–2035. doi: 10.1053/j.gastro.2008.02.085. [DOI] [PubMed] [Google Scholar]
- 12.Armaka M, et al. Mesenchymal cell targeting by TNF as a common pathogenic principle in chronic inflammatory joint and intestinal diseases. J Exp Med. 2008;205:331–337. doi: 10.1084/jem.20070906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8:411–420. doi: 10.1038/nri2316. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi KS, et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 15.Rioux JD, et al. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat Genet. 2007;39:596–604. doi: 10.1038/ng2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cario E. Innate immune signalling at intestinal mucosal surfaces: A fine line between host protection and destruction. Curr Opin Gastroenterol. 2008;24:725–732. doi: 10.1097/MOG.0b013e32830c4341. [DOI] [PubMed] [Google Scholar]
- 17.Cadwell K, et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature. 2008;456:259–263. doi: 10.1038/nature07416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCarroll SA, et al. Deletion polymorphism upstream of IRGM associated with altered IRGM expression and Crohn's disease. Nat Genet. 2008;40:1107–1112. doi: 10.1038/ng.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maeda S, et al. Nod2 mutation in Crohn's disease potentiates NF-kappaB activity and IL-1beta processing. Science. 2005;307:734–738. doi: 10.1126/science.1103685. [DOI] [PubMed] [Google Scholar]
- 20.Saitoh T, et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature. 2008;456:264–268. doi: 10.1038/nature07383. [DOI] [PubMed] [Google Scholar]
- 21.Lala S, et al. Crohn's disease and the NOD2 gene: A role for Paneth cells. Gastroenterology. 2003;125:47–57. doi: 10.1016/s0016-5085(03)00661-9. [DOI] [PubMed] [Google Scholar]
- 22.Turner JR. Molecular basis of epithelial barrier regulation: From basic mechanisms to clinical application. Am J Pathol. 2006;169:1901–1909. doi: 10.2353/ajpath.2006.060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeissig S, et al. Downregulation of epithelial apoptosis and barrier repair in active Crohn's disease by tumour necrosis factor alpha antibody treatment. Gut. 2004;53:1295–1302. doi: 10.1136/gut.2003.036632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madison BB, et al. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem. 2002;277:33275–33283. doi: 10.1074/jbc.M204935200. [DOI] [PubMed] [Google Scholar]
- 25.Ravi A, Garg P, Sitaraman SV. Matrix metalloproteinases in inflammatory bowel disease: Boon or a bane? Inflamm Bowel Dis. 2007;13:97–107. doi: 10.1002/ibd.20011. [DOI] [PubMed] [Google Scholar]
- 26.Baugh MD, et al. Matrix metalloproteinase levels are elevated in inflammatory bowel disease. Gastroenterology. 1999;117:814–822. doi: 10.1016/s0016-5085(99)70339-2. [DOI] [PubMed] [Google Scholar]
- 27.Saada JI, et al. Subepithelial myofibroblasts are novel nonprofessional APCs in the human colonic mucosa. J Immunol. 2006;177:5968–5979. doi: 10.4049/jimmunol.177.9.5968. [DOI] [PubMed] [Google Scholar]
- 28.Lebedeva T, Dustin ML, Sykulev Y. ICAM1 co-stimulates target cells to facilitate antigen presentation. Curr Opin Immunol. 2005;17:251–258. doi: 10.1016/j.coi.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Piguet PF, Vesin C, Guo J, Donati Y, Barazzone C. TNF-induced enterocyte apoptosis in mice is mediated by the TNF receptor 1 and does not require p53. Eur J Immunol. 1998;28:3499–3505. doi: 10.1002/(SICI)1521-4141(199811)28:11<3499::AID-IMMU3499>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 30.Victoratos P, et al. FDC-specific functions of p55TNFR and IKK2 in the development of FDC networks and of antibody responses. Immunity. 2006;24:65–77. doi: 10.1016/j.immuni.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 31.Pfeffer K, et al. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 32.Nenci A, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–561. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 33.Kajino-Sakamoto R, et al. Enterocyte-derived TAK1 signaling prevents epithelium apoptosis and the development of ileitis and colitis. J Immunol. 2008;181:1143–1152. doi: 10.4049/jimmunol.181.2.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marini M, et al. TNF-alpha neutralization ameliorates the severity of murine Crohn's-like ileitis by abrogation of intestinal epithelial cell apoptosis. Proc Natl Acad Sci USA. 2003;100:8366–8371. doi: 10.1073/pnas.1432897100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olson TS, et al. The primary defect in experimental ileitis originates from a nonhematopoietic source. J Exp Med. 2006;203:541–552. doi: 10.1084/jem.20050407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaser A, et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134:743–756. doi: 10.1016/j.cell.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hill DA, Artis D. Intestinal bacteria and the regulation of immune cell homeostasis. Annu Rev Immunol. 2010;28:623–667. doi: 10.1146/annurev-immunol-030409-101330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jung HC, et al. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qin J, et al. MetaHIT Consortium. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Irving PM, Gibson PR. Infections and IBD. Nat Clin Pract Gastroenterol Hepatol. 2008;5:18–27. doi: 10.1038/ncpgasthep1004. [DOI] [PubMed] [Google Scholar]
- 42.La Ferla K, Seegert D, Schreiber S. Activation of NF-kappaB in intestinal epithelial cells by E. coli strains isolated from the colonic mucosa of IBD patients. Int J Colorectal Dis. 2004;19:334–342. doi: 10.1007/s00384-004-0583-7. [DOI] [PubMed] [Google Scholar]
- 43.Taylor CT, Fueki N, Agah A, Hershberg RM, Colgan SP. Critical role of cAMP response element binding protein expression in hypoxia-elicited induction of epithelial tumor necrosis factor-alpha. J Biol Chem. 1999;274:19447–19454. doi: 10.1074/jbc.274.27.19447. [DOI] [PubMed] [Google Scholar]
- 44.Powell DW, et al. Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am J Physiol. 1999;277:C183–C201. doi: 10.1152/ajpcell.1999.277.2.C183. [DOI] [PubMed] [Google Scholar]
- 45.Andoh A, Bamba S, Brittan M, Fujiyama Y, Wright NA. Role of intestinal subepithelial myofibroblasts in inflammation and regenerative response in the gut. Pharmacol Ther. 2007;114:94–106. doi: 10.1016/j.pharmthera.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 46.Di Sabatino A, et al. Functional modulation of Crohn's disease myofibroblasts by anti-tumor necrosis factor antibodies. Gastroenterology. 2007;133:137–149. doi: 10.1053/j.gastro.2007.04.069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.