Abstract
Background
Depot delivery of antimicrobial agents is used for treatment and prevention of bacterial orthopaedic infections; there is little information regarding newer antifungal agents and their potential use in polymethylmethacrylate (PMMA) depot delivery.
Questions/purposes
We determined the percent of anidulafungin or voriconazole present after polymerization in PMMA beads loaded with anidulafungin or voriconazole, and we assessed elution of anidulafungin or voriconazole from beads loaded with anidulafungin or voriconazole.
Materials and Methods
Beads containing 7.5% anidulafungin or voriconazole were pulverized and incubated in Kreb’s ringer buffer for 48 hours; the buffer was assayed for anidulafungin or voriconazole concentration. The in vitro release of anidulafungin and voriconazole from PMMA beads loaded with 7.5% anidulafungin or voriconazole was determined in triplicate in a continuous flow chamber.
Results
0.7% of anidulafungin and 5.6% of voriconazole loaded in the beads were detected after polymerization. No anidulafungin was detected in the elution studies. The mean peak voriconazole concentration in the elution studies was 0.9 μg/mL.
Conclusions
Anidulafungin may not be suitable for depot delivery in PMMA.
Introduction
Various antimicrobial agents have been mixed with PMMA for United States Food and Drug Administration-approved use, and as clinician-directed applications for depot drug delivery for treatment of bone and joint infections. However, there are many unknowns associated with adding antimicrobials to PMMA and surgically implanting it: questions remain regarding whether the antimicrobial agent will remain biologically active when mixed with components of PMMA and exposed to the heat of polymerization, and how the antimicrobial agent will release from PMMA.
Few studies have examined the elution of antifungal agents from PMMA bone cement. Silverberg et al. measured diffusion of amphotericin B, fluconazole, and 5-flucytosine from PMMA bone cement using agar diffusion [8]. Amphotericin B and fluconazole had zones of inhibition, whereas 5-flucytosine did not. They interpreted their findings as supporting mixing amphotericin B or fluconazole in bone cement for the treatment of fungal orthopaedic implant infections. In accordance with this finding, Bruce et al. reported two cases of fungal infection of prosthetic joints which were treated successfully with fluconazole-impregnated PMMA beads, surgical debridement, and oral fluconazole [2]. However, Goss et al. measured the elution of amphotericin B from PMMA using ultraviolet-visible spectroscopy, and determined that less than 0.03% of the amphotericin B was released [3]. Sealy et al. showed that anidulafungin levels in fetal calf serum surrounding PMMA spheres rapidly became undetectable, suggesting that anidulafungin may not be appropriate for incorporation in PMMA [7].
Our goal was to confirm the rapidly undetectable levels of anidulafungin after release from PMMA and to determine whether voriconazole remains stable after incorporation in PMMA and elutes from PMMA in vitro, which we do not believe has been previously addressed.
Methods and Materials
We prepared PMMA beads containing anidulafungin or voriconazole (Fig. 1). Two analyses then were performed. First, three beads of each type (independent variables) were pulverized, placed in a buffer for 48 hours, and the concentration of anidulafungin or voriconazole determined in the buffer (dependent variables). Second, three anidulafungin or voriconazole-containing beads (independent variables) were placed in a previously established in vitro elution chamber [1, 4, 6], and eluted concentrations of the study antifungal agents (dependent variables) were measured with time.
Fig. 1.
The experimental design is shown.
Beads were prepared by adding anidulafungin or voriconazole crystals to Surgical Simplex® P radiopaque bone cement (Stryker Orthopedics, Mahwah, NJ, USA) and methyl methacrylate liquid (9.75 mL methyl methacrylate, 0.25 mL N, N-dimethyl-para-toluidine, 75 ± 15 ppm hydroquinone). Beads containing 7.5% (weight/weight) antifungal agent were prepared by mixing 153 mg voriconazole powder (98% pure) with 2000 mg cement powder, or 182 mg anidulafungin (82% pure) with 2000 mg cement powder. Then, 1 mL methyl methacrylate liquid monomer was added and mixed, using a spatula, to a putty-like consistency. The cement putty was spread in a sterile 3-mm bead mold and allowed to polymerize for 2 hours. The 3-mm diameter beads (weight, 20.3–25.2 mg each) were stored at 4°C until studied.
We first determined the amount of antimicrobial present after polymerization of PMMA. Three beads were weighed, crushed with a tissue pulverizer, homogenized to a particle size less than 50 μm in 5 mL Krebs-Ringer buffer, and incubated at 4°C for 48 hours. PMMA particles were removed from the buffer by centrifugation and the buffer was assayed for the antifungal agent. Results were expressed as percent of antifungal loaded into the bead detected after polymerization.
Second, we used the continuous flow chamber as described by Perry et al. [6]. Each antifungal-loaded PMMA bead was weighed and placed in a custom glass elution cell containing 1 mL of Krebs-Ringer buffer. Two ports in the closed cell allowed for inflow (1 mL/hour) and outflow (1 mL/hour) of buffer. Buffer was pumped into the cell with a variable speed pump (Masterflex C/L, Vernon Hills, IL, USA) and out of the chamber through Tygon® tubing (Saint-Gobain Performance Plastics, Akron, OH) to a fraction collector (Model 2110, Bio-Rad, Richmond, CA, USA), which rotated every 60 minutes. Samples of chamber outflow were collected in microcentrifuge tubes every hour for the first 24 hours and every 2 hours for the second 24 hours. Samples were stored at −70ºC until assays were performed.
The sample size was based on previous studies with antibacterial agents [1, 4, 6]. Data are presented as the mean and range for the pulverization and elution experiments. Elution data are expressed as the area under the curve, peak concentration detected, and time until the antifungal no longer was detected.
Anidulafungin and voriconazole concentrations were determined by high performance liquid chromatography at the Fungus Testing Laboratory at the University of Texas Health Science Center (San Antonio, TX, USA). The lower limits of detection of anidulafungin and voriconazole were 0.125 and 0.2 μg/mL, respectively.
Results
The amount of anidulafungin detected after polymerization of PMMA was 0.7% (range, 0.1–2.7%), and the amount of voriconazole detected after polymerization of PMMA was 5.6% (range, 3.5–7.3%).
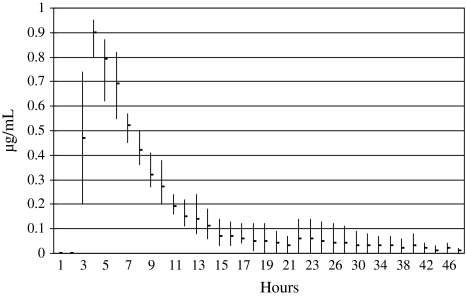
Voriconazole, but not anidulafungin, was detected in the continuous flow chamber experiments (Fig. 2). The peak voriconazole concentration detected in the continuous flow chamber experiments was 0.9 μg/mL and remained above the lower limit of detection of the assay for 10 hours. (For comparison, the voriconazole MIC90 for Candida albicans reported by Marco et al. was ≤ 0.03 μg/mL [5].) The voriconazole AUC0−∞ was 3.71 μg/mL/hour. No anidulafungin was detected in the effluent of the continuous flow chamber.
Fig. 2.
The mean ± range concentration of voriconazole at timed intervals of three continuous flow chamber release experiments are shown. The peak voriconazole concentration detected in the continuous flow chamber experiments was 0.9 μg/mL and remained above the lower limit of detection of the assay for 10 hours.
Discussion
Fungal bone and joint infections are rare, and little is known about PMMA depot delivery of antifungal agents for treatment. We determined the percent of anidulafungin or voriconazole present after polymerization using PMMA beads loaded with anidulafungin or voriconazole, and assessed elution of anidulafungin or voriconazole from beads loaded with anidulafungin or voriconazole.
Our study is subject to a major limitation. In previous studies [1, 4, 6], antimicrobial concentrations were assayed using bioassay; in this study, high performance liquid chromatography was used. Whether this affected the findings is unknown. Protein or other binding of anidulafungin may have interfered with the assay. However, in a recent study using a bioassay, Sealy et al. showed that anidulafungin levels in fetal calf serum surrounding PMMA spheres rapidly became undetectable in a static assay [7], corroborating our findings. Sealy et al. did not study voriconazole.
Compared with anidulafungin, the amount of voriconazole detected after polymerization was greater (0.7 versus 5.6%, respectively). The findings for anidulafungin and voriconazole are in contrast to those in previous studies with amikacin, tobramycin, gentamicin, cefazolin, ciprofloxacin, gatifloxacin, levofloxacin, linezolid, rifampin, and daptomycin, in which proportionally more antibacterial agent was detected after bead crushing [1, 4, 6] than we found with antifungal agents.
Using a dynamic elution model, we were unable to detect anidulafungin release from PMMA, using PMMA loaded with 7.5% anidulafungin. Conversely, we were able to detect a timed release of voriconazole in our continuous flow chamber. The findings are also in contrast to previous studies with amikacin, tobramycin, gentamicin, cefazolin, ciprofloxacin, gatifloxacin, levofloxacin, linezolid, rifampin, and daptomycin, which showed more substantial elution [1, 4, 6] than either of the antifungal agents observed in our current study. We found voriconazole had a similar release profile to the aforementioned antibacterial agents, but with a lower area under the concentration curve and maximum concentration. Our findings are in accord with those of Sealy et al. showing that anidulafungin levels in fetal calf serum surrounding PMMA spheres rapidly became undetectable [7].
Our findings raise concern regarding the clinical use of PMMA to deliver therapeutic doses of anidulafungin.
Acknowledgment
We acknowledge the technical assistance of Gary Nguyen.
Footnotes
One or more of the authors (MSR, AH, JMS, RP) has received funding from Pfizer Pharmaceuticals, Inc.
References
- 1.Anguita-Alonso P, Rouse MS, Piper KE, Jacofsky DJ, Osmon DR, Patel R. Comparative study of antimicrobial release kinetics from polymethylmethacrylate. Clin Orthop Relat Res. 2006;445:239–244. doi: 10.1097/01.blo.0000201167.90313.40. [DOI] [PubMed] [Google Scholar]
- 2.Bruce AS, Kerry RM, Norman P, Stockley I. Fluconazole-impregnated beads in the management of fungal infection of prosthetic joints. J Bone Joint Surg Br. 2001;83:183–184. doi: 10.1302/0301-620X.83B2.11444. [DOI] [PubMed] [Google Scholar]
- 3.Goss B, Lutton C, Weinrauch P, Jabur M, Gillett G, Crawford R. Elution and mechanical properties of antifungal bone cement. J Arthroplasty. 2007;22:902–908. doi: 10.1016/j.arth.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Hall EW, Rouse MS, Jacofsky DJ, Osmon DR, Hanssen AD, Steckelberg JM, Patel R. Release of daptomycin from polymethylmethacrylate beads in a continuous flow chamber. Diagn Microbiol Infect Dis. 2004;50:261–265. doi: 10.1016/j.diagmicrobio.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Marco F, Danes C, Almela M, Jurado A, Mensa J, la Bellacasa JP, Espasa M, Martinez JA, Jimenez de Anta MT. Trends in frequency and in vitro susceptibilities to antifungal agents, including voriconazole and anidulafungin, of Candida bloodstream isolates: results from a six-year study (1996–2001) Diagn Microbiol Infect Dis. 2003;46:259–264. doi: 10.1016/S0732-8893(03)00086-5. [DOI] [PubMed] [Google Scholar]
- 6.Perry AC, Rouse MS, Khaliq Y, Piper KE, Hanssen AD, Osmon DR, Steckelberg JM, Patel R. Antimicrobial release kinetics from polymethylmethacrylate in a novel continuous flow chamber. Clin Orthop Relat Res. 2002;403:49–53. doi: 10.1097/00003086-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Sealy PI, Nguyen C, Tucci M, Benghuzzi H, Cleary JD. Delivery of antifungal agents using bioactive and nonbioactive bone cements. Ann Pharmacother. 2009;43:1606–1615. doi: 10.1345/aph.1M143. [DOI] [PubMed] [Google Scholar]
- 8.Silverberg D, Kodali P, Dipersio J, Acus R, Askew M. In vitro analysis of antifungal impregnated polymethylmethacrylate bone cement. Clin Orthop Relat Res. 2002;403:228–231. doi: 10.1097/00003086-200210000-00033. [DOI] [PubMed] [Google Scholar]