The modern management of sickle cell disease (SCD) is based on three therapeutic approaches: blood transfusion (first used successfully in 1818), penicillin (discovered in 1928) and hydroxycarbamide (first synthesized in 1869).1 Dresler and Stein made this simple molecule from hydroxylamine, hydrochloric acid and potassium cyanide as a technical exercise in organic chemistry, as part of a series of experiments generating derivatives of urea. Hydroxycarbamide lay dormant for more than fifty years until it was studied as part of an investigation into the toxicity of protein metabolites and found to produce a mega-loblastic anemia, which was thought to mimic pernicious anemia.2 In the early 1960s further in vitro studies showed that hydroxycarbamide had activity against leukemia cell lines and some tumors3 and this led to clinical studies showing particular activity in myleoproliferative disorders.4
Increased fetal hemoglobin (HbF) production has long been recognized as one of the key factors which can ameliorate SCD5 and in the 1970s 5-azacytidine was investigated as an HbF promoting agent because of its potential ability to reactivate silenced γ-globin genes by inhibiting the methylation of deoxycytidine. Although 5-azacytidine successfully increased HbF levels in baboons, and subsequently in patients with SCD and thalassemia, it was relatively toxic.6 Hydroxycarbamide was also used in the early baboon experiments, partly as a cytotoxic control because it was known to have no effect on methylation and, perhaps surprisingly, was also found to promote HbF synthesis. Because of concerns about the toxicity of 5-azacytidine, hydroxycarbamide was developed as a safer alternative and an initial study in 2 adults with sickle cell anemia (HbSS) (SCA) showed significant increases in both HbF and total hemoglobin.7 Further observational studies followed, before the Multicenter Study of Hydroxyurea (MSH) study was published in 1995. In this double-blind randomized controlled study, 152 adult patients with SCA were assigned to hydroxycarbamide and 147 given placebo; the hydroxycarbamide group showed reductions in the rate of acute pain (median 2.5 vs. 4.5 episodes per year, P<0.001), acute chest syndrome (25 vs. 51, P<0.001) and blood transfusion (48 vs. 73, P<0.001).8 The study was stopped early because of the reduction in acute pain in the hydroxycarbamide arm. The only other published randomized controlled study involved a single-blind crossover study of 25 children and young adults with SCA treated for six months with hydroxycarbamide and for six months with placebo; hydroxycarbamide showed a treatment effect on reducing the number of hospitalizations (P=0.0016) and total days in hospital (P=0.0027).9 Since then a steady stream of registry, observational and follow-up studies have followed, all showing similar beneficial effects with increases in HbF levels and reductions in some acute complications. Most notably, two observational studies have suggested increased survival associated with long-term hydroxycarbamide use. A follow-up study of the patients in the original MSH study showed a 40% reduction in mortality in those who chose to continue hydroxycarbamide after nine years (P=0.04);10 a non-randomized study of patients in Greece with SCA, HbS/β0thalassemia and HbS/β+thalassemia showed the probability of 10-year survival was 86% for those taking hydroxycarbamide and 68% for those not taking it (P=0.001).11
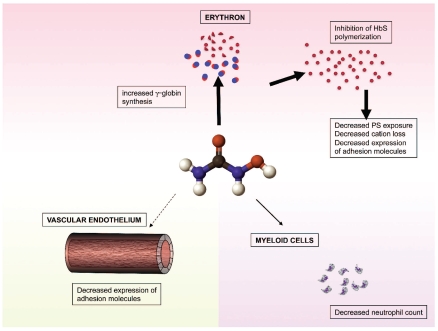
In parallel with clinical trials, laboratory studies have tried to identify how hydroxycarbamide works, although precise mechanisms of HbF promotion have not been defined. Hydroxycarbamide is an S-phase cytotoxic agent which does not demethylate DNA; it is thought to directly reduce DNA synthesis by inhibiting ribonucleotide reductase activity. This non-specific interruption of the cell cycle probably accounts for most of the HbF promoting activity. In cell culture systems hydroxycarbamide acts on both early and late erythroid progenitors to increase the total intracellular hemoglobin, γ-globin mRNA and HbF levels.12 There is also evidence that hydroxycarbamide can act as a nitric oxide donor and increase cGMP levels which accelerates translation of the γ globin gene.12 Despite uncertainty about its molecular mechanism of action, the in vivo effects of hydroxycarbamide on the blood in SCA are fairly well defined. The changes are dose-dependent and in addition to higher HbF levels include increased erythrocyte volume and hemoglobin content, decreased reticulocyte count, decreased white cell count and increased segmentation of the neutrophil nucleus. Further potentially beneficial erythrocyte changes have been noted, including reduced dehydration,13 reduced phosphatidylserine exposure, and reduced expression of adhesion molecules.14 These secondary erythrocyte changes, and most of the clinical benefit, seem likely to be directly related to the increased HbF levels and reduced hemoglobin polymerization within the red cell, resulting in reduced red cell damage (Figure 1); this interpretation is supported by studies in a mouse model of sickle cell disease in which HbF induction did not occur in response to hydroxycarbamide, and no hematologic or clinical benefit was seen.15 However, some therapeutic effects seem to occur independently of increases in HbF. The inevitable fall in white cell count which accompanies hydroxycarbamide use may be of rheological benefit, being significantly linked to clinical improvement in the MSH study, whereas HbF increase was not.16 Similarly, in this issue Laurance, et al. show that hydroxycarbamide acts directly on vascular endothelium to decrease the expression of some adhesion molecules, which is clearly independent of any effects on the β-globin gene family.17 Interestingly, Laurance et al. also show that the effects of hydroxycarbamide on adhesion molecules differed depending on whether the endothelial cells were from large or small blood vessels. This may be an important observation in explaining why hydroxycarbamide is more beneficial for some complications of SCD than others; for example, hydroxycarbamide may be more effective in preventing acute pain caused by microvascular complications than cerebrovascular disease in large blood vessels. Although the results of this study are potentially important, it is not clear how these findings will translate into clinical effects, and it seems likely that the effect of hydroxycarbamide on vascular endothelium is much less important than its promotion of γ-globin gene expression.
Figure 1.
Diagram showing three independent actions of hydroxycarbamide which are potentially of therapeutic benefit in preventing vaso-occlusion and vasculopathy. Most evidence exists for hydroxycarbamide’s action on erythroid cells (thick arrow), while observational studies have suggested that the reduced neutrophil count is also of therapeutic benefit (thin arrow). Laurance et al. in this issue showed in vitro evidence of hydroxycarbamide altering adhesion molecule expression in the vascular endothelium17 (dotted arrow) although the clinical significance of this is not yet known.
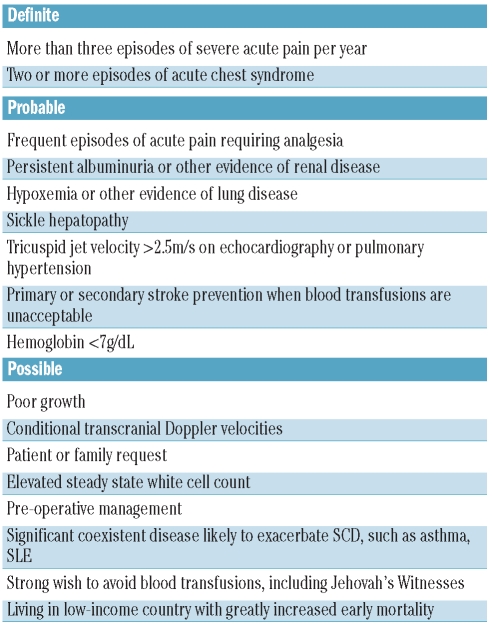
New clinical applications are beginning to emerge for hydroxycarbamide in SCD beyond its established use in reducing the frequency of acute pain and acute chest syndrome. There is observational evidence that hydroxycarbamide may improve hypoxemia, prevent cerebrovascular disease and reduce proteinuria.18 The evidence for these applications is provisional, although encouraging in some cases. However, a randomized controlled trial assessing hydroxycarbamide and venesection as an alternative to blood transfusion for secondary stroke prevention was stopped early last year with an excess of strokes in the hydroxycarbamide arm.5,18 A trial of hydroxycarbamide as primary stroke prevention in children with abnormal transcranial Doppler blood velocities is currently on-going.18 The suggested indications for hydroxycarbamide continue to expand, possibly ahead of the available supporting evidence (Table 1).
Table 1.
Definite, probable and possible indications for hydroxycarbamide in sickle cell disease.
Approximately 13% patients with SCA on the UK National Haemoglobinopathy Registry take hydroxycarbamide, with estimates in other countries varying from 10% to 30%,5 although precise figures are not available. In the USA, there is an emerging consensus that hydroxycarbamide is underused, with some suggesting that everyone with SCA should take hydroxycarbamide unless there is a definite contraindication. In Europe, there has generally been more caution. Short-term side-effects are few, but the theoretical possibilities of an increased risk of malignancy and reduced fertility continue to cause concern, particularly when prescribing the drug for young children who may take it for many decades. Anecdotal evidence suggests no increased risk of malignancy, and there is no convincing evidence of subfertility, although a small retrospective study found reduced sperm counts in men who had previously taken hydroxycarbamide.19
There is strong and increasing evidence that hydroxycarbamide is beneficial for many people with SCA, and probably also other types of sickle cell disease. It is certainly a much better drug than might have been expected when it was initially used to promote HbF in early studies. It has the great assets of being cheap, orally active and relatively free of side-effects, and also probably has some incidental therapeutic actions beyond its effects on HbF, as shown by Laurance et al.17 However, whilst it has been shown to alter the natural history of SCD, it is not in any sense curative and it is to be hoped that better drugs with more HbF promoting ability and other specific benefits are developed soon. For example, in this issue of Haematologica, Canalli et al. show interesting evidence that simvastatin may reduce the adhesiveness of leukocytes in SCD,20 and could potentially be useful as part of a multi-drug approach. Just as imatinib is now established as the treatment of choice in chronic myeloid leukemia, hopefully a new drug will be designed which specifically inhibits HbS polymerization and relegates hydroxycarbamide to the footnotes.
Footnotes
(Related Original Article on pages 526 and 534)
David Rees is a Senior Lecturer and Honorary Consultant in pediatric hematology at King’s College London and King’s College Hospital NHS Foundation Trust. He has a clinical and research interest in sickle cell disease and other inherited red cell disorders.
Financial and other disclosures provided by the author using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are available with the full text of this paper at www.haematologica.org.
References
- 1.Dresler WFC, Stein R. Ueber den Hydroxylharnstoff. Justus Liebigs Ann Chemie. 1869;150:1317–22. [Google Scholar]
- 2.Rosenthal F, Wislicki L, Kollek L. Uber die Bezeihungen von schwersten Blutgiften zu Abbauprodukten des Eiweisses. Ein Beitrag zum Entstehungsmecanismus der perniziosen Anamie. Klin Wochenschr. 1928;7:972–7. [Google Scholar]
- 3.Stearns B, Losee KA, Bernstein J. Hydroxyurea. A new type of potential antitumor agent. J Med Chem. 1963;6:201. doi: 10.1021/jm00338a026. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy BJ, Yarbro JW. Metabolic and therapeutic effects of hydroxyurea in chronic myelogenous leukemia. Trans Assoc Am Physicians. 1965;78:391–9. [PubMed] [Google Scholar]
- 5.Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010;376(9757):2018–31. doi: 10.1016/S0140-6736(10)61029-X. [DOI] [PubMed] [Google Scholar]
- 6.Charache S, Dover G, Smith K, Talbot CC, Jr, Moyer M, Boyer S. Treatment of sickle cell anaemia with 5-azacytidine results in increased fetal hemoglobin production and is associated with nonrandom hypomethylation of DNA around the γ-δ-β globin gene complex. Proc Natl Acad Sci USA. 1983;80:4842–6. doi: 10.1073/pnas.80.15.4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Platt OS, Orkin SH, Dover G, Beardsley GP, Miller B, Nathan DG. Hydroxyurea enhances fetal haemoglobin in sickle cell anaemia. J Clin Invest. 1984;74(2):652–6. doi: 10.1172/JCI111464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charache S, Terrin ML, Moore RD, Dover GJ, Barton FB, et al. Effect of hydroxyurea on frequency of painful crises in sickle cell anemia. N Engl J Med. 1995;332(20):1317–22. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- 9.Ferster C, Vermylen G, Cornu M, Buyse F, Corazza C, Devalck P, et al. Hydroxyurea for the treatment of severe sickle cell anaemia: a pediatric clinical trial. Blood. 1996;88:1960–4. [PubMed] [Google Scholar]
- 10.Steinberg MH, Barton F, Castro O, Pegelow CH, Ballas SK, Kutlar A, et al. Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: risks and benefits up to 9 years of treatment. JAMA. 2003;289(13):1645–51. doi: 10.1001/jama.289.13.1645. [DOI] [PubMed] [Google Scholar]
- 11.Voskaridou E, Christoulas D, Bilalis A, Plata E, Varvagiannis K, Stamatopoulos G, et al. The effect of prolonged administration of hydroxyurea on morbidity and mortality in adult patients with sickle cell syndromes: results of a 17-year, single-center trial (LaSHS) Blood. 2010;115(12):2354–63. doi: 10.1182/blood-2009-05-221333. [DOI] [PubMed] [Google Scholar]
- 12.Cokic VP, Smith RD, Beleslin-Cokic BB, Njoroge JM, Miller JL, Gladwin MT, Schechter AN. Hydroxyurea induces fetal hemoglobin by the nitric oxide-dependent activation of soluble guanylyl cyclase. J Clin Invest. 2003;111(2):231–9. doi: 10.1172/JCI16672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bridges KR, Barabino GD, Brugnara C, Cho MR, Christoph GW, Dover G, et al. A multiparameter analysis of sickle erythrocytes in patients undergoing hydroxyurea therapy. Blood. 1996;88(12):4701–10. [PubMed] [Google Scholar]
- 14.Styles LA, Lubin B, Vichinsky E, Lawrence S, Hua M, Test S, et al. Decrease of very late activation antigen-4 and CD36 on reticulocytes in sickle cell patients treated with hydroxyurea. Blood. 1997;89(7):2554–9. [PubMed] [Google Scholar]
- 15.Lebensburger JD, Pestina TI, Ware RE, Boyd KL, Persons DA. Hydroxyurea therapy requires HbF induction for clinical benefit in a sickle cell mouse model. Haematologica. 2010;95(9):1599–603. doi: 10.3324/haematol.2010.023325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charache S, Barton FB, Moore RD, Terrin ML, Steinberg MH, Dover GJ, et al. Hydroxyurea and sickle cell anemia. Clinical utility of a myelosuppressive “switching” agent. The Multicenter Study of Hydroxyurea in Sickle Cell Anemia. Medicine (Baltimore) 1996;75(6):300–26. doi: 10.1097/00005792-199611000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Laurance S, Lansiaux P, Pellay F-X, Hauchecorne M, Benecke A, Elion J, Lapoumeroulie C. Differential modulation of adhesion molecule expression by hydroxycarbamide in human endothelial cells from the micro- and macrocirculation: potential implications in sickle cell disease vasoocclusive events. Haematologica. 2011;96(4):534–42. doi: 10.3324/haematol.2010.026740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ware RE. How I use hydroxyurea to treat young patients with sickle cell anaemia. Blood. 2010;115(26):5300–11. doi: 10.1182/blood-2009-04-146852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berthaut I, Guignedoux G, Kirsch-Noir F, de Larouziere V, Ravel C, Bachir D, et al. Influence of sickle cell disease and treatment with hydroxyurea on sperm parameters and fertility of human males. Haematologica. 2008;93(7):988–93. doi: 10.3324/haematol.11515. [DOI] [PubMed] [Google Scholar]
- 20.Canalli AA, Proença RF, Franco-Penteado CF, Traina F, Sakamoto TM, Saad STO, et al. Participation of the Mac-1, LFA-1 and VLA-4 integrins in the in vitro adhesion of sickle cell disease neutrophils to endothelial layers, and reversal of adhesion by simvastatin. Haematologica. 2011;96(4):526–33. doi: 10.3324/haematol.2010.032912. [DOI] [PMC free article] [PubMed] [Google Scholar]