Abstract
Background
The Vertical Expandable Prosthetic Titanium Rib (VEPTR™; Synthes North America, West Chester, PA) reportedly controls spinal deformity associated with constrictive chest wall conditions.
Questions/purposes
We asked whether spine-to-spine constructs using VEPTR™ instrumentation in combination with standard spinal instrumentation could be deployed to salvage failed rib-to-spine constructs used originally in patients with constricted chest walls and to primarily treat progressive spinal deformity without chest wall abnormalities.
Patients and Methods
Fifty patients were treated with VEPTR™ constructs for thoracic insufficiency syndrome at our center between 2001 and 2007. Fourteen of these 50 patients had placement of a spine-to-spine construct using a VEPTR™ implant in combination with standard spinal implants and are the subject of this retrospective review. Five had prior rib-based VEPTR™ or growing implants with an average of two failures before this surgery. Radiographic variables, preceding treatment, complications, and changes in ambulatory status, were recorded. The minimum followup was 2 years (mean, 35 months; range, 2–4 years).
Results
After an average of five expansions in these 14 patients, positive changes were recorded for Cobb angle, T1–S1 height, sagittal balance, and space available for the lung. Complications included two rod fractures, two superficial infections, and one deep infection with rod removal.
Conclusions
VEPTR™ instrumentation as a spine-to-spine growing-rod construct demonstrated ease of implantation and expansion, with complication rates similar to other reported devices. This study suggests growing constructs using VEPTR™ can be used with relatively few complications and extends the potential uses of this instrumentation system.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Stabilization of progressive spinal and chest wall deformity and preservation of lung growth and function are the main goals of spinal deformity surgeons in the management of children with infantile and juvenile scoliosis. When nonoperative measures have been unable to control deformity progression, several different surgical approaches using either distracted growth or, more recently, guided growth techniques have been developed [1, 9]. These systems use proximal and distal spine-based anchors. Early use of a single rod was associated with early implant and anchor failures approaching 70%, but the introduction of dual-rod spine-to-spine constructs decreased the rates of early implant and anchor failures to less than 10%, with implant failures rising over time to 50% [2, 4, 10]. Campbell and Hell-Vocke [3] introduced the Vertical Expandable Prosthetic Titanium Rib (VEPTR™; Synthes North America, West Chester, PA) for the management of the constricted hemithorax. The device has US Food and Drug Administration (FDA)/Humanitarian Use Device (HUD) approval for the management of thoracic insufficiency syndrome plus the anatomic presence of flail chest, constrictive chest syndrome (which includes fused ribs and scoliosis), hypoplastic thorax, and neuromuscular or congenital scoliosis without chest wall abnormality. The system uses proximal rib-to-distal rib, spine, or pelvis constructs for these patients and has reported failures of the proximal rib anchors as the most common complication (26%) during treatment [6].
Distraction growth constructs (growing rods) can be manufactured from any existing spinal implant system using connectors and overlapping rods in an off-label use of the device. The dual-rod construct as described by Akbarnia et al. [1] uses a prefabricated sleeve and rod system (ISOLA®; DePuy Spine, Raynham, MA) construct. Fusion of the proximal and distal anchors provided a more stable anchor point compared to an isolated single fixation anchor point [8]. The senior author (KMS) has been using the VEPTR™ system in the treatment of infantile and juvenile neuromuscular and congenital scoliosis since the initial Investigational Device Exemption study in 2001. A subset of the patients treated at our institution using the original method described by Campbell et al. [4] had repeated proximal rib anchor failures and did not have primary chest wall deformities (Fig. 1). Because distractions using the VEPTR™ implants were very simple, highly predictable, and largely performed as outpatient procedures, these patients were managed using the VEPTR™ implants and the techniques described by Akbarnia et al. [1]. Subsequently, other patients meeting these criteria have been treated similarly.
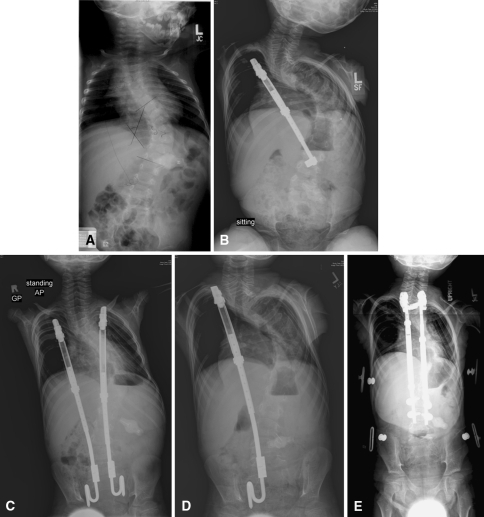
Fig. 1A–E.
Radiographs illustrate the case of a female patient with neuromuscular scoliosis (Patient 14). (A) A pretreatment radiograph shows the initial deformity. (B) The first rib-based construct resulted in a progression of the deformity 1 year after implant. (C) The first construct was converted to a rib-to-pelvis construct. (D) The left rod was removed after three proximal rib fractures, distal anchor migration, and revisions. (E) The construct was converted to a VEPTR™ spine-based construct using conventional spine implants for anchors.
VEPTR™ constructs reportedly control spinal deformities associated with constrictive chest wall conditions while allowing for continued spinal growth and preservation of respiratory function as measured by capillary blood gases and respiratory assistance [3–6].
We asked whether spine-to-spine constructs using VEPTR™ instrumentation in combination with standard spinal instrumentation could be deployed successfully to salvage failed (defined as loss of stable fixation) rib-to-spine constructs and to primarily treat progressive spinal deformity without chest wall abnormalities We then identified the complications and whether the surgery changed the ambulatory status.
Patients and Methods
Fifty patients were treated with VEPTR™ constructs for thoracic insufficiency syndrome at our center between 2001 and 2007. Fourteen of these 50 patients had placement of a spine-to-spine construct using VEPTR™ implant in combination with standard spinal implants using a modification of the method described by Akbarnia et al. [1] (Table 1). The indications for the spine-to-spine constructs using dual rods were (1) absence of a primary chest wall deformity, (2) progression of spinal deformity to a Cobb angle of greater than 50°, and (3) migration of a previously placed proximal rib anchor or of a prior non-VEPTR™ growing rod to the point of loss of stable fixation. The contraindications were (1) severe proximal kyphosis preventing successful control of the proximal deformity and (2) lack of sufficient soft tissue coverage to allow for safe placement of a proximal device as judged by our team’s general and thoracic surgeon. Ten patients were female and four were male. One patient had prior growing implants placed at another center with two distal rod breakages and distal hook migration, and four had a VEPTR™ rib-to-spine construct with an average of two rib anchor failures before conversion to a VEPTR™ spine-to-spine construct. All patients classified as “anchor failures” were symptomatic with pain at the anchor failure site, clinically noticeable loss of correction of their deformity, and/or skin at risk due to implant prominence. Average age at primary implantation of a spine-to-spine construct was 6.4 years (range, 2.1–10.8 years). Minimum followup was 24 months (mean, 35 months; range, 24–48 months). All patients have been continuously followed without loss of followup. No patients were recalled specifically for this study; all data were obtained from medical records and radiographs. Data were collected under a prospective Institutional Review Board (IRB)-approved protocol.
Table 1.
Clinical data on 14 patients treated by spine-to-spine constructs
| Patient | Gender | Age (months) | Diagnosis | Prior surgery | Reason for implantation | Followup (months) | Complications/comments |
|---|---|---|---|---|---|---|---|
| 1 | Female | 47 | Neuromuscular scoliosis | VEPTR™ | Rib fractures, progression of curve | 48 | Recurrent superficial infections, dehiscence, advancement flap; left rod removed |
| 2 | Female | 88 | Neuromuscular scoliosis | VEPTR™ | Progressive kyphosis | 45 | Implants removed; definitive fusion |
| 3 | Female | 73 | Neuromuscular scoliosis | VEPTR™ | Rib fractures | 44 | Rods fracture at third expansion; rod fracture after fifth expansion |
| 4 | Female | 104 | Neuromuscular scoliosis | No | Progressive curve (unresponsive to bracing) | 44 | None |
| 5 | Male | 63 | Neuromuscular scoliosis | No | Progressive curve (unresponsive to bracing) | 39 | Superficial infection, treated with antibiotics; progression of kyphosis; revision to VEPTR™ 2 |
| 6 | Male | 31 | Neuromuscular scoliosis | No | Progressive curve (unresponsive to bracing) | 38 | None |
| 7 | Female | 102 | Neuromuscular scoliosis | No | Progressive curve (unresponsive to bracing) | 36 | None |
| 8 | Female | 80 | Neuromuscular scoliosis | No | Progressive curve (unresponsive to bracing) | 36 | Superficial skin infection |
| 9 | Female | 74 | Congenital scoliosis | AP fusion, hemivertebrectomy | Progressive curve (unresponsive to bracing) | 30 | None |
| 10 | Female | 95 | Neuromuscular scoliosis | VEPTR™ | Rib fractures | 29 | None |
| 11 | Female | 128 | Neuromuscular scoliosis (tethered cord) | No | Progressive curve (unresponsive to bracing) | 27 | None |
| 12 | Male | 25 | Neuromuscular scoliosis | No | Progressive curve (unresponsive to bracing) | 24 | Wound infection, treated with irrigation and débridement × 2; rod fracture; implants removed after second expansion |
| 13 | Male | 89 | Neuromuscular scoliosis | VEPTR™ | Rib fractures, skin breakdown, superficial infection | 24 | None |
| 14 | Female | 86 | Neuromuscular scoliosis | Growing rod construct | Intrapelvic migration of rod | 24 | None |
VEPTR™ = Vertical Expandable Prosthetic Titanium Rib.
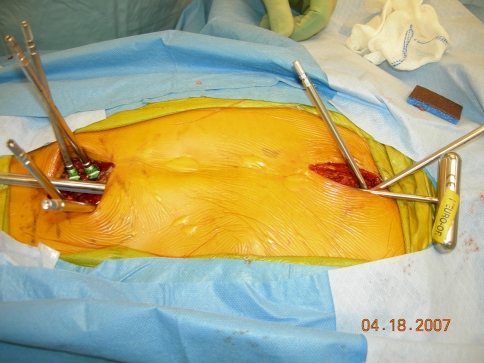
All surgery was performed by a single surgeon (KMS). The specific surgical technique used in these cases included removal of pre-existing implants when warranted. Subperiosteal exposure of the top and bottom two vertebrae was achieved for implant placement and fusion (Fig. 2). Proximal and distal anchor levels typically included measured end vertebrae extending distally to one or two levels above the measured end vertebra. A superior anchor was established using a claw technique with transverse process and pedicle hooks from the Dual-Opening Universal Spine System (Synthes North America). Caudally, a two-level pedicle screw anchor was created. A ventriculoperitoneal shunt passer was used to create a subfascial opening between the incisions through which a 20-Fr chest tube was passed. The assembled implants were then advanced subfascially with the female rod end left long by approximately 2 cm at the caudal pedicle screw anchor. The overlapping male and female components were maximally overlapped for future lengthening potential. Implants were engaged proximally and distally, and an initial lengthening of 1 to 2 cm was achieved by distraction at the caudal anchor. Decortication and placement of allograft were performed at the anchor sites. Drains were not placed. The patient was molded for a custom thoracolumbosacral orthosis that was applied 2 to 3 days later and worn full time for 3 months. The first lengthening was performed at 3 months with subsequent lengthenings at scheduled 6-month intervals. Initial implantation was always performed using comprehensive neuromonitoring (transcranial motor-evoked potentials, somatosensory evoked potentials, and free-run EMGs). No monitoring was used for lengthenings or exchanges unless there were changes at the initial implantation or the child had a history of a neural axis abnormality such as a tethered spinal cord. Average implantation time was 2.5 hours (range, 1 hour 20 minutes to 3 hours) and average blood loss was 75 mL (range, 25–150 mL). No patient received a transfusion. Patients were discharged home once they were tolerating oral intake and only taking oral pain medications.
Fig. 2.
Limited exposure is required at the upper and lower ends of the spinal construct. Implants are passed subfascially through a space created by a shunt passer and chest tube.
An underarm custom-molded thoracolumbosacral orthosis was applied the second postoperative day and continued for 3 months whenever the child was in an upright position. No physical therapy was recommended except for the initial mobilization after surgery. All children were supervised by their parents in returning to their preoperative mobility status.
Patients had a wound check by a nurse at 1 to 2 weeks postoperatively and returned for an initial radiograph in a brace at 4 to 6 weeks after surgery. Initial expansion was planned for and performed at 12 weeks after initial implantation. Bracing was discontinued just before the first expansion.
Information related to patient age, diagnosis, preceding treatment, and ambulatory status before and after treatment was collected (Table 1). Length of surgery, intraoperative blood loss, neuromonitoring changes, and postoperative complications including infections, implant failures, unplanned admissions to this or other hospitals, and unplanned returns to the operating room were recorded.
Three of us (KKW, BKD, KMS) independently measured the following variables on the preoperative and last followup radiographs: maximum coronal and sagittal Cobb angles; coronal T1–S1 length (superior end plate of T1 to superior end plate of S1); coronal balance (horizontal distance in millimeters from the center of T1 to the center sacral vertebral line); sagittal balance (distance in millimeters from a vertical line from the center of the superior end plate of S1 to the midpoint of the superior end plate of C7); and space available for the lung (SAL) as described by Campbell et al. [4] (ratio of length of the line from the midportion of the first rib of the concave hemithorax to the midportion of the dome of the ipsilateral diaphragm to the length of the line from the midportion of the first rib of the convex hemithorax to the midportion of the dome of the ipsilateral diaphragm). We used the average from the three independent observers for each variable (Table 2). An analysis of interobserver variability for all variables was performed using the intraclass correlation coefficient, with all being greater than 0.92.
Table 2.
Summary of results for 14 patients treated with spine-to-spine constructs
| Measurement | Number | Preoperative* | Postoperative* | Final* | p Value |
|---|---|---|---|---|---|
| Scoliosis (°) | 13 | 74 ± 12 | 53 ± 10 | 57 ± 15 | 0.001 |
| Kyphosis (°) | 9 | 71 ± 21 | 58 ± 18 | 69 ± 21 | 0.032 |
| T1–S1 length (mm) | 12 | 260 ± 43 | 297 ± 41 | 296 ± 62 | 0.002 |
| Coronal balance (mm) | 11 | 27 ± 17 | 32 ± 22 | 31 ± 32 | 1.00 |
| Sagittal balance (mm) | 7 | 31 ± 59 | 23 ± 43 | −7 ± 47 | 0.012 |
| SAL (%) | 12 | 85 ± 17 | 91 ± 16 | 92 ± 14 | 0.005 |
* Values are expressed as mean ± SD; SAL = space available for the lung.
Descriptive statistics were used for nominal data. Radiographic measurements were compared over the selected time points using Friedman’s test for analysis of repeated measurements of nonparametric, continuous data.
Results
Sixty-four expansions and four revision surgeries were performed during the followup time. All patients have had at least three expansions after initial implantation, with an average of five expansions per child (range, two to seven). There were no monitoring signal changes or neurologic deficits with initial implantation or any of the subsequent expansion or revision procedures. Average operative time for expansions was 37 minutes (median, 34 minutes; range, 19–123 minutes), including hardware revisions when necessary, with 50% performed as day surgery (n = 34) and 44% overnight observation (n = 30). Three patients stayed for two nights and one patient stayed more than two nights after revision surgery for exchange of implants as a result of reaching the maximum length available for expansion.
The Cobb angle improved (p = 0.001) from an average of 74° to 57° at final followup. We observed no changes in kyphosis: the patients had a mean kyphosis of 71º before and 69º after surgery. T1–S1 height increased (p = 0.002) from 260 to 296 mm at final followup. The sagittal balance changed from a mean of 31 mm anterior to the center of S1 to 7 mm posterior to the center of S1, but there was no change in coronal balance. The SAL increased (p = 0.005) from 85% to 92% (Table 2).
Complications occurred in six of 14 patients (Table 1). These included three rod fractures in two patients, one superficial infection managed with local débridement, one treated with antibiotics, and one requiring a local advancement flap after multiple recurrences and eventual rod removal. There have been no failures of proximal or distal anchors. One patient underwent elective implant removal and definitive fusion 4 years after initial implantation as a result of prominent hardware that threatened skin integrity.
All but one child with severe amyoplasia were community ambulators before surgery. All maintained their preoperative ambulatory status at final followup, with none reporting pain with ambulation or sitting.
Discussion
We investigated whether a VEPTR™ spine-to-spine construct could be used to stabilize spinal deformities for children with thoracic insufficiency syndrome who did not have chest wall deformities. Our initial question was whether rib anchor migration in rib-to-spine constructs in children with progressive spinal deformity without chest wall abnormalities could be replaced and could undergo successful distractions with a spine-to-spine dual-rod construct. The second question was whether children with progressive early-onset scoliosis without chest wall abnormality could have successful primary spine-to-spine constructs using VEPTR™ hybrids with existing spinal implants and undergo successful and minimally morbid distractions.
This study is limited by several factors. First, we had a small number of patients with limited followup and we did not intend to draw a comparison to existing studies using growing rods beyond comparing our early results with those studies. Longer followup and greater numbers of patients will shed more light on the indications and limitations of these methods. Second, we also had a heterogeneous population of children and cannot draw conclusions on the merits of growing systems versus early spinal fusion.
Our observations suggest spine-to-spine growing constructs using the VEPTR™ implant system with conventional spine anchors used in an off-label manner can be applied with low complication rates and with good short-term control of spinal deformity for progressive infantile and juvenile neuromuscular scoliosis. This method of implementation (spine to spine) extends the potential uses of this instrumentation system and provides a technique for salvage of anchor failures of traditional VEPTR™ rib-based implant constructs. Four of the 14 patients in this series had been previously treated with traditional VEPTR™ constructs with loss of proximal fixation at the proximal rib attachments requiring unplanned revision, whereas one other had a linked-rod system implanted elsewhere with migration of the distal anchor and rod fracture. In each of these cases, there were multiple failures with poor alternatives for further anchor points. In our experience, all patients with anchor migration are symptomatic, having pain at the anchor site, clinically evident loss of correction of their spinal deformity, or skin at risk due to prominence of the failed anchor. For each of these subjects, use of the dual-rod spine-to-spine anchor method has stabilized the deformity and eliminated anchor failure problems since conversion. Rod fractures using this technique were early in our experience and we believe resulted from bending of the hybrid component near the rod-sleeve juncture. The manufacturer’s warning label now includes avoiding bending of the device at this location in their package insert (Fig. 3). We have not had any device fractures since these early occurrences but note growing-rod series [1, 7, 9] report rod failures in a higher number than the rib-based reports by Campbell et al. [6]. The failures increase over time, raising a question of whether spine-based systems have higher implants stresses than rib-based systems.
Fig. 3.
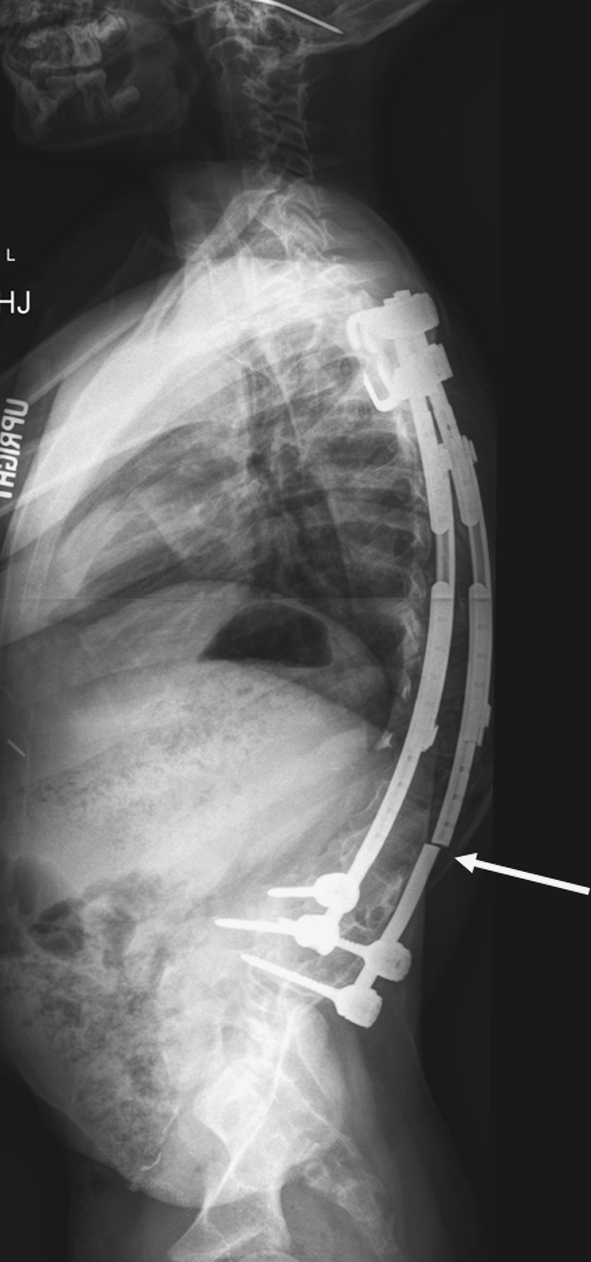
Two patients had fracture of the implant that occurred as a result of contouring of the hybrid rod near the rod sleeve attachment.
We have also found VEPTR™ instrumentation can be reliably linked to an existing spinal implant system for spine-to-spine anchors and utilized as a growing-rod construct with a low rate of complications, good control of spinal deformity, and return of children to their preoperative ambulatory status. Exposure is limited to the proximal and distal anchors with subfascial passage of a preassembled construct enabling easy linkage to the anchors. The technique of establishing solid anchor points as described by Akbarnia et al. [1, 8, 10] is critical and we recommend its use. They reported a 54% correction of the initial curve for children with infantile and juvenile idiopathic scoliosis and an average T1–S1 growth of 1.2 cm per year. Their complications rate was similar at 48%, with two deep infections, four superficial wound problems, two rod fractures, two hook dislodgements, one screw pullout, one case of crankshaft, and one case of junctional kyphosis [1]. Reporting this series of nonidiopathic scoliosis, we had a larger deformity on average and less overall correction of the Cobb angle (22%) but attained growth averaging 1.6 cm per year. Six of 14 patients in our cohort (43%) had complications, of which two were deep infections.
Surgeons with experience using the VEPTR™ system will find implementation of this instrumentation as a dual spine-to-spine construct to be a familiar approach like that with other growing systems. It should also be noted there are anecdotal cases of paraparesis resulting from breakout of proximal pedicle screw anchors (verbal communication, David Skaggs, MD, 2010), leading us to recommend the proximal construct be secured with hooks rather than pedicle screws. Although the VEPTR™ does have FDA approval for use in the growing spine for patients with thoracic insufficiency syndrome, the use of VEPTR™ with pedicle screws and spinal hooks from standard spine instrumentation systems is viewed as an off-label use of these devices and the consent process for these surgeries needs to include this. Users of this HUD device are also encouraged to contact their own IRB as to the required reporting and regulatory requirement of their institution.
In conclusion, we found the early preservation of spinal growth and control of spinal deformity using a VEPTR™ system in combination with conventional spine implants systems as a spine-to-spine growing construct similar to growing-rod results previously reported by others [1, 7, 10]. Regardless of which system is used, the high complication rates, need for multiple procedures in growing children, and small relative gains in radiographic parameters still challenge proof of efficacy of all such treatment methods. Much work remains to identify the structural correlates to respiratory insufficiency and develop effective strategies for changing these. The short followup of these patients mandates continuing assessment of these children to maturity.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Seattle Children’s Hospital.
References
- 1.Akbarnia BA, Marks DS, Boachie-Adjei O, Thompson AG, Asher MA. Dual growing rod technique for the treatment of progressive early-onset scoliosis: a multicenter study. Spine (Phila Pa 1976). 2005;30(Suppl):S46–S57. doi: 10.1097/01.brs.0000175190.08134.73. [DOI] [PubMed] [Google Scholar]
- 2.Blakemore LC, Scoles PV, Poe-Kochert C, Thompson GH. Submuscular Isola rod with or without limited apical fusion in the management of severe spinal deformities in young children: preliminary report. Spine (Phila Pa 1976). 2001;26:2044–2048. doi: 10.1097/00007632-200109150-00021. [DOI] [PubMed] [Google Scholar]
- 3.Campbell RM, Jr, Hell-Vocke AK. Growth of the thoracic spine in congenital scoliosis after expansion thoracoplasty. J Bone Joint Surg Am. 2003;85:409–420. doi: 10.2106/00004623-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Campbell RM, Jr, Smith MD, Hell-Vocke AK. Expansion thoracoplasty: the surgical technique of opening-wedge thoracostomy. Surgical technique. J Bone Joint Surg Am. 2004;86(Suppl 1):51–64. [PubMed] [Google Scholar]
- 5.Campbell RM, Jr, Smith MD, Mayes TC, Mangos JA, Willey-Courand DB, Kose N, Pinero RF, Alder ME, Duong HL, Surber JL. The characteristics of thoracic insufficiency syndrome associated with fused ribs and congenital scoliosis. J Bone Joint Surg Am. 2003;85:399–408. doi: 10.1302/0301-620X.85B3.13429. [DOI] [PubMed] [Google Scholar]
- 6.Campbell RM, Jr, Smith MD, Mayes TC, Mangos JA, Willey-Courand DB, Kose N, Pinero RF, Alter ME, Duong HL, Surber JL. The effect of opening wedge thoracostomy on thoracic insufficiency syndrome associated with fused ribs and congenital scoliosis. J Bone Joint Surg Am. 2004;86:1659–1674. doi: 10.2106/00004623-200408000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Klemme WR, Denis F, Winter RB, Lonstein JW, Koop SE. Spinal instrumentation without fusion for progressive scoliosis in young children. J Pediatr Orthop. 1997;17:734–742. doi: 10.1097/00004694-199711000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Mahar AT, Bagheri R, Oka R, Kostial P, Akbarnia BA. Biomechanical comparison of different anchors (foundations) for the pediatric dual growing rod technique. Spine (Phila Pa 1976) 2008;8:933–939. doi: 10.1016/j.spinee.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 9.McCarthy RE, Luhmann SJ, Lenke LG. Shilla growth enhancing system for the treatment of scoliosis in children. J Child Orthop. 2009;3:150. [Google Scholar]
- 10.Thompson GH, Akbarnia BA, Kostial P, Poe-Kochert C, Armstrong DG, Roh J, Lowe R, Asher MA, Marks DS. Comparison of single and dual growing rod techniques followed through definitive surgery: a preliminary study. Spine (Phila Pa 1976). 2005;30:2039–2044. doi: 10.1097/01.brs.0000179082.92712.89. [DOI] [PubMed] [Google Scholar]