Abstract
Background
In contrast with treatment recommendations for adolescent idiopathic scoliosis, there are no clear algorithms for treating patients with early-onset scoliosis. There has been rapid expansion of treatment options for children with early-onset scoliosis, including casting, growth rods, the vertical expandable prosthetic titanium rib, and anterior vertebral stapling.
Questions/purposes
Given the range of treatment options, we assessed variability in decision making regarding treatment of patients with early-onset scoliosis.
Methods
We presented 12 clinical and radiographic vignettes about patients with early-onset scoliosis to 13 experienced spine surgeons who are members of the Chest Wall and Spine Deformity Study Group. The reviewers were asked to choose type of treatment, type of construct, construct location, and whether a thoracotomy should be performed.
Results
All 13 surgeons agreed regarding the need for surgery in eight of the 12 cases. When the reviewers chose surgery, 76% (40%–100%) selected the vertical expandable prosthetic titanium rib; of those selecting that approach, 61% (0%–100%) coincided on using it bilaterally. Agreement was 20% (0%–60%) for growing rods and 4% (0%–25%) for fusions. Among all cases, agreement regarding whether instrumentation should extend to the pelvis was 71% (50%–100%). In all but two cases, at least 85% of surgeons recommended against a thoracotomy.
Conclusions
Although most surgeons agreed about the indication for surgery, we found wide variability in choice of construct type, number of constructs, and level of instrumentation.
Electronic supplementary material
The online version of this article (doi:10.1007/s11999-010-1540-0) contains supplementary material, which is available to authorized users.
Introduction
Although there are numerous options for treatment of progressive spinal deformity in the young child, no solution has emerged as the single most appropriate treatment for children with early-onset scoliosis (EOS). Historically, early fusion frequently was recommended for treatment of EOS [16]. However, there is a growing consensus that early spinal fusion results in negative thoracopulmonary consequences to children [15], and most surgeons now use fusion as a last resort in the young child. Hemiepiphysiodesis, hemivertebral resection, and short segment fusions continue to be used in selected patients. Growing rods [7] and the Vertical Expandable Prosthetic Titanium Rib (VEPTR™; Synthes Spine Co, West Chester, PA) [8] are commonly used to treat EOS. The literature supports the use of both techniques but also documents complications in this population including proximal migration of devices with rib fractures, and skin problems secondary to recurrent surgery and hardware [3, 8, 10, 13]. The complication rates ranged from 8% to 50% [2, 3, 8, 10, 13]. The literature also suggests the growing rod systems are associated with a high complication rate (ie, 8% to 50%) with the majority being minor; these systems appear to halt curve progression but not reduce curve magnitude [10, 13]. For example, Moe et al. [13] reported a complication rate of 50% owing to hook dislodgment, and Klemme et al. [10] reported an 8% complication rate attributable to implant failure. The VEPTR™ has been studied under an Investigational Device Exemption (IDE) that resulted in the US Food and Drug Administration (FDA) granting a Humanitarian Device Exemption (HDE) as FDA requirements for safety and efficacy were satisfied. The complications reported were frequent (ie, 9% to 21%) but not life threatening and deformities were corrected or controlled [3, 8]. Full approval of the VEPTR™ was not granted because the FDA believed there was insufficient control data.
The authors of one report recommend placement of growing rod instrumentation on the concave side of the spine only to expand the smallest hemithorax [2], whereas others report better radiographic correction and fewer complications with bilateral constructs [15]. An opening wedge thoracostomy sometimes is performed as part of distraction thoracoplasty [10], but some surgeons prefer not opening the thoracic cavity as they are concerned by the potential for scarring and subsequent restriction this could cause [8].
Several reports have described remarkable variability in construct patterns for adolescent idiopathic scoliosis (AIS) and adult deformity correction [1, 5, 9]. Aubin et al. [1] documented large variability among experienced surgeons in surgery and instrumentation strategies, including selection of the vertebral levels to instrument and fuse, and the number and type of implants. Donaldson et al. [5] suggested surgeons’ ratings of physical appearance on patients with AIS varied widely and suggested these inconsistencies of assessment contributed to inconsistencies in treatment recommendations. Irwin et al. studied variability for c-spine procedures and observed strong agreement for one-level disc herniation but high disagreement for the treatment of other degenerative conditions [10]. Other studies have shown variability among surgeons and radiographic techniques to accurately measure Cobb angles and how these differences can influence decision making, particularly in patients with congenital scoliosis [4, 6, 11, 12]. Intuitively, one would expect even more variability in surgical decision making in the relatively immature field of EOS.
We assessed variability in decision making for patients with EOS in four areas: (1) whether to recommend surgery; (2) what type of construct to recommend; (3) where to locate the construct; and (4) whether to perform thoracotomy.
Materials and Methods
Thirteen pediatric orthopaedic surgeons who are members of the Chest Wall and Spinal Deformity Study Group participated in this study. The surgeons were asked to submit a case of progressive EOS. All of the patients were younger than 8 years. For each case, a clinical vignette described socioclinical variables, including associated diagnoses, age, gender, and relevant medical history. This was accompanied by a set of preoperative posteroanterior and lateral spine radiographs, Cobb angles for each curve, and if available, three-dimensional CT reconstructions. All information was distributed in electronic files to each participant.
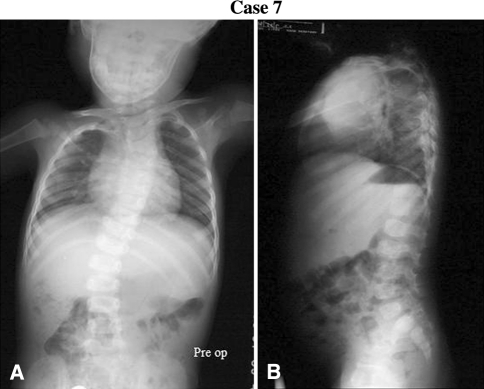
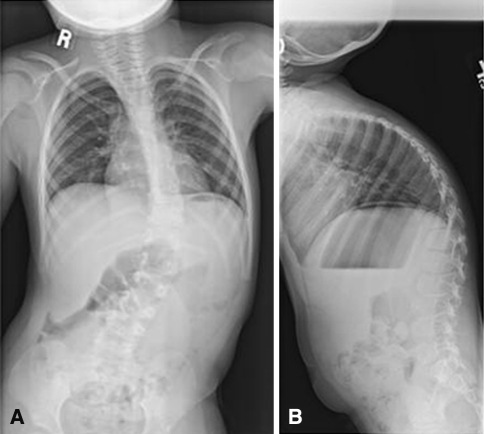
Each surgeon reviewed 12 cases (Table 1), excluding the one they submitted (Appendix 1; supplemental material available with the online version of CORR). This was the only clinical information provided (Table 1). Each reviewer recorded the following data as part of their preoperative planning: (1) type of treatment: surgical or nonsurgical (bracing or observation); (2) type of construct: VEPTR™, growing rods, definitive fusion, or other; (3) construct location: unilateral or bilateral; rib to rib, rib to pelvis, or rib to spine; and (4) thoracotomy: yes or no. Substantial agreement existed among investigators when viewing the radiographs for Patient 7 (Fig. 1; Tables 2, 3) and major disagreement existed when they viewed the radiographs for Patient 1 (Fig. 2; Tables 2, 3).
Table 1.
Description of 12 cases
| Patient number | Age (years) | Diagnosis | Type of curve | Notations |
|---|---|---|---|---|
| 1 | 2 | Congenital muscular dystrophy | Kyphoscoliosis 96°/26° | Bending 56°/18° |
| 2 | 5 | Complex congenital scoliosis | Scoliosis 82° | Previous fusion (T11–L1) 11 ribs bilaterally, sacralized L5 |
| 3 | 1.5 | Mitochondrial disorder | Scoliosis 77° | Bending 56° |
| 4 | 4.5 | Congenital scoliosis | Scoliosis 70° (55° at 3 months, 60° at 21 months) | Fused ribs, 4 hemivertebrae T9–L1, concave bar T11–L1, only nine ribs on right with Ribs 6 and 7 medially fused, SAL 80% |
| 5 | 8.5 | Congenital scoliosis | Scoliosis 89° (70° at 6.5 years, 84° 7.5 years) | Fused ribs, pelvic obliquity, after diastematomyelia repair at 9 months, hemiepiphysiodesis at 2 years of age, LLD, PFT: mild restrictive and reversible obstructive, semisegment T12 hemivertebrae, severe pelvic obliquity, conus at L3–L4, split cord starts at apex, thoracic syrinx |
| 6 | 2.5 | Infantile idiopathic scoliosis | Scoliosis 70° | |
| 7 | 3.5 | Congenital scoliosis–VACTER | Scoliosis 55° (37° at birth, 40° at 14 months, 55° at 18 months) | Fused ribs (T1–4, T6–7, T8–10), concave bar with convex T8 hemivertebrae, hemimetameric shift T4–5, spine anomalies and imperforate anus at birth, anoplasty at DOL 2 |
| 8 | 6 | Infantile idiopathic scoliosis | Scoliosis 60° | Grade 2 spondylolisthesis (painless and progressive), clubfoot at birth, TLSO intermittently for 3 years |
| 9 | 8 | Myelodysplasia/ hydrocephalus | Scoliosis 70° | Wheelchair-dependent sitter, moderate restrictive lung disease |
| 10 | 6.9 | SCIWORA in MVA at 18 months | Scoliosis 105° | T3/T4 level paraplegia |
| 11 | 4 | Congenital scoliosis | Scoliosis 63° | Freeman Sheldon syndrome |
| 12 | 8 | Infantile idiopathic scoliosis | Scoliosis 65° | Community ambulator |
VACTER = vertebral anomalies; anal atresia; cardiovascular anomalies; tracheoesophageal fistula; esophageal atresia; renal; SCIWORA = spinal cord injury without radiologic abnormality; MVA = motor vehicle accident; SAL = space available for lung; LLD = leg length discrepancy; PFT = pulmonary function test; DOL = day of life; TLSO = thoracolumbar spinal orthosis.
Fig. 1A–B.
The preoperative (A) coronal and (B) sagittal radiographs of Case 7 are shown.
Table 2.
Decision regarding surgery
| Patient number | First assessment | Second assessment | ||||
|---|---|---|---|---|---|---|
| Surgery | Nonsurgery | Surgery | Nonsurgery | |||
| Brace/cast | Observation | Brace/cast | Observation | |||
| 1 | 12/12 (100%) | 0/12 (0%) | 0/12 (0%) | 8/11 (73%) | 3/11 (27%) | 0/11 (0%) |
| 2 | 11/11 (100%) | 0/11 (0%) | 0/11 (0%) | 11/11 (100%) | 0/11 (0%) | 0/11 (0%) |
| 3 | 11/12 (92%) | 1/12 (8%) | 0/12 (0%) | 8/9 (89%) | 1/9 (11%) | 0/9 (0%) |
| 4 | 11/12 (92%) | 0/12 (0%) | 1/12 (8%) | 11/11 (100%) | 0/11 (0%) | 0/11 (0%) |
| 5 | 12/12 (100%) | 0/12 (0%) | 0/12 (0%) | 11/11 (100%) | 0/11 (0%) | 0/11 (0%) |
| 6 | 12/13 (92%) | 1/13 (8%) | 0/13 (0%) | 11/12 (92%) | 1/12 (8%) | 0/12 (0%) |
| 7 | 12/13 (92%) | 0/13 (0%) | 1/13 (8%) | 8/11 (73%) | 0/11 (0%) | 3/11 (27%) |
| 8 | 11/11 (100%) | 0/11 (0%) | 0/11 (0%) | 11/11 (100%) | 0/11 (0%) | 0/11 (0%) |
| 9 | 12/12 (100%) | 0/12 (0%) | 0/12 (0%) | 11/11 (100%) | 0/11 (0%) | 0/11 (0%) |
| 10 | 12/12 (100%) | 0/12 (0%) | 0/12 (0%) | 10/11 (91%) | 0/11 (0%) | 1/11 (9%) |
| 11 | 11/11 (100%) | 0/11 (0%) | 0/11 (0%) | 11/11 (100%) | 0/11 (0%) | 0/11 (0%) |
| 12 | 10/11 (91%) | 1/11 (9%) | 0/11 (0%) | 9/9 (100%) | 0/9 (0%) | 0/9 (0%) |
Table 3.
Types of surgical treatment
| Patient number | First assessment | Second assessment | ||||
|---|---|---|---|---|---|---|
| VEPTR™ | Growing rods | Fusion | VEPTR™ | Growing rods | Fusion | |
| 1 | 7/12 (58%) | 5/12 (42%) | 0/12 (0%) | 4/8 (50%) | 4/8 (50%) | 0/8 (0%) |
| 2 | 9/11 (82%) | 2/11 (18%) | 0/11 (0%) | 9/11 (82%) | 2/11 (18%) | 0/11 (0%) |
| 3 | 8/11 (73%) | 3/11 (27%) | 0/11 (0%) | 5/8 (63%) | 3/8 (37%) | 0/8 (0%) |
| 4 | 9/11 (82%) | 0/11 (0%) | 2/11 (18%) | 9/11 (82%) | 0/11 (0%) | 2/11 (18%) |
| 5 | 11/12 (72%) | 0/12 (0%) | 1/12 (8%) | 9/11 (82%) | 0/11 (18%) | 2/11 (18%) |
| 6 | 11/12 (92%) | 1/12 (8%) | 0/12 (0%) | 4/11 (36%) | 7/11 (64%) | 0/11 (0%) |
| 7 | 12/12 (100%) | 0/12 (0%) | 0/12 (0%) | 8/8 (100%) | 0/8 (0%) | 0/8 (0%) |
| 8 | 8/11 (73%) | 3/11 (27%) | 0/11 (0%) | 7/11 (64%) | 4/11 (36%) | 0/11 (0%) |
| 9 | 7/12 (58%) | 2/12 (17%) | 3/12 (25%) | 9/11 (82%) | 1/11 (9%) | 1/11 (9%) |
| 10 | 7/12 (58%) | 5/12 (42%) | 0/12 (0%) | 5/10 (50%) | 5/10 (50%) | 0/10 (0%) |
| 11 | 9/11 (82%) | 2/11 (18%) | 0/11 (0%) | 8/11 (73%) | 3/11 (27%) | 0/11 (0%) |
| 12 | 3/10 (30%) | 7/10 (70%) | 0/10 (0%) | 3/9 (33%) | 5/9 (56%) | 1/9 (11%) |
VEPTR™ = Vertical Expandable Prosthetic Titanium Rib.
Fig. 2A–B.
The preoperative coronal (A) and (B) sagittal radiographs of Case 1 are shown.
The participants completed the information either on the electronic file or on printed handouts and mailed them back to the principle investigator (MGV). Six months after the initial assessment, all surgeons repeated the case review using the same instructions and then submitted their second set of responses to the principle investigator.
A descriptive analysis of each set of results was performed. All analyses were conducted using SPSS® software (SPSS Inc, Chicago, IL).
Results
In the first assessment of type of treatment, all surgeons chose to recommend surgery for seven of 12 patients (Table 2). For the remaining five, only one of the surgeons chose nonoperative treatment with casting/bracing (three patients) or observation (two patients), whereas the others chose surgery. The percent agreement for surgical treatment of each patient was 91% to 100% (average, 97%). In the second assessment, all surgeons recommend surgery in seven of 12 patients. For three patients, only one surgeon opted for nonoperative treatment with casting/bracing (two patients) or observation (one patient). For the remaining two patients, three surgeons chose to treat with casting/bracing (one patient) or observation (one patient).
For the patients in whom surgical treatment was recommended, 25% to 92% (average, 72%) of surgeons agreed on the use VEPTR™ and 0% to 70% (average, 22%) of them agreed to use growing rods in the first assessment (Table 3). Fusion was the first option for treatment of three patients by some surgeons. In the second assessment, the recommendation of VEPTR™ decreased to an average of 66% of surgeons (range, 33%–100%), whereas an average of 30.4% (range, 0%–64%) agreed to use growing rods. Fusion was the option chosen by some surgeons for four patients at the second assessment.
Among surgical cases using the VEPTR™, bilateral implants were chosen by an average of 60% of surgeons (range, 8%–100%) and whether instrumentation should be extended to the pelvis was agreed on by an average of 67% (range, 8%–100%) in the first assessment (Table 4). Among those who chose growing rods, bilateral implants were selected 100% of the time and recommendations for extending the instruments to the pelvis averaged 18% (range, 0%–66%). In the second evaluation, among surgeons opting for the VEPTR™, 56% (range, 0%–100%) agreed on using a bilateral construct and 58% (range, 0%–100%) chose to extend the instrumentation to the pelvis. Among surgeons who chose to use the growing rods, all chose to use them bilaterally and 28% (range, 0%–100%) agreed to extend the instrument to the pelvis.
Table 4.
Location of instruments
| Patient number | First assessment | Second assessment | ||||||
|---|---|---|---|---|---|---|---|---|
| VEPTR™ | Growing rods | VEPTR™ | Growing rods | |||||
| Bilateral | To pelvis | Bilateral | To pelvis | Bilateral | To pelvis | Bilateral | To pelvis | |
| 1 | 7/7 (100%) | 7/7 (100%) | 5/5 (100%) | 1/5 (20%) | 4/4 (100%) | 4/4 (100%) | 4/4 (100%) | 1/4 (25%) |
| 2 | 3/9 (33%) | 8/9 (89%) | 2/2 (100%) | 0/2 (0%) | 3/9 (33%) | 6/9 (67%) | 2/2 (100%) | 0/2 (0%) |
| 3 | 6/8 (75%) | 7/8 (88%) | 3/3 (100%) | 1/3 (33%) | 3/5 (60%) | 3/5 (60%) | 3/3 (100%) | 1/3 (33%) |
| 4 | 1/9 (11%) | 4/9 (44%) | 0/0 | 0/0 | 2/9 (22%) | 2/9 (22%) | 0/0 | 0/0 |
| 5 | 3/11 (27%) | 6/11 (54%) | 0/0 | 0/0 | 2/9 (22%) | 6/9 (66%) | 0/0 | 0/0 |
| 6 | 7/1 (64%) | 4/11 (36%) | 1/1 (100%) | 0/1 (0%) | 3/4 (75%) | 2/4 (50%) | 7/7 (100%) | 1/7 (14%) |
| 7 | 1/12 (8%) | 1/12 (8%) | 0/0 | 0/0 | 0/8 (0%) | 2/8 (25%) | 0/0 | 0/0 |
| 8 | 8/8 (100%) | 8/8 (100%) | 3/3 (100%) | 2/3 (66%) | 5/7 (71%) | 5/7 (71%) | 4/4 (100%) | 0/4 (0%) |
| 9 | 7/7 (100%) | 7/7 (100%) | 2/2 (100%) | 0/2 (0%) | 9/9 (100%) | 9/9 (100%) | 1/1 (100%) | 1/1 (100%) |
| 10 | 4/7 (57%) | 7/7 (100%) | 5/5 (100%) | 2/5 (40%) | 3/5 (60%) | 3/5 (60%) | 5/5 (100%) | 4/5 (80%) |
| 11 | 7/9 (78%) | 5/9 (56%) | 2/2 (100%) | 0/2 (0%) | 8/8 (100%) | 6/8 (75%) | 3/3 (100%) | 0/3 (0%) |
| 12 | 2/3 (66%) | 1/3 (33%) | 7/7 (100%) | 0/7 (0%) | 1/3 (33%) | 0/3 (0%) | 5/5 (100%) | 0/5 (0%) |
VEPTR™ = Vertical Expandable Prosthetic Titanium Rib.
Regarding recommending thoracotomy in the first assessment, none recommended it for five patients, only one (8%–9%) recommended it for four patients, 50% recommended it for two patients, and 85% recommended it for one patient (Table 5). The recommendation of thoracotomy in the second assessment decreased compared with that in the first assessment. For eight of 12 patients, none of the surgeons recommended thoracotomy. Only one surgeon recommended it for one patient (10%), three did so for two patients (27%), and eight did so for one patient (73%).
Table 5.
Thoracotomy or no thoracotomy
| Patient number | First assessment | Second assessment |
|---|---|---|
| Yes | Yes | |
| 1 | 0/11 (0%) | 0/11 (0%) |
| 2 | 1/11 (9%) | 0/10 (0%) |
| 3 | 1/12 (8%) | 0/9 (0%) |
| 4 | 6/12 (50%) | 3/11 (27%) |
| 5 | 6/12 (50% | 3/11 (27%) |
| 6 | 1/13 (8%) | 0/12 (0%) |
| 7 | 11/13 (85%) | 8/11 (73%) |
| 8 | 0/12 (0%) | 0/11 (0%) |
| 9 | 0/12 (0%) | 0/11 (0%) |
| 10 | 0/12 (0%) | 0/10 (0%) |
| 11 | 1/12 (8%) | 1/10 (10%) |
| 12 | 0/11 (0%) | 0/9 (0%) |
Discussion
There has been an expansion of treatment options for the young child with scoliosis. No single algorithm for treating EOS has been proposed. Numerous obstacles, including small, heterogeneous patient populations and regulatory challenges in prospectively studying off-label devices, make rigorous clinical research challenging in this area. In part for these reasons, the comparative effectiveness of various treatment strategies is not well elucidated. Variability in treatment recommendations follow from the lack of evidence guiding decision making in this area. We assessed variability in decision making for patients with EOS in four areas: (1) whether to recommend surgery; (2) what type of construct to recommend; (3) where to locate the construct; and (4) whether to perform thoracotomy.
We acknowledge several limitations. First is limiting the study to members of the Chest Wall and Spinal Deformity Study Group. While this group of surgeons is familiar with nonoperative and operative techniques to treat EOS, they are specialized surgeons and may be biased toward surgery. This likely would bias the study toward lesser, not greater variability in agreement. Although this study group investigates all treatment options for EOS, members of this study group likely have more experience with the VEPTR™ than a general sample of pediatric spine surgeons. Second, assessment of the complex patient with EOS requires a more in-depth analysis than can be transmitted with a socioclinical and radiographic vignette, and perhaps variability would be lessened if the patients actually were examined by the participants.
We found surgeons tended to agree on whether any type of surgery was indicated. These findings support the utility of clinical and radiographic evaluations when assessing curve severity [14].
When evaluating the type of construct selected by each surgeon, we found poor intraobserver and fair interobserver agreement. This is not unexpected as we lack precise indications for the surgical constructs available to treat EOS, including spinal fusion, hemiepiphysiodesis with staples or tethers, growing rods, and the VEPTR™. Additionally, these findings reflect the incredible variability in this group of patients in etiology, curve morphology, and comorbidity.
Opinions regarding where spine implants should be affixed varied substantially. Intraobserver agreement was only fair and interobserver agreement substantial when surgeons decided to use the VEPTR™ bilaterally.
As highlighted in this study, rigid indications for surgical interventions currently do not exist for this patient population. Treatment options made by the group of surgeons experienced in treating EOS reflect individual preferences and opinions. The point of this study is not to make treatment recommendations but to document variability in treatment preference in a group of experienced EOS surgeons. Our study highlights the difficulty in choosing among treatment options in children with EOS and highlights several priority areas where better evidence needs to be developed to help surgeons formulate optimal strategies. Additional research is needed to develop and validate classification systems that can guide operative indications.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Members of Chest Wall and Spine Deformity Study Group: Randal R. Betz MD, Robert M. Campbell Jr, MD, John B. Emans MD, John M. Flynn MD, John M. Flynn MD, Norman Ramirez-Lluch MD, Brian Snyder MD, PhD, Peter F. Sturm MD, Kit M. Song MD, John T. Smith MD, and Jeffrey S. Shilt MD.
Footnotes
This study was not funded but supported by Morgan Stanley Children’s Hospital of New York-Presbyterian at Columbia University Medical Center. The Chest Wall and Spinal Deformity Study Group was supported by Synthes Spine Co (West Chester, PA). Michael G. Vitale, MD, is a member of the Chest Wall and Spinal Deformity Study Group, which is supported by Synthes Spine. He is not a consultant for Synthes nor is he a member of a speakers' bureau. He has received research support from Synthes Spine, unrelated to this research project. Jaime A. Gomez, MD, has received research support from Synthes Spine, unrelated to this research project. Hiroko Matsumoto, MA, has received research support from Synthes Spine unrelated to this research project. David P. Roye, MD, is a member of the Chest Wall and Spinal Deformity Study Group, which is supported by Synthes Spine. He is not a consultant for Synthes nor is he a member of a speakers' bureau. He has received research support from Synthes Spine, unrelated to this research project.
This work was performed at Columbia University Medical Center.
References
- 1.Aubin CE, Labelle H, Ciolofan OC. Variability of spinal instrumentation configurations in adolescent idiopathic scoliosis. Eur Spine J. 2007;16:57–64. doi: 10.1007/s00586-006-0063-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blakemore LC, Scoles PV, Poe-Kochert C, Thompson GH. Submuscular Isola rod with or without limited apical fusion in the management of severe spinal deformities in young children: preliminary report. Spine (Phila Pa 1976). 2001;26:2044–2048. [DOI] [PubMed]
- 3.Campbell RM, Jr, Smith MD, Mayes TC, Mangos JA, Willey-Courand DB, Kose N, Pinero RF, Alder ME, Duong HL, Surber JL. The effect of opening wedge thoracostomy on thoracic insufficiency syndrome associated with fused ribs and congenital scoliosis. J Bone Joint Surg Am. 2004;86:1659–1674. doi: 10.2106/00004623-200408000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Carvalho A, Vialle R, Thomsen L, Amzallag J, Cluzel G, le Pointe HD, Mary P. Reliability analysis for manual measurement of coronal plane deformity in adolescent scoliosis. Are 30 × 90 cm plain films better than digitized small films? Eur Spine J. 2007;16:1615–1620. doi: 10.1007/s00586-007-0437-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donaldson S, Hedden D, Stephens D, Alman B, Howard A, Narayanan U, Wright JG. Surgeon reliability in rating physical deformity in adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 2007;32:363–367. [DOI] [PubMed]
- 6.Gupta MC, Wijesekera S, Sossan A, Martin L, Vogel LC, Boakes JL, Lerman JA, McDonald CM, Betz RR. Reliability of radiographic parameters in neuromuscular scoliosis. Spine (Phila Pa 1976). 2007;32:691–695. [DOI] [PubMed]
- 7.Harrington PR. Treatment of scoliosis. Correction and internal fixation by spine instrumentation. J Bone Joint Surg Am. 1962;44:591–610. [PubMed] [Google Scholar]
- 8.Hell AK, Campbell RM, Hefti F. The vertical expandable prosthetic titanium rib implant for the treatment of thoracic insufficiency syndrome associated with congenital and neuromuscular scoliosis in young children. J Pediatr Orthop B. 2005;14:287–293. doi: 10.1097/01202412-200507000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Irwin ZN, Hilibrand A, Gustavel M, McLain R, Shaffer W, Myers M, Glaser J, Hart RA. Variation in surgical decision making for degenerative spinal disorders. Part II: cervical spine. Spine (Phila Pa 1976). 2005;30:2214–2219. [DOI] [PubMed]
- 10.Klemme WR, Denis F, Winter RB, Lonstein JW, Koop SE. Spinal instrumentation without fusion for progressive scoliosis in young children. J Pediatr Orthop. 1997;17:734–742. doi: 10.1097/00004694-199711000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Kuklo TR, Potter BK, Polly DW, Jr., O’Brien MF, Schroeder TM, Lenke LG. Reliability analysis for manual adolescent idiopathic scoliosis measurements. Spine (Phila Pa 1976). 2005;30:444–454. [DOI] [PubMed]
- 12.Loder RT, Urquhart A, Steen H, Graziano G, Hensinger RN, Schlesinger A, Schork MA, Shyr Y. Variability in Cobb angle measurements in children with congenital scoliosis. J Bone Joint Surg Br. 1995;77:768–770. [PubMed] [Google Scholar]
- 13.Moe JH, Kharrat K, Winter RB, Cummine JL. Harrington instrumentation without fusion plus external orthotic support for the treatment of difficult curvature problems in young children. Clin Orthop Relat Res. 1984;185:35–45. [PubMed] [Google Scholar]
- 14.Motoyama EK, Deeney VF, Fine GF, Yang CI, Mutich RL, Walczak SA, Moreland MS. Effects on lung function of multiple expansion thoracoplasty in children with thoracic insufficiency syndrome: a longitudinal study. Spine (Phila Pa 1976). 2006;31:284–290. [DOI] [PubMed]
- 15.Thompson GH, Akbarnia BA, Kostial P, Poe-Kochert C, Armstrong DG, Roh J, Lowe R, Asher MA, Marks DS. Comparison of single and dual growing rod techniques followed through definitive surgery: a preliminary study. Spine (Phila Pa 1976). 2005;30:2039–2044. [DOI] [PubMed]
- 16.Winter RB, Moe JH. The results of spinal arthrodesis for congenital spinal deformity in patients younger than five years old. J Bone Joint Surg Am. 1982;64:419–432. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.