Abstract
The role of the fibronectin receptor, α5β1-integrin, as an adhesion receptor and in angiogenesis, is well established. However, its role in cancer cell invasion and metastasis is less clear. We describe a novel mechanism by which fibronectin regulates ovarian cancer cell signaling and promotes metastasis. Fibronectin binding to α5β1-integrin led to a direct association of α5-integrin with the receptor tyrosine kinase, c-Met, activating it in a hepatocyte growth factor/scatter factor (HGF/SF) independent manner. Subsequently, c-Met associated with Src and activated Src and focal adhesion kinase (FAK). Inhibition of α5β1-integrin decreased the phosphorylation of c-Met, FAK and Src, both in vitro and in vivo. Independent activation of c-Met by its native ligand, HGF/SF, or overexpression of a constitutively active FAK in HeyA8 cells could overcome the effect of α5β1-integrin inhibition on tumor cell invasion, indicating that α5β1-integrin is upstream of c-Met, Src and FAK. Inhibition of α5β1-integrin on cancer cells in two xenograft models of ovarian cancer metastasis resulted in a significant decrease of tumor burden, which was independent of the effect of α5β1-integrin on angiogenesis. These data suggest that fibronectin promotes ovarian cancer invasion and metastasis through an α5β1-integrin/c-Met/FAK/Src dependent signaling pathway, transducing signals through c-Met in a HGF/SF independent manner.
Keywords: ovarian cancer, α5β1-integrin, c-Met, Src, FAK, fibronectin
Introduction
Ovarian cancer is the fifth leading cause of cancer related death among women in the United States and has the highest mortality rate of all gynecologic malignancies (Jemal et al., 2009). It involves a unique metastatic process, which rarely takes the hematogenous route commonly observed in other cancers. The cancer cells exfoliate from the surface of the ovary by shedding from the primary tumor, or through hydroflotation caused by circulating peritoneal fluid. They subsequently attach to the peritoneal surfaces and invade to form metastatic tumors. This mode of metastasis places unique demands on the ovarian cancer cells and may require a specific molecular mechanism different from the intra- and extravasation involved in hematogenous metastasis (Lengyel,2010).
Integrins are among the genes reported to be involved in ovarian cancer metastasis. They are heterodimeric transmembrane proteins comprised of various combinations of an α- and β-subunit, with each heterodimer possessing one or more ligands (Hynes,2002). Integrins anchor the cancer cells to the extracellular matrix (ECM) which provides a contact point for invasion and metastasis. Signals from the ECM are transmitted via integrins through interactions with multiple proteins which eventually determine the cellular response to the ECM, including changes in cell shape, motility, proliferation etc. (Mitra et al., 2006; Giancotti et al., 2003). The fibronectin receptor (α5β1-integrin) primarily binds to fibronectin to anchor cells and subsequently activates non receptor tyrosine kinases, FAK and Src, which play an important role in tumorigenesis by promoting the proliferation and invasion of cancer and endothelial cells (Mitra et al., 2006; Caswell et al., 2007).
Knocking out α5β1-integrin is associated with major vascular defects, such as a marked decrease in capillary branching and incompletely formed vessels in mouse embryos, leading to embryonic lethality (Yang et al., 1993; Francis et al., 2002). Since α5β1-integrin is abundantly present on endothelial cells, research on this integrin has primarily focused on its role in angiogenesis and elucidated its function in vascular endothelial cells (Taverna et al., 2001; Kim et al., 2000; Magnussen et al., 2005). Endothelial cells in tumor vessels overexpress α5β1-integrin, which helps to anchor them to the basement membrane. When antibodies against α5β1-integrin are injected intravenously, they rapidly label tumor vessels (Magnussen et al., 2005), highlighting the importance of α5β1-integrin for tumor vasculature. However, the role of α5β1-integrin, when expressed on tumor cells, is less well understood. We have previously shown that, during ovarian cancer progression, loss of E-cadherin upregulated α5β1-integrin, which promoted the adhesion and invasion of ovarian cancer cells. Overexpression of α5β1-integrin indicated a very poor prognosis in patients with epithelial ovarian cancer (Sawada et al., 2008). However, the molecular mechanism by which α5β1-integrin promotes cancer cell metastasis is currently unknown. Given that fibronectin is one of the most abundant proteins in the ECM of both the peritoneum and omentum (Franke et al., 2003; Hafter et al., 1984; Kenny et al., 2009), an understanding of how fibronectin α5β1-integrin interactions regulate metastasis in the ovarian cancer cell might provide additional insights into the biology of ovarian cancer.
In the present study, we show that upon binding to fibronectin, α5β1-integrin interacts directly with the receptor tyrosine kinase c-Met, thereby activating Src and FAK. This is the first report to demonstrate that the fibronectin receptor transduces signals through c-Met, which is itself, a major player in ovarian cancer metastasis.
Results
Inhibition of α5β1-integrin inhibits ovarian cancer metastasis
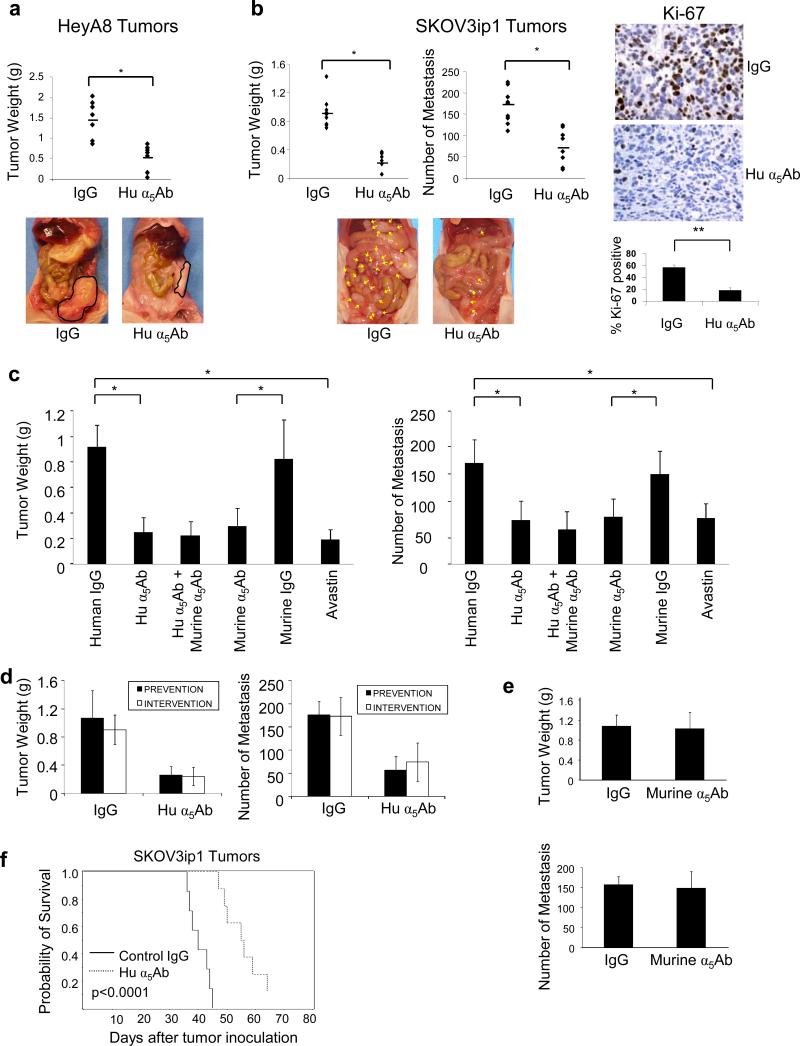
The ECM of the normal human omentum, the most common site of ovarian cancer metastasis expresses abundant fibronectin and vitronectin (Supplementary Figure S1). We and others have previously shown that the fibronectin receptor, α5β1-integrin, is upregulated in ovarian cancer cells during tumor progression (Sawada et al., 2008; Yokoyama et al., 2007). Therefore, we decided to inhibit α5β1-integrin to determine if this would result in a reduction of ovarian cancer dissemination. Two orthotopic xenograft models of ovarian cancer metastasis were treated with a monoclonal antibody blocking human α5β1-integrin (Hu α5Ab) (Bhaskar et al., 2007). SKOV3ip1 or HeyA8 human ovarian cancer cells, both of which have high expression levels of α5β1-integrin (Supplementary Figure S2), were injected intraperitoneally into nude mice. While SKOV3ip1 cells form many small metastases, mimicking milliary disseminated ovarian cancer, HeyA8 cells form a few, large metastases in the omentum and mesentery which resemble the more solid tumor growth found in a subgroup of patients. Tumors were counted, resected and the tumor weight measured. Treatment with the human α5β1-integrin blocking antibody reduced tumor weight in HeyA8 and SKOV3ip1 xenografts by 64% (p<0.001) and 73% (p<0.001), respectively, compared to isotype control treatment (Figure 1a and b). In the SKOV3ip1 model there was also a 57% (p<0.005) decrease in the number of metastases (Figure 1b). These mice also produced smaller tumors than the controls, a finding which was paralleled by reduced Ki-67 staining, suggesting a lower proliferation rate upon treatment with the α5-integrin antibody (Figure 1b right panel).
Figure 1. Blocking α5β1-integrin in ovarian cancer cells inhibits metastasis in xenograft models.
(a) HeyA8 cells (1×106) were injected intraperitoneally (i.p.) in nude mice which were randomized into 2 groups of 10/group receiving 10mg/kg human α5β1-integrin antibody (Hu α5Ab) or control IgG. The effect of Hu α5Ab on tumor weight was assessed on day 20. (b) SKOV3ip1 cells (1×106) were similarly injected i.p. treatment started on day 8 and tumor weight and the number of metastases assessed on day 35. Representative images of SKOV3ip1 tumors stained for Ki-67 (x400). Images of sections from 5 mice were quantified (**p<0.01). (c) SKOV3ip1 cells (1×106) were injected i.p. in nude mice randomized into 6 groups of 10 each and were treated with the indicated antibodies starting on day 8. Mice were euthanized on day 35 and the effect on tumor weight and number of metastases was assessed. (*p<0.001, **p<0.01,). (d) The effect of co-administering SKOV3ip1 cells with a single dose of Hu α5Ab injected i.p. in mice (prevention) was compared with treatment of SKOV3ip1 tumor bearing mice starting 8 days after injection of cancer cells (intervention). The effect of treatment on tumor weight and number of metastases was measured. (e) A single dose of murine α5Ab was co-injected with SKOV3ip1 cells i.p. in nude mice (prevention) and tumor weight and number of metastases was measured on day 35. (f) Kaplan-Meier curve for survival. SKOV3ip1 cells were injected i.p. and 10 mice/group were treated with the human α5Ab or control IgG twice a week starting from day 8 (p<0.0001; log-rank test).
Currently, the most completely characterized function of α5β1-integrin in cancer is its contribution to angiogenesis (Avaamides et al., 2008; Wingerter et al., 2005). However, the human α5-integrin antibody used in Figure 1a and b does not cross-react with murine α5β1-integrin (Bhaskar et al., 2007), indicating that the effects seen on metastasis and tumor growth are the result of a direct effect of the antibody on the human tumor cells rather than blocking host α5β1-integrin function. To compare the contribution of α5β1-integrin in cancer cells to that in host stromal cells, we took advantage of an anti-murine α5Ab which inhibits angiogenesis by blocking α5β1-integrin on mouse vascular endothelial cells (Bhaskar et al., 2007). The specificity of the human and murine α5-integrin antibody was confirmed by testing their effect on adhesion of the human ovarian cancer cell line HeyA8 and the mouse ovarian cancer cell line ID-8 on fibronectin (Supplementary figure S3). Treatment with the human or the murine α5-integrin antibody showed a comparable reduction in tumor weight (57% and 48% respectively) and tumor numbers (73% and 64%, respectively) in a SKOV3ip xenograft model (Figure 1c). This reduction was similar to the effect of bevacizumab (Avastin), a humanized anti-VEGF antibody. Combining the human and murine α5β1-integrin antibodies did not significantly augment the anti-tumor effect of each individual antibody (Figure 1c). Immunohistochemical staining of the tumors for CD31 confirmed an anti-angiogenic effect of murine α5Ab and bevacizumab, while, as expected, the human antibody had no effect on blood vessel density (Supplementary Figure S4) or on VEGF expression (data not shown).
Next, we sought to determine whether α5β1-integrin plays a more important role in early or in late metastasis (Figure 1d). To study early metastasis a single treatment of the integrin antibody was given to the nude mice simultaneously with the intraperitoneal injection of ovarian cancer cells, in order to block the initial attachment and invasion of tumor cells to the peritoneum (prevention study). Late metastasis was studied with the administration of repeated treatments beginning 8 days after tumors had established within the peritoneal cavity (intervention study). Interestingly, the prevention and intervention studies resulted in similar reductions in tumor weight and the number of metastases, (Figure 1d). Given that in vitro, the human α5-integrin antibody inhibited both ovarian cancer cell adhesion and proliferation (Supplementary Figure S5), our results suggest that α5β1-integrin is important for both the initial attachment/invasion and subsequent tumor growth of ovarian cancer cells. Repeating the prevention experiment described above with murine α5Ab, expectedly, did not alter metastasis or tumor growth (Figure 1e).
Based on these results, we performed a survival study after intraperitoneal injection of SKOV3ip1 by administering the α5β1-integrin antibody or control IgG twice a week. The median survival was 39 days in the control treated animals and 56 days when the fibronectin receptor was inhibited (log rank: P<0.0001, Figure 1f).
α5β1-integrin regulates and interacts with c-Met
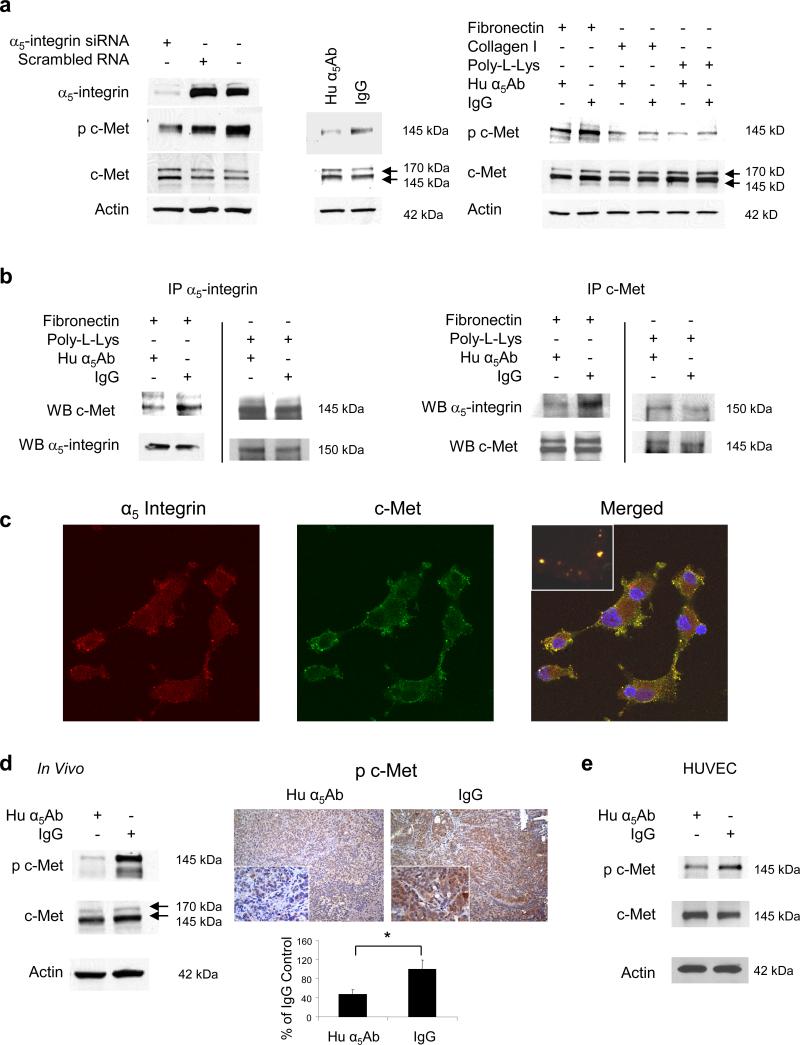
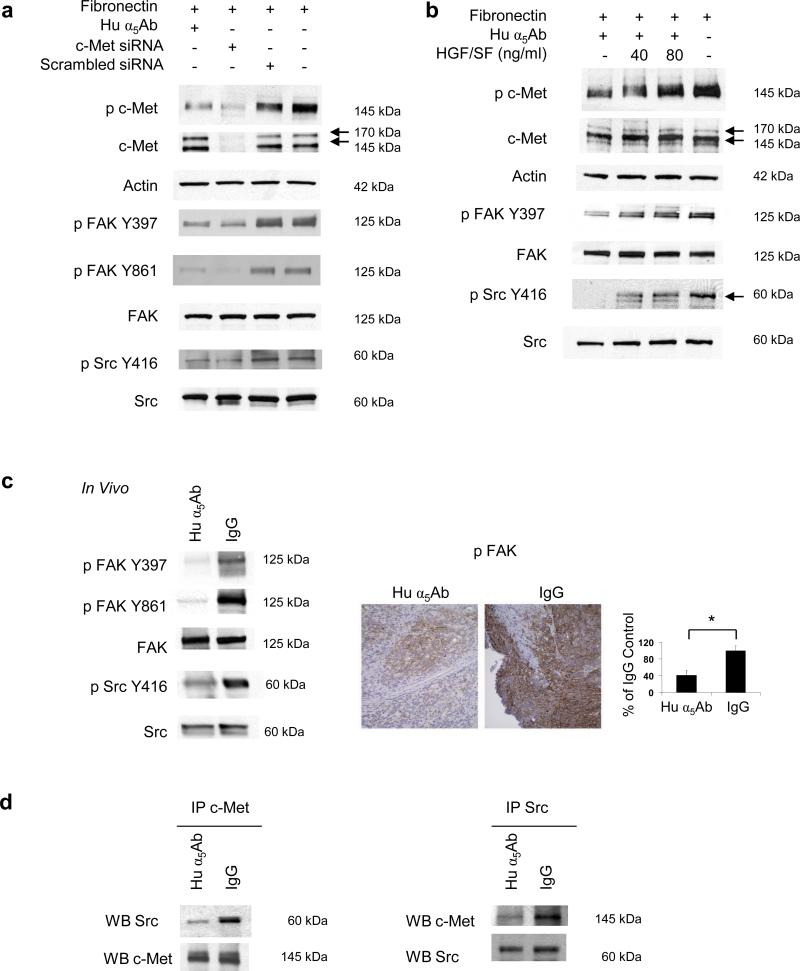
Since the role of α5β1-integrin in angiogenesis is well described (Taverna et al., 2001; Kim et al., 2000; Magnussen et al., 2005) we decided to further investigate how expression of α5β1-integrin on tumor cells regulates tumor cell function(s). Integrins can upregulate cellular signaling pathways directly, but there are reports that integrin mediated adhesion activates receptor tyrosine kinases (RTKs) (Ricono et al., 2009; Wang et al., 1996; Nakamura et al., 2008; Vuori et al., 1994). We and others have previously reported that c-Met contributes to the dissemination of ovarian cancer cells (Sawada et al., 2007; Wong et al., 2004; Saga et al., 2001), which is why we investigated the interesting possibility that fibronectin mediated activation of α5β1-integrin recruits c-Met signaling. To determine whether cell adhesion to fibronectin influences c-Met activation, HeyA8 cells, which express both α5β1-integrin and c-Met, were transfected with a siRNA against α5-integrin and plated on fibronectin. After 24 hours c-Met was detected by immunoblotting. Silencing α5-integrin resulted in decreased phosphorylation of c-Met in the activation loop of the tyrosine kinase domain (Tyr1230,1234,1235), but total c-Met expression was unchanged (Figure 2a, left panel). Treatment with the α5β1-integrin blocking antibody also blocked c-Met phosphorylation and confirmed this finding (Figure 2a, middle panel). The c-Met phosphorylation was specific to fibronectin adhesion and was not induced by tumor cell binding to collagen or poly-L-lysine (Figure 2a right panel). Thereafter, we considered the possibility that α5-integrin and c-Met associate, given previous reports (Nakamura et al., 2008; Chan et al., 2006) that integrins and RTKs can interact. Indeed, c-Met co-immunoprecipitated with α5-integrin in IgG treated cells, and inhibiting α5-integrin lead to a significant reduction in the association of the two proteins which was confirmed by immunoprecipitating both with α5-integrin and c-Met (Figure 2b). A direct interaction was confirmed by confocal microscopy showing that, in ovarian cancer cells plated on fibronectin, α5-integrin and c-Met co-localize (yellow overlay, Figure 2c). The interaction of α5-integrin with c-Met is also important in vivo, since mice bearing SKOV3ip1 xenografts treated with the inhibitory human α5β1-integrin antibody have lower c-Met phosphorylation (Tyr1230,1234,1235) in the tumor than mice treated with IgG (Figure 2d). Moreover blocking α5β1-integrin in normal human umbilical vein endothelial cells (HUVEC) also inhibited c-Met phosphorylation (Figure 2e) suggesting that regulation of c-Met by α5β1-integrin can also occur in untransformed cells. Analysis of nine ovarian cancer cell lines showed that both proteins are co-expressed, and that those cells that had high expression of α5-integrin also had high expression of c-Met and vice versa (Supplementary Figure S2). The Pearson correlation coefficient for co-expression of c-Met and α5-integrin was 0.61.
Figure 2. α5β1-Integrin signals through the receptor tyrosine kinase c-Met.
(a) Left: HeyA8 cells were transfected with α5-integrin siRNA or treated with human α5Ab and plated on fibronectin followed by western blots for α5-Integrin, p-c-MetY1230,1234,1235 and c-Met. Right: HeyA8 cells were plated on fibronectin, collagen or poly-L-Lysine and were treated with human α5Ab followed by immunoblotting for phospho-c-Met Y1230,1234,1235, c-Met and actin. (b) HeyA8 cells were plated on fibronectin or poly-L-Lysine and treated with human α5Ab for 24 h. Cell lysates were immunoprecipitated (IP) with an antibody against α5-integrin or c-Met, respectively followed by immunoblotting (WB) for α5-integrin or c-Met. (c) Confocal microscopy. HeyA8 cells were plated on fibronectin coated cover slips, detected with c-Met and α5-integrin antibodies followed by fluorescein or Alexa Fluor 594 labeled secondary antibodies respectively. (d) SKOV3ip1 xenografts were treated with Hu α5Ab or IgG as described in Figure 1a. Left: Western blots for p-c-Met Y1230,1234,1235 and c-Met using tumor lysates. Right: Image of tumor sections stained for p-c-Met Y1200,1234,1235 (x200 and x400). Images of sections from 5 mice were quantified using Image J with color deconvolution (*p<0.001). (e) Human umbilical vein endothelial cells (HUVEC) were plated on fibronectin and treated with human α5Ab followed by western blots for p-c-MetY1230,1234,1235 and c-Met.
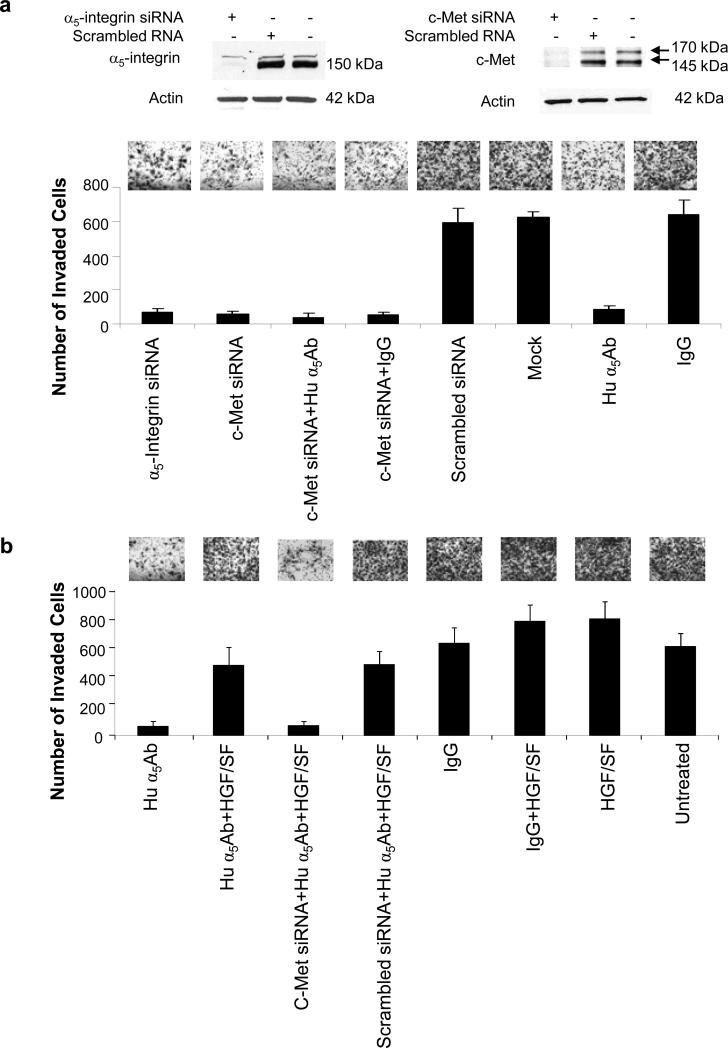
To understand the functional significance of α5β1-integrin signaling through c-Met, invasion assays were performed. HeyA8 cells were transfected with a siRNA against c-Met or α5-integrin and then compared with HeyA8 cells treated with the human α5β1-integrin antibody. After verifying silencing we found that the α5-integrin and the c-Met siRNA reduced invasion by 89% and 91% respectively (Figure 3a). Blocking the fibronectin receptor with α5β1-integrin antibody had a similar inhibitory effect (87%). There was no additional reduction of invasion when both α5β1-integrin and c-Met inhibition were combined, suggesting that both are part of the same pathway. The absence of any additive effect was also confirmed at lower concentrations of c-Met siRNA and the human α5-integrin antibody (Supplementary figure S6). Importantly, the inhibition of invasion by the antibody could be reversed by activating c-Met with its ligand HGF/SF, (Figure 3b). This supports the concept that α5β1-integrin is upstream of the c-Met receptor, but that c-Met can still be activated independently of α5β1-integrin by its ligand HGF/SF.
Figure 3. Inhibiting α5β1-integrin, silencing c-Met or both blocks invasion which can be overcome by activation of c-Met by HGF.
(a) HeyA8 cells were transfected with the indicated siRNAs and seeded on matrigel coated Boyden chambers, treated with human α5Ab and allowed to invade for 24 h. Silencing was confirmed by immunoblotting. (b) HeyA8 cells or HeyA8 cells transfected with c-Met siRNA were placed in matrigel coated Boyden chambers, treated with human α5Ab with or without HGF/SF or untreated control as indicated and allowed to invade for 24h. For (a) and (b) the average number of invaded cells/field was plotted against the treatment given. Results are from three independent experiments.
α5β1-integrin activates a c-Met/FAK/Src signaling pathway
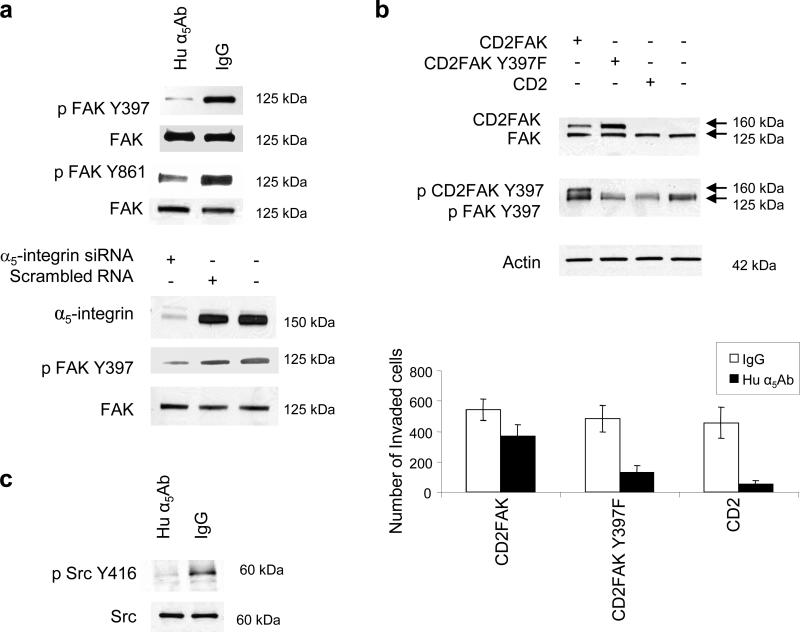
Having shown that α5β1-integrin associates with and activates c-Met, we next sought to identify downstream signaling pathways. Initial experiments focused on the MAPK, STAT-3, and AKT pathways by measuring their key signaling proteins. However, none of these pathways were affected by α5β1-integrin inhibition (Supplementary Figure S7 and data not shown). Given previous reports that integrins use FAK and Src to signal, we turned our attention to these non-receptor tyrosine kinases (Mitra et al., 2006). Indeed, blocking α5β1-integrin with the antibody in HeyA8 cells plated on fibronectin decreased FAK phosphorylation at Tyr397, which is the FAK auto-phosphorylation site, and at Tyr861, which is the Src phosphorylation site (Figure 4a). This was confirmed when α5-integrin was silenced with a siRNA (Figure 4a, lower panel) and also confirmed in the SKOV3ip1 ovarian cancer cell line (Supplementary Figure S8a). Ectopic expression of a constitutively active, membrane-bound form of FAK, CD2-FAK (Huttenlocher et al., 1998; Chan et al., 1994), reversed, at least in part, the inhibitory effect of the α5β1-integrin on invasion, while neither the mutated CD2-FAK (Y397F) plasmid nor the empty vector (CD2) had any effect (Figure 4b and Supplementary Figure S8b for SKOV3ip1 cells). Following the observation that α5β1-integrin inhibition decreased FAK phosphorylation at Tyr861, which is a Src phosphorylation site (Mitra et al., 2006), we determined whether blocking α5β1-integrin also inhibits Src phosphorylation and, indeed, found decreased Src phosphorylation after antibody treatment (Figure 4c).
Figure 4. Adhesion of ovarian cancer cells to fibronectin activates FAK and Src through α5β1-integrin.
(a) Top: HeyA8 cells were transfected with α5-integrin siRNA or treated with human α5Ab and plated on fibronectin for 24h. Western blots were performed for p-FAK-Y397, p-FAK-Y861, FAK, and α5-integrin. (b) HeyA8 cells were transfected with CD2FAK (constitutively active FAK), CD2FAK-Y397F (mutated/inactive FAK) or CD2 (vector). Top: Expression and phosphorylation (Y397) of CD2FAK (160kDa) was verified by western blotting. Bottom: Transfected HeyA8 cells were added to matrigel coated Boyden chamber with human α5Ab or IgG and allowed to invade for 24 h. The number of invaded cells was counted. (c) HeyA8 cells were plated on fibronectin and treated with human α5Ab or IgG for 24 h. Phosphorylated (p)-Src Y416 and Src was detected by immunoblotting.
While these experiments suggest that α5β1-integrin regulates FAK and Src, they do not indicate whether these two proteins are downstream of α5-integrin or c-Met. Both these interactions have been previously reported (Jin et al., 2007; Mitra et al., 2006; Chen et al., 2006). First, we tested whether α5β1-integrin interacted with FAK and/or Src when HeyA8 cells were plated on fibronectin but neither immunoprecipitation nor confocal microscopy showed any interaction (data not shown). Next, we tested whether FAK and Src are downstream of the fibronectin/α5β1-integrin/c-Met axis. HeyA8 cells were transfected with the c-Met siRNA, plated on fibronectin, and the effect of c-Met silencing on FAK and Src phosphorylation was compared to that of treatment with the α5β1-integrin antibody. Silencing of c-Met resulted in a decrease in FAK (Tyr397) and Src phosphorylation (Tyr416) (Figure 5a). The inhibitory effect of the α5β1-integrin antibody on c-Met, FAK, and Src phosphorylation could be overcome by stimulating cells with HGF/SF (Figure 5b). Addition of HGF/SF in the presence of the α5β1-integrin inhibitor resulted in a dose dependent re-phosphorylation of c-Met, FAK, and Src. The in vitro results were confirmed in the α5β1-integrin antibody treated SKOV3ip1 tumors. Western blotting showed a decrease in FAK (Tyr397, Tyr861) and Src (Tyr416) phosphorylation in tumor tissue from integrin antibody treated tumors (Figure 5c left). Immunohistochemical staining showed a reduction of FAK phosphorylation (Tyr397) in tumors treated with the α5β1-integrin inhibitory antibody, but not in the control IgG treated tumors (Figure 5c, right). To determine if c-Met directly associated with and activated FAK and Src, lysates from α5β1-integrin antibody or IgG, treated HeyA8 cells were immunoprecipitated with anti c-Met antibody. We observed that Src co-immunoprecipitated with c-Met (Figure 5d) but not FAK (data not shown). Taken together, these data indicate that fibronectin activates an α5β1-integrin/c-Met/Src/FAK dependent signaling pathway.
Figure 5. α5β1-Integrin signals through c-Met to activate FAK and Src.
(a) HeyA8 cells were transfected with c-Met siRNA or with scrambled siRNA, plated on fibronectin and treated with human α5Ab. Cells were lysed, resolved on SDS-PAGE, transferred to nitrocellulose membrane and probed with the indicated antibodies. (b) HeyA8 cells were plated on fibronectin and treated with human α5Ab as indicated for 24 h. The cells were then treated with HGF/SF for 30 min and immunoblotting performed with the specified antibodies. Results in (a) and (b) are representative of three independent experiments. (c) SKOV3ip1 xenografts were treated with human α5Ab or with IgG as described in Figure 1 (b) Left: Tumor lysates were immunoblotted with the indicated antibodies. Right: Representative image of tumor sections stained for p-FAK-Y397 (x200). Images of sections from 5 mice were quantified using Image J (*p<0.001). (d) Immunoprecipitation. HeyA8 cells were plated on fibronectin and treated with human α5Ab or control IgG for 24 h followed by immunoprecipitation with an antibody against c-Met or Src, respectively followed by immunoblotting (WB) Src and c-Met. All results are representative of at least three independent experiments.
Discussion
The ECM protein, fibronectin, is a potent mediator of cell adhesion, migration, and growth. It is also one of the most abundant ECM and basement membrane proteins in human omentum and peritoneum ((Franke et al., 2003) and Supplementary Figure S1), the most common sites of ovarian cancer metastasis. Our data provide evidence for a novel mechanism by which fibronectin regulates ovarian cancer cell signaling. Binding of α5β1-integrin to fibronectin recruits the RTK, c-Met, which then activates downstream signaling events. The presence of this mechanism is supported by experiments showing that inhibition of α5β1-integrin using a siRNA or a blocking antibody inhibits fibronectin mediated c-Met phosphorylation both in vitro and in vivo. Immunoprecipitation, and visualization of c-Met and α5-integrin using confocal microscopy, imply that the adhesion receptor and the RTK associate to form a molecular signaling unit. Inhibition of α5β1-integrin also led to a decrease in phosphorylation of c-Met in HUVEC cells, indicating that this process is not specific for cancer cells alone.
The first evidence that adhesion can regulate c-Met came from an observation in the Bishop Laboratory (Wang et al., 1996) at UCSF, showing that adherence of cancer cells to fibronectin and type IV collagen induces phosphorylation of c-Met. This observation was consistent with an inverse experiment using melanoma cancer cells, which showed that the constitutive activation of c-Met is lost upon cellular detachment (Rusciano et al., 1996). The data presented in our study indicate that cell adhesion regulates c-Met through direct activation by α5β1-integrin. When α5β1-integrin mediated c-Met activation was blocked, c-Met could still be stimulated by its ligand, HGF/SF, suggesting that in ovarian cancer cells, c-Met can be activated both in a ligand dependent and independent manner. The ligand dependent activation triggers the canonical pathways involving Erk and AKT (Birchmeier et al., 2003), which we and others have shown to be inhibited by small molecule inhibitors of c-Met (Zillhardt et al., 2010; Birchmeier et al., 2003). Fibronectin mediated activation resulted in signaling via an alternate pathway involving Src and FAK. The cell, therefore, has the flexibility to use the ECM as well as paracrine HGF/SF to gain access to c-Met directed signaling pathways. However, while c-Met is the signal mediator after α5β1-integrin activation, it can also initiate integrin mediated signaling. Activation of c-Met leads to association with α6β4-integrin and tyrosine phosphorylation of the β4-integrin cytoplasmic domain (Trusolino et al., 2001). In this case, the integrin does not act as the adhesion receptor, but rather as a mediator of c-Met signaling.
To determine whether inhibition of the fibronectin receptor on cancer cells plays a role in either early or late tumor metastasis, ovarian cancer cells received either a single pre-treatment with the α5β1-integrin antibody before i.p. injection (prevention) or were treated repeatedly after solid tumors were established within the peritoneal cavity (intervention). The results indicated that there are at least two distinct mechanisms by which the α5β1-integrin expression on tumor cells promotes ovarian cancer metastasis. It contributes to the initial adhesion/invasion on the fibronectin rich peritoneum and omentum and it later helps the cancer cells to metastasize and proliferate as the tumor progresses. In contrast, host α5β1-integrin contributes to tumor angiogenesis essential for later stages of metastasis (Kim et al., 2000; Magnussen et al., 2005).
The integrin mediated activation of Src and FAK is not a consequence of direct binding of these non RTK to the integrin, but occurs via c-Met. Expression of an activated form of FAK was able to overcome the inhibitory function of the α5-integrin blocking antibody on invasion, as was stimulation of cells by HGF/SF. These results were confirmed in vivo, since treatment with the human α5β1-integrin antibody blocked both FAK and Src activation in the tumor. We were never able to show a direct interaction between α5-integrin and FAK or Src (data not shown), despite reports that FAK can directly interact with integrins (Mitra et al., 2006). However, activation of c-Met by α5β1-integrin resulted in the direct binding of Src to c-Met (but not FAK). This suggests that upon binding to fibronectin, α5β1-integrin activates c-Met which then associates with Src and subsequently activates FAK. Consistent with our results, Chen and colleagues report that when c-Met is constitutively activated in aggressive lung cancer cells, FAK interacts with c-Met independent of stimulation with HGF/SF. This was not observed in less invasive cells (Chen et al., 2006). It is possible that most of what is seen as the ligand independent activation of c-Met is instead the regulation of c-Met by α5β1-integrin.Taking into consideration that c-Met can be activated in a ligand dependent as well as in a ligand independent manner, current strategies to inhibit c-Met could be reconsidered: Inhibition of the c-Met cytoplasmic domains (e.g. the kinase domain) could be more effective than targeting the HGF ligand binding site.
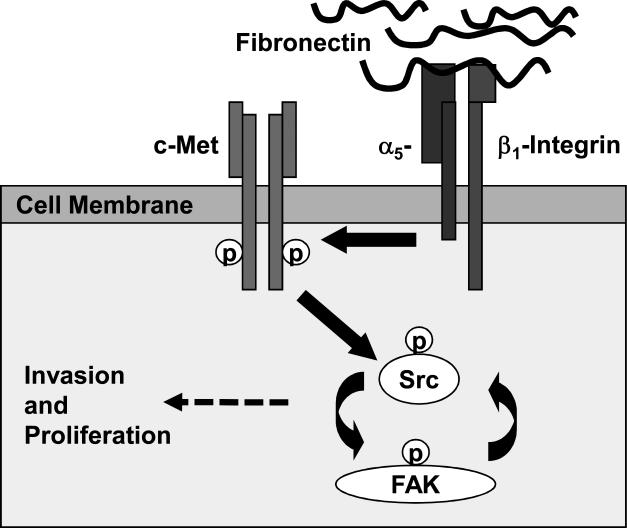
In summary, we define a signaling pathway in ovarian cancer that starts with the fibronectin mediated activation of α5β1-integrin and leads to direct contact with c-Met, followed by Src and FAK activation linking mitogenic RTK signaling with adhesion (Figure 6). This may be one of the mechanisms utilized by cancer cells to constitutively activate c-Met in a fibronectin rich microenvironment. In particular, this mechanism could be of special importance in ovarian cancer, because the first step of ovarian cancer metastasis begins with cancer cell adhesion. Indeed, it could provide an explanation for how, in ovarian cancer, the early adhesion of cancer cells to the fibronectin rich basement membranes instantly activates key mitogenic signaling pathways in the cell, allowing a single ovarian cancer cell or a small cluster of cells to survive, invade and subsequently thrive in the initially hostile environment on the surface of the abdominal cavity.
Figure 6. Fibronectin / α5β1-Integrin mediated regulation of c-Met.
Proposed role of ligand independent activation of c-Met involving a fibronectin/α5β1-integrin/c-Met/Src/FAK dependent signaling pathway.
Materials and Methods
Reagents and cell lines
Anti FAK and paxillin antibodies, growth factor reduced matrigel and human fibronectin were from BD Biosciences (San Jose, CA), phospho-FAK Y397, phospho-FAK Y861, phospho-c-Met Y1230,1234,1235 were from Biosource (Carlsbad, CA). Antibodies against c-Met, α5-integrin, CD31 and protein A/G agarose beads were from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). Anti Src and phospho-SrcY416 antibodies were purchased from Upstate (Billerica, MA). Antibodies against AKT, phospho-AKT S473, phospho-ERK1/2, ERK1/2, phospho-JNK, JNK, phospho-p38 MAPK, p38 MAPK were from Cell Signaling (Danvers, MA). The fibronectin antibody was from Sigma-Aldrich (St. Louis, MO), Ki-67 antibody from LabVision (Fremont, CA), vimentin, collagen IV and laminin antibodies were from Dako Cytomation (Carpinteria, CA). 5-chloromethylfluorescein diacetate (CMFDA) was from Invitrogen (Carlsbad, CA). The human ovarian cancer cell lines SKOV3ip1 (first described by Janet Price (Brader et al., 1997; Yu et al., 1992) and HeyA8 were from Gordon B. Mills (M.D. Anderson Cancer Center, Houston, TX). The function blocking antibodies against human α5β1-integrin (M200, referred to as Hu α5Ab), mouse α5β1-integrin (339.1, referred to as Murine α5Ab) and Avastin® (Genentech) were provided by Facet Biotech Corp (Redwood City, CA). Nonspecific human or mouse IgG was from Chrome Pure, Jackson ImmunoResearch, West Grove, PA.
Mouse orthotopic xenograft model of ovarian cancer
The xenograft model was described previously (Sawada et al., 2008; Sawada et al., 2007). Briefly, HeyA8 or SKOV3ip1 cells (1×106) were injected intraperitoneally (i.p.) into 6 week old, female, athymic nude mice. On day 3 (HeyA8) and day 8 (SKOV3ip1) post injection, by which time the cells had metastasized and visible metastatic tumors had developed, mice were randomized into treatment groups. All antibodies were injected i.p. at a dose of 10mg/kg, twice a week. Treatment continued until day 20 for HeyA8 and day 35 for SKOV3ip1 models.
Immunohistochemistry
Immunohistochemical studies were performed by using 5μm thick formalin-fixed deparaffinized sections as previously described (Sawada et al., 2008). Xenograft tumor tissue was probed with anti CD31 antibody (1:100), anti Ki-67 (1:300), anti p-c-Met Y1230, 1234, 1235 (1:50) and anti p-FAK Y397 (1:200). Specimens of normal human omentum which were obtained from patients undergoing surgery for benign conditions were stained for fibronectin (1:600), collagen IV (1:50, laminin (1:50), or vitronectin antibody (1:500).
Western blot analysis
Cells or tumor tissues were lysed, proteins were separated by 4-20% gradient SDS-PAGE and transferred to nitrocellulose and probed with the respective primary and secondary antibodies (Sawada et al., 2008).
Immunoprecipitation
Cell lysates were pre-cleared with protein A/G agarose beads and incubated with anti α5-integrin, c-Met or Src antibodies followed by protein A/G agarose beads (Radjabi et al., 2008). The beads were then spun down and washed, boiled for 5 min in SDS-PAGE gel loading buffer, chilled and spun down. Equal amounts of the supernatant were then separated by 4-20% SDS-PAGE, transferred to nitrocellulose membrane, probed for co-immunoprecipitation of c-Met, α5-integrin or src antibodies and later stripped and reprobed for the immunoprecipitated protein.
Immunofluorescence imaging
HeyA8 cells were plated in serum free medium on fibronectin coated cover slips (5ug/ml) and allowed to attach overnight. Thereafter, the cells were fixed with 4% paraformaldehyde in PBS, permeabilized with 0.1% Triton X-100 and probed with rabbit anti-α5-integrin and mouse anti-c-Met antibodies at 4°C overnight (1:200). The samples were washed and incubated with Alexa Fluor 594-labelled goat anti rabbit antibody (1:1000) and fluorescein-labelled goat anti mouse antibody (1:1000), mounted with Prolong Gold (Invitrogen, Carlsbad, CA) and analyzed using a Leica SP2 laser scanning confocal microscope.
Transient transfection
HeyA8 cells were transfected with 30nM siRNA against α5-integrin (Ambion) or against c-Met using Lipofectamine 2000 transfection reagent (Invitrogen). The plasmids for the constitutively activated FAK (CD2FAK), CD2FAK with a mutation at the autophosphorylation site (CD2FAK Y397F) and the control vector (CD2) not expressing FAK, were a kind gift from Dr. Alan Horwitz, University of Virginia School of Medicine. HeyA8 cells or SKOV3ip1 cells were seeded in 6 well plates and transfected with 1μg DNA using Fugene6 transfection reagent (Roche). The cells were subsequently used for invasion assays.
Matrigel invasion assay
Cellular invasion was assayed in vitro as described previously (Sawada et al., 2008). Wherever applicable the human α5β1-integrin antibody or control IgG (25μg/ml) was added to the top chamber. Cells were fixed with 4% paraformaldehyde after 24h, stained with Giemsa and enumerated. Experiments were performed in triplicate and 5 fields (200x magnification) per filter counted.
Adhesion Assay
Cellular adhesion was assayed in vitro as described previously (Sawada et al., 2008). Briefly, ovarian cancer cells were fluorescently labeled with 10μM CMFDA and treated with the human α5β1-integrin antibody or control IgG (25μg/ml) and plated in a 96-well plate precoated with fibronectin (5 μg/mL). Adherent cells were quantified by measuring fluorescent intensity (excitation, 490 nm; emission, 520 nm).
Statistic
Data analysis was done by unpaired, two-tailed Student's t-test assuming equal variance of the test and the control populations.
Supplementary Material
Acknowledgements
We thank Dr. Alan Horwitz, Department of Cell Biology, University of Virginia School of Medicine for providing us with the FAK constructs. We thank Gail Isenberg for critically reviewing the manuscript.
Funding: Ernst Lengyel holds a Clinical Scientist Award in Translational Research from the Burroughs Wellcome Fund and is supported by grants from the Ovarian Cancer Research Fund (Liz Tilberis Scholars Program), and the NCI (RO1 CA111882).
Footnotes
Conflicts of Interest: Facet Biotech Corp. provided funding and antibodies for the animal experiments (Fig. 1).
Supplementary Information is available at Oncogene's website.
References
- Avraamides C, Garmy-Susini B, Varner JA. Integrins in angiogenesis and lymphangiogenesis. Nature Rev Cancer. 2008:604–617. doi: 10.1038/nrc2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar V, Zhang D, Fox M, Seto P, Wong M, Wales PE, et al. A function blocking anti-mouse integrin α5β1 antibody inhibits angiogenesis and impedes tumor growth in vivo. J Transl Med. 2007;5:1–11. doi: 10.1186/1479-5876-5-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- Brader KR, Wolf JK, Hung M-C, Yu D, Crispens MA, van Golen KL, et al. Adenovirus E1A expression enhances the sensitivity of an ovarian cancer cell line to multiple cytotoxic agents through an apoptotic mechanism. Clin Cancer Res. 1997;3:2017–2024. [PubMed] [Google Scholar]
- Caswell PT, Spence HJ, Parsons M, White DP, Clark K, Cheng KW, et al. Rab25 associates with α5β1 integrin to promote invasive migration in 3D microenvironments. Developmental Cell. 2007;13:496–510. doi: 10.1016/j.devcel.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Chan P-C, Chen SY, Chen C-H, Chen HC. Crosstalk between hepatocyte growth factor and integrin signaling pathways. Journal of Biomedical Science. 2006;13:215–223. doi: 10.1007/s11373-005-9061-7. [DOI] [PubMed] [Google Scholar]
- Chan P-Y, Kanner SB, Whitney G, Aruffo A. A transmembrane-anchored chimeric focal adhesion kinase is constitutively activated and phosphorylated at tyrosine residues identical to pp125fak*. The Journal of Biological Chemistry. 1994;269:20567–20574. [PubMed] [Google Scholar]
- Chen SY, Chen H-C. Direct interaction of focal adhesion kinase (FAK) with met is required for FAK to promote hepatocyte growth factor-induced cell invasion. Mol Cell Biol. 2006;26:5155–5167. doi: 10.1128/MCB.02186-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis SE, Goh KL, Hodivala-Dilke KM, Bader BL, Stark M, Davidson D, et al. Central roles of α5β1 integrin and fibronectin in vascular development in mouse embryos and embryoid bodies. Arteriosclerosis Thrombosis Vascular Biology. 2002;22:927–933. doi: 10.1161/01.atv.0000016045.93313.f2. [DOI] [PubMed] [Google Scholar]
- Franke FE, Von George R, Zygmunt M, Münstedt K. Association between fibronectin expression and prognosis in ovarian carcinoma. Anticancer Res. 2003;23:4261–4268. [PubMed] [Google Scholar]
- Giancotti FG, Tarone G. Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu Rev Cell Dev Biol. 2003;19:173–206. doi: 10.1146/annurev.cellbio.19.031103.133334. [DOI] [PubMed] [Google Scholar]
- Hafter R, Klaubert W, Gollwitzer R, Graeff H. Crosslinked fibrin derivatives and fibronectin in ascitic fluid from patients with ovarian cancer compared to ascitic fluid in liver cirrhosis. Thromb Haemost. 1984;35:53–64. doi: 10.1016/0049-3848(84)90312-8. [DOI] [PubMed] [Google Scholar]
- Huttenlocher A, Lakonishok M, Kinder M, Wu S, Truong T, Knudsen K, et al. Integrin and cadherin synergy regulates contact inhibition of migration and motile activity. The Journal of Cell Biology. 1998;141:515–526. doi: 10.1083/jcb.141.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: Bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- Jin E, Choi Y, Park E, Bang O, Kang S. MMP-2 functions as a negative regulator of chondrogenic cell condensation via down-regulation of the FAK -integrin β1 interaction. Devel Biol. 2007;308:474–484. doi: 10.1016/j.ydbio.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Kenny HA, Lengyel E. MMP-2 functions as an early response protein in ovarian cancer metastasis. Cell Cycle. 2009;8:683–688. doi: 10.4161/cc.8.5.7703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Bell K, Mousa SA, Varner JA. Regulation of angiogenesis in vivo by ligation of integrin α5β1 with the central cell-binding domain of fibronectin. Am J Pathol. 2000;156:1345–1362. doi: 10.1016/s0002-9440(10)65005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010;177:1053–1064. doi: 10.2353/ajpath.2010.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnussen A, Kasman I, Norberg S, Baluk P, Murray R, McDonald D. Rapid access of antibodies to α5β1 integrin overexpressed on the luminal surface of tumor blood vessels. Cancer Res. 2005;65:2712–2721. doi: 10.1158/0008-5472.CAN-04-2691. [DOI] [PubMed] [Google Scholar]
- Mitra SK, Schlaepfer D. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006;18:516–523. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Matsubara D, Goto A, Ota S, Sachiko O, Ishikawa S, et al. Constitutive activation of c-Met is correlated with c-Met overexpression and dependent on cell-matrix adhesion in lung adenocarcinoma cell lines. Cancer Sci. 2008;99:14–22. doi: 10.1111/j.1349-7006.2007.00640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radjabi AR, Sawada K, Jagadeeswaran S, Eichbichler A, Kenny HA, Montag A, et al. Thrombin induces tumor invasion through the induction and association of matrix metalloproteinase-9 and β1-integrin on the cell surface. J Biol Chem. 2008;283:1463–1472. doi: 10.1074/jbc.M704855200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricono JM, Huang M, Barnes LA, Lau SK, Weis S, Schlaepfer D, et al. Specific crosstalk between epidermal growth factor receptor and integrin αvβ5 promotes carcinoma cell invasion and metastasis. Cancer Res. 2009;69:1383–1391. doi: 10.1158/0008-5472.CAN-08-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusciano D, Lorenzoni P, Burger MM. Constitutive activation of c-Met in liver metastatic B16 melanoma cells depends on both substrate adhesion and cell density and is regulated by a cytosolic tyrosine phosphatase activity. J Biol Chem. 1996;271:20763–20769. doi: 10.1074/jbc.271.34.20763. [DOI] [PubMed] [Google Scholar]
- Saga Y, Mizukami H, Suzuki H, Urabe M, Kume A, Nakamura T, et al. Expression of HGF/NK4 in ovarian cancer cells suppresses intraperitoneal dissemination and extends host survival. Gene Therapy. 2001;8:1450–1455. doi: 10.1038/sj.gt.3301553. [DOI] [PubMed] [Google Scholar]
- Sawada K, Mitra AK, Radjabi AR, Bhaskar V, Kistner E, Tretiakova MS, et al. Loss of E-cadherin promotes ovarian cancer metastasis via alpha 5-integrin, which is a therapeutic target. Cancer Res. 2008;68:2329–2339. doi: 10.1158/0008-5472.CAN-07-5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada K, Radjabi AR, Shinomiya N, Kistner E, Kenny HA, Salgia R, et al. C-Met overexpression is a prognostic factor in ovarian cancer and an effective target for inhibition of peritoneal dissemination and invasion. Cancer Res. 2007;67:1670–1680. doi: 10.1158/0008-5472.CAN-06-1147. [DOI] [PubMed] [Google Scholar]
- Taverna D, Hynes RO. Reduced blood vessel formation and tumor growth in α5-integrin-negative teratocarcinomas and embryoid bodies. Cancer Res. 2001;61:5255–5261. [PubMed] [Google Scholar]
- Trusolino L, Bertotti A, Comoglio PM. A signaling adapter function for α6β4 integrin in the control of HGF-dependent invasive growth. Cell. 2001;107:643–654. doi: 10.1016/s0092-8674(01)00567-0. [DOI] [PubMed] [Google Scholar]
- Vuori K, Ruoslahti E. Association of insulin receptor substrate-1 with integrins. Science. 1994;266:1576–1578. doi: 10.1126/science.7527156. [DOI] [PubMed] [Google Scholar]
- Wang R, Kobayashi R, Bishop M. Cellular adherence elicits ligand-independent activation of the Met cell -surface receptor. Proc Natl Acad Sci USA. 1996;93:8425–8430. doi: 10.1073/pnas.93.16.8425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingerter P, Kazman I, Norberg S, Magnussen A, Zanivan S, Rissone A, et al. Uniform overexpression and rapid accessibility of α5β1 integrin on blood vessels in tumors. Am J Pathol. 2005;167:193–211. doi: 10.1016/s0002-9440(10)62965-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AST, Roskelley CD, Pelech SL, Miller D, Leung PCK, Auersperg N. Progressive changes in Met-dependent signaling in a human ovarian surface epithelial model of malignant transformation. Experimental Cell Res. 2004;299:248–256. doi: 10.1016/j.yexcr.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Yang JT, Rayburn H, Hynes RO. Embryonic mesodermal defects in β5 integrin-deficient mice. Development. 1993;119:1093–1105. doi: 10.1242/dev.119.4.1093. [DOI] [PubMed] [Google Scholar]
- Yokoyama Y, Sedgewick G, Ramakrishnan S. Endostatin binding to ovarian cancer cells inhibits peritoneal attachment and dissemination. Cancer Res. 2007;67:10813–10822. doi: 10.1158/0008-5472.CAN-07-0172. [DOI] [PubMed] [Google Scholar]
- Yu D, Wolf JK, Scanlon M, Price JE, Hung M-C. Enhanced c-erb B-2/neu expression in human ovarian cancer cells correlates with more severe malignancy that can be suppressed by E1A. Cancer Res. 1992;53:891–898. [PubMed] [Google Scholar]
- Zillhardt M, Christensen J, Lengyel E. An orally available small molecule inhibitor of c-Met, PF-2341066, reduces tumor burden in a pre-clinical model of ovarian cancer metastasis. Neoplasia. 2010;12:1–10. doi: 10.1593/neo.09948. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.