Abstract
Receptor activator of NF-κB (RANK), known for controlling bone mass, has been recognized for its role in epithelial cell activation of the mammary gland. Because bone and the epidermo-pilosebaceous unit of the skin share a lifelong renewal activity where similar molecular players operate, and because mammary glands and hair follicles are both skin appendages, we have addressed the function of RANK in the hair follicle and the epidermis. Here, we show that mice deficient in RANK ligand (RANKL) are unable to initiate a new growth phase of the hair cycle and display arrested epidermal homeostasis. However, transgenic mice overexpressing RANK in the hair follicle or administration of recombinant RANKL both activate the hair cycle and epidermal growth. RANK is expressed by the hair follicle germ and bulge stem cells and the epidermal basal cells, cell types implicated in the renewal of the epidermo-pilosebaceous unit. RANK signaling is dispensable for the formation of the stem cell compartment and the inductive hair follicle mesenchyme, and the hair cycle can be rescued by Rankl knockout skin transplantation onto nude mice. RANKL is actively transcribed by the hair follicle at initiation of its growth phase, providing a mechanism for stem cell RANK engagement and hair-cycle entry. Thus, RANK–RANKL regulates hair renewal and epidermal homeostasis and provides a link between these two activities.
Keywords: osteoprotegerin, TRANCE, anagen, S100A8
Receptor activator of NF-κB (RANK), a member of the TNF receptor family (TNFRSF11a), was originally identified as a regulator of bone density. Mice deficient in Rank or Rankl display increased bone density owing to reduced osteoclast formation, but the loss of the RANK ligand (RANKL) decoy receptor osteoprotegerin (OPG) results in lower bone mass because of unchecked RANK activation (1, 2).
RANK has emerged as an important player in epithelial cell growth and differentiation. It is required for the formation of lactating mammary glands (3, 4), thymic medullary epithelial cells (5), and intestinal microfold cells (6) and has been implicated in the growth and metastasis of prostate (7) and mammary epithelial cancers (8, 9). Thus, RANK affects a great variety of epithelial cells of different organs. The skin is the largest epithelial surface, and its epidermo-pilosebaceous unit comprises the interfollicular epidermis (IFE), the hair follicle (HF), and the sebaceous gland. The HF has the particularity of undergoing cycles of growth (anagen), regression (catagen), and relative quiescence (telogen) (10), making it an excellent system for studying epidermal (stem) cells and organ remodeling (11–14). Each HF is composed of a permanent upper portion, which includes the sebaceous gland and the bulge region, and a temporary lower cycling portion. The local balance of hair growth stimulators and inhibitors is critical for initiation of new hair growth (15–17). Intriguingly, many of these molecular players also operate in bone development and remodeling (e.g., members of the TGF-β superfamily and their antagonists, parathyroid hormones, and hedgehog proteins) (18).
Because Rank and Rankl-null mice exhibit defects in mammary gland development (3) and in the eruption of teeth (19), which, like HFs, are skin appendages, it raises the question of the role of the RANK–RANKL–OPG triad in HF formation and cycling. The finding that Opg is transcribed by the bulge stem cells (20, 21) supports an important role of these proteins in HF biology. However, so far this question has not been investigated.
The IFE is intrinsically linked to the HF through the outer-root sheath, which merges distally the basal layer of IFE and proximally the HF bulge and the HF bulb (10). Like HFs, the IFE undergoes renewal (homeostasis). In this process, keratinocytes of the basal layer divide and terminally differentiate to replace upper-lying cells. RANKL is expressed by the activated IFE, and the epidermal Langerhans dendritic cells carry RANK (22, 23). RANK engagement on Langerhans cells leads to an increase in their cell numbers and to immune response modulation (22, 23). However, the role of RANK in epidermal homeostasis has not been addressed.
We have studied whether RANK plays a functionally important role in the stimulation of epithelial cells of the HF and the IFE. Using Rankl knockout (Rankl-KO) and Rank transgenic (Rank-Tg) mice, as well as recombinant RANKL and anti-RANKL mAb, we here present previously unreported evidence that RANK functions in HF growth and in epidermal renewal.
Results
RANK Stimulation Is Dispensable for Normal HF Development but Is Required for HF Anagen.
Many molecular regulators of HF cycling also drive HF development (morphogenesis) (17). Therefore, we first investigated the importance of RANK stimulation in HF morphogenesis. We analyzed the expression of RANK, RANKL, and OPG mRNA by RT-PCR in total skin of wild-type (WT) mice at postnatal day 5 (P5) and P15 and found that the genes were transcriptionally active (Fig. S1A). In situ hybridization of RANK and RANKL mRNA at P5 showed that the genes were transcribed in the IFE and the HF (Fig. S1B). This finding confirms previous data of RANK and RANKL expression in the embryonic IFE and HF (24). We then compared HF morphogenesis of Rankl−/− mice and control littermates. At P5, the back skin HFs of WT mice were in postnatal HF morphogenesis and by P15 began to enter catagen. Exactly the same observation was made in Rankl−/− mice (Fig. S1C), whose postnatal HF morphogenesis and macroscopic hair phenotype appeared indistinguishable from those of WT littermates (25). Furthermore, all four hair types (monotrich, awl, auchene, and zigzag) were found (Fig. S1D), indicating that RANK stimulation is dispensable for HF morphogenesis.
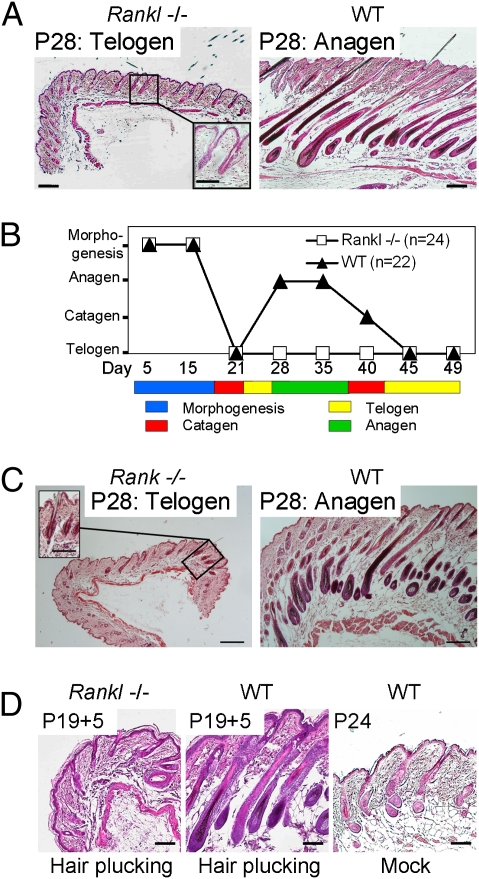
Next, we studied whether absent RANK stimulation affected the HF renewal cycle with entry into the hair growth phase at around P24 in C57BL/6 mice (26). Strikingly, Rankl−/− HFs did not enter anagen by P28 through to P49, whereas WT littermates had long proceeded into anagen or had already reached their second catagen (Fig. 1 A and B and Fig. S2A). Also at P25, there was no sign of anagen initiation, showing a genuine block of HF cycling rather than the occurrence of an abortive, drastically shortened growth phase (Fig. S2B). As expected, given that RANKL is the only ligand for RANK (27), Rank−/− mice also failed to undergo transition from telogen to anagen (Fig. 1C).
Fig. 1.
RANKL is required for HF anagen. (A) At P28, Rankl−/− HFs are arrested in telogen, whereas WT littermates are in anagen. (B) Schematic representation of HF morphogenesis and cycling in Rankl−/− and controls, and the failure of all Rankl−/− mice to enter anagen. (C) Arrest of Rank−/− HFs in telogen. (D) At 5 d after hair plucking at P19, HFs were in advanced anagen in WT mice but remained in telogen in Rankl−/− mice. Mock-treated WT mice displayed early anagen. (Scale bars, 100 μm; in Insets, 50 μm.) The paraffin-embedded sections were stained with hematoxylin/eosin.
Because hair plucking can experimentally provoke hair-cycle activation (28), we tested this stimulus on Rankl−/− mice. Five days after hair plucking, 24-d-old control mice were in advanced anagen, but the Rankl-KO animals still remained in telogen (Fig. 1D). Thus, in the absence of RANK stimulation by RANKL, the mouse HF is arrested in first postnatal telogen and is unable to enter its anagen growth phase.
Telogen Arrest in the Absence of RANK Stimulation Does Not Result from Defects of the HF Stem Cell Compartment or from Defective HF Mesenchyme.
At least two distinct subpopulations of HF epithelial cells are needed for normal anagen formation, the bulge stem cells (11, 29) and the secondary hair germ (SHG) cells (21, 30). Therefore, we looked for the presence of these cells in the Rankl−/− animals.
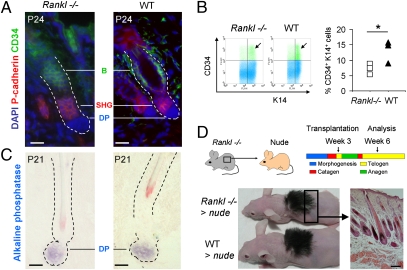
Immunofluorescence for CD34, a marker of murine bulge stem cells (31), showed that they were preserved in the Rankl−/− telogen HF (Fig. 2A). Their quantification by flow cytometry revealed that their number was only slightly reduced (Fig. 2B). Immunofluorescence for P-cadherin (21) showed that the SHG was also present (Fig. 2A). Thus, the absence of RANKL does not deplete these cells sufficiently to explain the HF arrest in telogen.
Fig. 2.
The HF stem cell and the mesenchymal cell compartment is RANK-signaling independent. (A) Identification of CD34+ bulge (B) and P-cadherin+ SHG cells in Rankl−/− and control telogen HFs by immunofluorescence. Nuclei were colored with DAPI. (Scale bars, 20 μm.) (B Left) Flow cytometry of CD34+ bulge cells among keratin 14+ keratinocytes in Rankl−/− and WT telogen HFs. Arrows point to CD34+ keratin 14+ cells. (Right) Graph depicts the percentage of CD34+ and keratin 14+ cells in three KO mice and littermate controls (*P < 0.05). (C) Identification of follicular DP by their alkaline phosphatase activity. (Scale bars, 20 μm.) (D) Restored Rankl−/− hair renewal after transplantation onto nude mice at week 3 and analysis at week 6. A Rankl−/− skin section shows HFs in anagen. Image is representative for five transplantation experiments. (Scale bar, 100 μm.) The paraffin-embedded section was stained with hematoxylin/eosin.
Anagen development also requires inductive signals from the mesenchymal follicular dermal papilla (DP) (32). Therefore, we examined its presence by alkaline phosphatase enzyme histochemistry (33). The enzyme activity in the correct position beneath the HF revealed the presence of morphologically and enzyme-histochemically normal DP in Rankl−/− mice (Fig. 2C), which shows that that the Rankl−/− telogen HF contained all of the cellular elements required for telogen–anagen transition.
We therefore tested whether the hair-cycle arrest can be rescued. We transplanted shaved, 21-d-old Rankl−/− skin, whose HFs were all in telogen, onto age-matched nude mice. Because RANKL is produced during wound healing by neovascularization and by activated epidermal keratinocytes (22, 34), it could be expected that recipient RANKL is synthesized and complements the genetic deficiency. Hair regrowth and anagen development of the grafted skin was monitored. We observed that, by 10 d, both the WT and the Rankl−/− transplanted skin had acquired a dark appearance, a sign of anagen, and, by 3 wk, both transplants presented full hair regrowth (Fig. 2D). Skin cross-sections confirmed that HFs of the Rankl-null mice were in anagen. These findings established that the epithelial stem cell compartment and the inductive HF mesenchyme of Rankl−/− HFs are functional.
RANK Is Expressed by HF Epithelial Stem Cells.
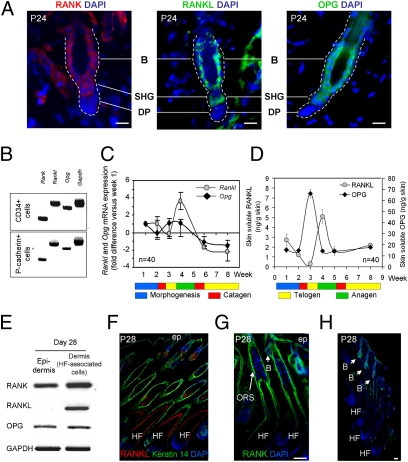
To better understand how RANK regulates the telogen–anagen transition, we determined the site of RANK expression in telogen HFs. Immunofluorescent labeling localized RANK to the bulge and the SHG (Fig. 3A). Also RANKL and the RANKL decoy receptor OPG were expressed at these sites (Fig. 3A). The immunoreactivity of RANK and RANKL was low, requiring signal amplification, whereas OPG could be visualized without amplification. This finding concurs with published gene profiling results that had shown abundant transcripts of Opg in murine bulge cells (20, 21). RANK, RANKL, or OPG were undetectable in the DP. RT-PCR of CD34+ and P-cadherin+ cells, which were isolated and sorted by FACS (21), confirmed transcriptional activity of Rank, Rankl, and Opg in the CD34+ bulge and the P-cadherin+ SHG epithelial cells (Fig. 3B).
Fig. 3.
Expression of RANK, RANKL, and OPG in HF stem cells. (A) Localization of RANK, RANKL, and OPG in WT telogen bulge (B) and SHG stem cells by immunofluorescence. Nuclei were colored with DAPI. (Scale bars, 20 μm.) Six mice were analyzed for RANK, RANKL, and OPG. (B) RT-PCR of Rank, Rankl, Opg, and Gapdh in FACS-sorted CD34+ bulge and P-cadherin+ SHG cells. (C and D) Measures (mean ± SEM) of Rankl and Opg gene transcription by quantitative RT-PCR in whole skin (C) and of soluble RANKL and OPG by ELISA in skin-organ cultures at the indicated ages (D). n, number of mice for the analysis, a minimum of six mice per time point. (E) RT-PCR of Rank, Rankl, Opg, and Gapdh transcripts in the IFE and the HF-associated dermal cells at P28. (F) Immunofluorescence of RANKL and keratin 14 on a P28 skin section. Nuclei were colored with DAPI. (G) Immunofluorescent labeling for RANK in a P28 skin section with DAPI counterstaining. (H) Immunofluorescent labeling for OPG with DAPI coloration in P28 anagen skin. (Scale bars, 50 μm.) ORS, outer root sheath; B, bulge; ep, IFE.
We then investigated whether RANKL and OPG expression changed during the hair cycle by measuring their mRNA by quantitative RT-PCR in total skin. OPG gene expression was maximal in early to mid-telogen, whereas that of RANKL peaked in early anagen (Fig. 3C). Because RANKL and OPG are soluble proteins, we measured their protein levels by ELISA in the supernatant of a 24-h skin culture. Fig. 3D shows that the RANKL and OPG protein levels reflected their mRNA changes with maximal levels of OPG at telogen followed by a peak of RANKL. To uncover the site of RANKL expression during its peak, we performed RT-PCR on separated IFE and dermal HF-associated cells (Fig. 3E); it disclosed that RANKL expression was restricted to the HF-associated cells. Anti-RANKL/keratin 14 immunofluorescent labeling of the whole skin revealed RANKL in the lower HF (Fig. 3F), and costaining with GATA-3, a marker for the HF inner root sheath (35), showed that RANKL is localized to the inner root sheath and the cuticle layer (Fig. S3A). Because RANKL is a soluble protein and the detection at these sites could be the consequence of binding to extracellular components, we visualized its mRNA on sections by in situ hybridization. Again, we saw the RANKL gene activity in the lower HF (Fig. S3B). There was no RANKL in the IFE except for some rare cells, in accord with earlier findings (22) (Fig. S3C). In anagen skin, RANK was seen in the bulge, the outer root sheath, and the IFE (Fig. 3G). Double labeling for RANK with CD34 and integrin α6, both markers for bulge cells (20, 31), validated RANK expression in the anagen bulge stem cells (Fig. S3D). Staining of OPG in anagen skin showed the protein in the bulge but not in the lower HF part (Fig. 3H). OPG can also be observed in basal keratinocytes (Fig. S3E).
Together, these findings showed that RANK, RANKL, and OPG are expressed by bulge and SHG cells. As the hair cycle progresses from telogen to anagen, OPG expression decreases but that of RANKL increases. Thus, at the telogen–anagen transition, more OPG-free RANKL is available to engage RANK and drive the HF stem cells into proliferation.
RANK Stimulation Triggers Anagen Entry.
To further address the importance of RANK activation in anagen induction, we used a mouse Tg for Rank under the control of the S100A8 gene promoter. This promoter had already been noted as active in the human HF (36) and, recently, was found transcribed in the SHG (21). Immunofluorescent labeling of telogen HFs confirmed that RANK was overexpressed in the SHG (Fig. S4A). Endogenous RANK was not seen because its visualization required signal amplification. Expression of the Tg RANK construct in HEK 293T cells showed that RANK was localized at the cell surface and that it activated NF-κB signaling as expected (37) (Fig. S4B). We also monitored RANKL and OPG production in these mice by ELISA of skin cultures from weeks 3 to 5. The Tg mice displayed an increase of RANKL, particularly during its peak production at anagen, but showed a reduced OPG level (Fig. S4C). Thus, with RANK overexpressed in the HF and a more favorable RANK-activating RANKL/OPG ratio, we expected an increased HF RANK engagement.
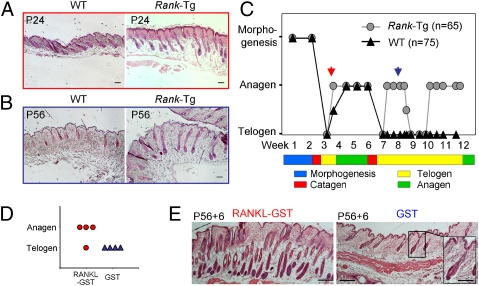
Indeed, we saw that the first anagen entry was advanced by 1 or 2 d (Fig. 4 A and C). Moreover, two rapidly evolving premature anagen cycles occurred at weeks 8 and 11, whereas control littermates were in telogen (Fig. 4 B and C). Three-month-old Tg mice showed mild alopecia (Fig. S4D), similar to other mutant mice with abnormally fast HF cycling (38).
Fig. 4.
Precocious anagen entry in Rank-Tg mice and in response to recombinant RANKL. (A) At P24, WT mice HFs are in anagen II/IIIa, whereas HFs of Rank-Tg mice are already in anagen IIIc. (Scale bars, 100 μm.) (B) At P56, WT mice HFs are in telogen, whereas HFs of Rank-Tg mice are in anagen IV/V. (Scale bars, 100 μm.) (C) Schematic representation of hair morphogenesis and the hair-cycle telogen-to-anagen transitions in Rank-Tg and control mice. Red arrow points to P24 and blue arrow points to P56, corresponding to images in A and B. (D) At 8 wk, WT mice received two s.c. injections of 100 μg of RANKL-GST or control GST with a 12-h interval. Then, 6 d later, their back skin was analyzed for anagen. Graphic representation that three of four treated mice precociously entered anagen, in contrast to control mice remaining in telogen. (E) Image of HFs in anagen of a RANKL-GST–treated mouse, whereas they are in telogen in the GST control mouse. (Scale bars, 100 μm; in Inset, 50 μm.) The paraffin-embedded sections were stained with hematoxylin/eosin.
To test whether hair renewal can be prematurely induced in WT mice by RANK stimulation, we injected recombinant RANKL s.c. into 8-wk-old mice in telogen. The functionality of the recombinant RANKL fused to the GST tag was confirmed by NF-κB signaling in RANK-expressing HEK 293T cells (Fig. S4E). Control mice received GST only. Hair-cycle analysis 6 d after injection revealed that three of four mice responded to recombinant RANKL-GST by premature anagen (Fig. 4 D and E). These findings support the importance of RANK stimulation as a critical hair-cycle entry signal.
RANKL Regulates Epidermal Homeostasis.
Because Rank-Tg animals express more soluble RANKL but less OPG in the skin, we investigated whether this change would affect the IFE. First, we verified epidermal RANK expression in the Tg mice and found that it was expressed by the basal keratinocytes (Fig. S5A). We could exclude the detection of Tg RANK because the Ab is directed against an N-terminal peptide, which is absent from the cDNA construct (Fig. S6). It was apparent that the Rank-Tg epithelium was greatly thickened (Fig. 5 A and B). In contrast, the epidermal size of the Rankl-KO mice was slightly but significantly reduced (Fig. 5 A and B). We therefore determined the epidermal renewal rate by using the thymidine analog BrdU. Proliferating BrdU+ keratinocytes were virtually absent in the Rankl-null mice but highly abundant in the IFE of the Rank-Tg mice (Fig. 5C). Quantification of IFE BrdU+ cells by cell counting on sections uncovered a significant reduction for the Rankl-KO mice and a highly significant eightfold increase for the Tg mice (Fig. 5D). We next analyzed Tg skin for signs of cell activation by measuring the release of TNFα and IL-1β. As shown in Fig. S5B, there was a small but significant increase in TNFα and IL-1β in the Tg skin at P21 and P30, respectively. However, there was no dermal CD3+ T-cell infiltration (Fig. S5C), and culturing P21 epidermal keratinocytes resulted in three times more cell recovery after 2 wk compared with WT (Fig. S5D). This observation supports a cell-autonomous enhanced proliferation in the Tg mice; however, because of the increased inflammatory cytokine production, we cannot formally exclude their contribution to the accelerated epidermal renewal. Except for keratin 14, which extended beyond its normal restriction in the Tg animals and is likely secondary to the high proliferation rate (39, 40), the differentiation of the IFE in both mouse models was normal as judged by the expression of keratin 10, loricrin, involucrin, and filaggrin (Fig. S7A). Moreover, there was no toluidine blue dye penetration (Fig. S7B), demonstrating normal skin barrier function. Finally, we treated Tg mice with anti-RANKL mAb, which, as shown in Fig. 5 E–G, resulted in a delay in the hair cycle and a significantly reduced epidermal thickness.
Fig. 5.
RANK regulates epidermal homeostasis. (A) Different epidermal thickness of skin of 4-wk-old Rankl−/−, WT, and Rank-Tg mice, visualized on fixed, paraffin-embedded sections stained with Masson's trichrome. (Scale bars, 20 μm.) (B) Epithelial thickness was measured on 10 randomly chosen images from four mice of each genotype per time point. Data are mean ± SD. (C) Identification (arrowheads) of BrdU+ proliferating IFE cells on skin sections stained with eosin. (Scale bars, 20 μm.) (D) Graph shows the number of BrdU+ IFE keratinocytes for each mouse. Data are mean ± SD. (E) Tg mice received anti-RANKL mAb or were mock-injected (control) every 3 d from P10 to P45. Skins were analyzed for HF cycle and epidermal size on sections stained with hematoxylin/eosin. (Scale bars, 50 μm.) (F) Schematic representation of the HF stage of eight treated and seven control mice. (G) Epidermal thickness measures of eight treated and seven control Tg mice. (Bars = mean values.) ep, IFE. *P < 0.05; **P < 0.01; ***P < 0.001.
Collectively, these data show that IFE homeostasis occurs normally during anagen when HF RANKL production is high, but that IFE hyperproliferates when RANKL is overproduced.
Discussion
We showed that RANK regulates hair cycling by activating its growth phase: (i) although dispensable for HF development, RANK stimulation was required for anagen to normally occur during the fourth week after birth; (ii) hair plucking, known to trigger hair renewal, failed to stimulate anagen entry in Rankl-null mice; (iii) mice with Tg RANK expression in the HF and an altered skin RANKL/OPG ratio displayed advanced hair cycles; and (iv) recombinant RANKL induced anagen in WT mice. In addition, we showed that RANK regulates IFE renewal because (i) the proliferation rate is reduced in Rankl-KO mice but (ii) it is increased in the Rank-Tg mice and (iii) blocking RANKL in Rank-Tg mice results in a delay in the hair cycle and a reduced IFE thickness.
Although RANK, RANKL, and OPG are expressed in the IFE and the HF during morphogenesis, we found no visible effect of RANKL on HF and IFE development. This finding, together with the observation that RANK signaling does not affect development of the mammary gland until pregnancy (3), suggests a role of RANK post-embryogenesis. However, embryonic generation of thymic medullary epithelial cells depends on RANKL, yet CD40L can partially compensate after birth (5), suggesting that, if RANK is active in the developing skin, it may be masked by other TNF receptor family members such as Edar or Troy (41–43). Interestingly, lymphotoxin-α—which, like RANKL, functions in lymphoid organ development—also plays a role in HF morphogenesis (44) and is a ligand for Troy (45).
RANK or RANKL were required for the anagen phase of the hair cycle. It is unlikely that the hair-cycle arrest is a reflection of a sickness of these KO mice because they thrive well beyond 6 wk without signs of weakness. Moreover, the hair-plucking experiment was performed on young animals and further demonstrates a genuine block in anagen transition. It remains to be established whether vibrissae hair undergo cycling. The inability to regenerate pelage hair was not the result of defects in the HF stem cell and mesenchymal cell compartment because these cellular elements were present. Indeed, upon transplantation onto a RANKL-proficient environment, Rankl−/− skin HFs passed into anagen. The efficiency with which nude mice rescued the hair-cycle arrest may be a reflection of the robust RANKL production by activated epidermal keratinocytes or endothelial cells during the healing process (22, 34). In addition, we have found that the anagen HF is an important source of RANKL, and, although nude mice lack hair, they have a normal hair telogen–anagen transition.
The expression of RANK, RANKL, and OPG in the HF bulge and SHG supports the importance this protein triad in the control of HF cycling. OPG localization to the bulge and the SHG corroborates previous studies, which have found Opg transcription in these cells (20, 21). Rank or Rankl transcripts were not detected in these studies, probably owing to their low abundance. Indeed, signal amplification was needed to detect RANK and RANKL protein, and soluble RANKL levels were low during telogen. This finding suggests that OPG serves as an autocrine regulator by blocking engagement of RANK by RANKL during telogen.
It is tempting to speculate that, as in the mammary gland (3, 46), HF RANKL is under hormonal control because hair growth is influenced by the endocrine system (47). However, it is unlikely that prolactin or parathyroid hormone related peptide (PTHrp) up-regulate RANKL in the HF because these stimuli antagonize hair renewal and induce catagen in mice (48, 49). However, the Rankl promoter contains a number of cell-signaling regulatory elements (NF-κB, Jun/AP-1, vitamin D, and Runx2) (46), allowing it to respond to a multitude of signals known to act on the hair cycle (17, 50, 51). Further work will be necessary to elucidate the impact of these signals on HF RANKL synthesis.
We did not detect any RANK, RANKL, or OPG expression in the DP, which is similar to mammary glands, where stromal cells failed to express RANK or RANKL even after hormonal stimulation (46). However, the mammary gland stromal cells constitutively transcribe Opg (46). it is possible that Opg is induced in the DP in response to as-yet-unidentified stimuli.
Support for the idea that RANKL and OPG synthesis is subject to changes is provided by their mRNA and protein measures during the hair cycle. It is unlikely that these changes are influenced by tissue injury after the skin excision because RNA extractions were performed immediately on the freshly collected skin, and protein levels closely reflected the mRNA measures. A closer investigation into the site of RANKL production at P28 uncovered that it was synthesized by the lower anagen HF. RT-PCR, mRNA hybridization, and immunofluorescence point to the proliferating matrix and bulb cells as its source, which would concur with RANKL production by activated keratinocytes (22).
Evidence that RANK stimulation by RANKL induces anagen is provided by the precocious anagen phases in mice overexpressing RANK in the HF and in mice administrated with recombinant RANKL. The overexpression of RANK in the SHG may be particularly effective in inducing hair cycling in view of the importance of these cells in anagen entry (21, 30). In addition, there was an increase in RANKL levels and a reduction in OPG. The underlying reasons for the changes in RANKL and OPG synthesis are currently unclear. One possibility may be that the overexpression of RANK in the HF triggers RANKL through a positive feedback loop. Such a loop, not uncommon in the TNF family, may be mediated by NF-κB.
Because the IFE basal-layer keratinocytes also express RANK, we studied the IFE for changes in Rankl-KO and Rank-Tg animals. We found that, in the Rankl−/− mice, epidermal thickness and cell growth were reduced, whereas the epidermis was thickened and cell proliferation was greatly accelerated in the Rank-Tg mice. In neither model did we find a marked defect in epidermal differentiation or barrier function, demonstrating that RANK has little impact on this process. The extended keratin 14 expression to suprabasal layers in the Tg animals is likely owing to the hyperproliferation because suprabasal keratin 14 expression is also seen in other mouse models of accelerated epidermal growth (39, 40). We could detect an increase in TNFα and IL-1β production from Tg skin; however, more keratinocytes were recovered after a 15-d culture of primary cutaneous keratinocytes from Tg mice, which suggests a cell-autonomous enhanced keratinocyte proliferation. More work is needed to further clarify the role of these cytokines on keratinocyte activation.
The underlying molecular mechanism for epithelial activation of the epidermo-pilosebaceous unit by RANK is currently unclear. Because RANK is a potent NF-κB stimulator and because NF-κB has been implicated in the regulation of HF cycling (52) as well as in epidermal turnover (53), we had first postulated that RANK triggers the NF-κB pathway in skin epithelial cells. However, we could not obtain any evidence for NF-κB activation either in the HF or in the IFE. RANK has also been shown to engage the signaling cascades, implicating JNK, Id-2, Akt, and NFATc1 (54), but, again, we could not observe any evidence that these signaling flows were elicited. Moreover, mice deficient in TRAF6, a signaling module of the NF-κB and JNK pathway, display a phenotype similar to that of Eda-null mice but not of Rankl−/− animals (55). Therefore, the molecular signaling pathways engaged by RANK in the HF and the IFE remain to be uncovered. Our findings, together with the cyclic renewal of the HF on one side and the continuous homeostasis of the IFE on the other, provide a valuable model system to dissect the signaling pathways triggered by RANK in epithelial (stem) cells.
The herein revealed function of RANK in murine hair-cycle control and epidermal homeostasis further underpins the shared molecular controls of two seemingly distant organs, skin and bone (18), and introduces the RANK–RANKL–OPG triad as a key player in the control of epithelial stem cell biology.
Materials and Methods
Animals.
PCR primers for genotyping are listed in Table S1.
Histology/Immunofluorescence.
Primary Abs are listed in Table S2.
RT-PCR.
Classic and real-time PCR primers are listed in Table S3. Detailed experimental protocols for cell proliferation, ELISA, flow cytometry, in situ hybridization, RANK signaling assay, skin cytokine production, primary keratinocyte culture, and epidermal barrier tests are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Graham Anderson for Rank−/− skin samples and Raphael Doineau and Benjamin Voisin for technical help. We appreciate critical reading of the manuscript by Viljar Jaks, Maria Kasper, Ruth Schmidt-Ullirch, and Katja Fink. This work was supported by Centre National de la Recherche Scientifique (CNRS), University of Strasbourg, Société de Recherche en Dermatologie (C.G.M.); a grant from Deutsche Forschungsgemeinschaft (to R.P.); a German Academic Exchange Service (DAAD) short-term stipend (to V.D.); and La Ligue Contre le Cancer (E.H.). V.D. and E.H. were recipients of CNRS fellowships Bourses de Doctorat pour Ingénieur (CNRS-BDI) and Ministère de la Recherche et de la Technologie (CNRS-MRT), respectively.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1013054108/-/DCSupplemental.
References
- 1.Walsh MC, Choi Y. Biology of the TRANCE axis. Cytokine Growth Factor Rev. 2003;14:251–263. doi: 10.1016/s1359-6101(03)00027-3. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka S, Nakamura K, Takahasi N, Suda T. Role of RANKL in physiological and pathological bone resorption and therapeutics targeting the RANKL–RANK signaling system. Immunol Rev. 2005;208:30–49. doi: 10.1111/j.0105-2896.2005.00327.x. [DOI] [PubMed] [Google Scholar]
- 3.Fata JE, et al. The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell. 2000;103:41–50. doi: 10.1016/s0092-8674(00)00103-3. [DOI] [PubMed] [Google Scholar]
- 4.Beleut M, et al. Two distinct mechanisms underlie progesterone-induced proliferation in the mammary gland. Proc Natl Acad Sci USA. 2010;107:2989–2994. doi: 10.1073/pnas.0915148107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akiyama T, et al. The tumor necrosis factor family receptors RANK and CD40 cooperatively establish the thymic medullary microenvironment and self-tolerance. Immunity. 2008;29:423–437. doi: 10.1016/j.immuni.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 6.Knoop KA, et al. RANKL is necessary and sufficient to initiate development of antigen-sampling M cells in the intestinal epithelium. J Immunol. 2009;183:5738–5747. doi: 10.4049/jimmunol.0901563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo JL, et al. Nuclear cytokine-activated IKKα controls prostate cancer metastasis by repressing Maspin. Nature. 2007;446:690–694. doi: 10.1038/nature05656. [DOI] [PubMed] [Google Scholar]
- 8.Schramek D, et al. Osteoclast differentiation factor RANKL controls development of progestin-driven mammary cancer. Nature. 2010;468:98–102. doi: 10.1038/nature09387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez-Suarez E, et al. RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature. 2010;468:103–107. doi: 10.1038/nature09495. [DOI] [PubMed] [Google Scholar]
- 10.Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999;341:491–497. doi: 10.1056/NEJM199908123410706. [DOI] [PubMed] [Google Scholar]
- 11.Cotsarelis G. Epithelial stem cells: A folliculocentric view. J Invest Dermatol. 2006;126:1459–1468. doi: 10.1038/sj.jid.5700376. [DOI] [PubMed] [Google Scholar]
- 12.Fuchs E. Scratching the surface of skin development. Nature. 2007;445:834–842. doi: 10.1038/nature05659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blanpain C, Horsley V, Fuchs E. Epithelial stem cells: Turning over new leaves. Cell. 2007;128:445–458. doi: 10.1016/j.cell.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watt FM, Hogan BL. Out of Eden: Stem cells and their niches. Science. 2000;287:1427–1430. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- 15.Morgan BA. A glorious revolution in stem cell biology. Nat Genet. 2008;40:1269–1270. doi: 10.1038/ng1108-1269. [DOI] [PubMed] [Google Scholar]
- 16.Plikus MV, Widelitz RB, Maxson R, Chuong CM. Analyses of regenerative wave patterns in adult hair follicle populations reveal macro-environmental regulation of stem cell activity. Int J Dev Biol. 2009;53:857–868. doi: 10.1387/ijdb.072564mp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider MR, Schmidt-Ullrich R, Paus R. The hair follicle as a dynamic miniorgan. Curr Biol. 2009;19:R132–R142. doi: 10.1016/j.cub.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Ross FP, Christiano AM. Nothing but skin and bone. J Clin Invest. 2006;116:1140–1149. doi: 10.1172/JCI28605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kong YY, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 20.Morris RJ, et al. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- 21.Greco V, et al. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell. 2009;4:155–169. doi: 10.1016/j.stem.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loser K, et al. Epidermal RANKL controls regulatory T-cell numbers via activation of dendritic cells. Nat Med. 2006;12:1372–1379. doi: 10.1038/nm1518. [DOI] [PubMed] [Google Scholar]
- 23.Barbaroux JB, Beleut M, Brisken C, Mueller CG, Groves RW. Epidermal receptor activator of NF-κB ligand controls Langerhans cells numbers and proliferation. J Immunol. 2008;181:1103–1108. doi: 10.4049/jimmunol.181.2.1103. [DOI] [PubMed] [Google Scholar]
- 24.Kartsogiannis V, et al. Localization of RANKL (receptor activator of NFκB ligand) mRNA and protein in skeletal and extraskeletal tissues. Bone. 1999;25:525–534. doi: 10.1016/s8756-3282(99)00214-8. [DOI] [PubMed] [Google Scholar]
- 25.Paus R, et al. A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J Invest Dermatol. 1999;113:523–532. doi: 10.1046/j.1523-1747.1999.00740.x. [DOI] [PubMed] [Google Scholar]
- 26.Müller-Röver S, et al. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol. 2001;117:3–15. doi: 10.1046/j.0022-202x.2001.01377.x. [DOI] [PubMed] [Google Scholar]
- 27.Bossen C, et al. Interactions of tumor necrosis factor (TNF) and TNF receptor family members in the mouse and human. J Biol Chem. 2006;281:13964–13971. doi: 10.1074/jbc.M601553200. [DOI] [PubMed] [Google Scholar]
- 28.Wilson C, et al. Cells within the bulge region of mouse hair follicle transiently proliferate during early anagen: Heterogeneity and functional differences of various hair cycles. Differentiation. 1994;55:127–136. doi: 10.1046/j.1432-0436.1994.5520127.x. [DOI] [PubMed] [Google Scholar]
- 29.Panteleyev AA, Jahoda CA, Christiano AM. Hair follicle predetermination. J Cell Sci. 2001;114:3419–3431. doi: 10.1242/jcs.114.19.3419. [DOI] [PubMed] [Google Scholar]
- 30.Jaks V, et al. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet. 2008;40:1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- 31.Tumbar T, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jahoda CA, Reynolds AJ. Dermal-epidermal interactions. Adult follicle-derived cell populations and hair growth. Dermatol Clin. 1996;14:573–583. doi: 10.1016/s0733-8635(05)70385-5. [DOI] [PubMed] [Google Scholar]
- 33.Handjiski BK, Eichmüller S, Hofmann U, Czarnetzki BM, Paus R. Alkaline phosphatase activity and localization during the murine hair cycle. Br J Dermatol. 1994;131:303–310. doi: 10.1111/j.1365-2133.1994.tb08515.x. [DOI] [PubMed] [Google Scholar]
- 34.Collin-Osdoby P, et al. Receptor activator of NF-κB and osteoprotegerin expression by human microvascular endothelial cells, regulation by inflammatory cytokines, and role in human osteoclastogenesis. J Biol Chem. 2001;276:20659–20672. doi: 10.1074/jbc.M010153200. [DOI] [PubMed] [Google Scholar]
- 35.Kaufman CK, et al. GATA-3: An unexpected regulator of cell lineage determination in skin. Genes Dev. 2003;17:2108–2122. doi: 10.1101/gad.1115203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidt M, et al. Selective expression of calcium-binding proteins S100a8 and S100a9 at distinct sites of hair follicles. J Invest Dermatol. 2001;117:748–750. doi: 10.1046/j.0022-202x.2001.01485.x. [DOI] [PubMed] [Google Scholar]
- 37.Anderson DM, et al. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175–179. doi: 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- 38.Mecklenburg L, Tobin DJ, Cirlan MV, Craciun C, Paus R. Premature termination of hair follicle morphogenesis and accelerated hair follicle cycling in Iasi congenital atrichia (fzica) mice points to fuzzy as a key element of hair cycle control. Exp Dermatol. 2005;14:561–570. doi: 10.1111/j.0906-6705.2005.00343.x. [DOI] [PubMed] [Google Scholar]
- 39.Hobbs RM, Silva-Vargas V, Groves R, Watt FM. Expression of activated MEK1 in differentiating epidermal cells is sufficient to generate hyperproliferative and inflammatory skin lesions. J Invest Dermatol. 2004;123:503–515. doi: 10.1111/j.0022-202X.2004.23225.x. [DOI] [PubMed] [Google Scholar]
- 40.Pasparakis M, et al. TNF-mediated inflammatory skin disease in mice with epidermis-specific deletion of IKK2. Nature. 2002;417:861–866. doi: 10.1038/nature00820. [DOI] [PubMed] [Google Scholar]
- 41.Mikkola ML. TNF superfamily in skin appendage development. Cytokine Growth Factor Rev. 2008;19:219–230. doi: 10.1016/j.cytogfr.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt-Ullrich R, et al. NF-κB transmits Eda A1/EdaR signalling to activate Shh and cyclin D1 expression, and controls post-initiation hair placode down growth. Development. 2006;133:1045–1057. doi: 10.1242/dev.02278. [DOI] [PubMed] [Google Scholar]
- 43.Pispa J, Pummila M, Barker PA, Thesleff I, Mikkola ML. Edar and Troy signalling pathways act redundantly to regulate initiation of hair follicle development. Hum Mol Genet. 2008;17:3380–3391. doi: 10.1093/hmg/ddn232. [DOI] [PubMed] [Google Scholar]
- 44.Cui CY, et al. Ectodysplasin regulates the lymphotoxin-β pathway for hair differentiation. Proc Natl Acad Sci USA. 2006;103:9142–9147. doi: 10.1073/pnas.0509678103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hashimoto T, Schlessinger D, Cui CY. Troy binding to lymphotoxin-α activates NFκB mediated transcription. Cell Cycle. 2008;7:106–111. doi: 10.4161/cc.7.1.5135. [DOI] [PubMed] [Google Scholar]
- 46.Srivastava S, et al. Receptor activator of NF-κB ligand induction via Jak2 and Stat5a in mammary epithelial cells. J Biol Chem. 2003;278:46171–46178. doi: 10.1074/jbc.M308545200. [DOI] [PubMed] [Google Scholar]
- 47.Randall VA. Hormonal regulation of hair follicles exhibits a biological paradox. Semin Cell Dev Biol. 2007;18:274–285. doi: 10.1016/j.semcdb.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 48.Schilli MB, Ray S, Paus R, Obi-Tabot E, Holick MF. Control of hair growth with parathyroid hormone (7-34) J Invest Dermatol. 1997;108:928–932. doi: 10.1111/1523-1747.ep12294690. [DOI] [PubMed] [Google Scholar]
- 49.Foitzik K, et al. Prolactin and its receptor are expressed in murine hair follicle epithelium, show hair cycle-dependent expression, and induce catagen. Am J Pathol. 2003;162:1611–1621. doi: 10.1016/S0002-9440(10)64295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li M, et al. Skin abnormalities generated by temporally controlled RXRα mutations in mouse epidermis. Nature. 2000;407:633–636. doi: 10.1038/35036595. [DOI] [PubMed] [Google Scholar]
- 51.Demay MB, et al. Role of the vitamin D receptor in hair follicle biology. J Steroid Biochem Mol Biol. 2007;103:344–346. doi: 10.1016/j.jsbmb.2006.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y, et al. Reciprocal requirements for EDA/EDAR/NF-κB and Wnt/β-catenin signaling pathways in hair follicle induction. Dev Cell. 2009;17:49–61. doi: 10.1016/j.devcel.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pasparakis M. Regulation of tissue homeostasis by NF-κB signalling: Implications for inflammatory diseases. Nat Rev Immunol. 2009;9:778–788. doi: 10.1038/nri2655. [DOI] [PubMed] [Google Scholar]
- 54.Wada T, Nakashima T, Hiroshi N, Penninger JM. RANKL–RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med. 2006;12:17–25. doi: 10.1016/j.molmed.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 55.Naito A, et al. TRAF6-deficient mice display hypohidrotic ectodermal dysplasia. Proc Natl Acad Sci USA. 2002;99:8766–8771. doi: 10.1073/pnas.132636999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.