Introduction
The current classification of lymphoid neoplasms is based on the integrated utilization of morphological, immunohistochemical, genetic and clinical criteria to define disease entities, which putatively have their origin in a “normal counterpart” in the immune system.1 The application of modern immunophenotypic and molecular genetic techniques in routine diagnosis has made it possible for most lymphomas to be successfully diagnosed and classified as one of the currently recognized distinct disease entities. Nevertheless, there are cases that do not follow this principle and show overlapping or borderline morphological, biological and clinical features between various types of lymphomas. The updated 2008 World Health Organization (WHO) classification of tumors of hematopoietic and lymphoid tissues recognized this problem and introduced two new provisional categories of B-cell lymphoma, unclassifiable, one with features intermediate between diffuse large B-cell lymphoma (DLBCL) and Burkitt’s lymphoma and the second, which is the topic of this review, with features intermediate between DLBCL and classical Hodgkin’s lymphoma (CHL).2 This second provisional category recognizes cases that usually occur in the mediastinum and have features of both nodular sclerosis CHL (NSCHL) and primary mediastinal large B-cell lymphoma (PMBL). In this review PMBL will be briefly considered in order to provide the framework for understanding the concept of mediastinal gray zone lymphoma.
Primary mediastinal B-cell lymphoma
Primary mediastinal B-cell lymphoma arises in the mediastinum and presumably originates from a thymic B cell.1, 3 This lymphoma often presents in young women with a rapidly enlarging mediastinal mass with symptoms related to compression of intrathoracic structures. Histologically, this lymphoma is composed of an infiltrate of large cells with round or lobulated nuclei and abundant clear cytoplasm. Occasional pleomorphic, multilobated Reed-Sternberg-like cells can be seen raising the differential diagnosis of CHL. There is a characteristic compartmentalizing sclerosis in the background (Figure 1). Although most cases are diffuse, some show a focal nodularity mimicking NSCHL. The immunophenotype is that of a mature B cell expressing CD20, CD79a and PAX5 but the neoplastic cells lack surface immunoglobulin expression. In contrast to the situation in CHL, the B-cell-associated transcription factors OCT-2 and BOB.1 are strongly expressed.4 CD30 is often expressed, but weakly and heterogeneously compared to its expression in CHL. CD15 is always negative. The tumor cells usually express CD23 and MAL protein in 70% of the cases, both of which have been taken as evidence in favor of the thymic B-cell derivation.5–6 TRAF-1 and c-REL are expressed in PMBL, unlike other DLBCL, and reflect activation of the nuclear factor kappa B pathway.7 PMBL has characteristic genomic imbalances that include gains at chromosome 9p13.1–9p13.3 (70%), 9p23–9p24 (60%) and 2p15-p16.1 (50%).8 Candidate genes in these areas include REL and BCL11A, which are amplified in a proportion of cases. Further proof that PMBL is indeed a specific type of aggressive large B-cell lymphoma distinct from nodal DLBCL was provided by gene expression profiling studies by two independent groups.9–10 PMBL has a distinct gene signature with several highly expressed genes including MAL, CD23, FIG1, TARC, NFkB2 and PDL1/L2. Furthermore, the PMBL molecular signature was associated with a more favorable survival compared to those DLBCL cases with a non-germinal and germinal-center profile, supporting a distinct natural history for PMBL. Although all these findings helped to define PMBL as a distinct entity, perhaps the most surprising finding in these two studies was the fact that the molecular signature of PMBL was more closely related to CHL than to the other DLBCL subtypes.
Figure 1.
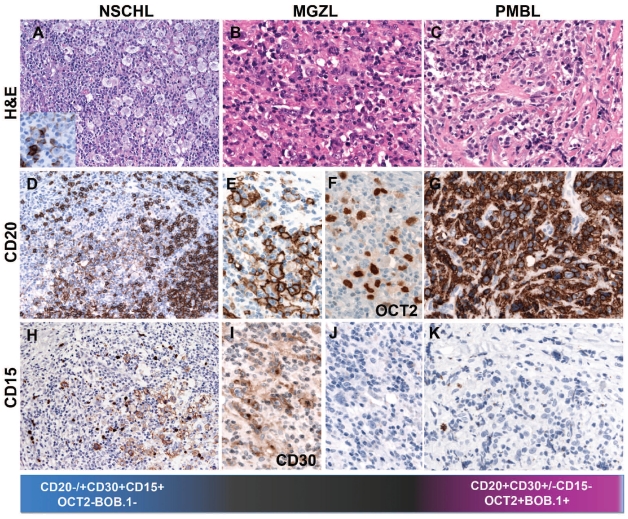
Biological continuum between CHL and PMBL. (A) Nodular sclerosis, classical Hodgkin’s lymphoma with abundant Hodgkin and Reed-Sternberg (RS) cells of lacunar type (H&E, 400x, insert: CD20 immunostain, 400x magnification). The RS cells are weakly and heterogeneously CD20 positive, in contrast to the strong CD20 positivity of the surrounding reactive B cells (immunostaining, 200x). The lacunar cells are CD15 positive (Immunostaining, 200x). (B) Mediastinal gray zone lymphoma with morphological features reminiscent of CHL but an immunophenotype more consistent with PMBL. Note the strong, homogeneous CD20 and OCT2 positivity. The tumor cells are CD15 negative (H&E and immunostaining, 400x). (C) Primary mediastinal B-cell lymphoma composed of an infiltrate of large cells with round or lobulated nuclei and abundant clear cytoplasm. In the background there is a characteristic compartmentalizing sclerosis (H&E, 400x) The tumor cells are CD20 positive and CD15 negative (immunostaining, 400x).
The relationship between primary mediatinal large B-cell lymphoma and nodular sclerosis classical Hodgkin’s lymphoma
The clinical, histopathological and molecular similarities between PMBL and NSCHL have long been recognized. Both entities predominantly affect young women who present with a mediastinal mass with involvement of the thymus and/or supraclavicular lymph nodes. The cell of origin in both disorders is a mature B cell with lack of immunoglobulin expression, presumably the thymic B cell. Further similarities between these two disorders are the amplification of the REL locus on chromosome 2p and the JAK2 locus on 9p found in both entities.8,11 Interestingly, gene-expression studies have shown striking molecular similarities between PMBL and NSCHL arising in the mediastinum, especially low expression of B-cell receptor components and the downstream signaling cascade and high expression of tumor necrosis factor family members, and extracellular matrix elements.9,10 These studies provided further evidence that PMBL and NSCHL arising in the mediastinum may represent related tumors with a common pathogenic pathway, at least in some stages of tumorigenesis. It is not, therefore, completely unexpected that some cases may show overlapping morphological and/or immunophenotypic features. Despite all these similarities, there are clear differences in the morphology and immunophenotype of the two disorders which make it possible to distinguish them in the vast majority of cases (Figure 1). Problems in the classification arise in tumors that show the morphologic features of PMBL but the expected immunophenotype is not found, e.g. absence of CD20 expression, strong expression of CD30 and CD15 or presence of Epstein-Barr virus. Equally problematic are those cases with typical morphology of NSCHL but with strong, homogeneous expression of CD20 and/or expression of the B-cell-associated transcription factors OCT2 and BOB.1 and absence of CD15 (Figure 1). Establishing the correct diagnosis has obvious clinical consequences, since historically CHL and NHL are treated differently. Nevertheless, cases with overlapping morphological and/or phenotypic features between NSCHL and PMBL are difficult to classify and represent a real challenge for pathologists who feel urged by their clinical colleagues to make a diagnosis. To reflect the morphological and biological difficulties in understanding these cases the term mediastinal gray-zone lymphoma (MGZL) was coined.12
Mediastinal gray-zone lymphoma
The 2008 WHO lymphoma classification introduced the provisional category of B-cell lymphoma, unclassifiable with features intermediate between DLBCL and CHL to encompass cases that do not fulfill the morphological and/or phenotypic criteria for PMBL or CHL in the mediastinum but exhibit transitional features between these two entities (MGZL).2 Another phenomenon related to MGZL is the occurrence of PMBL and CHL as composite or sequential lymphomas. Notably MGZL, in contrast to PMBL and CHL, is more common in young men and has a more aggressive clinical course and poorer outcome than either CHL or PMBL,13,14 which emphasizes the importance of keeping them separate with the hope that further studies will reveal whether these cases represent biologically true borderline cases or whether they can be assigned to a specific entity. In this issue of Haematologica, Eberle et al.,15 using methylation profiling provide further evidence for the existence of MGZL as a true biological phenomenon related to PMBL and CHL but with enough important differences to justify keeping it separate. The authors analyzed ten cases of NSCHL, ten cases of PMBL, ten cases of nodal DLBCL and eight cases of MGZL, one composite lymphoma and one case with sequential diagnoses of CHL and PMBL. The MGZL included four cases morphologically resembling NSCHL but with a PMBL phenotype (CD20+, CD15−) and four cases morphologically resembling PMBL but with a CHL phenotype (loss of B-cell markers and expression of CD30 and CD15). Regardless of the dominant morphology and/or phenotype, all analyzed cases clustered together in the methylation profile, indicating that biologically these cases were closely related. Interestingly, the authors developed a prediction model that could accurately distinguish between MGZL, NSCHL and PMBL. Of note, both components of one composite lymphoma whose two components were separately analyzed were predicted to be MGZL, suggesting that composite lymphomas do indeed most probably represent the two morphological ends of the spectrum of MGZL. This raises the important question of why some MGZL develop a phenotype closer to PMBL and others to CHL if they all originate from the thymic B cell. In recent years there has been a greater appreciation of lineage plasticity or reprogramming capabilities within the hematopoietic system, which opens the possibility that the phenotype of the transformed thymic B-cell might be primarily influenced by epigenetic changes leading the tumor to differentiate in one direction or the other. This might explain the discordant phenotypes of MGZL or why cases that originally present as CHL, recur as PMBL or vice versa. Accordingly, Eberle et al. found that NSCHL is characterized by de novo hypermethylation typically located within CpG islands, confirming previous findings indicating that the development of the Reed-Sternberg cell may be induced by gene silencing due to DNA methylation.16–17 In contrast, PMBL was characterized by both de novo hypomethylation and hypermethylation. MGZL showed a methylation profile related to but different from that of both PMBL and NSCHL and fell somewhere in the middle. A unique feature found in MGZL was the hypomethylation of HOXA5, but the importance of this finding for the pathogenesis of MGZL is still to be determined. One additional important finding was that the methylation profile of nodal DLBCL was completely different from that of both PMBL and CHL, supporting the conclusions of published gene expression studies. The study by Eberle et al.15 clearly demonstrates that MGZL is not just a result of diagnostic uncertainty but represents a true “gray zone”, indicating that there is a biological continuum between NSCHL and PMBL.
Diagnostic and clinical perspectives
As new studies dedicated to understanding the biology underlying MGZL become available, two issues remain that need to be resolved urgently. First, what are the minimal morphological and/or phenotypic criteria required to put a case into the unclassifiable category? Although most experts agree that the deviation of a single marker or criterion, e.g., CD20 expression in otherwise typical CHL, is not sufficient to put a case in the unclassifiable category, the expression of CD15 in an otherwise typical PMBL would be.14 One of the major unresolved problems is the meaning and interpretation of CD20 expression in CHL. As antigen retrieval methods for immunohistochemistry have improved, CD20 positivity in Reed-Sternberg cells is no longer a rare finding although its expression is usually weak and heterogeneous. From the practical point of view, strong and uniform expression of CD20 in CHL should be regarded as abnormal and prompt the pathologist to determine the complete phenotype (looking at other B-cell markers and the B-cell transcription factors BOB.1 and OCT2) to exclude the possibility of a hybrid phenotype not in line with current diagnostic and biological concepts. This exercise is not academic but rather clinically relevant since it will influence the treatment and prognosis of these patients. Although the prognostic importance of CD20 expression in CHL is still controversial, two independent studies have shown that patients with CD20+ CHL do poorly in clinical trials compared to cases with the classical phenotype (CD20− CD30+CD15+).18,19 It should also be noted that, in the light of current knowledge, some of these patients would very probably now be given different diagnoses, including MGZL. The results cannot, therefore, be extrapolated to those obtained with modern standards and used to draw conclusions about the poor prognosis or optimal treatment for MGZL.
This brings us to the second question: what is the best treatment for MGZL? Are these tumors more aggressive because of their biology or have they appeared more aggressive because they have been treated suboptimally? Ideally, a clinical trial for MGZL should answer these questions; however, due to the rareness of the disorder and the lack of uniform diagnostic criteria it is improbable that such a trial can be performed, at least in the near future. Nevertheless, the tendency, more anecdotal than evidence-based, is to treat these patients with therapy for aggressive B-cell non Hodgkin’s lymphoma. More studies like the one from Eberle et al.15 are needed to understand the epigenetic and genetic events underlying the pathogenesis of MGZL in order to facilitate the future development of novel and more successful therapeutic options for this disease.
Footnotes
(Related Original Article on page 558)
Financial and other disclosures provided by the author using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are available with the full text of this paper at www.haematologica.org.
References
- 1.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al., editors. WHO Classification of Tumour of Haematopoietic and Lymphoid Tissues. Lyon: 2008. [Google Scholar]
- 2.Jaffe ES, Stein H, Swerdlow SH, Campo E, Pileri S, Harris NL IARC. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: 2008. B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and classical Hodgkin Lymphoma; pp. 267–8. [Google Scholar]
- 3.Möller P, Moldenhauer G, Momburg F, Lämmler B, Eberlein-Gonska M, Kiesel S, Dörken B. Mediastinal lymphoma of clear cell type is a tumor corresponding to terminal steps of B cell differentiation. Blood. 1987;69(4):1087–95. [PubMed] [Google Scholar]
- 4.Pileri SA, Gaidano G, Zinzani PL, Falini B, Gaulard P, Zucca E, et al. Primary mediastinal B-cell lymphoma: high frequency of BCL-6 mutations and consistent expression of the transcription factors OCT-2, BOB.1, and PU.1 in the absence of immunoglobulins. Am J Pathol. 2003;162(1):243–53. doi: 10.1016/s0002-9440(10)63815-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calaminici M, Piper K, Lee AM, Norton AJ. CD23 expression in mediastinal large B-cell lymphomas. Histopathology. 2004;45(6):619–24. doi: 10.1111/j.1365-2559.2004.01969.x. [DOI] [PubMed] [Google Scholar]
- 6.Copie-Bergman C, Plonquet A, Alonso MA, Boulland ML, Marquet J, Divine M, et al. MAL expression in lymphoid cells: further evidence for MAL as a distinct molecular marker of primary mediastinal large B-cell lymphomas. Mod Pathol. 2002;15(11):1172–80. doi: 10.1097/01.MP.0000032534.81894.B3. [DOI] [PubMed] [Google Scholar]
- 7.Rodig SJ, Savage KJ, LaCasce AS, Weng AP, Harris NL, Shipp MA, et al. Expression of TRAF1 and nuclear c-Rel distinguishes primary mediastinal large cell lymphoma from other types of diffuse large B-cell lymphoma. Am J Surg Pathol. 2007;31(1):106–12. doi: 10.1097/01.pas.0000213334.40358.0e. [DOI] [PubMed] [Google Scholar]
- 8.Joos S, Otaño-Joos MI, Ziegler S, Brüderlein S, du Manoir S, Bentz M, et al. Primary mediastinal (thymic) B-cell lymphoma is characterized by gains of chromosomal material including 9p and amplification of the REL gene. Blood. 1996;87(4):1571–8. [PubMed] [Google Scholar]
- 9.Rosenwald A, Wright G, Leroy K, Yu X, Gaulard P, Gascoyne RD, et al. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med. 2003;198(6):851–62. doi: 10.1084/jem.20031074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savage KJ, Monti S, Kutok JL, Cattoretti G, Neuberg D, De Leval L, et al. The molecular signature of mediastinal large B-cell lymphoma differs from that of other diffuse large B-cell lymphomas and shares features with classical Hodgkin lymphoma. Blood. 2003;102(12):3871–9. doi: 10.1182/blood-2003-06-1841. [DOI] [PubMed] [Google Scholar]
- 11.Joos S, Küpper M, Ohl S, von Bonin F, Mechtersheimer G, Bentz M, et al. Genomic imbalances including amplification of the tyrosine kinase gene JAK2 in CD30+ Hodgkin cells. Cancer Res. 2000;60(3):549–52. [PubMed] [Google Scholar]
- 12.Rüdiger T, Jaffe ES, Delsol G, deWolf-Peeters C, Gascoyne RD, Georgii A, et al. Workshop report on Hodgkin's disease and related diseases ('grey zone' lymphoma) Ann Oncol. 1998;9 (Suppl 5):S31–8. doi: 10.1093/annonc/9.suppl_5.s31. [DOI] [PubMed] [Google Scholar]
- 13.Traverse-Glehen A, Pittaluga S, Gaulard P, Sorbara L, Alonso MA, Raffeld M, Jaffe ES. Mediastinal gray zone lymphoma: the missing link between classic Hodgkin's lymphoma and mediastinal large B-cell lymphoma. Am J Surg Pathol. 2005;29(11):1411–21. doi: 10.1097/01.pas.0000180856.74572.73. [DOI] [PubMed] [Google Scholar]
- 14.Quintanilla-Martinez L, de Jong D, de Mascarel A, Hsi ED, Kluin P, Natkunam Y, et al. Gray zones around diffuse large B cell lymphoma. Conclusions based on the workshop of the XIV meeting of the European Association for Hematopathology and the Society of Hematopathology in Bordeaux, France. J Hematop. 2009;2(4):211–36. doi: 10.1007/s12308-009-0053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eberle FC, Rodriguez-Canales J, Wei L, Hanson JC, Killian JK, Sun H-W, et al. Methylation profiling of mediastinal gray zone lymphoma reveals a distinctive signature with elements shared by classical Hodgkin's lymphoma and primary mediastinal large B-cell lymphoma. Haematologica. 2011;96(4):558–66. doi: 10.3324/haematol.2010.033167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ehlers A, Oker E, Bentink S, Lenze D, Stein H, Hummel M. Histone acetylation and DNA demethylation of B cells result in a Hodgkin-like phenotype. Leukemia. 2008;22(4):835–41. doi: 10.1038/leu.2008.12. [DOI] [PubMed] [Google Scholar]
- 17.Ushmorov A, Ritz O, Hummel M, Leithäuser F, Möller P, Stein H, Wirth T. Epigenetic silencing of the immunoglobulin heavy-chain gene in classical Hodgkin lymphoma-derived cell lines contributes to the loss of immunoglobulin expression. Blood. 2004;104(10):3326–34. doi: 10.1182/blood-2003-04-1197. [DOI] [PubMed] [Google Scholar]
- 18.von Wasielewski R, Mengel M, Fischer R, Hansmann ML, Hübner K, Franklin J, et al. Classical Hodgkin's disease. Clinical impact of the immunophenotype. Am J Pathol. 1997;151(4):1123–30. [PMC free article] [PubMed] [Google Scholar]
- 19.Portlock CS, Donnelly GB, Qin J, Straus D, Yahalom J, Zelenetz A, et al. Adverse prognostic significance of CD20 positive Reed-Sternberg cells in classical Hodgkin's disease. Br J Haematol. 2004;125(6):701–8. doi: 10.1111/j.1365-2141.2004.04964.x. [DOI] [PubMed] [Google Scholar]