Abstract
In human disorders, the genotype-phenotype relationships are often complex and influenced by genetic and/or environmental factors. Wilson disease (WD) is a monogenic disorder caused by mutations in the copper-transporting P-type ATPase ATP7B. WD shows significant phenotypic diversity even in patients carrying identical mutations; the basis for such diverse manifestations is unknown. We demonstrate that the 2623A/G polymorphism (producing the Gly875→Arg substitution in the A-domain of ATP7B) drastically alters the intracellular properties of ATP7B, whereas copper reverses the effects. Under basal conditions, the common Gly875 variant of ATP7B is targeted to the trans-Golgi network (TGN) and transports copper into the TGN lumen. In contrast, the Arg875 variant is located in the endoplasmic reticulum (ER) and does not deliver copper to the TGN. Elevated copper corrects the ATP7B-Arg875 phenotype. Addition of only 0.5–5 μM copper triggers the exit of ATP7B-Arg875 from the ER and restores copper delivery to the TGN. Analysis of the recombinant A-domains by NMR suggests that the ER retention of ATP7B-Arg875 is attributable to increased unfolding of the Arg875-containing A-domain. Copper is not required for the folding of ATP7B-Arg875 during biosynthesis, but it stabilizes protein and stimulates its activity. A chemotherapeutical drug, cisplatin, that mimics a copper-bound state of ATP7B also corrects the “disease-like” phenotype of ATP7B-Arg875 and promotes its TGN targeting and transport function. We conclude that in populations harboring the Arg875 polymorphism, the levels of bioavailable copper may play a vital role in the manifestations of WD.
Keywords: trafficking, cisplatin, phenotypic variability
Wilson disease (WD) is an autosomal recessive disorder, caused by mutations in the copper-transporting ATPase ATP7B. WD manifests as hepatolenticular degeneration associated with copper accumulation in the liver and other organs (1). Although WD follows a simple Mendelian pattern of inheritance (monogenic disorder), the phenotypic manifestations of WD are diverse and strong genotype-phenotype correlations have not yet been found. Different severity of the disease and a variable time of onset for the same causative mutations suggest involvement of additional genetic and/or environmental factors (2). Furthermore, several ATP7B mutations have a dual designation as a “disease variant” and “nondisease variant” depending on the population in which they were identified. One such substitution is Gly875→Arg (c2623A/G) within the A-domain of ATP7B (Fig. S1A).
Arg at position 875 was originally detected in the ATP7B sequence generated using the cDNA library from the brain of a patient with a neurological (non-WD) disease (3). Parallel studies using a human kidney cDNA library identified Gly at this position (4); Gly is also conserved in ATP7B orthologs (Fig. S1B). In vitro, both the Arg- and Gly-ATP7B variants hydrolyze ATP with the formation of a phosphorylated intermediate (5, 6). This observation is consistent with the designation of c2623A/G as a polymorphism, which was made in genetic studies of the Han Chinese population (7). In the Indian population, however, Arg875 is a WD-causing mutation (8). To identify reasons for this dual behavior, we investigated the intracellular targeting and activity of ATP7B-Arg875 under various conditions. We demonstrate that unlike the common Gly variant, ATP7B-Arg875 is exquisitely sensitive to variations in available copper levels, which modulate its properties. We also found that a well-known chemotherapeutical drug, cisplatin (DDP), can mimic effects of copper and improve the “disease-like” phenotype.
Results
ATP7B-Arg875 Does Not Show Transport Activity Under Basal Conditions.
The physiological function of human copper-ATPases is to transport copper into the trans-Golgi network (TGN) for biosynthetic incorporation into copper-dependent enzymes, such as ceruloplasmin, tyrosinase, or dopamine-β-hydroxylase. To evaluate the transport activity of ATP7B-Arg875, we used Menkes fibroblasts transfected with tyrosinase (YSTT cells). These cells lack endogenous copper-ATPase and are unable to transport copper into the TGN, thus producing inactive tyrosinase (9, 10). The expression of ATP7B-Gly875 in YSTT cells restores copper delivery to the TGN and activates tyrosinase, as evident from the formation of black pigment [levo-3,4-dihydroxy-l-phenylalanine (l-DOPA) quinone]. In contrast, ATP7B-Arg875 does not activate tyrosinase (Fig. 1A) even though ATP7B-Arg875 is expressed in cells (Fig. 1B). Thus, under standard conditions, ATP7B-Arg875 does not transport copper to the TGN (i.e., behaves as a disease variant).
Fig. 1.
Transport activity and expression of ATP7B-Arg875 and ATP7B-Gly875 in YSTT cells. (A) Tyrosinase assay under basal copper conditions shows pigment formation for ATP7B-Gly875 and no pigment for ATP7B-Arg875. (B) Immunostaining illustrates that both variants are expressed. (Scale bar: 14 μm.)
ATP7B-Arg875 Is Mislocalized Under Basal Conditions but Traffics Normally in Response to Copper.
Normally, ATP7B resides in the TGN but traffics in response to changing copper levels. In elevated copper, ATP7B moves to vesicles to sequester excess copper, whereas copper depletion induces the return of ATP7B from vesicles to the TGN (11, 12). Therefore, the lack of copper delivery to the TGN could be attributable to either the retention of ATP7B-Arg875 in the endoplasmic reticulum (ER) or its inability to return to the TGN from vesicles. To discriminate between these scenarios, we characterized the ATP7B-Arg875 localization at different copper concentrations. (To minimize potential artifacts associated with protein overexpression, we used tet-regulated expression in Hek293Trex cells with 20 ng/mL doxycycline and expression time optimized before the experiments).
Control ATP7B-Gly875 showed expected TGN staining (Fig. 2A). In contrast, the ATP7B-Arg875 had a perinuclear ER-like pattern. Costaining with the ER marker calnexin and the TGN marker TGN38 confirmed that ATP7B-Arg875 was retained in the ER and was absent in the TGN (Fig. 2B). Unexpectedly, despite the loss of TGN targeting, the trafficking behavior of ATP7B-Arg875 appeared normal [i.e., in response to high (100 μM) copper, ATP7B-Arg875 relocalized to vesicles]. Furthermore, once in vesicles, ATP7B-Arg875 could cycle back upon copper depletion with the copper chelator bathocuproine disulfonate (BCS) (Fig. 2C). Following this two-step (copper→BCS) treatment, ATP7B-Arg875 acquired proper TGN targeting instead of returning to its initial ER location. [Without copper pretreatment, BCS (bathocuproine disulfonic acid disodium salt) did not induce TGN targeting.] Thus, once ATP7B-Arg875 escaped the ER, it was targeted normally.
Fig. 2.
Intracellular localization of ATP7B-Arg875 is copper-dependent. (A) Immunostaining with anti-Flag antibody (green) illustrates the marked difference in the localization patterns of the Flag-tagged ATP7B-Arg875 (diffuse ER-like) and ATP7B-Gly875 (TGN-like) in basal medium. (B) (Upper) Costaining with the ER marker calnexin (red) and anti-Flag antibody (green) shows ER localization for ATP7B-Arg875. (Lower) No colocalization is observed with the TGN marker (red). CNX, calnexin. (C) (Left) Following treatment with 100 or 200 μM copper (shown), ATP7B-Arg875 redistributes to vesicles. (Right) Subsequent treatment with 50 μM BCS causes the relocalization of ATP7B to the TGN. (Scale bar: 7 μm.)
Physiological Copper Levels Promote ATP7B-Arg875 Exit from the ER and Restore Copper Delivery to the TGN.
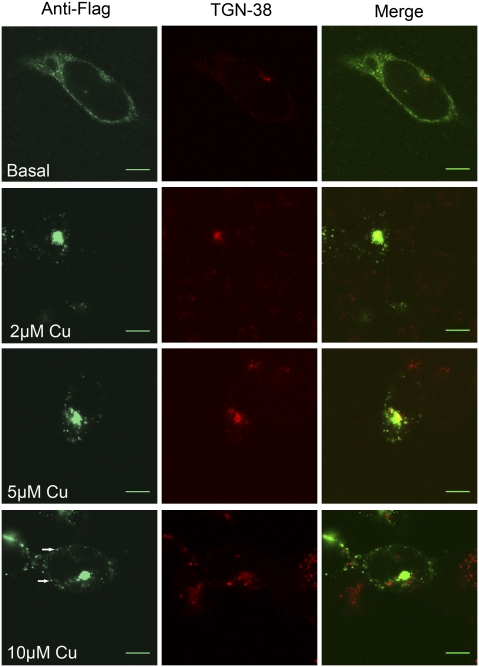
The trafficking of ATP7B-Arg875 from the ER to vesicles in response to high copper proceeds, presumably, via the TGN. This consideration led us to investigate whether low concentrations of copper would trigger ATP7B-Arg875 exit from the ER to the TGN. Overnight treatment of cells with 2 or 5 μM copper resulted in loss of the ER pattern and colocalization of ATP7B-Arg875 with TGN38 (Fig. 3). Even at 0.5 μM copper, partial TGN localization was observed. At 10 μM copper, in addition to TGN, ATP7B-Arg875 was seen in vesicles (i.e., it showed an expected trafficking response to increasing copper). [Treatment with 50–100 μM zinc did not alter ATP7B-Arg875 localization in the ER (Fig. S2)].
Fig. 3.
Low copper (Cu) promotes ATP7B-Arg875 exit from the ER. Costaining with anti-Flag (green) and TGN38 (red) antibodies was performed following overnight incubation of Hek293Trex cells with 0–10 μM copper. At 2 μM copper, ATP7B-Arg875 is found predominantly in the TGN, whereas at 10 μM copper, additional vesicular localization (arrows) becomes apparent. (Scale bar: 7 μm.)
Because 2–5 μM copper promoted normal TGN localization of ATP7B-Arg875, we were interested whether the same treatment would restore copper transport to the TGN. As in Hek293Trex cells, overnight treatment of YSTT cells with 0.5–10 μM copper resulted in the TGN targeting of Arg875-ATP7B (shown for 5 μM copper in Fig. 4A). The relocalization was coupled with the appearance of black pigment, indicative of tyrosinase activation (Fig. 4B). This activation was not attributable to nonspecific copper entry into the TGN, because cells treated with the same copper concentrations showed no tyrosinase activity in the absence of ATP7B-Arg875. Thus, once the Arg875 variant reaches the TGN, it transports copper to the cuproenzyme(s).
Fig. 4.
Relocalization to the TGN restores copper transport activity of ATP7B-Arg875. (A) Colocalization with TGN38 (red) staining confirms efficient targeting of Flag-ATP7B-Arg875 (green) to the TGN in the YSTT cells following treatment with 5 μM copper. (Scale bar: 14 μm.) (B) In the tyrosinase assay, pigment formation is observed in cells transfected with ATP7B-Arg875 and treated with 5 μM copper; control cells treated with 5 μM copper show no color.
Gly875→Arg Substitution Decreases Stability of the A-Domain.
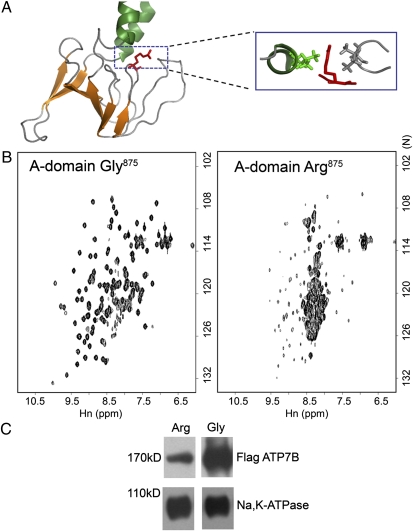
To understand the mechanism behind the copper-dependent behavior of ATP7B-Arg875, we first examined the consequences of Gly/Arg875 substitution on protein structure. In the A-domain structure (13), Gly875 is located in a flexible and partially exposed loop. The side chain of Arg in this position can be accommodated but may have negative effects because of its proximity to neighboring residues (Fig. 5A). To determine whether the Gly→Arg substitution alters the A-domain structure, we generated the recombinant A-domains with corresponding substitutions. Both domains showed robust expression in Escherichia coli, were soluble, and purified in milligram quantities, although the average yield of the Arg875 variant was lower (Fig. S3). The folding of the domains was examined by solution NMR at equal protein concentrations. The 1H,15N-HSQC (heteronuclear single quantum coherence) spectrum of the Gly875 A-domain showed excellent chemical shift dispersion, characteristic of a well-folded protein (Fig. 5B). In contrast, the Arg875 A-domain had poor signal dispersion indicative of the loss of structure. Thus, Arg875 promotes unfolding of the isolated A-domain. To evaluate the effect of Arg875 on the full-length ATP7B, we expressed ATP7B-Gly875 and ATP7B-Arg875 in HEK293Trex cells and compared protein amounts by Western blot analysis. In crude cell lysates, the ATP7B-Arg875 was consistently less abundant than the Gly variant. The difference became particularly pronounced after the isolation of microsomal membranes (Fig. 5C).
Fig. 5.
Unfolding of the A-domain correlates with the decreased levels of ATP7B-Arg875 in cells. (A) Structure of the A-domain (13) with Arg replacing Gly875 (red). The box shows the position of the Arg side chain with respect to neighboring residues (green and gray). (B) 1H,15N-HSQC spectra of the Gly875 or Arg875-containing A-domain at 27 °C. (C) Western blot of membrane preparations from HEK293Trex cells transfected with the Flag-ATP7B Gly875 or Arg875 variant. Staining for anti-FLAG antibody (Upper) and for Na,K-ATPase (Lower), used as a loading control, is shown.
A-Domain Interacts with the N-Terminal Copper-Binding Domain.
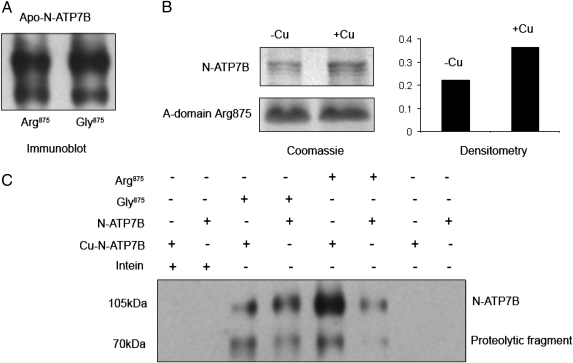
The A-domain does not bind copper; hence, to understand the effect of copper on the A-domain, we examined whether the A-domain interacted with the N-terminal copper-binding domain of ATP7B (N-ATP7B). By copurifying the recombinant N-ATP7B and the A-domain intein fusion from mixed cell lysates, we observed copurification indicative of interdomain interactions. No copurification was detected for intein alone, which was used as a control (Fig. 6). Apo-N-ATP7B interacted equally with the Gly875 and Arg875 variants, suggesting that before purification (or because of the presence of N-ATP7B), the Arg875 A-domain retained sufficient structure (Fig. 6A). That the two variants differed in their properties became apparent in experiments with a copper-bound form of N-ATP7B. Copper binding had no effect on N-ATP7B interactions with the Gly875 A-domain, whereas interaction with the Arg875 variant was noticeably increased (Fig. 6 B and C).
Fig. 6.
Interactions between the A-domain variants and the N-terminal copper-binding domain (N-ATP7B). The A-domain (Arg875 and Gly875) intein fusions and the N-ATP7B maltose-binding fusion were expressed in E. coli; cell lysates were then mixed, and proteins were copurified using chitin beads (for intein binding). After 16 column volume washes, bound proteins were eluted by cleaving the A-domain from the intein fusion using MESNA. The composition of eluates was then analyzed. (A) Western blotting using an anti-N-ATP7B antibody shows that apo-N-ATP7B is present in the eluates of both the Arg875 and Gly875 A-domains in comparable quantities. (B) Holoform of N-ATP7B was generated by growing cells in the presence of copper, and the binding of apo-N-ATP7B (−Cu) and holo-N-ATP7B (+Cu) to the Arg875 A-domain were compared, as above. The copper-bound N-ATP7B interacted more strongly with the Arg875 A-domain compared with the apo-N-ATP7B. (Left) Coomassie-stained gel showing equal loading of the Arg875 A-domain (Lower) and, in the same gel, a larger amount of copurified N-ATP7B in a (+Cu) form (Upper). (Right) Quantification of differences between the eluted N-ATP7B (−Cu) and (+Cu) by densitometry. (C) These results were verified by Western blot analysis in experiments with additional controls. When intein was used as bait, no binding of either apo- or copper-bound N-ATP7B was detected. Similarly, N-ATP7B maltose-binding fusion alone did not bind to chitin beads. As in A, similar interaction was observed for Arg875 and Gly875 with the apo-N-ATP7B. Similar to B, the interaction of the A-domain Arg875 (but not Gly875) is higher for the copper-bound N-ATP7B.
Extra Copper Is Not Required for ATP7B-Arg875 Biosynthesis but Stabilizes Folded Protein.
The above experiments suggested that copper influences ATP7B-Arg875 properties by facilitating interdomain interactions. Consequently, we examined whether extra copper was needed during ATP7B-Arg875 biosynthesis or if it stabilized an already folded protein, allowing its exit from the ER. ATP7B-Arg875 was expressed in basal copper, and protein synthesis was then blocked with cycloheximide (CHX). Subsequent treatment with 5 μM copper resulted in relocalization of ATP7B-Arg875 to the TGN (Fig. S4). Thus, during biosynthesis, ATP7B-Arg875 was folded sufficiently well and extra copper simply stabilized it in a form that could pass ER quality control.
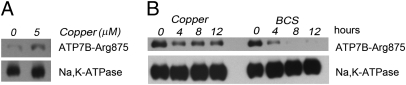
Overnight treatment with 5 μM copper also increases ATP7B-Arg875 amounts by approximately threefold (Fig. 7A). [Copper had no effect on ATP7B-Gly875 level (Fig. S5)]. To investigate whether the increase in ATP7B-Arg875 abundance was solely attributable to protein escaping the ER-associated degradation or to general protein stabilization, we examined ATP7B-Arg875 stability after it reached the TGN. Following relocation of ATP7B-Arg875 to the TGN, cells were placed in medium with CHX and treated with either BCS or copper for different periods of time (0–12 h). The ATP7B-Arg875 levels were measured in membrane preparations at each time point. After an initial decrease in protein levels at 4 h (common for both conditions), further decrease was evident for ATP7B-Arg875 from the BCS-treated cells, whereas no significant change was observed in the copper-treated samples (Fig. 7B). Thus, copper stabilizes ATP7B-Arg875 even after it leaves the ER.
Fig. 7.
Copper increases ATP7B-Arg875 abundance and diminishes its post-ER degradation. (A) Western blots of membrane preparations from HEK293Trex cells transfected with Flag-ATP7B-Arg875 and treated overnight with or without 5 μM copper. (B) ATP7B-Arg875 was first targeted to the TGN by overnight treatment with copper; cells were then transferred to fresh medium with 5 μM copper or 50 μM copper chelator BCS, and protein levels were measured over time in the presence of CHX. Staining of Na,K-ATPase is used as a loading control.
Bioavailable Copper Is Essential for ATP7B-Arg875 Rescue.
WD is a disease of copper accumulation. One may expect that high cellular copper would stabilize ATP7B-Arg875, precluding abnormal behavior, except that extra copper may not be easily available to ATP7B because of up-regulation of the endogenous high-affinity copper chelator metallothionein (14). To clarify this issue, we used Menkes fibroblasts, which, similar to WD liver, accumulate copper on prolonged exposure and up-regulate metallothionein (15). We first compared copper levels (by atomic absorption spectroscopy) in Menkes fibroblasts grown in basal medium (when ATP7B-Arg875 is targeted to the ER) after treatment for 4 h with 5 μM copper (when ATP7B traffics to the TGN) and after treating cells for 24 h with 50–100 μM copper [to overload cells with copper and induce metallothionein (15)]. We then transfected copper-loaded cells with ATP7B-Arg875 using our standard protocol and characterized its intracellular localization. Although total copper was higher in the overloaded cells compared with cells in basal medium or cells treated with low copper (Fig. S6A), ATP7B-Arg875 was localized in the ER (Fig. S6B). Thus, bioavailable copper, rather than accumulated copper, rescues ATP7B-Arg875.
Chemotherapeutical Drug DDP Facilitates TGN Targeting of ATP7B-Arg875.
Finally, we tested whether drugs that mimic the effect of copper on ATP7B can influence ATP7B-Arg875 localization and activity. We have previously found that a chemotherapeutical drug, DDP, binds to N-ATP7B (16, 17) and stabilizes the copper-bound–like state of ATP7B without competing with copper for transport sites (5). Therefore, we examined whether DPP would trigger ATP7B-Arg875 trafficking to the TGN and restore copper delivery to tyrosinase in the absence of copper additions.
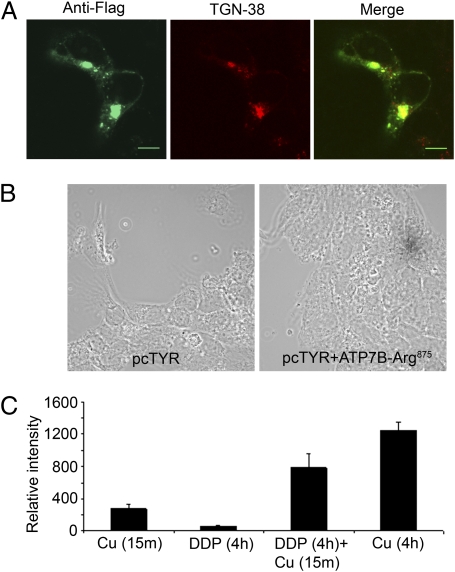
Treatment with 10 μM DDP for 4 h induced ATP7B-Arg875 trafficking to the TGN (Fig. 8A) and activated tyrosinase (Fig. 8B). Fewer cells showed pigment formation, however, and the pigment color intensity was lower compared with cells treated with copper (Fig. 4B). To examine whether the lower transport activity was attributable to the low affinity of ATP7B-Arg875 for basal copper, cells were first treated with DPP to relocalize ATP7B-Arg875 to the TGN and then briefly (for 15 min) exposed to copper to stimulate transport (without altering protein amounts). In this case, the pigment intensity and the number of cells showing pigment formation were significantly higher than in cells exposed only to DDP (4 h). This observation is consistent with ATP7B-Arg875 requiring extra copper for its transport activity. At the same time, the color intensity, although increased, still did not reach that of cells exposed to copper for longer times (4 h, when ATP7B is protected against degradation) (Fig. 8C). Thus, the full transport activity of ATP7B-Arg875 also requires protein stabilization by copper.
Fig. 8.
DDP partially mimics the effects of copper (Cu). (A) Colocalization of Flag-ATP7B-Arg875 (green) and TGN marker (red) in YSTT cells treated with 10 μM DDP for 4 h. (Scale bar: 7 μm.) (B) In the tyrosinase assay, pigment formation was observed in DDP-treated cells transfected with ATP7B-Arg875. (C) Comparison of the color intensity of pigment for cells transfected with ATP7B-Arg875 and treated with copper (15 min), DDP (4 h), DDP (4 h) plus copper (15 min), and copper (4 h). Pigment intensity was quantified using Image J software (National Institutes of Health); the effect of dual-DDP/copper treatment exceeds the additive.
Discussion
The Arg875 variant of ATP7B was previously found to be a disease-causing mutation in patients with WD as well as a polymorphism in the normal population. We demonstrate that cellular localization, stability, and activity of ATP7B-Arg875 greatly depend on available copper. At a basal copper level, Arg875-ATP7B resides in the ER and shows no copper delivery to the TGN-located enzymes, whereas when more copper is available, it traffics to the TGN, restoring copper delivery. Thus, copper fluctuations may greatly influence the phenotype of this variant. In human populations, individuals heterozygous for WD mutations on a common Gly875 background typically do not show disease manifestations. Our results suggest that individuals with the same mutation on the Arg875 background may have the WD phenotype under certain conditions, such as copper deficiency. Additionally, patients homozygous for the WD mutation in combination with the Arg875 background may have a more severe disease phenotype or an earlier onset.
We demonstrate that the recombinant A-domain with Arg875 is unstable and unfolds during purification, likely because of the unfavorable effects of a bulky Arg side chain on neighboring residues. In the full-length ATP7B, interactions with other domains prevent complete protein unfolding (as evident from the preservation of ATP7B trafficking and activity). Nevertheless, under basal conditions, imperfections of the ATP7B-Arg875 structure are detected by the ER quality control, causing protein retention. Defects in protein folding have been reported for numerous human disorders, and there is a significant need for identifying factors that can enhance protein folding and/or stability (18). We demonstrate that in the case of ATP7B-Arg875, the native ligand copper markedly improves the transporter's properties.
How does elevated copper correct the Arg875 phenotype? We observed that the Arg875 A-domain interacted with N-ATP7B and that copper binding to N-ATP7B enhanced this interaction. Although the structural basis for this increase is not yet known, higher stability of ATP7B-Arg875 in copper-treated cells suggests that interdomain interactions impart extra rigidity to the protein. In vitro, copper binding to copper-ATPases stabilizes a distinct protein conformation (5, 19). Thus, we speculate that copper-induced restriction of conformational mobility allows the ATP7B-Arg875 to exit from the ER, the initial key step in overcoming the disease phenotype. DDP, which binds to N-ATP7B and stabilizes ATP7B in a conformation resembling the copper-bound form (5), mimics the effects of copper.
In summary, our studies reveal how genetic and nongenetic factors can work together in determining the WD phenotype. DDP-mediated rescue of ATP7B-Arg875 encourages the search for drugs that stabilize ATP7B and improve protein targeting and function.
Materials and Methods
Additional details are provided in SI Materials and Methods.
Cell Lines and Microscopy.
Hek293TREx cells were grown in DMEM supplemented with 10% (vol/vol) FBS, 15 μg/mL blasticidin, and 100 μg/mL zeocin; protein expression was induced with 20 ng/mL doxycycline. YSTT cells were maintained in DMEM with 10% (vol/vol) FBS, 200 μg/mL G418, and 0.5 μg/mL puromycin. The pcTYR plasmid was described earlier (10). For localization studies, the N-terminal FLAG tag was added to ATP7B by PCR and the construct was cloned into the pcDNA5 FRT/TO plasmid (Invitrogen). The 2623A→G substitution was introduced using a QuikChange protocol. The immunodetection of Flag tag, calnexin, and TGN-38 was performed using commercial antibodies as described in SI Materials and Methods; images were acquired using confocal microscopy.
Copper, DDP, and Zinc Treatments.
CuCl2 (0.5–200 μM) was added to the growth medium for different time intervals; DDP (Sigma) was dissolved in DMSO and used at 10 μM for 4 h. Cells treated with an equal volume of DMSO were used as a control. Atomic absorption spectroscopy confirmed the lack of copper in the DDP solutions. ZnCl2 (100 μM) was added for 4 h.
Studies of the A-Domain.
The ATP7B fragment (residues 789–907) encoding the A-domain with Arg or Gly at position 875 was cloned into the pTXB1 vector and expressed in E. coli. Proteins were metabolically labeled with 15NH4Cl; purified on chitin beads; cleaved using 2-mercaptoethane sulfonate sodium (Sigma); and dialyzed into 50 mM Na-phosphate (pH 7.0), 50 mM arginine, and 50 mM glutamate. The 1H,15N-HSQC spectra were recorded with 0.2 mM protein.
Tyrosinase Activation Assay.
YSTT cells transfected with tyrosinase with or without ATP7B were incubated with or without copper/DPP (see above) and fixed for 30 s in cold acetone-methanol (1:1). l-DOPA was added as originally described (9), and formation of black pigment was detected by phase microscopy.
ATP7B Stability in Cells.
HEK293TRex cells in 6-cm plates were transfected with 6 μg of ATP7B cDNAs and treated overnight with doxycycline (20 ng/mL) in basal medium. Cells were then transferred to fresh medium containing 50 μM CHX and incubated for 4 h with or without 5 μM copper. Microsomal fractions were isolated, and the protein amounts were analyzed by Western blot using the anti-Flag and anti-Na,K-ATPase antibodies.
Supplementary Material
Acknowledgments
We thank Lawrence Gray for help with copper measurements. This study was supported by National Institutes of Health Grants DK071865 and P01GM067166. NMR data were collected at the Saskatchewan Structural Sciences Center.
Footnotes
*This Direct Submission article had a prearranged editor.
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1014959108/-/DCSupplemental.
References
- 1.Das SK, Ray K. Wilson's disease: An update. Nat Clin Pract Neurol. 2006;2:482–493. doi: 10.1038/ncpneuro0291. [DOI] [PubMed] [Google Scholar]
- 2.Gupta A, et al. Molecular pathogenesis of Wilson disease: Haplotype analysis, detection of prevalent mutations and genotype-phenotype correlation in Indian patients. Hum Genet. 2005;118:49–57. doi: 10.1007/s00439-005-0007-y. [DOI] [PubMed] [Google Scholar]
- 3.Petrukhin K, et al. Mapping, cloning and genetic characterization of the region containing the Wilson disease gene. Nat Genet. 1993;5:338–343. doi: 10.1038/ng1293-338. [DOI] [PubMed] [Google Scholar]
- 4.Bull PC, Thomas GR, Rommens JM, Forbes JR, Cox DW. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat Genet. 1993;5:327–337. doi: 10.1038/ng1293-327. [DOI] [PubMed] [Google Scholar]
- 5.Leonhardt K, Gebhardt R, Mössner J, Lutsenko S, Huster D. Functional interactions of copper-ATPase ATP7B with cisplatin and the role of ATP7B in the resistance of cells to the drug. J Biol Chem. 2009;284:7793–7802. doi: 10.1074/jbc.M805145200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsivkovskii R, Eisses JF, Kaplan JH, Lutsenko S. Functional properties of the copper-transporting ATPase ATP7B (the Wilson's disease protein) expressed in insect cells. J Biol Chem. 2002;277:976–983. doi: 10.1074/jbc.M109368200. [DOI] [PubMed] [Google Scholar]
- 7.Wu ZY, et al. Mutation analysis and the correlation between genotype and phenotype of Arg778Leu mutation in Chinese patients with Wilson disease. Arch Neurol. 2001;58:971–976. doi: 10.1001/archneur.58.6.971. [DOI] [PubMed] [Google Scholar]
- 8.Santhosh S, et al. Genotype phenotype correlation in Wilson's disease within families—A report on four south Indian families. World J Gastroenterol. 2008;14:4672–4676. doi: 10.3748/wjg.14.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petris MJ, Strausak D, Mercer JF. The Menkes copper transporter is required for the activation of tyrosinase. Hum Mol Genet. 2000;9:2845–2851. doi: 10.1093/hmg/9.19.2845. [DOI] [PubMed] [Google Scholar]
- 10.Braiterman L, et al. Apical targeting and Golgi retention signals reside within a 9-amino acid sequence in the copper-ATPase, ATP7B. Am J Physiol Gastrointest Liver Physiol. 2009;296:G433–G444. doi: 10.1152/ajpgi.90489.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo Y, Nyasae L, Braiterman LT, Hubbard AL. NH2-terminal signals in ATP7B copper-ATPase mediate its Cu-dependent anterograde traffic in polarized hepatic cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:G904–G916. doi: 10.1152/ajpgi.00262.2005. [DOI] [PubMed] [Google Scholar]
- 12.Roelofsen H, et al. Copper-induced apical trafficking of ATP7B in polarized hepatoma cells provides a mechanism for biliary copper excretion. Gastroenterology. 2000;119:782–793. doi: 10.1053/gast.2000.17834. [DOI] [PubMed] [Google Scholar]
- 13.Banci L, et al. Solution structures of the actuator domain of ATP7A and ATP7B, the Menkes and Wilson disease proteins. Biochemistry. 2009;48:7849–7855. doi: 10.1021/bi901003k. [DOI] [PubMed] [Google Scholar]
- 14.Nartey NO, Frei JV, Cherian MG. Hepatic copper and metallothionein distribution in Wilson's disease (hepatolenticular degeneration) Lab Invest. 1987;57:397–401. [PubMed] [Google Scholar]
- 15.Labadie GU, Hirschhorn K, Katz S, Beratis NG. Increased copper metallothionein in Menkes cultured skin fibroblasts. Pediatr Res. 1981;15:257–261. doi: 10.1203/00006450-198103000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Dolgova NV, Olson D, Lutsenko S, Dmitriev OY. The soluble metal-binding domain of the copper transporter ATP7B binds and detoxifies cisplatin. Biochem J. 2009;419:51–56, 3, 56. doi: 10.1042/BJ20081359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mangala , et al. Therapeutic targeting of ATP7B in ovarian carcinoma. Clin Cancer Res. 2009;15:3770–3780. doi: 10.1158/1078-0432.CCR-08-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- 19.Hatori Y, Lewis D, Toyoshima C, Inesi G. Reaction cycle of Thermotoga maritima copper ATPase and conformational characterization of catalytically deficient mutants. Biochemistry. 2009;48:4871–4880. doi: 10.1021/bi900338n. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.