Abstract
Background
Kyphoscoliosis is considered a relative contraindication to treatment with the Vertical Expandable Prosthetic Titanium Rib (VEPTR®; Synthes Inc, Paoli, PA). Nevertheless, patients do present with early-onset kyphoscoliosis and thoracic insufficiency syndrome, and no suitable alternative treatments are currently available. However, it is unclear whether VEPTR® is reasonable for treating patients with kyphoscoliosis.
Questions/purposes
We determined whether VEPTR® controls progression in patients with kyphoscoliosis and, if so, what methods might be used to improve control of deformity progression in these patients.
Patients and Methods
We retrospectively reviewed 14 patients who had VEPTR® treatment of early-onset kyphoscoliosis. Degrees of kyphosis and scoliosis before, during, and after treatment were measured, and levels of instrumentation, thoracic dimensions, and complications were recorded. Minimum followup was 1.7 years (average, 5.8 years; range, 1.7–12.8 years).
Results
While scoliosis was stabilized, kyphosis increased a mean of 22° at last followup. Supple kyphosis became rigid during treatment. Proximal cradle cutout was a recurring problem. Distal anchors placed too proximally had inadequate lever arms to control kyphosis.
Conclusions
Progression of kyphosis can be minimized during VEPTR® treatment by early extension of the construct to the second ribs bilaterally, distal extension of hybrid constructs to the pelvis, use of bilateral hybrid VEPTR® implants, and use of redesigned VEPTR® constructs that enhance fixation at the upper end. While our early results suggest these devices control progression of kyphosis, longer followup with more patients will be required to confirm the concept in these patients.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
The treatment of early-onset scoliosis continues to evolve as the importance of maintaining thoracic spine, rib cage, and lung growth becomes better documented [7]. Vertebral resections and spinal fusion continue to have their place in the overall armamentarium available to the pediatric spine surgeon. However, with the growing appreciation of the importance of spinal length to lung growth and development, techniques that attempt to preserve spinal growth have gained in popularity, although increase of thoracic length has not yet been proven to correlate with improved alveolar number, size, or function. Growing rods [25] and the Vertical Expandable Prosthetic Titanium Rib (VEPTR®; Synthes Inc, Paoli, PA) [2–4] both promote spinal growth during the important early years (0–5 years) when lung growth is maximal [3, 4, 7, 10]. Excessive kyphosis has presented special challenges to both techniques [2, 9, 25], including poor control of kyphotic deformity, failure of the instrumentation to fit closely to the spine or thorax and still be expandable, and failure of fixation either proximally or distally. Nevertheless, patients do present who have severe kyphosis as part of their deformity. Unfortunately, the best method of treatment for these patients is unclear at present. The degree to which either growing rod or VEPTR® treatment is contraindicated by hyperkyphosis has not been characterized in the literature.
In an effort to further define one option in dealing with these challenging patients, we document the radiographic response and complications of patients with severe kyphoscoliosis treated with VEPTR®. We asked the following questions: (1) How well does VEPTR® treatment control hyperkyphosis in patients who have this as a compounding factor in their deformity? (2) How can surgeons utilizing VEPTR® in such patients enhance control of overall spinal deformity, including hyperkyphosis? (3) Is VEPTR® contraindicated in patients with hyperkyphosis and, if so, what degree or pattern of kyphosis should be considered a contraindication?
Patients and Methods
The Spinal and Thoracic Treatment and Research Center at Christus Santa Rosa Children’s Hospital maintains an IRB-approved, prospectively gathered database of children who have undergone VEPTR® treatment. All parents of patients included in this database have given informed consent for participation in this study. More than 300 patients are currently in this database. From this database, we identified 14 patients with thoracic kyphoscoliosis treated with VEPTR®. These patients were chosen for study because, at some point in their treatment, we altered our surgical plan to specifically treat thoracic kyphosis. We did not include patients with nonprogressive minor (< 60°) thoracic kyphosis or only lumbar kyphosis. However, we did include two patients (Patients 3 and 7) who had normal kyphosis initially but developed hyperkyphosis during treatment. Furthermore, patients who had kyphosis that could not be accommodated by VEPTR® instrumentation were rejected for VEPTR® care. Thus, this series is limited to those who met initial criteria for VEPTR® instrumentation but subsequently developed major difficulty with thoracic kyphosis. We reviewed all charts to determine demographics and recorded any complications of treatment, including cradle or hook dislodgements, infections, and rod revisions. Diagnoses varied, with five patients having nonsyndromic congenital kyphoscoliosis, two having Vater syndrome, and one each having Williams syndrome, Freeman-Sheldon syndrome, arthrogryposis, mitochondrial encephalomyopathy, central core disease, cleidocranial dysostosis, and infantile idiopathic scoliosis. Before seeking our care, two patients had had unsuccessful anterior and posterior spinal fusions, one after spinal osteotomies. One patient had a prior cervical fusion for instability. The cohort included 10 males and four females. The mean age at initial VEPTR® surgery was 3.9 years (range, 1.4–23.3 years) (Table 1). Minimum followup was 1.7 years (average, 5.8 years; range, 1.7–12.8 years). No patients were lost to followup. No patients were recalled specifically for this study; all data were obtained from medical records and radiographs.
Table 1.
Patient summary
| Patient | Diagnosis | Age at onset (years) | Followup (years) | Initial scoliosis Curve 1/Curve 2 | End scoliosis Curve 1/Curve 2 | Initial kyphosis | End kyphosis |
|---|---|---|---|---|---|---|---|
| 1 | Vater syndrome | 2.7 | 1.7 | 60°/20° | 58°/27° | 63° | 56° |
| 2 | Williams syndrome | 3.0 | 3.1 | 53°/84° | 64°/48° | 62° | 78° |
| 3 | Congenital kyphoscoliosis | 2.6 | 2.1 | 83°/78° | 72°/62° | 10° | 43° |
| 4 | Freeman-Sheldon syndrome | 4.6 | 4.9 | 81°/63° | 73°/29° | 68° | 90° |
| 5 | Congenital kyphoscoliosis | 8.3 | 4.0 | 70°/82° | 90°/78° | 85° | 124° |
| 6 | Mitochondrial myopathy | 4.4 | 6.0 | 72°/72° | 90°/44° | 53° | 98° |
| 7 | Congenital kyphoscoliosis | 2.6 | 4.3 | 58°/52° | 54°/33° | 24° | 69° |
| 8 | Arthrogryposis | 4.3 | 2.8 | 73°/126° | 43°/100° | 138° | 113° |
| 9 | Central core disease | 1.4 | 3.8 | 76°/68° | 54°/48° | 84° | 103° |
| 10 | Congenital kyphoscoliosis | 3.0 | 12.8 | 42°/65° | 55°/65° | 95° | 94° |
| 11 | Vater syndrome | 6.0 | 10.0 | 47°/102° | 72°/81° | 62° | 85° |
| 12 | Cleidocranial dysostosis | 2.7 | 9.3 | 30°/60° | 38°/34° | 74° | 104° |
| 13 | Infantile idiopathic kyphoscoliosis | 6.2 | 7.1 | 42°/80° | 90°/65° | 80° | 114° |
| 14 | Congenital kyphoscoliosis | 23.3 | 9.5 | 48°/75° | 40°/53° | 56° | 99° |
Four patients had associated rib fusions, and one had absence of multiple ribs. Comorbidities included congenital heart disease in four patients, failure to thrive in three, and tracheoesophageal fistulas in two. Single patients had cleft palate with Pierre Robin syndrome, pectus excavatum, a hypoplastic left lung, esophageal atresia, diaphragmatic hernia, a herniated kidney with renal failure, renal atresia, clubfoot, bilateral dislocated hips, necrotizing enterocolitis, laryingomalacia, cord tethering, and syringomyelia.
Initial surgical technique varied greatly due to variations in patient needs, advances in instrumentation and imaging modalities, and development of better techniques based upon past experience. We utilized a team approach, with an orthopaedic surgeon, a thoracic surgeon, a pulmonologist, and an anesthesiologist participating in the care of every patient. Preoperative evaluation was performed by all team members and included CT of chest, MRI of spine, pulmonary function assessment (when possible), and blood gases. Dynamic MRI and, recently, dynamic CT were used to assess pulmonary expansion and chest wall mobility. At surgery, open thoracostomy was used when indicated. Rib-to-rib or rib-to-spinal constructs were selected in such a way as to best solve the patient’s deformity problem. Two to three VEPTR® constructs were routine on either side of the chest cage. We used hook fixation to the lumbar spine rather than screw fixation as we found too rigid fixation distally increased the risk of failure of instrumentation. For the same reason, we used Dunn-McCarthy pelvic hook fixation rather than screw fixation in the pelvis.
Postoperatively, we did not use any braces or casts, and we encouraged the children to be fully active. After initial VEPTR® placement, patients were kept in the intensive care unit until stable. We generally used physical therapy in the inpatient setting but not routinely after discharge from the hospital. Ambulatory patients were ambulated as soon as pain management allowed.
Most patients came from far away and were followed by their local practitioners between expansions. They were not seen by us until the day before scheduled expansions unless there were problems requiring a change in plan. The patients had three to 16 expansions, with a mean of 8.9. Expansions were performed every 6 to 12 months, with younger children having more frequent expansions. They were performed under general anesthesia, usually with 23-hour admissions.
These patients were exposed to many radiographs during their course of treatment. We therefore tried to limit radiographs to those absolutely necessary for patient care. Early in the series, patients had routine lung CTs before expansions. We no longer perform any “routine” radiographic analysis. Regular radiographs are obtained before expansions only if needed. The image intensifier is utilized during surgery, and standard radiographs are obtained for documentation afterward.
Pulmonary status was assessed at each visit, with the method most appropriate for age. Pulmonary measurement has evolved over the years, with the addition of dynamic MRI and, most recently, dynamic CT to our armamentarium. We believe quantitative assessment of the dynamics of lung function in this population is still inadequate. Development of better quantification of pulmonary volume and function is a major research focus of our center.
Two of us (KR, ZS) reviewed all radiographs. Neither of the two authors measuring the curves participated in the care of any patient in the series. We measured Cobb angles of major scoliotic curves, degree of kyphosis from T1 to T5 and T1 to T12, thoracic spinal length on AP radiographs from the center of the T1 vertebral body to the center of the T12 vertebral body, thoracic cage width at the diaphragms, and thoracic depth from the lower sternum to the adjacent vertebral body. We also recorded the apical vertebrae of kyphoses. Curve 1 was designated as the upper thoracic scoliotic curve, while Curve 2 was a lower thoracic or thoracolumbar curve. In all patients, both curves were believed to be structural curves. For consistency, all measurements were made on erect films, either sitting or standing. Six patients had pretreatment supine lateral radiographs to determine the degree of flexibility of the hyperkyphosis.
Results
At latest followup, total kyphosis ranged from 43° to 124° and averaged 90.7°. Total kyphosis (from T2 to T12) ranged from 10° to 138° initially and averaged 68.1°. Change in total kyphosis ranged from −24° to +45°, with a mean of +22.6°. The initial apex of kyphotic deformity was at T10 in one patient, T9 in two, T8 in two, T7 in three, T6 in five, and T5 in one. Final apices of curvature were the same in eight patients, more proximal in four, and more distal in two. Only two patients shifted their apex of kyphosis more than one level, both of these being more proximal by two segments and three segments, respectively. Initial upper thoracic kyphosis, measured from T1 to T5, averaged 5.9° and ranged from −50° to +63°. Upper thoracic kyphosis after initial surgery averaged −3.1° and ranged from −30.0° to +20.0°. Final upper thoracic kyphosis averaged −0.4° and ranged from −83° to +77°. Four of these patients had supple kyphosis that was at least 25° less than their upright kyphosis, and two had kyphosis within normal limits (< 45°) on their supine films, although they had quite excessive kyphosis on their erect films. The kyphosis in the four patients with supple kyphosis on supine films but excessive kyphosis on erect films became rigid during the course of treatment, the behavior of their kyphosis not differing substantially from the behavior of curves initially more rigid. The two who had correctability of their kyphosis to less than 45° ended followup with kyphoses of 90° and 103°, respectively. Thoracic length gain ranged from −1 to 7.5 cm, with a mean of 2.6 cm. Thoracic width gain ranged from −1.5 to 6.6 cm and averaged 2.2 cm. Thoracic depth gain ranged from −2.0 to 10 cm and averaged 3.2 cm (Table 1). Initially, Curve 1 averaged 59.6° of scoliosis (range, 30°–83°) and Curve 2 averaged 73.4° (range, 20°–126°). Curve 1 gained an average of 4.1° (range, +40° to −38°) during treatment while Curve 2 improved an average of 18.6° (range, −36° to +7°). At the end of treatment, Curve 1 averaged 63.8° (range, 38°–90°) and Curve 2 averaged 73.4° (range, 27°–100°). After the initial VEPTR® placement, there was a mean gain of 8.3° of lumbar lordosis (range, −20° to +53.0°), and final lordotic change was 6.2° (range, −34° to +62°).
Six patients had initial proximal anchor placement below the third rib with subsequent increase of kyphosis. While some improvement in the kyphosis was seen after more proximal cradle placement, the increase in kyphosis prompting the change to a higher level did not entirely resolve with the resetting of cradles at the second rib. In seven patients, an inadequate distal lever arm was noted, with distal anchor placements above L3. Improvement of the kyphosis occurred after extension of one or more implants distally to the pelvis or, in one case, to L5. The proximal cradle had to be reseated more than once in seven of our patients. One patient had removal of his hybrid implant after eroding through the rib four times within 2 years. Two others had to have removal of implants due to infection. Kyphosis progressed in all of these patients.
Discussion
Kyphoscoliosis is considered a relative contraindication to VEPTR® treatment. Nevertheless, patients do present with early-onset kyphoscoliosis and thoracic insufficiency syndrome, and no suitable alternative treatments are currently available. No literature yet documents the efficacy of VEPTR® in patients with kyphoscoliosis, and the treating physician thus has no data on which to base treatment decisions. We therefore assessed the efficacy of treating patients with severe kyphosis with VEPTR® technology, determined how radiographic control of kyphosis might be optimized while using this technology, and clarified the degree to which hyperkyphosis should be currently viewed as a contraindication to VEPTR® treatment.
This study has several weaknesses. First, although these patients were a subgroup of a prospective study of VEPTR® treatment, the prospective study was not designed to consider the specific problem of kyphosis. Therefore, some data that might have been valuable to assess were not available. In particular, supine lateral radiographs were not present for most patients. Second, the effects of treatment on pulmonary function could not be assessed due to an initial lack of pulmonary evaluation techniques applicable for young children. Third, the number of patients is small, reflecting the rarity of the conditions leading to VEPTR® treatment and some selection bias due to the inability of VEPTR® implants to fit snugly on spines or thoraces with midthoracic hyperkyphosis. Fourth, accurate measurement of kyphosis in the presence of scoliosis, and vice versa, is difficult due to the problems of tilting of disc spaces out of the plane of the radiograph and the difficulty of seeing vertebrae in the upper thoracic spine. The senior author (KR), who has more than 30 years’ experience, was assisted in these measurements by an orthopaedic resident (ZS). Nevertheless, the measurements are subject to error. Finally, this is not an end outcome study. Patients were still undergoing VEPTR® treatment at the conclusion of the study.
Kyphosis increased moderately (mean, 22°) in these patients during the course of treatment. Correctability of the kyphosis with supine positioning was not predictive of a good outcome in the four patients who had a supple kyphosis. Hyperkyphosis results in subsidence of T1, a decrease of thoracic length, and a decline in the space available for lung. This is detrimental to the main purpose of VEPTR® treatment—the increase of thoracic cage dimensions to give more space for the lungs to grow. In our patients, thoracic length increased an average of 2.5 cm during the course of treatment. Campbell et al. [4] documented a mean thoracic spinal height gain of 0.71 cm per year. Using this number in our series, 4.1 cm of increase would have been expected. Thus, kyphosis did appear to have a detrimental effect upon our ability to influence thoracic growth.
Three factors seemed to contribute to the development of increased kyphosis. The first was initial upper cradle placement at levels below the third rib (Fig. 1). Such placement was sometimes mandated by earlier versions of VEPTR® implants. Redesigned VEPTR® implants allowed better proximal extension above the kyphosis and seemed to stabilize kyphosis when utilized. We might have seen better results if these had been available at the onset of treatment. The second factor was a diminished distal lever arm due to distal anchor placement at too proximal a level (Fig. 2). In general, rib-to-rib constructs were ineffective in controlling kyphosis. Only hybrid constructs were effective, and these worked best when extended to the pelvis. The third factor was recurrent erosion and loss of fixation on the proximal ribs. The second and third ribs are small, and in growing children, the ribs are biologically plastic and will remodel in response to sustained force. The cradle may then lose fixation, usually with reformation of the rib below the cradle. This is particularly a problem in the presence of kyphosis, where cantilever forces increase the normal pressure on the rib. If kyphosis is encountered during VEPTR® treatment, it should be addressed early. Possible strategies include (1) resetting the proximal cradles to the highest possible rib, (2) more distal placement of the lower hybrid hook, (3) use of redesigned VEPTR® implants with more than one cradle to give more firm fixation proximally, and (4) use of a hybrid rod on the opposite thorax, so that dual hybrid VEPTR® implants are used. The upper limit for VEPTR® is the second rib. The optimal distal site and fixation to control sagittal deformity are more controversial [6, 18]. In these patients, however, arthrodesis is not performed and the spinal deformity is dynamic. The pelvis clearly provides the longest lever arm for fixation. However, other considerations, such as balance in the frontal plane or sagittal imbalance due to lumbar hypolordosis, may make pelvic fixation of the implant undesirable. In the end, it is always an individual decision.
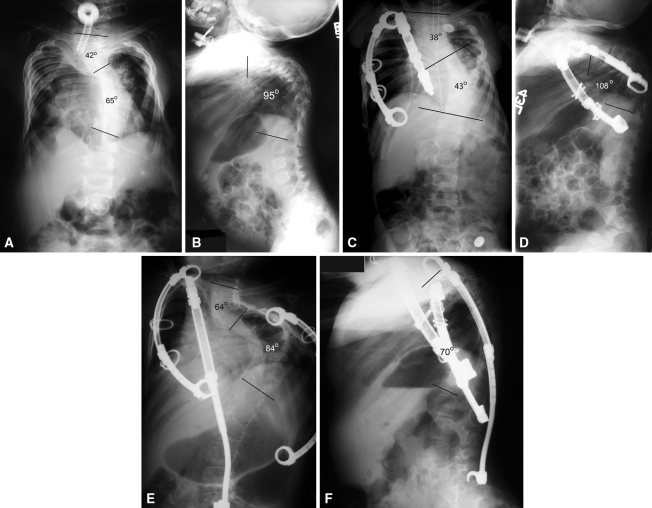
Fig. 1A–F.
Initial (A) posteroanterior and (B) lateral radiographs of Patient 10 show severe scoliosis and kyphosis. (C) Scoliosis in Patient 10 has been improved by initial VEPTR® placement, (D) but kyphosis has worsened. (E) Addition of a hybrid VEPTR® extending to L5 has improved the kyphosis (F) but has resulted in some lateral decompensation.
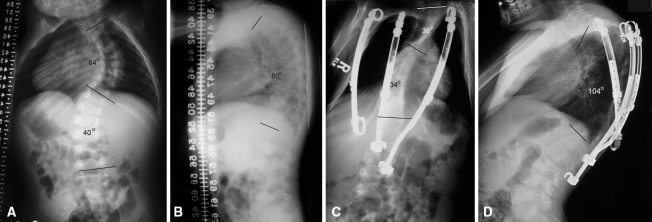
Fig. 2A–D.
Patient 12 has (A) a moderately severe scoliosis and (B) a supple 80° kyphosis before VEPTR® treatment. At latest followup, (C) Patient 12 has excellent control of scoliosis, (D) but his kyphosis has increased to 104°. The VEPTR® cradled at T2 has too proximal a distal seating to control the kyphosis and the two hybrid VEPTR® implants do not bridge the apex of kyphosis.
Were these patients good candidates for VEPTR® treatment to begin with? Not all patients with kyphosis can be treated with VEPTR®. The instrumentation must fit flush to the spine or rib cage to avoid erosion through the skin. The extensile segment of the implant has a fixed radius that cannot be contoured and still function. Thus, fitting the instrumentation flush to the spine or rib cage can only be performed in the ductile segments above and below the sliding portion of the instrumentation. While improvements in instrumentation have allowed more difficult curves to be accommodated, the surgeon must still be selective in using this implant in the presence of kyphosis. These patients were accepted for care after taking these considerations into account. We have demonstrated, in these kyphoscoliotic patients, less thoracic length was obtained than expected. Thus, kyphosis, even in the patients in whom VEPTR® is technically feasible, may compromise the end result of VEPTR® treatment. Whether VEPTR® is contraindicated in the presence of kyphosis, however, requires a comparison of natural history to the results obtained. We do not have a control group and must rely upon the depiction of natural history in the literature to attempt to answer this question. McMaster and Singh [19] have shown congenital kyphoscoliosis progresses throughout growth. Similarly, Fernandes and Weinstein [10] have documented the progressive natural history of infantile scoliosis. In contrast, multiple authors have documented improvements in thoracic dimensions, spinal height, and scoliotic curves with the use of VEPTR® in thoracic deformity causing thoracic insufficiency syndrome [3, 4, 9, 13, 20, 22, 24]. We too demonstrate modest improvements in scoliosis and spinal height. VEPTR® is primarily used to address thoracic insufficiency syndrome; control of scoliosis or kyphosis is important but is secondary to this goal. VEPTR® may still be indicated in some patients even when improvement or control of hyperkyphosis with kyphoscoliosis is not possible. One also must consider the limitations of alternative techniques. One alternative that might be considered is to perform kyphosis correction by spinal fusion earlier, improving the original deformity, gaining control of the apex of deformity, and perhaps making subsequent VEPTR® treatment more successful. However, Goldberg et al. [12] and Karol et al. [14] have documented the detrimental effects of early thoracic spinal arthrodesis on the growing spine. Thompson et al. [25] noted worse results when limited fusions were performed before growing rod insertion. Vertebral resections are technically demanding procedures with substantial associated risk [5]. Furthermore, according to O’Shaughnessy et al. [21], pedicle subtraction osteotomy yields less correction than would be necessary for correction of kyphotic deformity of the magnitude described in this article. Two of our patients did have attempts at such surgery at other institutions before being referred to our institution. Their outcomes were poor, with one having 124° of kyphosis at the end of treatment and the other having 94°. Kyphotic patients have complex problems presenting challenges to all currently used methods of treatment. Proximal junctional kyphosis is a potential problem with any fixation extending into the upper spine, being reported in approximately 30% of patients who have surgery for Scheuermann’s kyphosis [1, 8, 17], 39% of adult patients who had five or more segments fused [11], and 9% to 27% of adolescent idiopathic scoliotic fusions [15, 16, 18, 23]. Growing rod techniques have also been kyphogenic, with a reported mean 40% increase in kyphosis over the course of treatment [2, 25]. Proximal fixation in the presence of kyphosis is a problem not limited to VEPTR®; hook dislodgements and pedicle screw erosion are also well-documented in growing rod techniques [2, 25]. We conclude VEPTR® is comparable or superior to other reported techniques in the control of hyperkyphosis.
In summary, kyphosis presents special problems during VEPTR® treatment. These problems can be minimized but not eliminated by instrumenting as high as possible and as low as possible and by taking measures to improve and maintain proximal fixation. It is clear there is room for improvement in our methods for dealing with early-onset kyphosis and kyphoscoliosis. The management of severe kyphosis, once established, is difficult. Kyphosis in association with early-onset scoliosis remains an unsolved problem.
Acknowledgments
The authors acknowledge the contributions of Robert Campbell, MD, and Melvin Smith, MD (deceased) whose surgical skills and inventiveness made this study possible, and William Koeck, MD, and Ajeya Joshi, MD, who contributed ideas to the paper, improving its quality and design.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Christus Santa Rosa Children’s Hospital.
References
- 1.Atici T, Aydinli U, Akesen B, Serifoglu R. Results of surgical treatment for kyphotic deformity of the spine secondary to trauma or Scheuermann’s disease. Acta Orthop Belg. 2004;70:344–348. [PubMed] [Google Scholar]
- 2.Campbell RM, Jr, Smith MD. Thoracic insufficiency syndrome and exotic scoliosis. J Bone Joint Surg Am. 2007;89(1):108–122. doi: 10.2106/JBJS.F.00270. [DOI] [PubMed] [Google Scholar]
- 3.Campbell RM, Jr, Smith MD, Mayes TC, Mangos JA, Willey-Courand DB, Kose N, Pinero RF, Alder ME, Duong HL, Surber JL. The characteristics of thoracic insufficiency syndrome associated with fused ribs and congenital scoliosis. J Bone Joint Surg Am. 2003;85:399–408. doi: 10.1302/0301-620X.85B3.13429. [DOI] [PubMed] [Google Scholar]
- 4.Campbell RM, Jr, Smith MD, Mayes TC, Mangos JA, Willey-Courand DB, Kose N, Pinero RF, Alder ME, Duong HL, Surber JL. The effect of opening wedge thoracostomy on thoracic insufficiency syndrome associated with fused ribs and congenital scoliosis. J Bone Joint Surg Am. 2004;86:1659–1674. doi: 10.2106/00004623-200408000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Cheh G, Lenke LG, Padberg AM, Kim YJ, Daubs MD, Kuhns C, Stobbs G, Hensley M. Loss of spinal cord monitoring signals in children during thoracic kyphosis correction with spinal osteotomy: why does it occur and what should you do? Spine (Phila Pa 1976) 2008;33:1093–1099. doi: 10.1097/BRS.0b013e31816f5f73. [DOI] [PubMed] [Google Scholar]
- 6.Cho KJ, Lenke LG, Bridwell KH, Kamiya M, Sides B. Selection of the optimal distal fusion level in posterior instrumentation and fusion for thoracic hyperkyphosis: the sagittal stable vertebra concept. Spine (Phila Pa 1976). 2009;34:765–770. doi: 10.1097/BRS.0b013e31819e28ed. [DOI] [PubMed] [Google Scholar]
- 7.Conner AN. Early onset scoliosis: a call for awareness. Br Med J (Clin Res Ed) 1984;289:962–963. doi: 10.1136/bmj.289.6450.962-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denis F, Sun EC, Winter RB. Incidence and risk factors for proximal and distal junctional kyphosis following surgical treatment for Scheuermann kyphosis: minimum five-year follow-up. Spine. 2009;34:E729–E734. doi: 10.1097/BRS.0b013e3181ae2ab2. [DOI] [PubMed] [Google Scholar]
- 9.Emans JB, Caubet JF, Ordonez CL, Lee EY, Ciarlo M. The treatment of spine and chest wall deformities with fused ribs by expansion thoracostomy and insertion of vertical expandable prosthetic titanium rib: growth of thoracic spine and improvement of lung volumes. Spine (Phila Pa 1976) 2005;30(17):S58–S68. doi: 10.1097/01.brs.0000175194.31986.2f. [DOI] [PubMed] [Google Scholar]
- 10.Fernandes P, Weinstein SL. Natural history of early onset scoliosis. J Bone Joint Surg Am. 2007;89(1):21–33. doi: 10.2106/JBJS.F.00754. [DOI] [PubMed] [Google Scholar]
- 11.Glattes RC, Bridwell KH, Lenke LG, Kim YJ, Rinella A, Edwards C., II Proximal junctional kyphosis in adult spinal deformity following long instrumented posterior spinal fusion: incidence, outcomes, and risk factor analysis. Spine (Phila Pa 1976) 2005;30:1643–1649. doi: 10.1097/01.brs.0000169451.76359.49. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg CJ, Moore DP, Fogarty EE, Dowling FE. Long-term results from in situ fusion for congenital vertebral deformity. Spine (Phila Pa 1976) 2002;27:619–628. doi: 10.1097/00007632-200203150-00011. [DOI] [PubMed] [Google Scholar]
- 13.Hell AK, Campbell RM, Hefti F. The vertical expandable prosthetic titanium rib implant for the treatment of thoracic insufficiency syndrome associated with congenital and neuromuscular scoliosis in young children. J Pediatr Orthop B. 2005;14:287–293. doi: 10.1097/01202412-200507000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Karol LA, Johnston C, Mladenov K, Schochet P, Walters P, Browne RH. Pulmonary function following early thoracic fusion in non-neuromuscular scoliosis. J Bone Joint Surg Am. 2008;90:1272–1281. doi: 10.2106/JBJS.G.00184. [DOI] [PubMed] [Google Scholar]
- 15.Kim YJ, Bridwell KH, Lenke LG, Kim J, Cho SK. Proximal junctional kyphosis in adolescent idiopathic scoliosis following segmental posterior spinal instrumentation and fusion: minimum 5-year follow-up. Spine(Phila Pa 1976) 2005;30:2045–2050. doi: 10.1097/01.brs.0000179084.45839.ad. [DOI] [PubMed] [Google Scholar]
- 16.Lee GA, Betz RR, Clements DH, 3rd, Huss GK. Proximal kyphosis after posterior spinal fusion in patients with idiopathic scoliosis. Spine(Phila Pa 1976) 1999;24:795–799. doi: 10.1097/00007632-199904150-00011. [DOI] [PubMed] [Google Scholar]
- 17.Lee SS, Lenke LG, Kuklo TR, Valente L, Bridwell KH, Sides B, Blanke KM. Comparison of Scheuermann kyphosis correction by posterior-only thoracic pedicle screw fixation versus combined anterior/posterior fusion. Spine (Phila Pa 1976) 2006;31:2316–2321. doi: 10.1097/01.brs.0000238977.36165.b8. [DOI] [PubMed] [Google Scholar]
- 18.Lowe TG, Kasten MD. An analysis of sagittal curves and balance after Cotrel-Dubousset instrumentation for kyphosis secondary to Scheuermann’s disease: a review of 32 patients. Spine. 1994;19:1680–1685. doi: 10.1097/00007632-199408000-00005. [DOI] [PubMed] [Google Scholar]
- 19.McMaster MJ, Singh H. Natural history of congenital kyphosis and kyphoscoliosis: a study of one hundred and twelve patients. J Bone Joint Surg Am. 1999;81:1367–1383. doi: 10.2106/00004623-199910000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Motoyama EK, Deeney VF, Fine GF, Yang CI, Mutich RL, Walczak SA, Moreland MS. Effects on lung function of multiple expansion thoracoplasty in children with thoracic insufficiency syndrome: a longitudinal study. Spine (Phila Pa 1976) 2006;31:284–290. doi: 10.1097/01.brs.0000197203.76653.d0. [DOI] [PubMed] [Google Scholar]
- 21.O’Shaughnessy BA, Kuklo TR, Hsieh PC, Yang BP, Koski TR, Ondra SL. Thoracic pedicle subtraction osteotomy for fixed sagittal spinal deformity. Spine (Phila Pa 1976) 2009;34:2893–2899. doi: 10.1097/BRS.0b013e3181c40bf2. [DOI] [PubMed] [Google Scholar]
- 22.Ramirez N, Flynn JM, Serrano JA, Carlo S, Cornier AS. The Vertical Expandable Prosthetic Titanium Rib in the treatment of spinal deformity due to progressive early onset scoliosis. J Pediatr Orthop B. 2009;18:197–203. doi: 10.1097/BPB.0b013e32832bf5e0. [DOI] [PubMed] [Google Scholar]
- 23.Rhee JM, Bridwell KH, Won DS, Lenke LG, Chotigavanichaya C, Hanson DS. Sagittal plane analysis of adolescent idiopathic scoliosis: the effect of anterior versus posterior instrumentation. Spine (Phila Pa 1976) 2002;27:2350–2356. doi: 10.1097/00007632-200211010-00008. [DOI] [PubMed] [Google Scholar]
- 24.Samdani AF, St Hilaire T, Emans JB, Smith JT, Song K, Campbell RJ, Jr, Betz RR. The usefulness of VEPTR in the older child with complex spine and chest deformity. Clin Orthop Relat Res. 2010;468:700–704. doi: 10.1007/s11999-009-0886-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson GH, Akbarnia BA, Campbell RM., Jr Growing rod techniques in early-onset scoliosis. J Pediatr Orthop. 2007;27:354–361. doi: 10.1097/BPO.0b013e3180333eea. [DOI] [PubMed] [Google Scholar]