Abstract
Background
To understand how myelodysplastic syndrome cells evolve from normal stem cells and gain competitive advantages over normal hematopoiesis, we established a murine xenograft model harboring bone marrow cells from patients with myelodysplastic syndromes or acute myeloid leukemia with myelodysplasia-related changes.
Design and Methods
Bone marrow CD34+ cells obtained from patients were injected, with or without human mesenchymal stem cells, into the bone marrow of non-obese diabetic/severe combined immunodeficient/IL2Rγnull hosts. Engraftment and differentiation of cells derived from the patients were investigated by flow cytometry and immunohistochemical analysis.
Results
Co-injection of patients’ cells and human mesenchymal stem cells led to successful engraftment of patient-derived cells that maintained the immunophenotypes and genomic abnormalities of the original patients. Myelodysplastic syndrome-originated clones differentiated into mature neutrophils, megakaryocytes, and erythroblasts. Two of the samples derived from patients with acute myeloid leukemia with myelodysplasia-related changes were able to sustain neoplastic growth into the next generation while these cells had limited differentiation ability in the murine host. The hematopoiesis of mice engrafted with patients’ cells was significantly suppressed even when human cells accounted for less than 1% of total marrow mononuclear cells. Histological studies revealed invasion of the endosteal surface by patient-derived CD34+ cells and disruption of extracellular matrix architecture, which probably caused inhibition of murine hematopoiesis.
Conclusions
We established murine models of human myelodysplastic syndromes using cells obtained from patients: the presence of neoplastic cells was associated with the suppression of normal host hematopoiesis. The efficiency of engraftment was related to the presence of an abnormality in chromosome 7.
Keywords: xenograft, MDS, NOG mouse, niche, MSC
Introduction
Myelodysplastic syndromes (MDS) are a heterogeneous group of clonal hematopoietic disorders originating from primitive hematopoietic cells and some of the least studied hematopoietic malignancies due largely to difficulties in creating an in vivo model suitable for studying the biology of MDS since these syndromes cause variable degrees of morphological dysplasia in non-lymphoid lineages and accompanying hematopoietic failure.1,2 The prognosis of MDS patients is generally poor with an approximately 25% risk of the disease evolving into acute myeloid leukemia (AML).3 A wide variety of cytogenetic abnormalities is recognized in nearly half of MDS patients.4,5 Although a multi-step process of disease development has been proposed,6–9 the current understanding of the molecular pathogenesis of this disease is limited and, consequently, the precise mechanisms of how MDS cells evolve from normal hematopoietic cells remain unclear.
Mouse models of human diseases have been proven to be useful tools for elucidating the biology of various diseases and for evaluating the efficacy of evolving therapy.10 The successful establishment of murine xenograft models for human AML has yielded empirical evidence for the existence of so-called ‘cancer stem cells’, a minor subpopulation of cells responsible for maintenance of neoplastic proliferation.11–13 In addition, recent studies demonstrated that chemotherapy-resistant leukemic stem cells reside in the endosteal region of bone marrow.14,15 These findings helped to clarify how acute leukemia cells are maintained and propagated in vivo; however, little is known about the behavior of MDS cells in the bone marrow microenvironment partly because of the difficulties in obtaining a suitable in vivo model for this disease. The reason for the selective outgrowth of MDS clones and the concurrent decrease in normal hematopoietic stem cells in patients does, therefore, remain elusive.
To establish a murine model of human MDS, which would undoubtedly be of benefit in the study of the pathology and biology of MDS, we transplanted bone marrow CD34+ cells from patients with MDS and acute myeloid leukemia with myelodysplasia-related changes (AML-MRC) and human mesenchymal stem cells (MSC) as auxiliary cells in murine bone marrow using an established intramedullary co-transplantation method.
Design and Methods
Patients and preparation of human cells
The experimental protocol of this study was approved by the Institutional Review Board of Tokai University, School of Medicine, and all human samples were handled accordingly. Bone marrow samples were obtained from six patients with MDS, eight patients with AML-MRC, and four healthy individuals after obtaining written informed consent. The clinical characteristics and immunophenotypes of the patients are summarized in Online Supplementary Tables S1 and S2, respectively. CD34+ cells were selected using the CD34 Progenitor Cell Isolation Kit (Miltenyi Biotec, Sunnyvale, CA, USA) according to the manufacturer’s instructions as described previously.16 The purity of the selected bone marrow CD34+ cells was always more than 95%. Human MSC were purchased from Lonza Walkersville Inc. (Walkersville, MD, USA) and cultured according to the directions supplied by the company. In some experiments, MSC were established from the CD34− fraction of patients’ cells. The ability of the cells to differentiate into adipocytes, chondrocytes and osteoblasts was assessed and confirmed in vitro as described previously17 before the cells were used for this study (data not shown).
Antibodies
The following monoclonal antibodies were used for flow cytometry: anti-CD7 (4H9), -CD11b (D12), -CD13 (L138), -CD14 (MφP9), -CD19 (SJ25C1), -CD36 (CB38(NL07)), -CD38 (HB7), -CD56 (MY31), -CD61 (VI-PL2), -CD64 (10.1), and -HLA-DR (L243, all from BD Biosciences, San Jose, CA, USA); anti-CD33 (WM53), -CD34 (581), -CD41b (P2), -CD45 (J.33), and CD117 (95C3, all from Coulter/Immunotech, Marseille, France); and MPO (MPO-7, DACO, Denmark).
The following antibodies were used for tissue immunostaining: anti-CD15 (80H5, 1:150, Coulter/Immunotech); anti-CD31 (1:100, TECNE Corporation, Minneapolis, MN, USA); anti-CD34 (My10, 1:20), -CD45 (2D1, 1:200), and -CD38 (HIT2, 1:100, all from BD Biosciences); anti-glycophorin A (JC159, 1:400) and -CD61(Y2/51, 1:1000, both from DACO); anti-fibronectin (1:400, Sigma, St Louis, MO, USA); and anti-PCNA (1:200, abcam, Cambridge, UK).
Experimental animals, lentiviral gene transduction, and cell transplantation
Non-obese diabetic/severe combined immunodeficient/IL2Rγnull (NOG) mice were maintained in sterile microisolator cages in the animal facility of Tokai University School of Medicine. The mice were irradiated with 250 cGy from an X-ray irradiator (HW-300, Hitex, Osaka, Japan) 24 h prior to intramedullary transplantation of cells. All procedures were approved by the Animal Care Committee of Tokai University. The MSC were transduced with the GFP gene as described previously.17
Analysis of human cells
The mice were killed humanely 8 to 16 weeks after transplantation, and the entire bone marrow contents of the injected tibiae were collected in phosphate-buffered saline containing 0.5% bovine serum albumin and 0.5 M EDTA. The total number of bone marrow mononuclear cells was counted for each bone of individual experimental animals. The number of non-human bone marrow cells was obtained by calculation. Aliquots of cells were used to examine the percentages of cells expressing human cell surface antigens. A four-color flow cytometric analysis was conducted using FACSCaliber. Quadrants were set to include at least 97% of the isotype-negative cells. The proportion of each lineage was calculated from 10,000 events acquired using the CELLQuest software package. The remaining cells were saved for secondary transplantation, cytospin preparation for morphological examination, chromosomal analysis and fluorescence in situ analysis (FISH). Chromosomal analysis was conducted using a conventional method in the clinical laboratory of the University Hospital, while the FISH analysis was performed at SRL Inc. (Tokyo, Japan). The preparation of the bone marrow for histological studies, immunofluorescent staining and enzyme immunohistochemistry were performed as described previously.18 Images of stained slides were captured using an LSM510 META confocal microscope with a 63X/1.2 numeric aperture c-Apochromat objective lens (Carl Zeiss, Jena, Germany) and an Olympus Ax80 microscope with a 20X/0.70 numeric aperture UplanApo lens equipped with a DP71 digital camera (Olympus, Japan). Images were transferred to Adobe Photoshop CS4 (Adobe Systems, San Jose, CA, USA)
Histological analysis of bone
For serial transplantation experiments, the percentage of cells in the endosteal region (within 5 cells’ distance) was obtained by counting the cells in the entire field of bone specimens under the light field microscope. More than five slides were examined for each transplant.
Statistics
Data are presented as the mean ± standard deviation. The two-sided P value was determined by testing the null hypothesis that the two population medians are equal. P values less than 0.05 were considered to be statistically significant.
Results
Engraftment of myelodysplastic syndrome-originated human hematopoietic cells in murine bone marrow
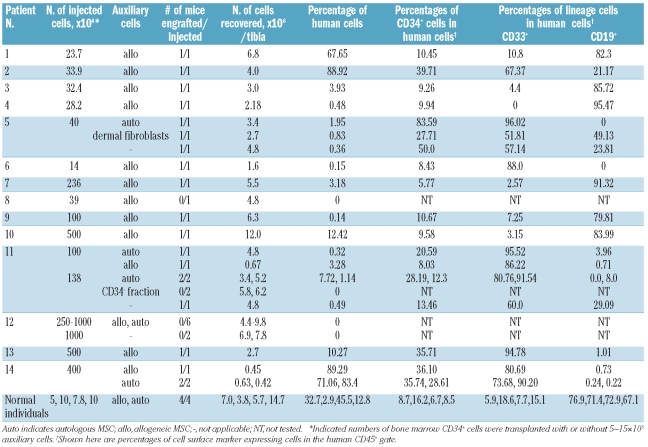
We previously reported that intramedullary injection of cord blood hematopoietic stem cells along with human MSC improved engraftment of human cells in the murine microenvironment.18 We, therefore, transplanted bone marrow CD34+ cells, which included hematopoietic stem cells and primitive progenitors, obtained from six patients with MDS, eight patients with AML-MRC and four healthy individuals, into the bone marrow of NOG mice with or without human MSC. Flow cytometric analysis detected the presence of human CD45+ cells, at varying frequencies, in the bone marrow of 8/8, 12/23, and 4/4 recipient mice injected with bone marrow CD34+ cells from the MDS patients, the AML-MRC patients, and the healthy individuals, respectively (Table 1). As expected, transplantation of MSC alone did not result in hematopoietic engraftment (data not shown). Further lineage analysis revealed a CD33+ myeloid dominant differentiation, 60% or more, in three of six MDS cases (3/8 mice engrafted) and three of eight AML-MRC cases (9/12 mice engrafted), suggesting the engraftment of MDS-originated cells (Table 1; patients 2, 5, 6, 11, 13, and 14, and Figure 1A). Human cells recovered from transplanted animals were positive for cell surface markers found on the original patients’ cells (Online Supplementary Table S2), such as CD13 (74.90%) and CD56 (32.08%) for patient 11, CD7 (72.13%) and CD41b (60.12%) for patient 13, and CD13 (81.92%, 37.86% of which co-expressed CD34) and CD117 (31.41%) for patient 14.
Table 1.
Engraftment of bone marrow CD34+ cells obtained from MDS and AML-MRC patients.
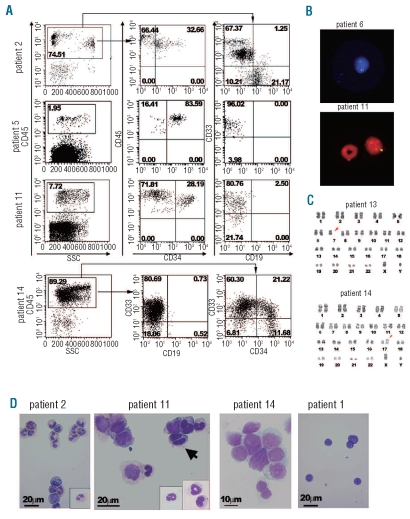
Figure 1.
Engraftment of human MDS-originated hematopoietic cells in the bone marrow (BM) of NOG mice. (A) Representative flow cytometric profiles of BM cells recovered from mice engrafted with patients’ BM cells. The majority of human CD45-expressing cells were positive for a myeloid marker CD33 in patients 5, 11, and 14, while some CD19+ cells were present in BM cells recovered from the mouse engrafted with cells from patient 2. For patient 14, approximately one quarter of CD33+ cells co-expressed CD34. The percentages of cells in the respective regions are shown. (B) FISH detection of a partial deletion of chromosome 7 and monosomy 7. Human cells recovered from the mice engrafted with BM cells from patient 6 and patient 11 were subjected to FISH analysis using D7Z1 (green signal for centromere of chromosome 7) plus D7S486 (red signal for 7q31 region) probes for patient 6 and D7Z1 (yellow signal) probe for patient 11. In a lower panel, a murine granulocyte with a ring-shaped nucleus which did not hybridize with the human probe is located adjacent to the human cell hybridized with D721. All cells analyzed (10 cells for patient 6 and 100 cells for patient 11) demonstrated the same outcome. (C) Chromosomal analysis of cells recovered from the mice transplanted with MDS-originated cells obtained from the BM of patient 13 and patient 14 demonstrated the maintenance of the original abnormal karyotype, namely isochromosome 17 and monosomy 7 (arrows), respectively. Eight cells were analyzed for patient 13 and 20 cells for patient 14. (D) Wright-Giemsa-stained cytospin preparations made of CD45-sorted human cells. In the cytospin samples for patient 2, various stages of myeloid lineage cells and an eosinophil are shown. An insert shows a myelocyte with pseudo-Pelger anomaly. For patient 11, an arrow indicates a bi-nucleated myelocyte. Inserts show differentiated neutrophils. The majority of cells found in a cytospin preparation of BM cells obtained from the mice engrafted with cells from patient 14 demonstrated fine chromatin formation and conspicuous nucleoli. Cytospin samples of a normal cell-engrafted mouse (patient 1) were composed of lymphocytes.
To confirm that this was indeed engraftment of MDS-originated cells, we performed cytogenetic and morphological analyses on human cells recovered from the mice engrafted with patients’ bone marrow cells. FISH analysis confirmed cytogenetic abnormalities of original bone marrow in the human cells isolated from mice engrafted with cells from patients 6, 11, and 13 (Figure 1B and Online Supplementary Table S3) in 100% of the cells analyzed. In addition, patient-specific chromosomal abnormalities (monosomy 7 for patient 13 and isochromosome 17 for patient 14) were detected in 100% of cells analyzed (Figure 1C). Morphological observations of cytospin samples and bone marrow histology of mice engrafted with bone marrow cells from patients 2 and 11 showed dysplasia typically associated with MDS, such as bi-nucleated myelocytes and megakaryocytes with separated nuclei (Figures 1D and 2A, and data not shown). In samples prepared with cells recovered from mice engrafted with bone marrow cells from patient 2, myelocytes with variable degrees of normal differentiation were easily seen, but there were also sporadic cells with dysplasia which were not seen in samples from animals engrafted with normal human bone marrow cells. Large blastic cells were prominent in cytospin samples of bone marrow cells prepared from mice engrafted with cells from patients 13 and 14 (Figure 1D and data not shown). Considering these findings collectively, mice injected with bone marrow cells from patients 2, 5, 6, 11, 13, and 14 were engrafted with MDS-originated cells. Five of these patients harbored one or more genetic abnormalities, most of which were abnormalities in chromosome 7 (Online Supplementary Table S1). In contrast, human cell engraftment of mice transplanted with cells obtained from normal individuals and patients 1, 3, 4, 7, 9, and 10 consisted mainly of B-lineage cells, typical of normal human cell differentiation in the NOG mice environment.19–21 Analyses of cytospin samples prepared from human cells recovered from the bone marrow of these mice confirmed a B-cell dominant differentiation (Figure 1D). In addition, no clonal markers specific to patients’ phenotype were detected by FISH analysis (Online Supplementary Table S3, patients 9 and 10). The human engraftment in mice injected with cells from these patients was, therefore, considered to come from a minor population of normal hematopoietic stem cells co-existing in the patients’ bone marrow CD34+ cells.
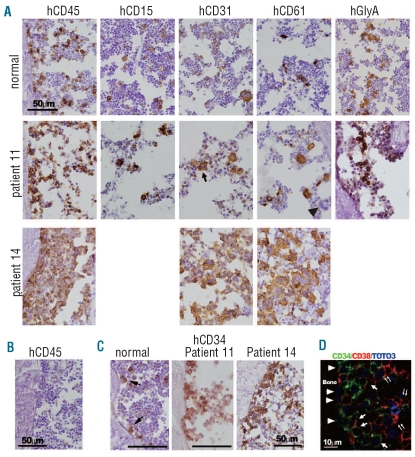
Figure 2.
Histological analysis of human MDS-originated cells in murine bone marrow (BM). (A) Immunohistochemical staining of bone sections of the mice engrafted with BM cells from normal individuals and patients 11 and 14. Human cells were recognized by specific staining for human antigens. Cells that reacted with antibodies specific to human CD45, CD15, CD31, CD61, and glycophorin A (GlyA) were detected throughout the murine BM compartment in the bone samples of normal individuals and patient 11. Relatively small megakaryocytes with separated nuclei (an arrow) were often observed in the CD31 stained sections of the mice engrafted with cells from patient 11. Note, murine megakaryocytes are negative for human CD61 (an arrowhead), confirming the specificity of the antigen-antibody reaction. In bone samples of patient 14, human CD45-expressing cells occupied most of the marrow compartment. Cells expressing megakaryocytic markers, CD31 and CD61, were noticeable. (B) A human CD45-stained bone section of contra-lateral tibia of the mice intramedullary injected with MDS-originated bone marrow CD34+ cells from patient 11 and MSC. Neither obvious hypocellularity nor human CD45+ cells were detected. (C) In bone samples of patient 11, MDS-originated CD34+ cells proliferated along the surface of the endosteum, while individual CD34+ cells (arrows) attached to the endosteum in normal cell-engrafted mice. Invasion of CD34+ cells was prominent in bones engrafted with cells from patient 14. (D) The cells expressing only CD34 (arrowheads) attached to the endosteum, but the cells expressing both CD34 and CD38 did not (arrows). The cells expressing only CD38 (double arrows) were located distant from the bone.
Co-transplantation of bone marrow CD34+ cells along with human MSC facilitated the engraftment of MDS-originated cells (Table 1; patients 5 and 11). In mice transplanted with bone marrow CD34+ cells and MSC, more than 80% of human cells expressed CD33 while less than 4% expressed CD19 (5/5), in contrast to the mice transplanted with bone marrow CD34+ cells alone (23.81% and 29.09% CD19+ cells) or in combination with dermal fibroblasts (49.13% CD19+ cells) in which B-cell proliferation was more notable (3/3), thus suggesting that normal human cells were also engrafted. The co-injection of the CD34−fraction of bone marrow cells did not yield any human cell engraftment (2/2). These results indicate the unique property of MSC of facilitating the engraftment of MDS-originated cells.
Histological analysis of the bone marrow compartment of mice engrafted with myelodysplastic syndrome-originated cells
Successful engraftment of MDS-originated cells from six patients prompted us to dissect out the phenotypes of MDS-originated cells in the murine bone marrow environment. Although MDS is normally a disease of normal- to hyper-cellularity, the total number of bone marrow cells recovered from injected tibiae of the mice engrafted with MDS-originated cells was significantly lower than that of the mice engrafted with normal cells (Table 1, MDS-originated cell engrafted tibiae: 2.48±1.81×106, n=11, normal cell engrafted tibiae: 5.59±3.14×106, n=13, P=0.004). We then analyzed histological sections of mice engrafted with bone marrow cells from patients 11, 13, and 14. Consistent with the above finding, the marrow of the animals engrafted with MDS-originated cells from patient 11 appeared distinctly hypocellular compared to the marrow of normal cell-engrafted animals (Figure 2A). Human cells expressing human CD45, CD15, CD31, CD61, or glycophorin A (GlyA) were scattered throughout the marrow compartment in the injected tibiae of mice engrafted with normal cells or cells from patient 11. Neither human hematopoietic cells nor mesenchymal cells were observed in the contralateral tibiae of the same mice (Figure 2B and data not shown), consistent with our previous findings that intramedullary injected cells, especially when a limited number of cells were used, had a tendency to stay in the injected tibia.16,18
The cytology and bone histology of the mice engrafted with cells from patients 13 and 14 revealed that most of the marrow compartment was filled with large human CD45+ leukemic blasts with prominent nucleoli (Figures 1D and 2A, and data not shown), leaving little space for normal murine hematopoiesis. Consistently, the number of non-human cells in the tibiae of mice engrafted with MDS-originated cells was significantly lower than that of the mice engrafted with normal cells (MDS-originated cell-engrafted tibia: 1.98±1.8×106, n=11, normal cell-engrafted tibia: 4.59±2.7×106, n=13, P=0.006). Although all experimental animals were irradiated equally, to exclude a possibility of heterogeneous response to sublethal irradiation as a cause of this decreased bone marrow cellularity in tibiae engrafted with MDS-originated cells, the number of non-human cells was also compared with that in the contralateral tibia of the same mice. The number of non-human cells in the injected tibia was significantly lower in the mice engrafted with MDS-originated cells (injected tibia: 3.08±1.35×106, contralateral tibia: 5.12±0.84×106, n=4, P=0.02), while there was no difference in non-human cell cellurality in mice engrafted with normal cells (injected tibia: 5.4±0.8×106, contralateral tibia: 5.2±1.2×106, n=3, P=0.4). The cell surface phenotypes of cells from patients 13 and 14 in murine bone marrow were primarily CD34+, CD31+, or CD61+. Unlike the mice engrafted with normal cells or bone marrow cells from patient 11, the cells derived from patients 13 and 14 in the bone marrow rarely expressed CD15 or GlyA, two lineage markers used in this histological study.
Interestingly, proliferating primitive CD34+ cells were prominently clustered along the endosteum, contrasting with the bone marrow of animals transplanted with normal CD34+ cells in which individual CD34+ cells attached to the endosteum (patient 11, Figure 2C). The endosteal surface of primary recipient mice for patient 14 was covered with CD34+ cells, indicating an invasion of the putative hematopoietic stem cell niche by MDS-originated CD34+ cells (Figure 2C). Consistent with a previous report,15 many of the MDS-originated CD34+ cells adhering to the endosteal surface lacked CD38 expression. The immunophenotypes of human cells gradually changed from CD34+CD38− to CD34+CD38+, and eventually to CD34−CD38+ as cells were located further away from the endosteum (Figure 2D).
Sequential engraftment of myelodysplastic syndrome-originated cells
Serial transplants were conducted using bone marrow cells recovered from mice engrafted with bone marrow CD34+ cells from patients 11, 13, and 14. MDS-originated cells from patients 13 and 14, two patients whose cells demonstrated limited differentiation ability in murine bone marrow, but not from patient 11, successfully engrafted in secondary recipient mice (Figure 3A). In addition, it was possible to maintain the MDS-originated bone marrow cells from patient 14 for more than 2 years in vivo through passaging until the 8th recipient. The immunophenotypes of the engrafted cells were basically maintained throughout the experiments despite a gradual decline in the percentages of human cells over the period of the serial transplants. Interestingly, the frequency of CD34-expressing cells increased in the later transplant animals (Online Supplementary Table S4 and Figure 3B), even though the frequency of CD34+ cells (%) in the endosteal area (within the distance of 5 cells) declined (53.79 ± 3.23, 40.56 ± 1.95, 38.85±0.87, and 29.88±6.74, for the 3rd, 5th, 6th, and 7th transplants, respectively), indicating a widespread distribution of primitive CD34+ cells and, thus, the selection or overgrowth of blastic cells against lineage differentiation. As expected, CD34+ cells, but not CD34− cells, were able to sustain neoplastic cell growth into the next generation (Online Supplementary Table S4). Importantly, fluorescent activated cells sorting (FACS) analysis of human cells recovered from the engrafted mice demonstrated the maintenance of approximately the same proportion of CD34+CD38− cells, a sub-population of cells that includes leukemic stem cells,13,15 until the 7th engraftment even though the overall human cell chimerism declined (Figure 3B).
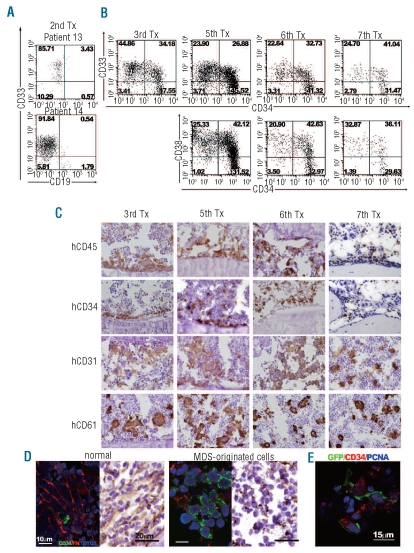
Figure 3.
Serial transplantation of MDS-originated cells and the bone marrow microenvironment (A) Flow cytometric profiles of bone marrow (BM) cells recovered from secondary hosts. Tx indicates transplantation. (B) Flow cytometric profiles of BM cells recovered from mice that had undergone serial transplants with BM cells from patient 14. (C) Immunohistochemical staining of bones obtained from mice serially transplanted with cells of patient 14 demonstrated a lineage cell staining pattern similar to that of the primary recipient mice, while CD45 and CD34-expressing cells were more confined to the endosteal region. (D) Immunofluorescent and immunohisotochemical staining for fibronectin (FN) of bones of normal cell- and MDS-originated cell-engrafted mice. Murine and human cells in normal cell-transplanted mice were tightly enveloped by fibronectin while the fibronectin network of MDS-originated cell-engrafted mice was disrupted. A light field photograph confirmed the well-structured fibronectin network in the BM of normal cell-engrafted mice, but only fibronectin fibrils were detected in the MDS-originated cell-engrafted mice. Stained sections of the mice engrafted with cells from patient 11 are shown. The same staining patterns were confirmed in bone sections of mice engrafted with cells from patients 13 and 14. (E) MDS-originated CD34+ cells expressing a proliferating marker, PCNA, interacted with human MSC marked with green fluorescent protein.
Human cells were localized in the endosteal region in the bone marrow of serially transplanted mice, in contrast to the situation in the primary recipient mice in which human CD45+ cells were ubiquitously located (Figures 2A and 3C). Even so, the MDS-originated CD34+ cells occupied the murine endosteal surface, in striking resemblance to the situation in the bones of the primary recipients, thus indicating the persistence of leukemic stem cells, at least until the 6th transplant. Although the FACS analysis indicated the maintenance of a CD34+CD38− subpopulation that included leukemic stem cells until the 7th serial transplant (Figure 3B), the 8th transplant did not result in obvious human cell engraftment (Online Supplementary Table S4). Consistent with this, in histological studies of the bone marrow of the recipient of the 7th transplantation, MDS-originated CD34+ cells attached to the bone surface had disappeared (Figure 3C). These results are also consistent with our previous finding that CD34+CD38− fractions are heterogeneous and stem cells reside at the endosteal surface.22
The microenvironment of mice engrafted with myelodysplastic syndrome-originated cells
The proliferation and survival of neoplastic cells are influenced by the host microenvironment. In this study, decreased bone marrow cellularity was a distinctive feature of all the MDS-originated cell engrafted samples analyzed (Table 1), thus indicating the suppression of murine hematopoiesis when neoplastic cells were present. To shed light on how neoplastic cells gained competitive advantages over normal host cells, the marrow compartments of animals engrafted with MDS-originated cells and normal bone marrow cells were examined. In the mice engrafted with normal bone marrow cells, the fibronectin network was well-structured throughout the marrow cavity (Figure 3D). In contrast, fibronectin network formation was irregular or disrupted in the mice engrafted with MDS-originated cells. Clusters of human CD34+ cells were proliferating around the disrupted fibronectin fibrils in the central medulla of the bone marrow compartment. These observations, combined with an earlier histological finding that contralateral tibiae of the mice engrafted with MDS-originated cells exhibited normal cellularity (Figure 2B), suggest that the disruption of fibronectin network favors survival and proliferation of MDS-originated cells, while making it difficult to sustain normal hematopoiesis. In addition, CD34+ cells with a proliferation marker, PCNA+, were associated with co-transplanted MSC (Figure 3E) in MDS-originated cell engrafted bone marrow, which suggests the involvement of transplanted MSC in the engraftment, survival and proliferation of MDS-originated cells in the murine microenvironment.
Discussion
The recent development of mouse models of human acute leukemia are helping us to understand the phenotypes and physiology of leukemic stem cells. Although the engraftment of clonal MDS cells has been reported,23,24 little is known about the behavior of MDS stem cells in vivo because of the difficulties of propagation of MDS cells up to a level that allows detailed investigation of MDS biology. In this study, in order to establish a reliable murine model for human MDS, bone marrow CD34+ cells from patients with MDS and AML-MRC were co-injected with human MSC into the bone marrow of mice, a method proven to help engraftment and differentiation of human hematopoietic cells in the murine bone marrow microenvironment.16,18,25 Successful engraftment of MDS-originated cells was observed in three out of six MDS cases (3 out of 8 mice engrafted with patients’ cells) and three out of eight AML-MRC cases (9 out of 12 mice engrafted with patients’ cells). Much to our surprise, the mice engrafted with MDS-originated cells uniformly exhibited significant decreases in the number of non-human bone marrow cells in the injected tibiae in comparison to the number of such cells in mice engrafted with normal cells, even when the percentage of human cells was less than 1% of total mononuclear cells. This is consistent with the fact that suppression of normal hematopoiesis can occur even when the tumor burden is relatively low. We found that most of the endosteal surface was covered with MDS-originated CD34+ cells, a phenomenon also seen in murine models of acute leukemia,14,15 which could be the underlying basis for the selective outgrowth of MDS-originated clones in patients over time.
The MDS-originated cells recovered from the human cell-engrafted mice maintained many characteristics of the original patients’ cells, such as the cell surface phenotype and cytogenetic abnormalities. Unlike the previous two studies in which clonal abnormalities were detected in a fraction of cells,23,24 we confirmed the presence of abnormalities in all cells examined by either FISH or chromosomal analysis, probably because purified bone marrow CD34+ cells were used, rather than bone marrow mononuclear cells or T-cell-depleted blood or bone marrow cells. This makes our method more attractive for studying the behavior of MDS-originated cells in vivo because the majority, if not all, of the engrafted cells can be assumed to originate from clonal MDS cells. The majority of cells capable of engrafting in murine hosts were derived from patients carrying one or more genetic abnormalities (5/6; Online Supplementary Table S1). It is also noteworthy that four of those patients harbored an abnormality in chromosome 7, monosomy 7 or a partial deletion of chromosome 7, which is commonly found in Asian MDS cases and an indicator of aggressive disease with a poor prognosis.26,27 On the other hand, five of eight cases that did not engraft or engrafted with normal cells were genetically normal. Taken together, the engraftment ability of MDS-originated cells positively correlated with the cytogenetic abnormalities of the patients.
In the current study, we attempted to explore the importance of MSC in engraftment of MDS-originated cells by comparison to non-bone marrow-derived stromal cells and the non-stromal component of bone marrow cells as auxiliary cells of transplantation. The presence of non-bone marrow-derived stromal cells (dermal fibroblasts) or non-stromal cells (cells of the CD34− fraction) did not help engraftment of MDS-originated cells. In contrast, the presence of MSC consistently improved engraftment of MDS-originated bone marrow CD34+ cells. The pro-engraftment effect observed in our study could, therefore, be uniquely attributed to the presence of bone marrow-derived MSC. An investigation of how MSC help engraftment of human hematopoietic cells was beyond the scope of this study, but our observation of a physical interaction between MDS-originated CD34+ cells and MSC (Figure 3E) suggests that MSC create a favorable environment for human MDS-originated cells to survive in the murine microenvironment, possibly through the physical interaction itself and production of human cytokines, as indicated by our previous study.18 It has been speculated that microenvironmental changes are involved in the pathogenesis of MDS.28–30 However, since there was no significant difference in the engraftment of MDS-originated cells between normal (allogeneic-) and patient-derived (autologous-) MSC transplanted groups tested in this study, humanization of the microenvironment appeared more important, at least in our present study. One interesting finding in the serial transplantation study was that AML-MRC cells that had already engrafted in mice no longer required auxiliary cells or an intramedullary route of administration in subsequent transplants, perhaps because the cells with an ability to overcome hurdles to homing and engraftment in a murine host were selected during the serial transplants.
Local regulatory signals from the surrounding microenvironment to stem/progenitor cells play restrictive roles not only for normal cell development but also for tumorigenesis and metastasis.31 It has also been reported that leukemic cells disrupt the behavior of normal hematopoietic progenitor cells by creating an abnormal microenvironment.32 The microenvironment consists of heterogeneous types of cells and extracellular matrix proteins. Fibronectin is one of the major components of microenvironment structure. It was shown that mice lacking the enzyme needed to produce galactocerebrosides, a class of glycolipids in the nervous system, had an altered fibronectin network in the marrow microenvironment, which resulted in defective intramedullary lymphopoiesis and a hypocellular bone marrow.33 Several studies found that interactions between leukemic cells and fibronectin prevented the apoptosis of leukemic cells from patients with AML, acute lymphocytic leukemia, and B-cell chronic lymphocytic leukemia, as well as leukemic cell lines in vitro.34–37 In this study, mice engrafted with MDS-originated cells had an overall disruption of the fibronectin network in the bone marrow compartment and a striking decrease in the number of non-human bone marrow cells, indicating the importance of the three-dimensional structure of the fibronectin network in normal hematopoiesis. The reason for this fibronectin disruption and decrease in bone marrow cellularity are currently undetermined, but matrix metalloproteinases may play a role in creating a microenvironment that favors neoplastic cell growth over normal hematopoietic development. This idea is consistent with the results from previous studies demonstrating the increased matrix metalloproteinase production in cells obtained from MDS patients38,39 and degradation of fibronectin by matrix metalloproteinases in a number of pathological processes.40–43
In summary, this study describes the establishment of a xenograft model of human MDS, using cells obtained from patients with low-risk MDS, high-risk MDS, and AML-MRC. Our murine xenograft MDS model consisted of human cell progeny, the majority of which, if not all, originated from clonal MDS cells and which demonstrated the defective cytological features of the original patients’ cells. The efficiency of engraftment in the AML-MRC group was lower than that of de novo AML study.15,44 This is probably because our AML-MRC group included patients with low percentages of blasts, formally categorized as having refractory anemia with excess blasts in transformation according to the French-American-British criteria. The study shows that the transplantability of neoplastic MDS-originated cells is partly determined by genetic abnormality and clinical aggressiveness of disease, which reflects the pathogenesis and progression of MDS towards leukemia.
Acknowledgments
The authors would thank members of the animal facility of Tokai University for their care of animals and all members of the Research Center for Regenerative Medicine for their support.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
Funding: this work was supported by grants to KA and YM, a Research Grant of the Scientific Frontier Program a Basic Research Grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1.Steensma DP, Tefferi A. The myelodysplastic syndrome(s): a perspective and review highlighting current controversies. Leuk Res. 2003;27(2):95–120. doi: 10.1016/s0145-2126(02)00098-x. [DOI] [PubMed] [Google Scholar]
- 2.Nimer SD. Myelodysplastic syndromes. Blood. 2008;111 (10):4841–51. doi: 10.1182/blood-2007-08-078139. [DOI] [PubMed] [Google Scholar]
- 3.Heaney ML, Golde DW. Myelodysplasia. N Engl J Med. 1999;340(21):1649–60. doi: 10.1056/NEJM199905273402107. [DOI] [PubMed] [Google Scholar]
- 4.Haase D, Fonatsch C, Freund M, Wormann B, Bodenstein H, Bartels H, et al. Cytogenetic findings in 179 patients with myelodysplastic syndromes. Ann Hematol. 1995;70(4):171–87. doi: 10.1007/BF01700373. [DOI] [PubMed] [Google Scholar]
- 5.Parlier V, van Melle G, Beris P, Schmidt PM, Tobler A, Haller E, et al. Hematologic, clinical, and cytogenetic analysis in 109 patients with primary myelodysplastic syndrome. Prognostic significance of morphology and chromosome findings. Cancer Genet Cytogenet. 1994;78(2):219–31. doi: 10.1016/0165-4608(94)90094-9. [DOI] [PubMed] [Google Scholar]
- 6.Raskind WH, Tirumali N, Jacobson R, Singer J, Fialkow PJ. Evidence for a multistep pathogenesis of a myelodysplastic syndrome. Blood. 1984;63(6):1318–23. [PubMed] [Google Scholar]
- 7.Tsukamoto N, Morita K, Maehara T, Okamoto K, Karasawa M, Omine M, et al. Clonality in myelodysplastic syndromes: demonstration of pluripotent stem cell origin using X-linked restriction fragment length polymorphisms. Br J Haematol. 1993;83(4):589–94. doi: 10.1111/j.1365-2141.1993.tb04695.x. [DOI] [PubMed] [Google Scholar]
- 8.Nilsson L, Astrand-Grundstrom I, Arvidsson I, Jacobsson B, Hellstrom-Lindberg E, Hast R, et al. Isolation and characterization of hematopoietic progenitor/stem cells in 5q-deleted myelodysplastic syndromes: evidence for involvement at the hematopoietic stem cell level. Blood. 2000;96(6):2012–21. [PubMed] [Google Scholar]
- 9.Van Etten RA, Shannon KM. Focus on myeloproliferative diseases and myelodysplastic syndromes. Cancer Cell. 2004;6 (6):547–52. doi: 10.1016/j.ccr.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Uckun FM. Severe combined immunodeficient mouse models of human leukemia. Blood. 1996;88(4):1135–46. [PubMed] [Google Scholar]
- 11.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 12.Lapidot T. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367(6464):645–8. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 13.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3(7):730–7. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 14.Ninomiya M, Abe A, Katsumi A, Xu J, Ito M, Arai F, Suda T, et al. Homing, proliferation and survival sites of human leukemia cells in vivo in immunodeficient mice. Leukemia. 2007;21(1):136–42. doi: 10.1038/sj.leu.2404432. [DOI] [PubMed] [Google Scholar]
- 15.Ishikawa F, Yoshida S, Saito Y, Hijikata A, Kitamura H, Tanaka S, et al. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol. 2007;25(11):1315–21. doi: 10.1038/nbt1350. [DOI] [PubMed] [Google Scholar]
- 16.Yahata T, Ando K, Sato T, Miyatake H, Nakamura Y, Muguruma Y, et al. A highly sensitive strategy for SCID-repopulating cell assay by direct injection of primitive human hematopoietic cells into NOD/SCID mice bone marrow. Blood. 2003;101(8):2905–13. doi: 10.1182/blood-2002-07-1995. [DOI] [PubMed] [Google Scholar]
- 17.Muguruma Y, Reyes M, Nakamura Y, Sato T, Matsuzawa H, Miyatake H, et al. In vivo and in vitro differentiation of myocytes from human bone marrow-derived multi-potent progenitor cells. Exp Hematol. 2003;31(12):1323–30. doi: 10.1016/j.exphem.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Muguruma Y, Yahata T, Miyatake H, Sato T, Uno T, Itoh J, et al. Reconstitution of the functional human hematopoietic microenvironment derived from human mesenchymal stem cells in the murine bone marrow compartment. Blood. 2006;107(5):1878–87. doi: 10.1182/blood-2005-06-2211. [DOI] [PubMed] [Google Scholar]
- 19.Hiramatsu H, Nishikomori R, Heike T, Ito M, Kobayashi K, Katamura K, et al. Complete reconstitution of human lymphocytes from cord blood CD34+ cells using the NOD/SCID/gammacnull mice model. Blood. 2003;102(3):873–80. doi: 10.1182/blood-2002-09-2755. [DOI] [PubMed] [Google Scholar]
- 20.Ishikawa F, Saito Y, Yoshida S, Harada M, Shultz LD. The differentiative and regenerative properties of human hematopoietic stem/progenitor cells in NODSCID/IL2rgamma(null) mice. Curr Top Microbiol Immunol. 2008;324:87–94. doi: 10.1007/978-3-540-75647-7_5. [DOI] [PubMed] [Google Scholar]
- 21.Giassi LJ, Pearson T, Shultz LD, Laning J, Biber K, Kraus M, et al. Expanded CD34+ human umbilical cord blood cells generate multiple lymphohematopoietic lineages in NOD-scid IL2rgamma(null) mice. Exp Biol Med (Maywood) 2008;233(8):997–1012. doi: 10.3181/0802-RM-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yahata T, Muguruma Y, Yumino S, Sheng Y, Uno T, Matsuzawa H, et al. Quiescent human hematopoietic stem cells in the bone marrow niches organize the hierarchical structure of hematopoiesis. Stem Cells. 2008;26 (12):3228–36. doi: 10.1634/stemcells.2008-0552. [DOI] [PubMed] [Google Scholar]
- 23.Thanopoulou E, Cashman J, Kakagianne T, Eaves A, Zoumbos N, Eaves C. Engraftment of NOD/SCID-beta2 microglobulin null mice with multilineage neoplastic cells from patients with myelodysplastic syndrome. Blood. 2004;103(11):4285–93. doi: 10.1182/blood-2003-09-3192. [DOI] [PubMed] [Google Scholar]
- 24.Kerbauy DM, Lesnikov V, Torok-Storb B, Bryant E, Deeg HJ. Engraftment of distinct clonal MDS-derived hematopoietic precursors in NOD/SCID-beta2-microglobulindeficient mice after intramedullary transplantation of hematopoietic and stromal cells. Blood. 2004;104(7):2202–3. doi: 10.1182/blood-2004-04-1518. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura Y, Yahata T, Muguruma Y, Uno T, Sato T, Matsuzawa H, et al. Angiopoietin-1 supports induction of hematopoietic activity in human CD34- bone marrow cells. Exp Hematol. 2007;35 (12):1872–83. doi: 10.1016/j.exphem.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Haase D, Germing U, Schanz J, Pfeilstocker M, Nosslinger T, Hildebrandt B, et al. New insights into the prognostic impact of the karyotype in MDS and correlation with subtypes: evidence from a core dataset of 2124 patients. Blood. 2007;110(13):4385–95. doi: 10.1182/blood-2007-03-082404. [DOI] [PubMed] [Google Scholar]
- 27.Pozdnyakova O, Miron PM, Tang G, Walter O, Raza A, Woda B, et al. Cytogenetic abnormalities in a series of 1,029 patients with primary myelodysplastic syndromes: a report from the US with a focus on some undefined single chromosomal abnormalities. Cancer. 2008;113 (12):3331–40. doi: 10.1002/cncr.23977. [DOI] [PubMed] [Google Scholar]
- 28.Borojevic R, Roela RA, Rodarte RS, Thiago LS, Pasini FS, Conti FM, et al. Bone marrow stroma in childhood myelodysplastic syndrome: composition, ability to sustain hematopoiesis in vitro, and altered gene expression. Leuk Res. 2004;28(8):831–44. doi: 10.1016/j.leukres.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 29.Flores-Figueroa E, Arana-Trejo RM, Gutirrez-Espindola G, Perez-Cabrera A, Mayani H. Mesenchymal stem cells in myelodysplastic syndromes: phenotypic and cytogenetic characterization. Leuk Res. 2005;29(2):215–24. doi: 10.1016/j.leukres.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 30.Raaijmakers MH, Mukherjee S, Guo S, Zhang S, Kobayashi T, Schoonmaker JA, et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2010;464(7290):852–7. doi: 10.1038/nature08851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mueller MM, Fusenig NE. Friends or foes -bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4(11):839–49. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 32.Colmone A, Amorim M, Pontier AL, Wang S, Jablonski E, Sipkins DA. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science. 2008;322(5909):1861–5. doi: 10.1126/science.1164390. [DOI] [PubMed] [Google Scholar]
- 33.Katayama Y, Frenette PS. Galacto-cerebrosides are required postnatally for stromal-dependent bone marrow lymphopoiesis. Immunity. 2003;18(6):789–800. doi: 10.1016/s1074-7613(03)00150-x. [DOI] [PubMed] [Google Scholar]
- 34.Bendall LJ, Makrynikola V, Hutchinson A, Bianchi AC, Bradstock KF, Gottlieb DJ. Stem cell factor enhances the adhesion of AML cells to fibronectin and augments fibronectin-mediated anti-apoptotic and proliferative signals. Leukemia. 1998;12 (9):1375–82. doi: 10.1038/sj.leu.2401136. [DOI] [PubMed] [Google Scholar]
- 35.de la Fuente MT, Casanova B, Garcia-Gila M, Silva A, Garcia-Pardo A. Fibronectin interaction with alpha4beta1 integrin prevents apoptosis in B cell chronic lymphocytic leukemia: correlation with Bcl-2 and Bax. Leukemia. 1999;13(2):266–74. doi: 10.1038/sj.leu.2401275. [DOI] [PubMed] [Google Scholar]
- 36.Mudry RE, Fortney JE, York T, Hall BM, Gibson LF. Stromal cells regulate survival of B-lineage leukemic cells during chemotherapy. Blood. 2000;96(5):1926–32. [PubMed] [Google Scholar]
- 37.Matsunaga T, Takemoto N, Sato T, Takimoto R, Tanaka I, Fujimi A, et al. Interaction between leukemic-cell VLA-4 and stromal fibronectin is a decisive factor for minimal residual disease of acute myelogenous leukemia. Nat Med. 2003;9 (9):1158–65. doi: 10.1038/nm909. [DOI] [PubMed] [Google Scholar]
- 38.Ries C, Loher F, Zang C, Ismair MG, Petrides PE. Matrix metalloproteinase production by bone marrow mononuclear cells from normal individuals and patients with acute and chronic myeloid leukemia or myelodysplastic syndromes. Clin Cancer Res. 1999;5(5):1115–24. [PubMed] [Google Scholar]
- 39.Travaglino E, Benatti C, Malcovati L, Della Porta MG, Galli A, Bonetti E, et al. Biological and clinical relevance of matrix metalloproteinases 2 and 9 in acute myeloid leukaemias and myelodysplastic syndromes. Eur J Haematol. 2008;80 (3):216–26. doi: 10.1111/j.1600-0609.2007.01012.x. [DOI] [PubMed] [Google Scholar]
- 40.Nakahara H, Howard L, Thompson EW, Sato H, Seiki M, Yeh Y, et al. Transmembrane/cytoplasmic domain-mediated membrane type 1-matrix metalloprotease docking to invadopodia is required for cell invasion. Proc Natl Acad Sci USA. 1997;94(15):7959–64. doi: 10.1073/pnas.94.15.7959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsumura S, Iwanaga S, Mochizuki S, Okamoto H, Ogawa S, Okada Y. Targeted deletion or pharmacological inhibition of MMP-2 prevents cardiac rupture after myocardial infarction in mice. J Clin Invest. 2005;115(3):599–609. doi: 10.1172/JCI22304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clark ES, Whigham AS, Yarbrough WG, Weaver AM. Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia. Cancer Res. 2007;67(9):4227–35. doi: 10.1158/0008-5472.CAN-06-3928. [DOI] [PubMed] [Google Scholar]
- 43.Hsu CC, Lai SC. Matrix metalloproteinase- 2, -9 and -13 are involved in fibronectin degradation of rat lung granulomatous fibrosis caused by Angiostrongylus cantonensis. Int J Exp Pathol. 2007;88(6):437–43. doi: 10.1111/j.1365-2613.2007.00554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saito Y, Uchida N, Tanaka S, Suzuki N, Tomizawa-Murasawa M, Sone A, et al. Induction of cell cycle entry eliminates human leukemia stem cells in a mouse model of AML. Nat Biotechnol. 2010;28 (3):275–80. doi: 10.1038/nbt.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]