Abstract
Zebrafish were proposed as an alternative to mammalian models to assess the efficacy and toxicity of antileukemic drugs. Due to the limited number of transgenic zebrafish leukemia models, we explored human leukemic cell xenograft in zebrafish embryos. Human leukemic cell lines and blast cells sorted from patients with acute myelogenous leukemia were injected 48 hours post-fertilization and remained in the circulation of zebrafish embryos for several days without affecting their development. Imatinib and oxaphorines did not demonstrate any toxicity on normal zebrafish embryos and decreased the leukemic burden in animals xenografted with sensitive leukemic cell lines. Two other molecules, all-trans retinoic acid and the translation inhibitor 4EGI-1, demonstrated teratogenic effects at concentrations shown to be efficient in vitro, which precluded investigation of their antileukemic activity in such models. Altogether, xenografted leukemic cells in zebrafish embryos are a pharmacologically relevant model for screening non-teratogenic drugs.
Keywords: Zebrafish model, acute leukemia, antileukemic drugs, pharmacology, teratogenicity
Introduction
Evaluation of new therapeutic regimen efficacy in acute and chronic leukemia is a long and complex process that usually requires validation in animal models before clinical trials. In recent years, zebrafish have been proposed as a cost-effective alternative to mammals such as rodents and dogs.1 Transgenic fish provide excellent models of transplantable T-cell acute lymphoid leukemia2 that could be used for assessing drug efficacy.3 Transgenic zebrafish models of acute or chronic myeloid leukemia are still rare, and the models obtained so far could not be used for drug screening.4–6 Tumor cells xenografted in zebrafish embryos may constitute an alternative to transgenic animals and were used to explore angiogenesis7 or radiation sensitizers8 whereas transplantation of tumor cells in transparent adult zebrafish was used to characterize stem cell transplantation9 and tumor cell extravasation10 and invasion.11 We explored leukemic cell xenograft in zebrafish embryos as a model for studying antileukemic drug efficacy.
Design and Methods
Animal care and xenograft procedure
A zebrafish (Danio rerio) breeding colony (wild-type AB strain) was maintained at 28°C as previously described.12 The age of embryos was indicated as hours post-fertilization (hpf) and days post-fertilization (dpf). Dechorionated embryos, anesthetized with Tricaine methanesulfonate, were injected at 48 hpf through the yolk sac into the posterior cardinal vein (PVC) with 50 to 200 human leukemic cells per embryo using a Femtojet microinjector (Eppendorf, Hamburg, Germany). At 1 dpi, injected embryos (50 per point) were examined by fluorescence microscopy to select for those that were morphologically normal and bearing fluorescent cells (50–60% of the injected larvae). Twenty-five positive embryos were transferred into E3 medium containing 0.3% Phenylthiourea (PTU) (25 animals in each 100 mm Petri dish) and treated with indicated concentrations of drugs. Photographs and movies were obtained using a Leica MZ FL III Fluo Combi™ fluorescence stereomicroscope bearing appropriate filters and a fluorescence microscope Leitz Aristoplan coupled with a Leica D-LUX 3 camera. Photoshop software was used to process images.
Cell cultures and labeling
K562, Jurkat and NB4 human leukemia cell lines were obtained from the American Tissue Culture Collection and grown in RPMI 1640 medium with glutamax-I (Lonza, Basel, Switzerland) supplemented with 10% (vol/vol) fetal bovine serum (Lonza). Imatinib-resistant K562 cells (K562R) have been described previously.13 Human umbilical cord blood was supplied by the Etablissement Français du Sang, and blast cells were sorted from peripheral blood samples collected from patients with acute myeloid leukemia at diagnosis. These patients provided their informed consent according to recommendations of the independent ethical board of Dijon Hospital. CD34+ cells were enriched by immunomagnetic methods (Miltenyi Biotec, Köln, Germany) to obtain over 97% purity, and grown in H3000 medium (StemCell) supplemented with 100 ng/mL SCF, 100 ng/mL FLT3L, 20 ng/mL IL-3 and 20 ng/mL IL-6 for two days. Before injection into zebrafish larvae, all cells were labeled with CM-Dil, a lipophilic fluorescent tracking dye (Invitrogen, Paisley, UK) according to the manufacturer’s instructions.
Chemicals
Imatinib and all-trans retinoic were purchased from Enzo life sciences, mafosmamide and cyclophosphamide from Sigma-Aldrich. 4EGI-1 was synthesized by Chembridge and kindly provided by Dr Gerhard Wagner (Harvard Medical School, Boston, MA, USA).
Whole-mount immunohistochemistry embryos were fixed in 10% formalin, embedded in paraffin and whole-mount antibody stained for Ki67 using an anti-human Ki67 mouse monoclonal antibody (Abcam ab6526, Cambridge, MA, USA).
Reverse-transcription and real-time polymerase chain reaction
Total RNA from mixtures of 25 embryos was isolated with Trizol (Invitrogen, Cergy Pontoise, France) and reverse transcribed by Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI, USA) with random hexamers (Promega). Specific forward and reverse primers are accessible upon request. Real-time quantitative PCR (QPCR) was performed with AmpliTaq Gold polymerase in an Applied Biosystem 7500 Fast thermocycler using the standard SyBr Green detection protocol (Applied biosystems, Foster City, CA, USA). Briefly, 12 ng of total cDNA, 50 nM (each) primers, and 1x SyBr Green mixture were used in a total volume of 20 μL. QPCR was performed in triplicates and one representative out of three independent experiments was shown.
Statistical analyses
The Kruskall Wallis test was used and significance was considered when P values were lower than 0.05. Results are expressed as mean plus or minus SEM.
Results and Discussion
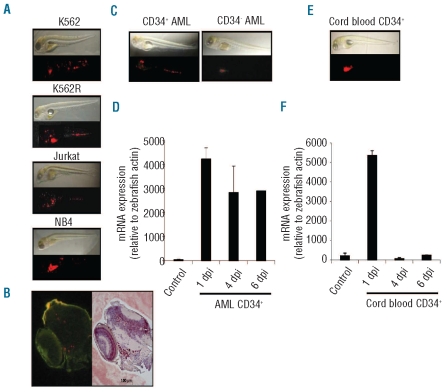
Zebrafish are usually maintained at 28°C, develop normally up to 34°C, and die before four days post-fertilization (dpf) at higher temperature (Online Supplementary Figure S1A). K562, Jurkat and NB4 human leukemic cell lines are usually grown at 37°C in vitro and their survival (data not shown), proliferation (Online Supplementary Figure S1B), and drug sensitivity (Online Supplementary Figure S1C) are poorly affected by decreasing the temperature to 34°C. These cells were labeled with CM-Dil and injected into 2-day old zebrafish larvae that were incubated for 1 h at 28°C, then at 34°C. Human metastatic melanoma cells placed in zebrafish embryos survive, exhibit motility and divide but do not form tumors, nor integrate into host organs.14 At one day post-injection (dpi), leukemic cells were easily observed in the circulation, especially in the caudal part, the heart and the head (data not shown). At 1 dpi, the presence of leukemic cells was observed in 12–15 out of the 25 injected embryos for each point (see movie in Online Supplementary Figures S2 and S3). At 4 dpi (Figure 1A), fluorescent cells remain easily detectable in all the animals, indicating their ability to survive. Ki67 labeling at 1 dpi indicated the ability of injected cells to proliferate after engraftment (Figure 1B). CD34-positive and -negative blast cells sorted from peripheral blood of patients with acute myeloblastic leukemia (AML), were also detected in injected larvae at 4 dpi (Figure 1C) and 6 dpi (data not shown) and L32 human gene remained expressed at 6 dpi (Figure 1D). Transplantation of metastatic melanoma cells into zebrafish blastula-stage embryos (between 2 and 5 hpf) disrupts development through secretion of Nodal, a member of the TGFβ superfamily that promotes body axis duplication.15 Transplantation of leukemic cells in later stage embryos (48 hpf) did not affect the animal development, at least until 6 dpi (Figure 1A-D).
Figure 1.
Engraftment of human leukemia cells in zebrafish embryos. (A) Dechorionated embryos were injected with 50-200 cells from indicated human leukemic cell lines (K562, K562-R, Jurkat, NB4) and examined at day 4 post-injection (dpi) by contrast phase and fluorescence microscopy. (B) Embryos were similarly injected with CD34-positive and -negative blast cells sorted from AML patient peripheral blood samples and examined at 4 dpi (one representative of 6 independent experiments). (C) RT-qPCR analysis of L32 human gene expression, relative to zebrafish actin gene, in embryos injected with medium (Control) or with CD34-positive cells sorted from an AML patient sample examined at indicated days post-injection (Mean +/− SEM of one representative of 6 independent experiments). (D) Embryos were injected with CD34-positive cells sorted from human umbilical cord blood and examined at 1 dpi as in A. (E) RT-qPCR analysis of L32 human gene expression, relative to zebrafish actin gene, in non-injected (Control) and in embryos injected with CD34-positive cells sorted from human umbilical cord blood. In each panel, one representative experiment of at least 3 is shown. For each experiment, at least 25 homogenously injected or control larvae were used per point.
Normal human melanocytes transplanted in zebrafish embryos are frequently found in the skin, indicating that the embryo contains possible homing cues that can be interpreted by normal human cells as their normal microenvironment.16 CD34-positive hematopoietic cells sorted from human cord blood and CM-Dil labeled are detected by fluorescence microscopy in all injected larvae at 1 dpi (Figure 1E), then rapidly disappear, as confirmed by L32 gene expression analysis (Figure 1F). Thus, zebrafish hematopoietic environment may not provide efficient signals for homing of normal hematopoietic progenitors.
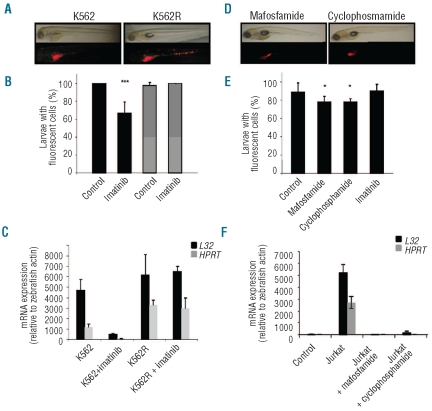
The K562 cell line, established from the acute blastic transformation of a chronic myelogenous leukemia (CML), is a well-described model for testing the activity of imatinib mesylate (Gleevec), a competitive inhibitor of the ATP-binding site of the Bcr-Abl enzyme that characterizes CML.17–18 Zebrafish larvae are permeable to small molecules.1 Addition of 3 μM imatinib to the water of larvae at 1dpi is non-toxic to control animals observed during eight days, and induces a decrease in the number of fluorescent cells detected in the fish, including the disappearance of any fluorescent cell in 34.7±12.5 % of the animals at 4 and 6 dpi (Figure 2A and B). In the K562-xenografted animals in which fluorescent cells were still visible, imatinib induces a dramatic decrease in the number of fluorescent cells. Flow cytometry analysis was not sensitive enough to reproducibly quantify the number of CM-Dil labeled residual leukemic cells in dispersed embryos (data not shown). Therefore, the ability of the drugs to decrease the leukemic cell burden in the animals with residual fluorescence was measured by examining human L32 and HPRT gene expression, which strongly decreased upon drug exposure (Figure 2C). Thus, the model easily detects the ability of imatinib to decrease the leukemic burden in fish larvae. Neither analysis of fluorescent animals (Figure 2A and B), nor analysis of human gene expression (Figure 2C), could detect any efficacy of imatinib on xenografted imatinib-resistant K562 cells. The Jurkat cell line, established from a T-cell acute lymphoblastic leukemia, is sensitive to oxazaphorines. Cyclophosphamide (10 μg/mL), a prodrug that requires conversion by cytochrome P450 into 4-hydroxy-cyclophosphamide to become active, and mafosfamide (10 μg/mL), an active compound, did not affect zebrafish development and survival when tested alone (data not shown) and improved the percentage of Jurkat-cell injected animals alive at day 5, indicating cyclophosphamide metabolism. For example, 54.7±10.5 % untreated larvae survived at day 5, compared to 82.7±8.2% and 90.7±3,8% in cyclophosphamide- and mafosfamide-treated larvae, respectively. These drugs did not induce the complete disappearance of the fluorescent cells in the animals, which explains the low decrease in the percentage of positive embryos (Figure 2E). Therefore, the ability of the drugs to decrease the leukemic cell burden was assessed by measuring human L32 and HPRT mRNA levels (Figure 2F). As anticipated, imatimib mesylate did not demonstrate any efficacy in this leukemia model. Altogether, these results confirmed that leukemic cells xenografted in zebrafish larvae could be used to explore cytotoxic drug efficacy.
Figure 2.
Antileukemic drugs eliminate xenografted leukemia cells. (A) Xenografted zebrafish embryos were exposed to imatinib at 1 dpi and examined three days later by contrast phase and fluorescence microscopy. (B) Percentage of larvae with remaining fluorescent cells five days after the beginning of drug treatment. (One representative of 3 experiments, mean ± SEM of at least 25 larvae/point) (***P<0.001). Black bars: embryos injected with K562 cells, gray bars: K562R cells. (C) qRT-PCR analysis of L32 and HPRT human gene expression, relative to zebrafish actin gene, in embryos injected with K562 and K562R cells, treated when indicated with 3 μM imatinib for five days. (Mean ± SEM of 3 experiments performed on 25 larvae/point). Control: embryos injected with medium. (D) Zebrafish embryos xenografted with Jurkat cells were exposed to mafosfamide (10 μg/mL) or cyclophosphamide (10 μg/mL) at 1 dpi and examined three days later by contrast phase and fluorescence microscopy. (E) Percentage of larvae with remaining fluorescent cells five days after the beginning of indicated drug treatment (mean ± SEM of 3 experiments, each performed on 25 larvae). (F) RT-qPCR analysis of L32 and HPRT human gene expression, relative to zebrafish actin gene, in embryos injected with Jurkat cells, treated when indicated with 10 μg/mL or 10 μg/mL cyclophos-phamide for five days. (Mean ± SEM of 3 experiments performed on 25 larvea).
The NB4 cell line, derived from an acute promyelocytic leukemia (APL) patient and expressing the PML-RARα fusion protein, has been used to explore the responsiveness to retinoid/rexinoid that allows cells to embark on neutrophil granulocyte terminal maturation.19 A unique exposure to all-trans retinoic acid (ATRA), up to 500 nM, did not affect NB4 cell growth in zebrafish embryos, whereas repeated exposure to ATRA for up to five days demonstrated a dose-dependent teratogenic effect including phenotypic alterations and death (Online Supplementary Figure S4), which is a well-known effect of this drug precluding identification of any antileukemic activity in the model.20 The dose of ATRA inducing the death of 50% of treated embryos (LD50, daily repeated exposure) at day 4 was 0.24 μM. Similarly, xenografted leukemia did not allow exploration in vivo of the efficacy of a small molecule inhibitor of the translation initiating complex eIF4F, 4EGI-1. 21 This molecule, which is one of the drugs currently being developed that target signaling pathways deregulated in AML cells,22 inhibits the eIF4E/eIF4G interaction, mimics the 4E-BP1 translation repressor activity, and prevents cap-dependent mRNA translation.21 4E-BP1 translation repressor activity decreases the growth of leukemic progenitors and promotes blast cell apoptosis with limited toxicity against normal hematopoiesis when tested in vitro.23 When added at 50 or 100 μM in the water of zebrafish embryo, which was the efficient concentration in vitro, this molecule induced the death of all embryos in less than 24 h. Experiments performed at lower doses confirmed the teratogenic effect of a unique exposure to the compound (LD50 1.2 μM when measured at 96 h; Online Supplementary Figure S4E).
Recent studies have shown that chemicals had in many cases very similar toxicological and teratological effects in zebrafish embryos and humans.24 Teratogenic indices calculated in zebrafish embryos demonstrated an acceptable level of predictivity when compared to previous studies with other animals, supporting the application of zebrafish embryos as an alternative method for developmental toxicity studies that predict effects in mammals.25 Altogether, these results demonstrate that leukemia cells xenografted in zebrafish embryos could be used to explore the pharmacological activity of antileukemic drugs, pending the lack of teratogenic effect of the tested compound when added to the water.
Acknowledgments
We are grateful to the facilities of the Federative Institute of Research (IFR100) and the biological hematology department, Assistance Publique-Hôpitaux de Paris (AP-HP), Hôpital Cochin-Hôtel-Dieu, Paris, France with special thanks to Jean-Christophe Deschemins.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
Funding: this work was supported by the Ligue Nationale Contre le Cancer (Labeled group, ES), the Agence Nationale de la Recherche (LACAM) and the National Institute of Cancer (INCa – ACM07). BP received support of the Fondation pour la Recherche Médicale (Prix Jean et Ana Paneboeuf).
References
- 1.Zon LI, Peterson RT. In vivo drug discovery in the zebrafish. Nat Rev Drug Discov. 2005;4(1):35–44. doi: 10.1038/nrd1606. [DOI] [PubMed] [Google Scholar]
- 2.Smith AC, Raimondi AR, Salthouse CD, Ignatius MS, Blackburn JS, Mizgirev IV, et al. High-throughput cell transplantation establishes that tumor-initiating cells are abundant in zebrafish T-cell acute lymphoblastic leukemia. Blood. 2010;115(16):3296–303. doi: 10.1182/blood-2009-10-246488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mizgirev IV, Revskoy S. A new zebrafish model for experimental leukemia therapy. Cancer Biol Ther. 2010;9(11):895–902. doi: 10.4161/cbt.9.11.11667. [DOI] [PubMed] [Google Scholar]
- 4.Kalev-Zylinska ML, Horsfield JA, Flores MV, Postlethwait JH, Vitas MR, Baas AM, et al. Runx1 is required for zebrafish blood and vessel development and expression of a human RUNX1-CBF2T1 transgene advances a model for studies of leukemogenesis. Development. 2002;129(8):2015–30. doi: 10.1242/dev.129.8.2015. [DOI] [PubMed] [Google Scholar]
- 5.Onnebo SM, Condron MM, McPhee DO, Lieschke GJ, Ward AC. Hematopoietic perturbation in zebrafish expressing a tel-jak2a fusion. Exp Hematol. 2005;33(2):182–8. doi: 10.1016/j.exphem.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 6.Zhuravleva J, Paggetti J, Martin L, Hammann A, Solary E, Bastie JN, et al. MOZ/TIF2-induced acute myeloid leukaemia in transgenic fish. Br J Haematol. 2008;143(3):378–82. doi: 10.1111/j.1365-2141.2008.07362.x. [DOI] [PubMed] [Google Scholar]
- 7.Nicoli S, Ribatti D, Cotelli F, Presta M. Mammalian tumor xenografts induce neo-vascularization in zebrafish embryos. Cancer Res. 2007;67(7):2927–31. doi: 10.1158/0008-5472.CAN-06-4268. [DOI] [PubMed] [Google Scholar]
- 8.Lally BE, Geiger GA, Kridel S, Arcury-Quandt AE, Robbins ME, Kock ND, et al. Identification and biological evaluation of a novel and potent small molecule radiation sensitizer via an unbiased screen of a chemical library. Cancer Res. 2007;67(18):8791–9. doi: 10.1158/0008-5472.CAN-07-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White RM, Sessa A, Burke C, Bowman T, LeBlanc J, Ceol C, et al. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell. 2008;2(2):183–9. doi: 10.1016/j.stem.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stoletov K, Kato H, Zardouzian E, Kelber J, Yang J, Shattil S, et al. Visualizing extravasation dynamics of metastatic tumor cells. J Cell Sci. 2010;123(13):1332–2341. doi: 10.1242/jcs.069443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiss FU, Marques IJ, Woltering JM, Vlecken DH, Aghdassi A, Partecke LI, et al. Retinoic acid receptor antagonists inhibit miR-10a expression and block metastatic behavior of pancreatic cancer. Gastroenterology. 2009;137(6):2136–45. doi: 10.1053/j.gastro.2009.08.065. [DOI] [PubMed] [Google Scholar]
- 12.Fishman MC, Stainier DY, Breitbart RE, Westerfield M. Zebrafish: genetic and embryological methods in a transparent vertebrate embryo. Methods Cell Biol. 1997;52:67–82. doi: 10.1016/s0091-679x(08)60374-x. [DOI] [PubMed] [Google Scholar]
- 13.Jacquel A, Colosetti P, Grosso S, Belhacene N, Puissant A, Marchetti S, et al. Apoptosis and erythroid differentiation triggered by Bcr-Abl inhibitors in CML cell lines are fully distinguishable processes that exhibit different sensitivity to caspase inhibition. Oncogene. 2007;26(17):2445–58. doi: 10.1038/sj.onc.1210034. [DOI] [PubMed] [Google Scholar]
- 14.Haldi M, Ton C, Seng WL, McGrath P. Human melanoma cells transplanted into zebrafish proliferate, migrate, produce melanin, form masses and stimulate angiogenesis in zebrafish. Angiogenesis. 2006;9 (3):139–51. doi: 10.1007/s10456-006-9040-2. [DOI] [PubMed] [Google Scholar]
- 15.Topczewska JM, Postovit LM, Margaryan NV, Sam A, Hess AR, Wheaton WW, et al. Embryonic and tumorigenic pathways converge via Nodal signaling: role in melanoma aggressiveness. Nat Med. 2006;12(8):925–32. doi: 10.1038/nm1448. [DOI] [PubMed] [Google Scholar]
- 16.Lee LM, Seftor EA, Bonde G, Cornell RA, Hendrix MJ. The fate of human malignant melanoma cells transplanted into zebrafish embryos: assessment of migration and cell division in the absence of tumor formation. Dev Dyn. 2005;233(4):1560–70. doi: 10.1002/dvdy.20471. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Ren R. Bcr-Abl efficiently induces a myeloproliferative disease and production of excess interleukin-3 and granulocyte-macrophage colony-stimulating factor in mice: a novel model for chronic myelogenous leukemia. Blood. 1998;92(10):3829–40. [PubMed] [Google Scholar]
- 18.Kantarjian H, Sawyers C, Hochhaus A, Guilhot F, Schiffer C, Gambacorti-Passerini C, et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med. 2002;346(9):645–52. doi: 10.1056/NEJMoa011573. [DOI] [PubMed] [Google Scholar]
- 19.Roussel MJ, Lanotte M. Maturation sensitive and resistant t(15;17) NB4 cell lines as tools for APL physiopathology: nomenclature of cells and repertory of their known genetic alterations and phenotypes. Oncogene. 2001;20(49):7287–91. doi: 10.1038/sj.onc.1204863. [DOI] [PubMed] [Google Scholar]
- 20.Collins MD, Mao GE. Teratology of retinoids. Annu Rev Pharmacol Toxicol. 1999;39:399–430. doi: 10.1146/annurev.pharmtox.39.1.399. [DOI] [PubMed] [Google Scholar]
- 21.Moerke NJ, Aktas H, Chen H, Cantel S, Reibarkh MY, Fahmy A, et al. Small-molecule inhibition of the interaction between the translation initiation factors eIF4E and eIF4G. Cell. 2007;128(2):257–67. doi: 10.1016/j.cell.2006.11.046. [DOI] [PubMed] [Google Scholar]
- 22.Park S, Chapuis N, Tamburini J, Bardet V, Cornillet-Lefebvre P, Willems L, et al. Role of the PI3K/AKT and mTOR signaling pathways in acute myeloid leukemia. Haematologica. 2010;95(3):819–28. doi: 10.3324/haematol.2009.013797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamburini J, Green AS, Bardet V, Chapuis N, Park S, Willems L, et al. Protein synthesis is resistant to rapamycin and constitutes a promising therapeutic target in acute myeloid leukemia. Blood. 2009;114(8):1618–27. doi: 10.1182/blood-2008-10-184515. [DOI] [PubMed] [Google Scholar]
- 24.Selderslaghs IW, Van Rompay AR, De Coen W, Witters HE. Development of a screening assay to identify teratogenic and embryotoxic chemicals using the zebrafish embryo. Reprod Toxicol. 2009;28(3):308–20. doi: 10.1016/j.reprotox.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Eimon PM, Rubinstein AL. The use of in vivo zebrafish assays in drug toxicity screening. Expert Opin Drug Metab Toxicol. 2009;5(4):393–401. doi: 10.1517/17425250902882128. [DOI] [PubMed] [Google Scholar]