Abstract
A comprehensive panel of clinical-biological parameters was prospectively evaluated at presentation in 112 patients with chronic lymphocytic leukemia (<65 years), to predict the risk of progression in early stage disease. Eighty-one percent were in Binet stage A, 19% in stages B/C. Treatment-free survival was evaluated as the time from diagnosis to first treatment, death or last follow up. In univariate analysis, advanced stage, hemoglobin, platelets, white blood cell, leukemic lymphocyte count, raised beta 2-microglobulin and LDH, unmutated immunoglobulin variable region genes, CD38, del(17p), del(11q) and +12, were significantly associated with a short treatment-free survival; the T/leukemic lymphocyte ratio was associated with a better outcome. Multivariate analysis of treatment-free survival in stage A patients selected a high white blood cell count and unmutated immunoglobulin variable region genes as unfavorable prognostic factors and a high T/leukemic lymphocyte ratio as a favorable one. At diagnosis, these parameters independently predict the risk of progression in stage A chronic lymphocytic leukemia patients.
Keywords: chronic lymphocytic leukemia, prognosis, immunoglobulin heavy chain variable region gene, stage A, young
Introduction
Although Binet and Rai clinical staging systems for chronic lymphocytic leukemia (CLL) remain the most powerful prognosticators to identify advanced stages for which treatment-free survival (TFS) and overall survival (OS) are usually short, they provide no risk stratification in early stages, nowadays the most represented at diagnosis. Binet stage A CLL patients normally undergo clinical observation up to disease progression and treatment requirement, for a time frame ranging from a few months up to decades.1–3
A number of new phenotypic, molecular and genetic parameters in addition to the traditional clinical features have enabled clinicians to better predict TFS and OS of CLL patients.1,4,5 However, it is still not clear which is the relative importance of the various clinical-biological parameters in the assessment of early stage CLL prognosis.5
In 2002, two multivariate analyses6,7 showed that the immunoglobulin heavy chain variable region gene (IGHV) mutational status, TP53 abnormalities and in one study also del(11q)6 were independent predictors of OS in stage A CLL. On the contrary, CD38 had no significant impact. In 2003, ZAP-70 expression was proposed as a surrogate marker of unmutated IGHV,8 although 20–30% of “discordant” cases were recognized. Since then, a number of studies have tried to demonstrate the superiority of a given parameter in terms of prognostication.8–12
The timing of the evaluation of these parameters is also important, as it is now clear that genetic abnormalities can be acquired over time.13
In this study, we assessed the distribution and clinical significance of a comprehensive panel of clinical-biological parameters prospectively evaluated at diagnosis in all young patients sequentially diagnosed with CLL at our institution, focusing on their predictive impact on the progression of early stage CLL.
Design and Methods
Patients
From November 2002 to December 2008, 112 young patients (<65 years) diagnosed with CLL at our institution were included in the study. There were 62 males and 50 females, with a median age of 52 years (range 29–68). Eighty-one percent were in Binet stage A, 19% were in stages B/C. Rai stage 0 was recorded in 61% of patients, I/II in 33.5%, III/IV in 5.5%.
Clinical features included: age, gender, Binet and Rai stage, hemoglobin (Hb), platelets (PLTs), white blood cell (WBC), lymphocyte counts, amount and distribution of T cells, amount of CLL cells. Serological parameters included: beta2-microglobulin (β2-m), LDH, IgG, IgA and IgM levels. Biological parameters included: lymphocyte morphology, IGHV mutations, CD38, ZAP-70 expression, cytogenetic abnormalities evaluated by fluorescence in situ hybridization (FISH) and TP53 gene sequencing.
Details on the immunophenotype, IGHV mutations,14,15 TP53 sequencing16 and FISH analyses15 are available in the Online Supplementary Appendix.
Statistics
Prognosis was evaluated as TFS, calculated from the time of diagnosis to the first treatment, death or last follow up. Since no patient died before treatment, the TFS probability has been estimated using the Kaplan-Meier method instead of the Cumulative Incidence Estimation, considering death before treatment as competing risk- and using the log rank test to evaluate differences between factors.
The Cox’s model has been used for the multivariate analysis; first, all factors with a clinical relevance or a statistical significance/trend for significance (P≤0.07) were included in the model; subsequently, factors which lost significance and were considered less important by a clinical prospective were excluded by the model. The WBC count and the T/CLL ratio cut-off points were estimated by means of martingale residuals.
Results and Discussion
Clinical and biological features of CLL at diagnosis
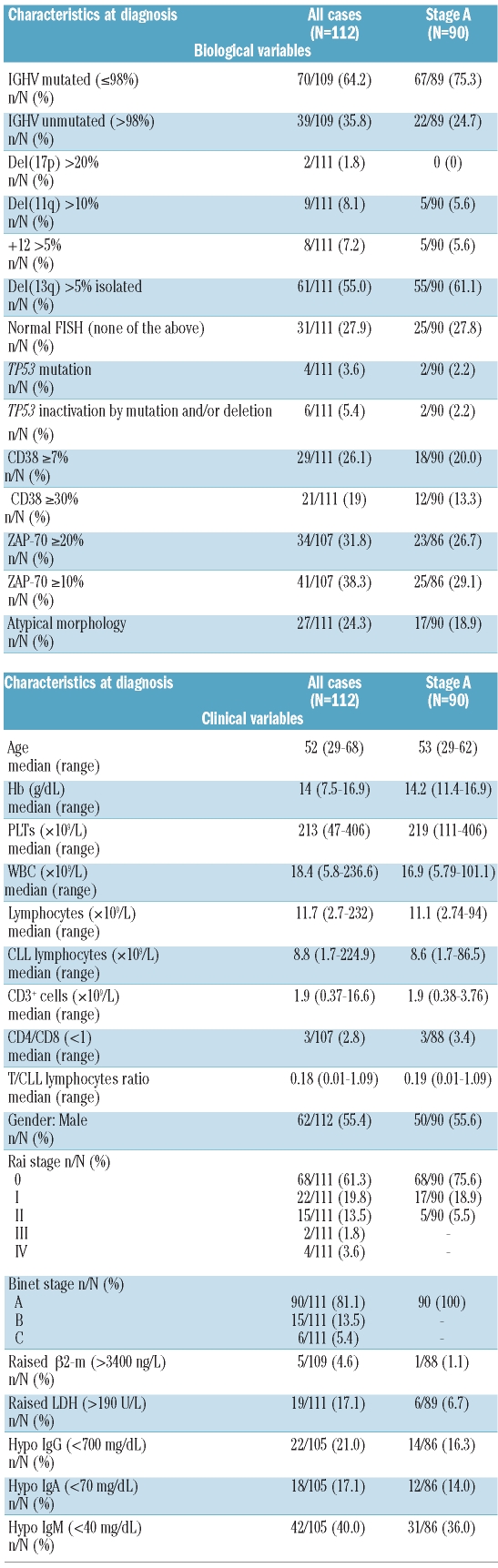
The distribution of the clinical-biological markers of all 112 patients and of 90 stage A CLL is summarized in Table 1 and Online Supplementary Table S1. We focused our study on patients younger than 65 years because of the importance of prognostic information in individuals with a long life expectancy, whose expected survival is more affected by the direct effect of the disease than in older patients.17 However, our results could be applied to the entire CLL population, as no difference related to age is reported regarding the presence of unmutated IGHV or del(17p),6 the prognostic value of ZAP70, IGHV, CD38,9 and the CD49d expression.18 Moreover, age appears to affect more OS than TFS.6,12,18–21
Table 1.
Biological and clinical characteristics of 112 CLL at diagnosis.
Unmutated IGHV cases represented 36% of the overall cohort and 25% of stage A CLL. CD38+ (≥7%) cases were 26% and 20%, and ZAP-70+ (≥20%) were 32% and 27% in the two cohorts, respectively. Thus, unfavorable biological features such as unmutated IGHV, CD38 and ZAP-70 positive expression, can be found in about one-third of young CLL at diagnosis and not exclusively in the advanced stages.
In contrast, prognostically adverse FISH abnormalities are present only in a small subgroup of cases at diagnosis. The incidence of del(17p) (cut off >20% cells) and del(11q) (cut off >10% cells) was 2% and 8% in the entire cohort and 0% and 6% in stage A CLL, respectively. The 11 patients with del(17p) or del(11q) all showed unmutated IGHV, ZAP-70+ in 8 of 11 and CD38+ in 7 of 11. In the analysis, they were pooled with the 8 trisomy 12 cases, because of their small number.
TP53 mutation was present in 4 cases (3.6%), 2 in stage A: 3 showed unmutated IGHV and del(17p), and one mutated IGHV and no del(17p).
There was no difference in median WBC count at presentation between the entire cohort and stage A CLL, being 18.4 and 17∞109/L, respectively. The median number of total lymphocytes, CLL cells, and T cells was 11.7 and 11.1∞109/L, 8.8 and 8.6∞109/L, and 1.9 and 1.9∞109/L, respectively, in the two groups.
It is notable that raised β2-m and LDH levels were present only in 5% and 17% of the entire cohort, and in 1% and 7% of stage A CLL, questioning the real utility of β2-m in identifying early stage CLL at high risk of progression. Hypogammaglobulinemia was present in a large proportion of patients (Table 1).
Risk factors for TFS in univariate analysis
The relevance of clinical and biological markers as predictors of TFS was investigated in univariate and multivariate analysis. During the follow up (median 35.4 months, range 1.1–93.8), 46 patients (41%) underwent treatment. The median TFS was 45.2 months (Online Supplementary Figure S1) in the entire cohort and 62.4 months in stage A CLL.
In univariate analysis (Online Supplementary Table S2), the following variables were associated with a short TFS: advanced stage Binet B/C and Rai intermediate/high (<0.0001), Hb, PLTs, WBC count (<0.0001) as continuous variables, raised β2-m (<0.0001) and LDH (<0.0001), unmutated IGHV (<0.0001), CD38+ (<0.0001), adverse cytogenetic abnormalities (del(17p)>20%, del(11q)>10%, +12) (<0.0001). The absolute CLL lymphocyte count was also a significant adverse prognostic parameter (<0.0001), reflecting the impact of disease burden, while the T/CLL lymphocyte ratio was a significant favorable variable (0.0004). The T-lymphocyte count showed a strong positive correlation with the WBC (Pearson’s correlation coefficient=0.68347, P<0.0001) and was not included in the analysis.
Atypical CLL morphology and ZAP-70+ (≥20%) were not significant (P 0.07 for both), as well as gender, age, hypo IgG and the CD4/CD8 ratio. The CD4/CD8 ratio was inverted only in 3 cases.
Particularly, TFS was significantly shorter in cases with unmutated versus mutated IGHV (at 36 months, 24.8% and 77%, respectively P<0.0001) (Figure 1A) and CD38+ versus CD38- (at 36 months 32.5% vs. 67.9%, P<0.0001) (Online Supplementary Figure S2).
Figure 1.
(A) TFS in CLL patients according to the IGHV mutational status. TFS of 39 IGHV unmutated and 70 IGHV mutated CLL patients. (B) TFS in CLL patients according to the WBC count. TFS of 33 CLL patients with a WBC ≥30x109/L and 79 with a WBC <30x109/L. (C) TFS in CLL patients according to T/CLL ratio. TFS of 62 CLL patients with a T/CLL ratio <0.2 and 50 with a T/CLL ratio ≥0.2.
Cases with del(17p) (>20% cells) or del(11q) (>10% cells) had a TFS at 36 months of 0% and 15.6% versus 41.7% for cases with +12, 66% for cases with no abnormalities and 66.4% for cases with del(13q) (P<0.0001) (Online Supplementary Figure S3). FISH abnormalities evaluated with a lower laboratory cut off were much less significant (P 0.0121), as patients with del(17p)>5% had a TFS at 36 months of 77.5% (median 47.1 months). Thus, we prefer to consider the higher cut off for prognostic purposes related to TFS.
We also explored different cut-off points for ZAP-70 and CD38 expression. ZAP-70 10% or more, present in 38.3% of cases, was more significant for TFS (P 0.0021) than ZAP-70 20% or more. The CD38 30% or more was as significant as 7% or more for TFS, but present only in 19% of all cases and in 13% of stage A, as previously described.22
The results of this univariate analysis are mostly in agreement with other published results, with the exception of ZAP-70.
Multivariate analysis of TFS in stage A CLL
The multivariate analysis of TFS was focused on the 90 patients with Binet stage A, those for whom the prediction of progression is most relevant, and included age, WBC count, Hb, PLTS, T/CLL lymphocyte ratio (as continuous variables), LDH, morphology, ZAP-70 and CD38 expression, IGHV mutations and FISH (del(13q) vs. normal vs. tris12+del(17p)>20%+del(11q)>10%).
High WBC count and unmutated IGHV were shown to be independent unfavorable prognostic factors (Table 2, model 1). From this model, we excluded the WBC count to verify whether this parameter could show a masking effect on the T/CLL lymphocyte ratio, due to the strong correlation between these variables. Excluding the WBC, a high T/CLL ratio was significantly associated with a better outcome (Table 2, model 2); the IGHV status was still significant.
Table 2.
Multivariate Cox’s regression analysis of TFS in stage A CLL. Model 1: results from a multivariate analysis including WBC count, T/CLL lymphocyte ratio (as continuous variables), LDH, IGHV mutation status. Model 2: multivariate analysis excluding the WBC count. Model 3: multivariate analysis excluding the IGHV mutational status (“simplified” prognostic model).
We also performed a multivariate analysis excluding the IGHV status to identify a “simplified” prognostic model. WBC count and LDH emerged as the independent prognostic parameters of TFS (Table 2, model 3) in stage A CLL; this model could be easily employed in developing countries or wherever molecular biology facilities are not available.
Thus, we searched for a significant cut-off point for WBC and T/CLL ratio. A WBC count greater than 30∞109/L and T/CLL ratio less than 0.2 significantly identified patients with a short TFS (Figure 1B and C).
Excluding the WBC and T/CLL lymphocyte ratio and considering the absolute CLL cell number, the latter was an unfavorable significant factor in both the entire cohort (HR: 1.014, 95%CI: 1.002–1.026; P=0.0246) and in stage A cases (HR: 1.069, 95%CI: 1.039–1.099; P=<0.0001; data not shown).
The WBC count is always an independent prognostic marker in multivariate analysis including purely clinical19–21 or clinical-biological parameters of CLL.6 However, this simple measure of tumor burden is often not considered, in contrast to the prognostic studies in acute leukemias, where it is invariably included. Our WBC cut off of 30∞109/L is a more than reasonable proposal when compared to the 30∞109/L lymphocyte count limit which characterizes the “smoldering CLL”23 or the A′ and A″ subgroups,24 as well as the other proposed risk categories to stratify CLL patients.19–21 Our results on the T-cell compartment are strengthened by recent similar observations25 underlining the importance of the non-malignant host immune compartment in the evolution of the disease. In our study, neither ZAP-70, CD38 (with both cutoff values) nor FISH showed an independent prognostic value, whilst the significant impact of IGHV was reinforced.6,7 The scarce number of stage A CLL with del(17p) or del(11q) might account for the lack of significance of cytogenetics in our series. Whilst CD38 does not retain an independent prognostic value when IGHV mutations or other markers are considered,6,7,9,12,18 the value of ZAP-70 is proven by some studies,9 but not by ours or by others.18
Our findings have some limitations. First, a longer follow up is necessary to further validate our prognostic model as predictor of progression and survival for stage A CLL. Second, our sample size is relatively small and our results represent a proposal that needs to be confirmed in independent larger series. The topic is of interest, as witnessed by the recent efforts of the MDACC19 to define a widely applicable CLL prognostic index based on the clinical variables of age, gender, β2-m, lymphocyte count, stage and number of involved lymph nodal groups (independently validated by the Mayo Clinic20 and the Italian GIMEMA group21) and by ongoing European prospective studies on stage A CLL prognostication based on clinical and biological markers.
In conclusion, in a young patient with stage A CLL diagnosis, the IGHV mutational status, WBC count and T/CLL lymphocyte ratio are the most important parameters to predict TFS. Our results meet the need to divide CLL Binet stage A patients into different prognostic groups and may question the utility of performing FISH analysis in the work-up of these patients at first diagnosis, rather than at progression before treatment, as stated in the new IWCLL guidelines.26
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
Funding: this work was supported by Associazione Italiana per la Ricerca sul Cancro (AIRC), Milan, Ministero dell’Università e della Ricerca Scientifica, Progetto PRIN (Programmi di ricerca di Rilevante Interesse Nazionale), Rome, Ministero della Salute, Progetto “Oncologia”, Rome, Compagnia di San Paolo, Torino and Fondazione Cenci-Bolognetti, Rome.
References
- 1.Gentile M, Mauro FR, Guarini A, Foà R. New developments in the diagnosis, prognosis and treatment of chronic lymphocytic leukemia. Curr Opin Oncol. 2005;17(6):597–604. doi: 10.1097/01.cco.0000181403.75460.c7. [DOI] [PubMed] [Google Scholar]
- 2.Rai KR, Sawitsky A, Cronkite EP, Chanana AD, Levy RN, Pasternack BS. Clinical staging of chronic lymphocytic leukemia. Blood. 1975;46(2):219–34. doi: 10.1182/blood-2016-08-737650. [DOI] [PubMed] [Google Scholar]
- 3.Binet JL, Auquier A, Dighiero G, Chastang C, Piguet H, Goasguen J, et al. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer. 1981;48(1):198–206. doi: 10.1002/1097-0142(19810701)48:1<198::aid-cncr2820480131>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 4.Montserrat E. New prognostic markers in CLL. Hematology Am Soc Hematol Educ Program. 2006:279–84. doi: 10.1182/asheducation-2006.1.279. [DOI] [PubMed] [Google Scholar]
- 5.Kay NE, O’Brien SM, Pettitt AR, Stilgenbauer S. The role of prognostic factors in assessing ‘high-risk’ subgroups of patients with chronic lymphocytic leukemia. Leukemia. 2007;21(9):1885–91. doi: 10.1038/sj.leu.2404802. [DOI] [PubMed] [Google Scholar]
- 6.Krober A, Seiler T, Benner A, Bullinger L, Brückle E, Lichter P, et al. V(H) mutation status, CD38 expression level, genomic aberrations, and survival in chronic lymphocytic leukemia. Blood. 2002;100(4):1410–16. [PubMed] [Google Scholar]
- 7.Oscier DG, Gardiner AC, Mould SJ, Glide S, Davis ZA, Ibbotson RE, et al. Multivariate analysis of prognostic factors in CLL: Clinical stage, IGVH gene mutational status, and loss or mutation of the p53 gene are independent prognostic factors. Blood. 2002;100(4):1177–84. [PubMed] [Google Scholar]
- 8.Crespo M, Bosch F, Villamor N, Bellosillo B, Colomer D, Rozman M, et al. ZAP-70 expression as a surrogate for immunoglobulin-variable-region mutations in chronic lymphocytic leukemia. N Engl J Med. 2003;348(18):1764–75. doi: 10.1056/NEJMoa023143. [DOI] [PubMed] [Google Scholar]
- 9.Rassenti LZ, Jain S, Keating MJ, Wierda WG, Grever MR, Byrd JC, et al. Relative value of ZAP-70, CD38, and immunoglobulin mutation status in predicting aggressive disease in chronic lymphocytic leukemia. Blood. 2008;112(5):1923–30. doi: 10.1182/blood-2007-05-092882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orchard JA, Ibbotson RE, Davis Z, Wiestner A, Rosenwald A, Thomas PW, et al. ZAP-70 expression and prognosis in chronic lymphocytic leukaemia. Lancet. 2004;363(9403):105–11. doi: 10.1016/S0140-6736(03)15260-9. [DOI] [PubMed] [Google Scholar]
- 11.Del Giudice I, Morilla A, Osuji N, Matutes E, Morilla R, Burford A, et al. Zeta-chain associated protein 70 and CD38 combined predict the time to first treatment in patients with chronic lymphocytic leukemia. Cancer. 2005;104(10):2124–32. doi: 10.1002/cncr.21437. [DOI] [PubMed] [Google Scholar]
- 12.Del Principe MI, Del Poeta G, Buccisano F, Maurillo L, Venditti A, Zucchetto A, et al. Clinical significance of ZAP-70 protein expression in B-cell chronic lymphocytic leukemia. Blood. 2006;108(3):853–61. doi: 10.1182/blood-2005-12-4986. [DOI] [PubMed] [Google Scholar]
- 13.Shanafelt TD, Witzig TE, Fink SR, Jenkins RB, Paternoster SF, Smoley SA, et al. Prospective evaluation of clonal evolution during long-term follow-up of patients with untreated early-stage chronic lymphocytic leukemia. J Clin Oncol. 2006;24(28):4634–41. doi: 10.1200/JCO.2006.06.9492. [DOI] [PubMed] [Google Scholar]
- 14.Capello D, Cerri M, Muti G, Berra E, Oreste P, Deambrogi C, et al. Molecular histogenesis of posttrasplantation lympho-proliferative disorders. Blood. 2003;102 (10):3775–85. doi: 10.1182/blood-2003-05-1683. [DOI] [PubMed] [Google Scholar]
- 15.Guarini A, Gaidano G, Mauro FR, Capello D, Mancini F, De Propris MS, et al. Chronic lymphocytic leukemia patients with highly stable and indolent disease show distinctive phenotypic and genotypic features. Blood. 2003;102(3):1035–41. doi: 10.1182/blood-2002-12-3639. [DOI] [PubMed] [Google Scholar]
- 16.Gaidano G, Ballerini P, Gong JZ, Inghirami G, Neri A, Newcomb EW, et al. p53 mutations in human lymphoid malignancies: association with Burkitt lymphoma and chronic lymphocytic leukemia. Proc Natl Acad Sci USA. 1991;88(12):5413–7. doi: 10.1073/pnas.88.12.5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mauro FR, Foa R, Giannarelli D, Cordone I, Crescenzi S, Pescarmona E, et al. Clinical characteristics and outcome of young chronic lymphocytic leukemia patients: a single institution study of 204 cases. Blood. 1999;94(2):448–54. [PubMed] [Google Scholar]
- 18.Gattei V, Bulian P, Del Principe MI, Zucchetto A, Maurillo L, Buccisano F, et al. Relevance of CD49d protein expression as overall survival and progressive disease prognosticator in chronic lymphocytic leukemia. Blood. 2008;111(2):865–73. doi: 10.1182/blood-2007-05-092486. [DOI] [PubMed] [Google Scholar]
- 19.Wierda WG, O’Brien S, Wang X, Faderl S, Ferrajoli A, Do KA, et al. Prognostic nomogram and index for overall survival in previously untreated patients with chronic lymphocytic leukemia. Blood. 2007;109 (11):4679–85. doi: 10.1182/blood-2005-12-051458. [DOI] [PubMed] [Google Scholar]
- 20.Shanafelt TD, Jenkins G, Call TG, Zent CS, Slager S, Bowen DA, et al. Validation of a new prognostic index for patients with chronic lymphocytic leukaemia. Cancer. 2009;115(2):363–72. doi: 10.1002/cncr.24004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molica S, Mauro FR, Callea V, Giannarelli D, Lauria F, Rotoli B, et al. The utility of a prognostic index for predicting time to first treatment (TFT) in early chronic lymphocytic leukemia (CLL): the GIMEMA (Gruppo Italiano Malattie Ematologiche dell’ Adulto) experience. Haematologica. 2010;95(3):464–9. doi: 10.3324/haematol.2009.011767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gentile M, Mauro FR, Calabrese E, De Propris MS, Giammartini E, Mancini F, et al. The prognostic value of CD38 expression in chronic lymphocytic leukaemia patients studied prospectively at diagnosis: a single institute experience. Br J Haematol. 2005;130(4):549–57. doi: 10.1111/j.1365-2141.2005.05659.x. [DOI] [PubMed] [Google Scholar]
- 23.Montserrat E, Viñolas N, Reverter JC, Rozman C. Natural history of chronic lymphocytic leukemia: on the progression and progression and prognosis of early clinical stages. Nouv Rev Fr Hematol. 1988;30(5–6):359–61. [PubMed] [Google Scholar]
- 24.Natural history of stage A chronic lymphocytic leukaemia untreated patients. French Cooperative Group on Chronic Lymphocytic Leukaemia. Br J Haematol. 1990;76(1):45–57. doi: 10.1111/j.1365-2141.1990.tb07835.x. [DOI] [PubMed] [Google Scholar]
- 25.Palmer S, Hanson CA, Zent CS, Porrata LF, Laplant B, Geyer SM, et al. Prognostic importance of T and NK-cells in a consecutive series of newly diagnosed patients with chronic lymphocytic leukaemia. Br J Haematol. 2008;141(5):607–14. doi: 10.1111/j.1365-2141.2008.07070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, et al. International Workshop on Chronic Lymphocytic Leukemia. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–56. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]