Abstract
Background
Spinal deformity, a common problem in children with myelodysplasia, is associated with alterations in pulmonary function and sitting balance. Sitting imbalance causes areas of high pressure in patients already at high risk for developing pressure ulcers due to insensate skin.
Questions/purposes
We asked: Does spinal deformity affect pulmonary function tests in children with myelodysplasia? Does the magnitude of spinal curvatures and pelvic obliquity affect seating pressures? Does spinal deformity and seated pressures correlate with a history of pressure ulcers?
Patients and Methods
We retrospectively reviewed 32 patients with myelodysplasia and scoliosis (mean age, 14 years). The mean thoracic scoliosis was 64° with a mean pelvic obliquity of 15°. The mean forced vital capacity was 59% of predicted. The mean of the average and peak seated pressures were 24 and 137 mm Hg, respectively. We examined spinal radiographs, pulmonary function tests, and seated pressure maps and evaluated correlations of spinal deformity measures, pulmonary function, and seated pressures.
Results
The thoracic scoliosis inversely correlated with lung volume and weakly related with only the forced midexpiratory volume parameter (R2 = 31%). The curve magnitude was associated with % seated area with pressures of 38 to 70 mm Hg while lesser degrees of pelvic obliquity were associated with % seating area with pressures of less than 38 mm Hg (R2 = 25% and 24%, respectively). A history of pressure ulcers did not correlate with any spinal deformity or seated pressure measures.
Conclusions
All patients displayed a reduced forced vital capacity, but this reduction was not related to increasing scoliosis. The smaller scoliosis curves and lesser degrees of pelvic obliquity were associated with larger areas of low seated pressures.
Introduction
Scoliosis is common in patients with myelodysplasia. The incidence varies with the age of the child, but the major factor related to the occurrence of scoliosis is the level of spinal dysraphism [8]. Trivedi et al. [17] defined scoliosis in patients with myelomeningocele as a curve with a Cobb angle of more than 20°. Prevalence ranges from 50% to 88% [7, 11, 13, 14]. Shurtleff et al. [14] reported, by the age of 20 years, scoliosis was found in 88% of patients with thoracic paralysis, 81% with high lumbar paralysis, and 23% in the low lumbar area. More than 80% of patients older than 10 years will have a diagnosis of scoliosis [5].
Pulmonary function studies in patients with idiopathic scoliosis show a direct correlation between increasing thoracic curve severity and decreasing pulmonary function [9, 18]. In neuromuscular scoliosis, pulmonary function testing is complicated by inability of the patient to perform spirometry and the additional effects of the particular neuromuscular disorder on breathing mechanics. Children with thoracic and thoracolumbar levels of paralysis may have impaired function of the accessory respiratory muscles. Whether the degree of scoliosis and pulmonary function tests (PFTs) correlate in patients with myelomeningocele is unknown. Identifying patients at risk for pulmonary compromise would be important for timing and planning of surgical treatment.
Proper sitting balance is reflected by a center of gravity located between the ischial tuberosities so they experience equal pressure [15]. Many children with myelomeningocele have altered sitting balance due to paralysis of abdominal and thoracic and lumbar extensor musculature [12]. Lack of sensation in the buttock region puts children with myelomeningocele at risk for pressure ulceration when they spend all day sitting in a wheelchair, which can be difficult to avoid and provide relief for [6]. Scoliosis can also result in pelvic obliquity, which contributes to unequal pressure during sitting, often leading to skin breakdown [3]. Drummond et al. [2] defined four criteria for risk of ulcer formation in patients with paralysis, including more than 30% of weight distributed under one ischium, more than 11% distributed under the sacrum/coccyx, more than 55% of total weight distributed posteriorly, and nonambulatory status.
Assessing children with myelodysplasia, we determined how the magnitude of their scoliosis and kyphosis affects their PFTs as an aid to treatment planning for the deformities, how the magnitude of their spinal curvatures and pelvic obliquity affected their seating pressures because of their risk for pressure ulcers, and how these spinal deformity measures and seated pressures correlated with a history of pressure ulcers to identify those children who are at highest risk.
Patients and Methods
We retrospectively reviewed all 386 patients with myelodysplasia at the Shriners Hospital for Children–Lexington. Patients were identified by diagnosis from the hospital database as having been seen between 1998 and 2006 when seat maps became available at our center. Charts and radiographs were reviewed to identify those patients with myelomeningocele and spinal deformity. We identified 32 patients (mean age, 168 months [14 years]) who had a seated pressure map performed as part of their wheelchair evaluation and screening PFTs. Spinal radiographic examinations within 6 months of these seated pressure and PFTs were also required for study inclusion. Twenty-two of the 32 patients (70%) had a prior history of at least one pressure ulcer to the buttocks or back. No patient had thoracic congenital anomalies. Ten of the patients ultimately had spine fusions. Seven of these patients had their PFTs and seated pressure measurements after their spinal fusions. No patient had any history of pulmonary disease. No patients were recalled specifically for this study. All data came from the medical records.
Parameters evaluated were age, ambulatory status, and history of ulcer formation in either the sacral or back region. One of us (JP) analyzed sitting AP and lateral spine radiographs (taken within 6 months of measured seated pressures and PFTs). The radiographs were obtained with the child sitting on a level seat and the radiographic cassette resting level on this seat. The largest scoliotic curve involving the thoracic region was chosen as a parameter for statistical analysis as it should have the most effect on the lung function. Many of the patients had truncal obesity and lumbar anomalies that made imaging the lumbar spine difficult. Measurements of the lumbar curves were so variable that they were not included in the statistical analysis. Pelvic obliquity was more easily visualized and measured. Therefore, it was chosen as the radiographic parameter for analysis, reflecting the final alignment of the buttock to the seating surface, regardless of the spinal curvatures above it. The largest curves involving the thoracic region were noted and the magnitude of the scoliosis and the kyphosis was determined using the Cobb technique. Pelvic obliquity was determined using the method described by Osebold et al. [10] for use in spinal deformity in myelodysplasia. This method measures the angle of inclination of a line drawn between both posterior iliac crests to the horizontal edge of the radiograph and should relate to seating pressures measured from a horizontal seat (Fig. 1A). The maximal thoracic scoliotic curve had a mean Cobb angle of 63.8º of scoliosis (range, 14º–116º). The apex of curves chosen ranged from T5 to T12. The thoracic kyphosis measured from T4 to T12 in the sagittal plane was 66.5º (range, 12º–166º). The average pelvic obliquity measured 14.9º (range, 0º– 44º).
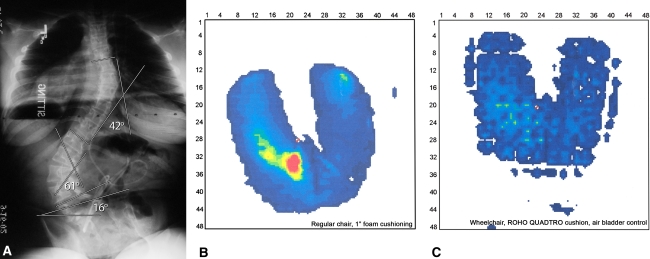
Fig. 1A–C.
(A) The sitting AP radiograph of the spine in this 13-year-old girl with lumbar myelodysplasia demonstrates thoracic and lumbar scoliotic curves with resultant pelvic obliquity. (B) The seat map pressures of the same patient sitting on a chair with 1-inch foam demonstrate an average pressure of 24 mm Hg and a peak pressure of 123 mm Hg. The areas of increased pressure are highlighted by red and yellow tones and the lower pressure areas with blue and green tones. (C) The seat map pressures are markedly reduced when the patient sits in her custom seating system with a ROHO Quadtro® cushion (ROHO, Inc, Belleville, IL) as indicated by the reduction of red and yellow areas.
Pulmonary function data were collected using the Puritan-Bennett Renaissance PB100 spirometer (Nellcor Puritan Bennett LLC, Boulder, CO) and flow was validated to comply with the American Thoracic Society Standard for Spirometry 1994 [16]. The percentage of predicted values for age, arm span as an assessment of height, weight, gender, race, barometric pressure, and temperature was used for analysis. We measured forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), ratio of FEV1:FVC, maximal midexpiratory volume (FEV 25%–75%), and peak expiratory flow.
A Seating Pressure Mapping system (X-36 Pad; Xsensor Technology Corp, Calgary, Alberta, Canada) was used to collect data on 29 of the patients. The system is based on measuring contact pressures between two opposing forces/surfaces. The sensor is a 48- × 48-cm pad with 1296 transducers placed between the patient and a 1-inch foam pad on the same wooden chair, using a computer to collect and analyze the recordings. The pressure and distribution curves are represented on the monitor via colors defining different levels of pressure measured in millimeters of mercury (Fig. 1B–C). The following data were then recorded: mean and peak pressures, total contact area, and % area with pressure distribution of less than 38 mm Hg and between 38 and 70 mm Hg.
In an effort to convert our pressure measuring systems to the same compatible software in our motion laboratory, we switched to a Pliance-HS wheelchair (Novel, St Paul, MN). Three patients had seating pressures using a new system. The pressure was measured on a 150- × 100- × 40-cm flexible elastic mat that connects from 256 to up to 1024 sensors and uses a Windows® XP application (Microsoft Corp, Redmond, WA) to analyze pressure distribution and record mean pressures, peak pressures, and total contact areas. Pressure in the new system was measured in kilopascals and contact area in square centimeters, which was converted to millimeters of mercury and square inches, respectively, to compare to the other system. Calculation of % seating area within a pressure range was not available with this system; thus, these three patients were excluded from calculations involving % area in high (38–70 mm Hg) or low (< 38 mm Hg) pressure. As seat maps were performed during a wheelchair evaluation, 80% of patients in this study used wheelchairs for all mobility; 20% were household ambulators and used wheelchairs for community mobility.
The parameters were first placed in a correlation analysis and relevant data were then placed in a stepwise regression analysis to determine whether the spinal deformity parameters predicted the changes observed in PFTs and seated pressures. An ANOVA was used to assess the association of magnitude of the scoliosis curve, kyphosis, pelvic obliquity, and all measures of seated pressures with the history of ulcers versus no ulcers. Statistical comparisons were assessed using StatView® Version 2.0 software (SAS Institute Inc, Cary, NC).
Results
The PFTs in patients with myelomeningocele demonstrated a restrictive pattern of lung function, and larger degrees of thoracic spinal deformity were correlated with lower measures of lung volume and peak expiratory flow (Table 1). The regression analysis identified only a relationship (R2 = 31%) between scoliosis curve magnitude and FEV 25%–75%.
Table 1.
Pulmonary function studies as related to scoliosis and kyphosis curve magnitude
| Variable | Value* | Scoliosis | Kyphosis | ||
|---|---|---|---|---|---|
| Correlation coefficient | Coefficient of determination (R2) | Correlation coefficient | Coefficient of determination (R2) | ||
| FVC | 58.8% (20%–135%) | 0.223 | 5% | 0.684 | 18% |
| FEV1 | 56.5% (21%–126%) | 0.335 | 11% | 0.647 | 13% |
| FEV1/FVC | 97.1% (78%–115%) | 0.409 | 17% | 0.657 | 16% |
| FEV 25%–75% | 61.9% (10%–137%) | 0.576 | 31% | 0.105 | 1% |
| Peak expiratory flow | 55.2% (23%–134%) | 0.552 | 24% | 0.074 | 0.5% |
All correlations are inverse in direction; * values are expressed as means, with ranges in parentheses; FVC = forced vital capacity; FEV1 = forced expiratory volume in 1 second; FEV 25%–75% = maximal midexpiratory volume.
Greater degrees of thoracic scoliosis and pelvic obliquity were correlated with greater average and peak seated pressures and larger % areas of high pressure (38–70 mm Hg) (Table 2). Smaller spinal deformities correlated with larger % areas of low pressure (< 38 mm Hg). The thoracic kyphosis was also directly correlated to average and peak seated pressures and % area of high pressure and inversely to % area of low pressure (Table 2). However, stepwise regression indicated the relationship of the spinal deformity parameters and seated pressures had a coefficient of determination of R2 = 25% or less.
Table 2.
Seated pressures as related to magnitude of scoliosis, kyphosis, and pelvic obliquity
| Variable | Value* | Scoliosis | Kyphosis | Pelvic obliquity | |||
|---|---|---|---|---|---|---|---|
| Correlation coefficient | Coefficient of determination (R2) | Correlation coefficient | Coefficient of determination (R2) | Correlation coefficient | Coefficient of determination (R2) | ||
| Average pressure (mm Hg) | 24.2 (12.2–37.4) | 0.425 | 16% | 0.292 | 8% | 0.413 | 12% |
| Peak pressure (mm Hg) | 137.13 (23–268.5) | 0.093 | 2% | 0.277 | 5% | 0.108 | 23% |
| % area < 38 mm Hg | 87.30% (64.7%–99.9%) | 0.501 | 24% | 0.240 | 4% | 0.706 | 24% |
| % area 38–70 mm Hg | 10.60% (0.2%–32%) | 0.503 | 25% | 0.179 | 2% | 0.461 | 23% |
* Values are expressed as means, with ranges in parentheses.
The magnitude of the thoracic scoliosis curve, kyphosis, pelvic obliquity, and all measures of seated pressures were all similar in patients with and without a history of ulcer.
Discussion
Children with myelodysplasia are at high risk for developing spinal deformity. In addition to their impaired muscular effort, that deformity compromises the thoracic cage and reduces their lung volumes. The spinal deformity also alters their sitting balance and puts them at risk for uneven pressure loading on insensate skin and resultant skin ulcers. Assessing children with myelodysplasia, we determined how the magnitude of their scoliosis and kyphosis affects their PFTs, how the magnitude of their spinal curvatures and pelvic obliquity affected their seating pressures, and how these spinal deformity measures and seated pressures correlated with a history of pressure ulcers.
We acknowledge limitations to our study. First, the retrospective nature of our study of existing data means full documentation of location of ulcers, seating system modification, and how they correlated to seating pressure measurements could not be made. A prospective study would have also allowed measurements of PFTs and seated pressures over time. Retrospectively, we were unable to evaluate how these parameters changed with increasing spinal deformity in an individual child. Second, the small number of subjects in this study did not allow for comparative analysis of PFTs and seated pressures with the more variable parameters of spinal deformity among a group of children with myelodysplasia, such as the apex and extent of their scoliosis or kyphosis, congenital/fused versus neuromuscular components, and the flexibility of their deformities. Third, the techniques used for measurement of seated pressures changed during our study. This meant three of our patients could not be used for analysis of the % area in high or low pressure. However, they were included because the two seat map systems were comparable in the measurement of average and peak pressures. Unfortunately, neither system could perform pressure localization calculations for comparison with the prior study on ulcers by Drummond et al. [2]. This may explain why we did not find strong relationships with seating pressures and history of ulcers. Fourth, while the static-positioned orthogonal radiographs and seated pressures are easy to measure and reproduce, they cannot fully describe how a three-dimensional spinal deformity affects the buttock skin of a child moving throughout the day. This may also explain the weak relationship between spinal curve measurements, seating pressures, and ulcers.
Most patients with myelomeningocele have a restrictive lung pattern, a finding we were able to support in our study. The PFT most related to thoracic scoliosis curve magnitude was FEV 25%–75% with 57.6% explained. For this correlation, as the Cobb angle increased, the FEV 25%–75% decreased. Previous studies in idiopathic scoliosis suggest FVC and FEV1 are minimally affected by magnitude of the thoracic curve, number of vertebrae, thoracic hypokyphosis, and coronal balance [9, 18]. Our regression analysis also showed a slight relationship between Cobb angle and FEV 25%–75%. No other PFT was related to curve magnitude. Our data suggest, unlike idiopathic scoliosis, pulmonary function in children with myelomeningocele does not change substantially with progressive Cobb angles. The effects of truncal and accessory respiratory muscle weakness, collapse of the abdominal region in sitting, and restricted movement due to lumbar scoliosis on PFTs were not studied. Additional studies to evaluate apex and extent of curve and coronal balance may show some association with pulmonary function.
We found kyphosis inversely correlated with FVC such that, as the kyphosis progressed, the FVC decreased. However, regression analysis involving all variables showed the two factors were not related. Kyphosis also inversely correlated with FEV1. While FEV1 is best for assessing asthma progression, it can also be decreased in patients with restrictive lung disease [4]. Similar findings have not been seen in idiopathic scoliosis.
Overall, the measured seating pressures were not correlated to spinal curve magnitude in our study. There was, however, a weak relationship in regard to % area with pressure of less than 38 mm Hg and % area with pressure of between 38 and 70 mm Hg. We found, as the scoliotic curve magnitude increased, there was a decrease in % area with low pressure but an increase in % area with higher pressures. Drummond et al. [1] studied the relationship between spinal deformity and pelvic obliquity on the distribution of seating pressure. Their study included a sitting pressure, but they did not specify at what magnitude of curve or pelvic obliquity they found ulcer formation or sitting imbalance. Our study showed, as the magnitude of the curve increased, we had an increase in % area with pressure of between 38 and 70 mm Hg. An assumption is, as the scoliotic curve progresses, acting through pelvic obliquity, there is sitting imbalance, leading to increasing seated pressure and ultimately to skin breakdown and ulcer formation. We did not determine where on the seat area (ischium, sacrum, or posterior) the pressure was highest. Therefore, we were not able to use the criteria of Drummond et al. [2] to define children with increased risk of ulcer formation. We found pelvic obliquity inversely correlated with % area with pressure of less than 38 mm Hg; as pelvic obliquity increased, % area with pressure of less than 38 mm Hg decreased, meaning, as pelvic obliquity progressed, seating pressures increased, which could in turn lead to increased skin breakdown. While the two are related, the relationship is weak.
History of ulcer formation in this study was not related to scoliosis, kyphosis, pelvic obliquity, or seated pressures. The causes of pressure ulcers are often multifactorial. Due to the limitations of this retrospective study regarding location of ulcers compared to areas of excessive pressure, the wheelchair seat modifications performed to prevent them, and other temporally related factors, such as nutritional status, hygiene, and changes in social or physical environment, we cannot provide any further conclusions on the variables causing ulcers in this patient population.
A larger-scale prospective study would allow us to draw conclusions about the changes occurring in pulmonary function and seated pressures over time with progressive spinal deformity and in response to spinal fusion in myelodysplasia. Nonetheless, we found restrictive lung function in patients with myelodysplasia only weakly related to the magnitude of their scoliosis or kyphosis, unlike what is documented in adolescents with idiopathic scoliosis. We also demonstrated, while seated pressure measures did not correlate with scoliosis or pelvic obliquity, smaller degrees of spinal deformity were associated with larger areas of low pressure. We observed no correlation between any of the seating pressure measures and history of ulcer formation. Seat pressure maps may have some clinical value in determining whether different seating options or adjustments reduce overall pressures or unload specific high pressure areas, thereby preventing ulcers for a particular patient (Fig. 1B–C).
Acknowledgments
The authors acknowledge the assistance of Dr. James Gardiner in study design, data collection, and data analysis. We additionally recognize the efforts of Christin Minter, MA, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, NC, for her work in managing the clinical study, data analysis, manuscript, and revision.
Footnotes
The institutions of one or more the authors (JLW, VRT, HJI, TAM) have received research funding from Kosair Charities and Shriners Hospitals research grants for projects unrelated to this study. The institutions of one or more of the authors (JP, JLW, VRT, HJI, TAM) have also received research funding from DePuy Orthopaedics, Inc (Warsaw, IN), Smith and Nephew, Inc (Memphis, TN), and Zimmer Inc (Warsaw, IN) for projects unrelated to this study.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the Shriners Hospital for Children–Lexington.
References
- 1.Drummond D, Breed AL, Narechania R. Relationship of spine deformity and pelvic obliquity on sitting pressure distributions and decubitus ulceration. J Pediatr Orthop. 1985;5:396–402. doi: 10.1097/01241398-198507000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Drummond D, Moreau M, Cruess R. The results and complications of surgery for the paralytic hip and spine in myelomeningocele. J Bone Joint Surg Br. 1980;62:49–53. doi: 10.1302/0301-620X.62B1.7351435. [DOI] [PubMed] [Google Scholar]
- 3.Drummond D, Narechania R, Rosenthal AN, Breed AL, Lange TA, Drummond DK. A study of pressure distributions measured during balanced and unbalanced sitting. J Bone Joint Surg Am. 1982;64:1034–1039. [PubMed] [Google Scholar]
- 4.Gal TJ. Pulmonary function testing. In: Miller RD, editor. Anesthesia. 5. Philadelphia, PA: Churchill Livingstone; 2000. pp. 886–887. [Google Scholar]
- 5.Guille JT, Sarwark JF, Sherk HH, Kumar SJ. Congenital and developmental deformities of the spine in children with myelomeningocele. J Am Acad Orthop Surg. 2006;14:294–302. doi: 10.5435/00124635-200605000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Henderson J, Price SH, Brandstater ME, Mendac BR. Efficacy of three measures to relieve pressure in seated persons with spinal cord injury. Arch Phys Med Rehabil. 1994;75:535–539. [PubMed] [Google Scholar]
- 7.Müller EB, Nordwall A. Prevalence of scoliosis in children with myelomeningocele in western Sweden. Spine (Phila Pa 1976) 1992;17:1097–1102. doi: 10.1097/00007632-199209000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Müller EB, Nordwall A, Oden A. Progression of scoliosis in children with myelomeningocele. Spine (Phila Pa 1976) 1994;19:147–150. doi: 10.1097/00007632-199401001-00005. [DOI] [PubMed] [Google Scholar]
- 9.Newton PO, Faro FD, Gollogly S, Betz RR, Lenke LG, Lowe TG. Results of preoperative pulmonary function testing of adolescents with idiopathic scoliosis. J Bone Joint Surg Am. 2005;87:1937–1946. doi: 10.2106/JBJS.D.02209. [DOI] [PubMed] [Google Scholar]
- 10.Osebold WR, Mayfield JK, Winter RB, Moe JH. Surgical treatment of paralytic scoliosis associated with myelomeningocele. J Bone Joint Surg Am. 1982;64:841–856. [PubMed] [Google Scholar]
- 11.Piggot H. The natural history of scoliosis in children with myelodysplasia. J Bone Joint Surg Br. 1980;62:54–58. doi: 10.1302/0301-620X.62B1.6985915. [DOI] [PubMed] [Google Scholar]
- 12.Rodgers WB, Frim DM, Emans JB. Surgery of the spine in myelodysplasia: an overview. Clin Orthop Relat Res. 1997;338:19–35. doi: 10.1097/00003086-199705000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Samuelsson L, Eklöf O. Scoliosis in myelomeningocele. Acta Orthop Scand. 1988;59:122–127. [PubMed] [Google Scholar]
- 14.Shurtleff DB, Goiney R, Gorden LD, Livermore N. Myelodysplasia: the natural history of kyphosis and scoliosis. A preliminary report. Dev Med Child Neurol Suppl. 1976;37:126–133. doi: 10.1111/j.1469-8749.1976.tb04294.x. [DOI] [PubMed] [Google Scholar]
- 15.Smith R, Emans J. Sitting balance and spinal deformity. Spine (Phila Pa 1976) 1992;17:1103–1109. doi: 10.1097/00007632-199209000-00016. [DOI] [PubMed] [Google Scholar]
- 16.Standardization of spirometry: 1994 update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152:1107–1136. [DOI] [PubMed]
- 17.Trivedi J, Thomson JD, Slakey JB, Banta JV, Jones PW. Clinical and radiographic predictors of scoliosis in patients with myelomeningocele. J Bone Joint Surg Am. 2002;84:1389–1394. doi: 10.2106/00004623-200208000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Weinstein SL. Adolescent idiopathic scoliosis: natural history. In: Weinstein SL, editor. The Pediatric Spine: Principles and Practice. 2. Philadelphia, PA: Lippincott Williams and Wilkins; 2001. pp. 355–369. [Google Scholar]