Abstract
Background
The use of bone grafting in orthopaedic surgery has increased dramatically in recent years. However, the degree to which immune responses are important for the survival of the allograft is not fully understood. In particular it remains unclear whether differences in the major histocompatibility complex (MHC) influence incorporation of bone allografts and their subsequent biologic performance.
Questions/purposes
Therefore, we asked whether isolated mismatch for MHC antigens of deep frozen bone allografts in the long-term causes (1) immune reactions, and whether these reactions have any effect on (2) morphologic features of the graft, (3) radiographic graft healing, and (4) graft strength.
Methods
We used an established orthotopic tibial segment transplantation technique that allows determination of mechanical strength, histologic evaluation, and immune responses. Tibial segments that had been deep-frozen at −80°C for 1 year were transplanted into 24 PVG (RT1 c) rats from either 12 syngeneic donors or 12 MHC congenic donors PVG.1U (RT1 u). We determined immune responses using an indirect Coombs reaction and determined graft healing radiographically and mechanically after 6 months.
Results
We detected no alloantibody production to graft MHC-I antigens, and found no differences between syngeneic and MHC mismatched grafts in terms of remodeling with host bone, graft healing, and mechanical strength.
Conclusions
Mismatches for MHC antigens do not seem to play a decisive role in healing of long-term, deep-frozen bone allografts.
Introduction
Bone is the second most transplanted tissue after blood and is used to provide support, fill voids, and enhance biologic repair of skeletal defects. Autologous bone provides the optimal source, but the harvest has considerable limitations, including donor-site morbidity, inappropriate form, and lack of sufficient quantities in procedures requiring large amounts of graft [1, 12]. This is of special concern in revision surgery of failed total hip and knee prostheses, and in limb salvaging resection of musculoskeletal tumors; cadaver allograft is the only alternative.
Generally, bone allografting is performed without tissue matching between donors and recipients, but in processing procedures, blood, bone marrow, and soft tissue are removed and freezing and thawing of the donor bone reduces the antigenicity. Antibodies against human leukocyte antigen (HLA) are induced by mismatched bone transplants [10, 30, 32], but it remains unknown whether donor-specific HLA sensitization influences the incorporation of bone allografts and their subsequent biologic performance. For example, Strong et al. [30] reported an increase in HLA antibody sensitization from 39% to 67% associated with bone allografts, but they were unsure if there was any associated effect on allograft incorporation.
In general, the rate of failure with allograft reconstruction owing to complications has been reported to range between 15% and 25% [3, 4, 6, 7, 9, 14, 15, 22]. However, results vary to some extent with the type of graft, anatomic site, and stage of the lesion, ie, the complexity of the surgery and the relative necessity for chemotherapy. The most important factors influencing graft survival are recurrence, infection, nonunion, and fracture. Mankin et al. [14] reported long-term results of allograft replacement in the management of bone tumors and found an infection rate of 11%, nonunion rate of 17% and fracture rate of 19%. The failure rate was highest in the first year and declined rapidly. Mankin and Hornicek reviewed 144 patients with giant cell tumors who had resection and implantation of cadaveric allografts from 1971 to 2001 [15]. Most procedures were done in the distal femur, proximal tibia, proximal femur, and proximal humerus. Seventy-eight percent of patients retained their grafts. Tumor complications included local recurrences in eight patients and metastases in three. None of the patients died. Allograft fracture occurred in 30 (21%) of the 144 patients, nonunion occurred in 12 (8%), and infection occurred in 12 (8%). Deijkers et al. [3] reported three infections, 12 fractures, and nine nonunion treatments in a 10-year followup of 35 grafts. Ultimately, six grafts failed, with infection and length of resection as predisposing factors. Muscolo et al. [17] reviewed 59 consecutive patients after segmental resection in 52 for malignant bone tumors and in seven for benign aggressive bone tumors. They reported an overall 5-year survivorship of the patients of 79%. Infection, nonunion, and fracture rates were 5%, 9%, and 7%, respectively.
Processed frozen bone allografts lack viable donor cells and may trigger an indirect pathway of alloantigen recognition in the recipient. The indirect pathway involves presentation of processed allogeneic HLA shed from foreign cells through cell necrosis and apoptosis. Recipient antigen presenting cells (APC) present the processed peptides in the context of self-HLA Class II to MHC Class II restricted CD4 + T cells [19, 25]. Degraded fragments in frozen bone allografts represent potential immunogenic proteins. This type of recognition may induce either a chronic type of rejection or an immunologic state of tolerance to grafted antigens that cannot be measured with human leukocyte blood tests. A chronic type of host-antigraft response may result in late deterioration of graft function characterized by inflammation and interstitial fibrosis [5].
Clinical studies have some disadvantages when studying HLA sensitization to bone allografts, such as blood transfusions during surgery and the variability of the grafts in terms of processing, implantation, fixation techniques, fixation quality, complications, soft tissue envelope, periosteal preservation, supplemental bone grafting at the junctions, anatomic distribution, and use of radiation or chemotherapy. Also, tissue injury in major surgery leads to the release of endogenous damage-associated molecular patterns that activate the innate immune cells, and one study suggests antibodies to degraded bone soluble proteins may develop in patients with osteoarthritis [31].
Therefore, we asked whether isolated mismatch for MHC antigens of deep frozen bone allografts in the long-term causes (1) immune reactions, and whether these reactions have any effect on (2) morphologic features of the graft, (3) radiographic graft healing, and (4) graft strength.
Materials and Methods
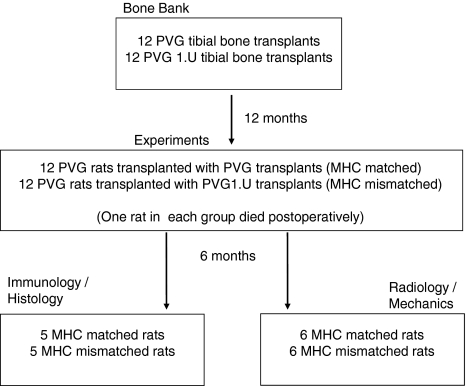
For the experiment we obtained 24 PVG rats that were operated on under general anesthesia (Hypnorm®/Dormicum®, VetaPharma, Sheburn, Leeds, England) (Fig. 1). Using an anterior incision, we exposed the proximal part of either the right or left tibia and carefully elevated the muscles. We then made two osteotomies at the shaft of the bone, 8 mm and 16 mm from the knee, using a fine-toothed circular saw blade mounted on an electric drill, and removed the segment. By random selection, the rats received a transplant of either a deep frozen syngeneic segment (PVG bone) or with a deep frozen allogeneic segment (PVG.1U bone), from the bone bank. We inserted a 0.8-mm steel pin (cannula), press fit, for intramedullary stabilization. The intact fibula secured rotational stability. We achieved fixation without radiographic control, but confirmed proper pin placement using radiographs taken at the end of the experiment. We performed the experiments using aseptic technique.
Fig. 1.
A diagram of the experimental design is shown.
The strength of deep frozen autogenous grafts in this model attained approximately 70% of intact bone at 16 weeks [23]. We therefore assumed that with a followup of 6 months the grafts would have attained the strength of the intact bone. With a difference between the two experimental groups of 15% as clinically interesting, six rats in each group for biomechanical testing would have a power of 80% with a two-tailed alpha of 0.05.
One year before the experiments, six PVG.1U (RT1 u) and six PVG (RT1 c) rats (Harlan UK Limited, Shaw’s Farm, Blackthorn, Bicester, England) were sacrificed and both tibial bones were excised for the grafts. To make the grafts we made two osteotomies at the shaft of the bones, 8 mm and 16 mm from the tibial plateau, using a fine-toothed circular saw blade mounted on an electric drill. By careful steel burring to the endosteal cortex, we emptied the bone segments of cancellous bone and bone marrow. We then sealed and froze the 24 bone segments at −80°C in our bone bank system.
The PVG.1U and PVG rats are identical except for the major histocompatibility complex (MHC of u haplotype versus MHC of c haplotype), where PVG.1U rats have their MHC (RT1) complex derived from the AO strain. Therefore, an isolated effect of MHC mismatches (Class I, Class II, and other polymorphic antigens in the MHC) on graft survival and other biologic parameters can be monitored. The experiment conformed to the Norwegian Council of Animal Research Code for the Care and Use for Experimental Purposes.
Postoperatively, one rat in each group died. We subcutaneously injected buprenorphine (Temgesic®; Reckitt & Benckiser, Slough, UK), 0.05 mg per kilo, twice daily for the first 3 postoperative days for analgesia, and observed the rats daily for limb function and signs of improper healing. They all resumed full weightbearing after a few days. At 6 months, we sacrificed the animals with Pentobarbital® (Ås produksjonslaboratorium, Ås, Norway), 100 mg/kg (0.1 mL/100 g) intraperitoneally (ie, 0.35 mL).
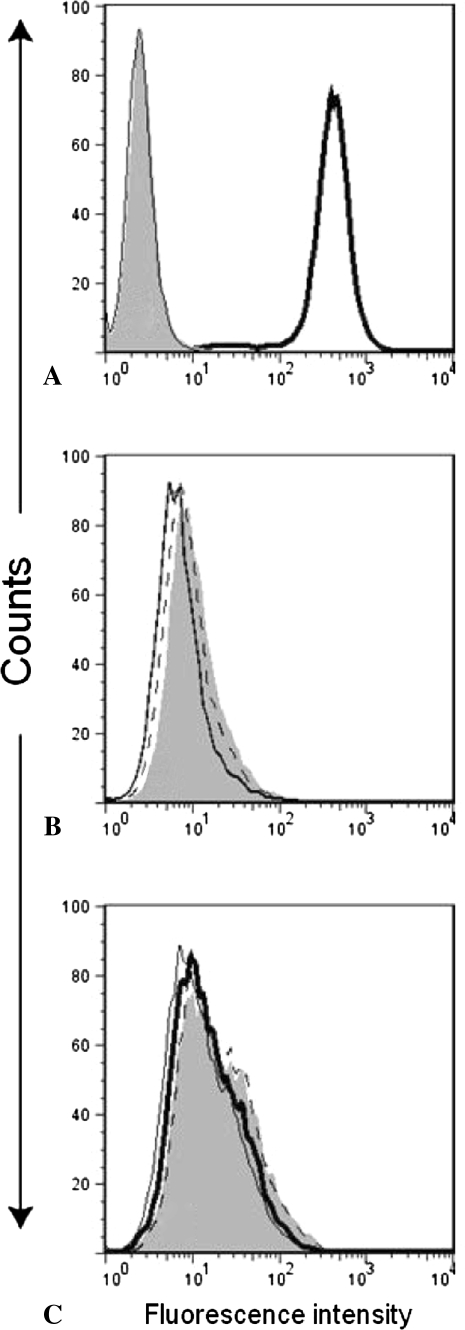
Postoperatively at the time of grafting and at termination of the experiments after 6 months, we obtained blood via a cannula from the tail vein of five rats from each group, and blood serum was examined for antibodies against MHC antigens. As described previously [20], we measured the antibody response in individual rats. In short, we incubated rat lymphoid cells, YB2/0, and expressing MHC-I (RT1 Class I) antigens of RTIuu haplotype with serum dilutions from the transplanted rats at 4ºC for 30 minutes, washed, then incubated with a FITC-labeled secondary goat anti-rat IgG heavy + light chain (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for another 30 minutes before washing again. We measured the fluorescence intensity in a FACScalibur (Becton Dickinson, San Jose, CA, USA), with gates set to exclude dead cells. In this test system, substantial antibody synthesis can be detected only when the peak fluorescence intensity has shifted so much to the right that there is only partly or no overlap between the two sets of curves. To show how an antibody response manifests itself in this system, we also included a standard mAb (NR5/10) against MHC Class I of the RT1u haplotype (positive control). We confirmed the validity of this method in a clear shift of the histogram to the right as evaluated by two of us (HS and BR).
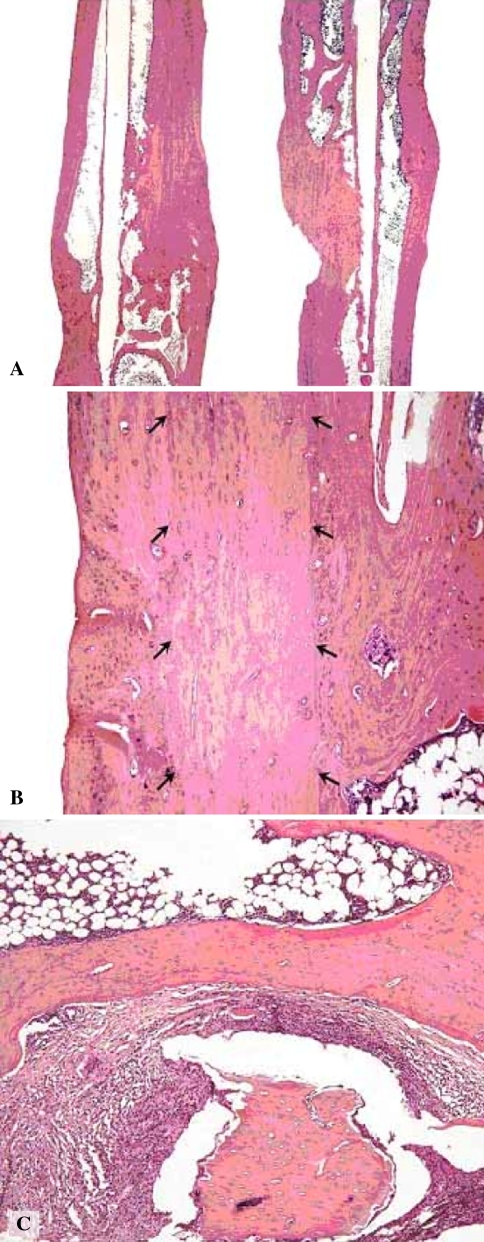
The transplanted bones of these five animals of each group then were harvested and fixed by immersion in 4% phosphate-buffered formaldehyde immediately after sacrifice, decalcified for 3 weeks in 7% ethylenediaminetetraacetic acid (EDTA), and embedded in paraffin according to a routine protocol. Sections were coded to blind the observer regarding the experimental group from which the section emanated. We used three to five hematoxylin and eosin-stained sections, including the central marrow area, to evaluate the tissue. One of us (FPR) used semiquantitative scoring with a three-grade scale (0, +, ++) to grade cortical integrity, identifiable remnants of graft, marrow fibrosis, and inflammation. The parameters were scored as follows: (1) cortical integrity = + one cortex intact, ++ both cortices intact; (2) identifiable remnants of graft = + remnants observed in less than 50% of the graft area, ++ remnants observed in greater than 50% of graft area; (3) marrow fibrosis = + fibrous tissue/collagen fibers in greater than 5% of the marrow area, ++ fibrous tissue/collagen fibers in greater than 20% of the marrow area; (4) inflammation = + one focus with more than 20 leukocytes in a high power field of vision, ++ two or more such foci (Fig. 2). The grading was performed twice by the same pathologist (FPR) at in interval greater than 1 month, and the reproducibility of the semiquantitative scoring was 90% (kappa 0.825).
Fig. 2A–C.
(A) A low-power photomicrograph is shown that includes the area of the graft, a bone with allogeneic graft on the left, and a syngeneic graft on the right. Both bones show ++ bone continuity (Stain, hematoxylin & eosin). Scale bar = 1.5 mm. (B) A medium-power photomicrograph shows an area with remnant of the graft outlined with arrows. The micrograph is from a bone with ++ remnants of implant (Stain, hematoxylin & eosin). Scale bar = 200 μm. (C) Another medium-power photomicrograph shows an area of bone marrow with dead bone (rounded structure in the lower middle of the photomicrograph) surrounded by inflammatory infiltrate and fibrosis. This is from a + inflammation bone (Stain, hematoxylin & eosin). Scale bar = 200 μm.
We subjected the remaining six rats in each group to radiographic and biomechanical examinations. We harvested their transplanted and their intact legs, and performed radiography of the transplanted leg with a standard clinical digital system (Siemens Axiom Aristos; Siemens AG, München, Germany) after removal of the intramedullary pin. The xray tube settings were 46 kV, 1.0 mA per second, and focus-to-film-distance (source-to-image receptor distance) of 115 cm. Two of us (OR, HS) assessed all radiographs for healing using the following criteria: (1) complete healing, defined as bone bridging without gaps, (2) incomplete healing, defined as a radiolucent gap with some areas of bridging bone trabeculae, and (3) no healing, defined as a continuous radiolucent gap at the osteotomy site with no bridging bone trabeculae. Interobserver agreement for radiographic healing was 100%.
After radiographic examinations, we examined the bone mineral content (BMC) and density (BMD) of the transplanted and contralateral intact bones using dual energy radiographic absorptiometry (DEXA). DEXA was performed on a GE Lunar Piximus (Lunar Corporation, Madison, WI, USA) with a tube current of 400 μA. The frequency of the scanning unit was 50/60 Hz, and the xray tube had a focal spot size of 0.25 mm by 0.25 mm with a coefficient variation of 1%. After scanning the whole tibia, we centralized a region of interest at the graft.
Thereafter, we tested the composite (transplanted) and the contralateral bones for torsional strength (MTS 858; MTS Systems Corporation, Eden Prairie, MN, USA). We fixed the tibial ends with a clamp and ran the instrument at a constant rate of 0.08 radii per second. The load values were recorded and transformed to a load deformation curve (Origin 7.5; OriginLab Corporation, Northampton, MA, USA). We defined the strength as the torsion necessary to produce fracture of the composite bone. We determined the torsion stiffness from the slope of the linear elastic part of the curve and defined the energy as the energy absorbed during loading to fracture.
Data for biomechanical parameters and BMC and BMD are presented as mean and standard deviation of the mean. Data were normally distributed and were analyzed using one-way ANOVA. Statistical analysis was performed with SPSS 16.0 (SPSS Inc, Chicago, USA.)
Results
None of the five allotransplanted rats exhibited any detectable antibody response as the FACS distribution curves were identical to the controls (Fig. 3).
Fig. 3A–C.
The FACS plots show no detectable antibody production in PVG rats transplanted with either frozen bone from PVG (syngeneic) or PVG.1U (allogeneic MHC mismatched) rats. The final serum dilution was 1/8. Propidium iodide was used for gating on live cells. The plots show (A) FITC labeled secondary Ab alone (gray filled), mAb OX6 against MHC-II (thin line) and a standard antibody against MHC-I of the RT1u haplotype mAb NR5/10 (thick line), (B) counts for three pretransplanted rats, and (C) from two representative rats of each group (syngeneic [gray filled and stapled line] and allogeneic [thin and thick lines]).
By histologic examinations, all samples showed intact cortical bone in the transplanted segment and adjacent bone, except for one focal defect in one of the animals in the allogeneic group (Fig. 2A; Table 1). We observed remnants of the graft in greater than 50% of the transplanted segment in four of five bones in each group (Fig. 2B). One animal in each group showed focal inflammation surrounding remnants of devitalized bone along with focal fibrosis (Fig. 2C).
Table 1.
Semiquantitative assessment of histologic features in 10 bones
| Animal type/number | Intact cortex* | Rest of implant† | Marrow fibrosis‡ | Inflammation§ |
|---|---|---|---|---|
| Allogeneic 1 | ++ | ++ | 0 | 0 |
| Allogeneic 2 | ++ | ++ | 0 | 0 |
| Allogeneic 3 | ++ | + | 0 | 0 |
| Allogeneic 4 | ++ | ++ | 0 | 0 |
| Allogeneic 5 | + | ++ | + | + |
| Syngeneic 1 | ++ | ++ | 0 | 0 |
| Syngeneic 2 | ++ | ++ | 0 | 0 |
| Syngeneic 3 | ++ | ++ | 0 | 0 |
| Syngeneic 4 | ++ | ++ | 0 | 0 |
| Syngeneic 5 | ++ | + | + | + |
* Intact cortex = + one cortex intact, ++ both cortices intact; †rest of implant = + remnants observed in less than 50% of the implant area, ++ remnants observed in greater than 50% of implant area; ‡marrow fibrosis = + fibrous tissue/collagen fibers in greater than 5% of the marrow area, ++ fibrous tissue/collagen fibers in greater than 20% of the marrow area; §inflammation = + one focus with greater than 20 leukocytes in a high power field of vision, ++ two or more such foci.
Complete radiographic healing of the grafted segment occurred in all transplanted bones (Fig. 4).
Fig. 4A–B.
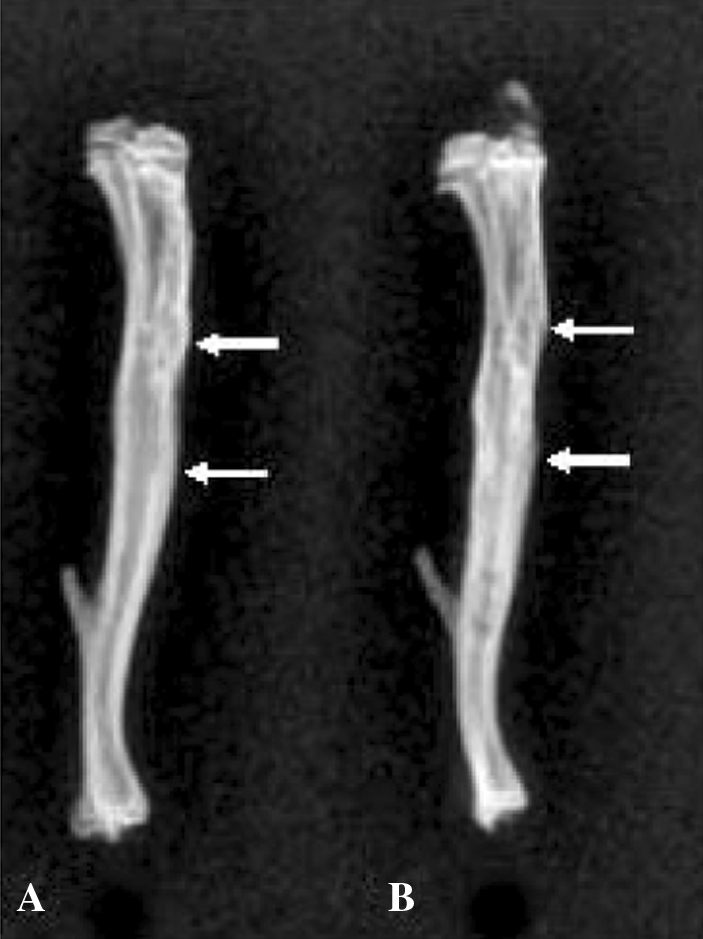
The radiographs show fully incorporated (A) frozen allogeneic and (B) syngeneic grafts (between arrows) after 6 months.
We observed no differences in either BMC or BMD between the two groups (Table 2), and bone mineralization was similar to that in the intact bones (Table 2). There were no differences in torsion strength between the groups, nor were there any differences in either stiffness or fracture energy (Table 3). Furthermore, the biomechanical parameters of the transplanted bones were similar to those of the intact bones (Table 3).
Table 2.
BMC and BMD of the transplanted and intact bones
| Mineralization | Syngeneic | Allogeneic | Intact | p Value |
|---|---|---|---|---|
| BMC (10−3 g) | 41.8 ± 2.1* | 44.6 ± 12.2 | 35.6 ± 3.4 | 0.364 |
| BMD (10−3 g/cm2) | 19.9 ± 2.2 | 19.5 ± 4.2 | 17.4 ± 0.7 | 0.473 |
BMC = bone mineral content; BMD = bone mineral density; * mean ± standard deviation.
Table 3.
Biomechanics of the transplanted and intact bones
| Biomechanics | Syngeneic | Allogenic | Intact | p Value |
|---|---|---|---|---|
| Moment (Nm × 10−2) | 13.6 ± 2.60* | 12.0 ± 2.54 | 14.0 ± 1.78 | 0.295 |
| Stiffness (Nm/degree × 10−3) | 3.06 ± 0.64 | 2.55 ± 0.60 | 3.06 ± 0.50 | 0.229 |
| Energy (Nm × degree × 10−1) | 66.7 ± 25.8 | 49.3 ± 13.6 | 50.5 ± 10.7 | 0.185 |
* Mean ± standard deviation.
Discussion
Bone allografting is being used increasingly in patients for bone reconstruction. However, on implantation of allogeneic bone, the host is expected to experience an intricate immune response [5]. Deep freezing may reduce the immunity, but also may alter the properties of the graft. How these processes affect the incorporation of massive bone allografts are not fully understood. Therefore, we asked whether isolated mismatch for MHC antigens of deep frozen bone allografts in the long-term causes (1) immune reactions, and whether these reactions have any effect on (2) morphologic features of the graft, (3) radiographic graft healing, and (4) graft strength.
Our study is associated with certain limitations. First, the results of experimental studies generally must be transferred to human clinical practice with caution, especially in this case, based on the magnitude of the difference in graft size and the inherent differences in the immune systems. However, the rats in this study were genetically identical except for the MHC region, and rats, in general, are biologically similar to humans when it comes to the biology of bone healing [21]. Second, we used intercalary allografts, and our findings may be restricted to these kinds of allografts. Third, the reproducibility of semiquantitative histologic scoring systems is reportedly low [11], but we believe semiquantitative scoring may be reasonable with a high degree of reproducibility if the parameters are kept basic and the grades are limited and well defined [13]. Fourth, we had no direct data to establish power analyses for continuous biomechanical data. However, our data were normally distributed with a power for the null hypothesis of 83% with a two-tailed alpha of 0.05. Fifth, a torsion test is not osteotomy site-specific, as it determines the weakest point of the bone rather than measuring the strength at the osteotomy sites. If the graft healed, it may be stronger than the entire bone. However, this is also why torsion tests usually are applied to bone segments and grafts.
In rats, the RT1 gene region on chromosome 20 encodes MHC Classes I and II molecules that are highly polymorphic and provoke strong alloimmune responses to organ transplantation, leading to their rejection. Based on differences solely in the MHC region, PVG rats reject organ grafts from PVG 1U rats. Strong antibody responses to allogeneic MHC-I molecules after MHC mismatched transplantation of fresh bone in this rat model have been observed [24]. Direct recognition occurs when recipient Th lymphocytes recognize MHC-II antigens on intact APC in the allograft. Animal and human studies show that the cellular components of bone express sufficient MHC molecules to alert the host immune system with activation of cytotoxic T cells, leading to destruction of cells in the graft [8, 18, 26]. Indirect recognition occurs when recipient APC ingests donor MHC antigens from broken down cells and present peptides of donor MHC in the groove of the recipient’s own MHC molecules. This latter scenario may be predominant when donor tissue is damaged by freezing, and releases peptides that can lead to a more chronic state of inflammation in the graft. To obtain a sufficient IgG response against MHC-I or II molecules, this will require help from sensitized T cells. In the current study, none of the five allotransplanted rats exhibited any detectable antibody response; the FACS-distribution curves at 6 months were identical to the controls. This implies that MHC-derived antigens from the donor were not present in quantities that provoked a measurable antibody response. However, we cannot formally exclude that low concentrations of donor antigens were taken up by host macrophages or antigen presenting cells, and could provoke an indirect antigen presentation to host T cells. Some experimental studies reported an antibody response after cryopreserved bone grafts [2, 19, 27, 29], but such reactions usually are weak. Bos et al. [2] performed iliac crest grafting of frozen bone with minor and major histocompatibility differences. Grafting in the face of a minor histocompatibility difference failed to generate second-set skin-graft reactivity rejection, whereas grafting across a major histocompatibility difference provided in vivo evidence of accelerated skin-graft rejection and in vitro evidence of cytolytic cells and antibodies. When grafting of frozen bone with MHC mismatch was performed without second-set skin grafting, antibodies were seen between Days 30 and 50 with a peak at Day 40, but never achieved high activity levels. There are some factors in experimental design that can explain this difference to our observations. First, Bos et al. performed iliac crest grafting with a high bone marrow concentration [2]. It is well recognized that bone marrow cells are strongly immunogenic. In our experiments on intercalary bone, we removed bone marrow content as much as possible in line with clinical practice. Second, in the experiments of Bos et al. the grafts were frozen to −20º for a minimum of 10 days, whereas our grafts were deep frozen to −80º for a year. Third, Bos et al. had an observation period of 50 days. At this time the antibody response declined, and they stated that longer followup studies would be highly desirable. We extended the followup to 6 months.
Although Bos et al. found immune activity, histologic evaluation at 50 days showed that frozen bone fared the same regardless of the genetic relationship, and neither was there any discernible difference in the scores of frozen grafts that were mismatched or syngeneic [2]. Our histologic results are in agreement with these observations, and these results may explain clinical difficulties in correlating HLA mismatches of frozen bone allografts with performance and failures. It is assumed that T cells primed by the indirect pathway may play the dominant role in the chronic state of inflammation and rejection of allografts. Histologic features of graft inflammation are well established as infiltration with large numbers of lymphocytes and macrophages, and a fibroproliferative process [18]. In a study of osteochondral allografts in dogs, the authors found that the ingrowths of fibrous connective tissue were directly proportional to the immunogenicity of the bone grafts [28]. It also was reported that revascularization was slow in mismatched grafts. In our study, the frozen allogeneic grafts did as well as the syngeneic grafts by histologic evaluations. The grafts consisted of a mixture of living and dead bone tissue, but complete healing at the osteotomy sites occurred in all cases. This reflects that the process of creeping substitution of grafts by the ingrowths of vessels is slow, and incorporation may be limited [5]. One rat in each group showed localized inflammation and marrow fibrosis related to small dead bone fragments, most likely emanating from the grafts. One may speculate that these fragments represent a sign of early fatigue microdamage, heralding a later graft fracture. But along with the antibody tests, our histologic data indicate that there was no indirect antigen presentation to the host T cells that might provoke any inflammation or affect the healing of the graft.
Bone graft incorporation is a complicated process with multiple variables influencing rate, pattern, and completeness; one method of evaluation would not adequately show the various aspects [27]. Our radiographic examinations were in agreement with the histologic findings and showed complete healing by complete bone bridging without gaps in all grafts. However, successful incorporation of bone grafts should result in a composite that can bear physiologic loads, and neither radiographic nor histologic methods can measure the biomechanical competence of grafted bones. Measurements of bone mineralization provide a valuable estimation of bone quantity and quality that correlates to biomechanical properties, but the technique cannot accurately predict biomechanical competence [16]. However, our data on bone mineralization and biomechanical testing showed that, at 6 months, the biomechanical constructions were equivalent to intact bone; either we used deep frozen grafts matched or mismatched for MHC antigens. Few studies on bone grafting report biomechanical data, but our findings agree with observations 11 months after fresh and frozen tissue antigen matched and mismatched osteochondral allografts in dogs [28].
Our observations suggest mismatches for MHC antigens are not serious obstacles to transplantation of long-term frozen-bone intercalary allografts. If these results can be translated to the human setting, HLA matching of the bone donor with the recipient does not, in itself, improve the outcome of the transplantation.
Acknowledgments
We thank the staff of Department of Comparative Medicine at Oslo University Hospital, Rikshospitalet for taking care of the animals.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the Oslo University Hospital.
References
- 1.Bauer TW, Muschler GF. Bone graft materials: an overview of the basic science. Clin Orthop Relat Res. 2000;371:10–27. doi: 10.1097/00003086-200002000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Bos GD, Goldberg VM, Zika JM, Heiple KG, Powell AE. Immune responses of rats to frozen bone allografts. J Bone Joint Surg Am. 1983;65:239–246. doi: 10.2106/00004623-198365020-00015. [DOI] [PubMed] [Google Scholar]
- 3.Deijkers RL, Bloem RM, Kroon HM, Van Lent JB, Brand R, Taminiau AH. Epidiaphyseal versus other intercalary allografts for tumors of the lower limb. Clin Orthop Relat Res. 2005;439:151–160. doi: 10.1097/00003086-200510000-00029. [DOI] [PubMed] [Google Scholar]
- 4.Donati D, Di Liddo M, Zavatta M, Manfrini M, Bacci G, Picci P, Capanna R, Mercuri M. Massive bone allograft reconstruction in high-grade osteosarcoma. Clin Orthop Relat Res. 2000;377:186–194. doi: 10.1097/00003086-200008000-00025. [DOI] [PubMed] [Google Scholar]
- 5.Enneking WF, Mindell ER. Observations on massive retrieved human allografts. J Bone Joint Surg Am. 1991;73:1123–1142. [PubMed] [Google Scholar]
- 6.Farid Y, Lin PP, Lewis VO, Yasko AW. Endoprosthetic and allograft-prosthetic composite reconstruction of the proximal femur for bone neoplasms. Clin Orthop Relat Res. 2006;442:223–229. doi: 10.1097/01.blo.0000181491.39048.fe. [DOI] [PubMed] [Google Scholar]
- 7.Fox EJ, Hau MA, Gebhardt MC, Hornicek FJ, Tomford WW, Mankin HJ. Long-term followup of proximal femoral allografts. Clin Orthop Relat Res. 2002;397:106–113. doi: 10.1097/00003086-200204000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Halloran PF, Lee EH, Ziv I, Langer F, Gross AE. Orthotopic bone transplantation in mice: II. Studies of the alloantibody response. Transplantation. 1979;27:420–426. doi: 10.1097/00007890-197903000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Hornicek FJ, Gebhardt MC, Tomford WW, Sorger JI, Zavatta M, Menzner JP, Mankin HJ. Factors affecting nonunion of the allograft-host junction. Clin Orthop Relat Res. 2001;382:87–98. doi: 10.1097/00003086-200101000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Horowitz MC, Friedlander GE. Induction of specific T-cell responsiveness to allogeneic bone. J Bone Joint Surg Am. 1991;73:1157–1168. [PubMed] [Google Scholar]
- 11.Hyllestad JL, Veje K, Ostergaard K. Histochemical studies of the extracellular matrix of human articular cartilage: a review. Osteoarthritis Cartilage. 2002;10:333–343. doi: 10.1053/joca.2002.0519. [DOI] [PubMed] [Google Scholar]
- 12.Keating JF, McQueen MM. Substitutes for autologous bone graft in orthopaedic trauma. J Bone Joint Surg Br. 2001;83:3–8. doi: 10.1302/0301-620X.83B1.11952. [DOI] [PubMed] [Google Scholar]
- 13.Loken S, Jakobsen RB, Arøen A, Heir S, Shahdadfar A, Brinchmann JE, Engebretsen L, Reinholt FP. Bone marrow mesenchymal stem cells in a hyaluronan scaffold for treatment of an osteochondral defect in a rabbit model. Knee Surg Sports Traumatol Arthrosc. 2008;16:896–903. doi: 10.1007/s00167-008-0566-2. [DOI] [PubMed] [Google Scholar]
- 14.Mankin HJ, Gebhardt MC, Jennings LC, Springfield DS, Tomford WW. Long-term results of allograft replacement in the management of bone tumors. Clin Orthop Relat Res. 1996;324:86–97. doi: 10.1097/00003086-199603000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Mankin HJ, Hornicek FJ. Treatment of giant cell tumors with allograft transplants: a 30-year study. Clin Orthop Relat Res. 2005;439:144–150. doi: 10.1097/01.blo.0000174684.85250.b5. [DOI] [PubMed] [Google Scholar]
- 16.Markel MD, Wikenheiser MA, Morin RL, Chao EY, Lewallen DG. Quantification of bone healing: comparison of QCT, SPA, MRI, and DEXA in dog osteotomies. Acta Orthop Scand. 1990;61:487–498. doi: 10.3109/17453679008993569. [DOI] [PubMed] [Google Scholar]
- 17.Muscolo DL, Ayerza MA, Aponte-Tinao L, Ranalletta M, Abalo E. Intercalary femur and tibia segmental allografts provide an acceptable alternative in reconstructing tumor resections. Clin Orthop Relat Res. 2004;426:97–102. doi: 10.1097/01.blo.0000141652.93178.10. [DOI] [PubMed] [Google Scholar]
- 18.Muscolo DL, Ayerza MA, Calabrese ME, Redal MA, Santini Araujo E. Human leukocyte antigen matching, radiographic score, and histologic findings in massive frozen bone allografts. Clin Orthop Relat Res. 1996;326:115–126. doi: 10.1097/00003086-199605000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Muscolo DL, Kawai S, Ray RD. In vitro studies of transplantation antigens present on bone cells in the rat. J Bone Joint Surg Br. 1977;59:342–348. doi: 10.1302/0301-620X.59B3.142775. [DOI] [PubMed] [Google Scholar]
- 20.Naper C, Ryan JC, Nakamura MC, Lambracht D, Rolstad B, Vaage JT. Identification of an inhibitory MHC receptor on alloreactive rat natural killer cells. J Immunol. 1998;160:219–224. [PubMed] [Google Scholar]
- 21.O’Loughlin PF, Morr S, Bogunovic L, Kim AD, Park B, Lane JM. Selection and development of preclinical models in fracture-healing research. J Bone Joint Surg Am. 2008;90(suppl 1):79–84. doi: 10.2106/JBJS.G.01585. [DOI] [PubMed] [Google Scholar]
- 22.Ortiz-Cruz E, Gebhardt MC, Jennings LC, Springfield DS, Mankin HJ. The results of transplantation of intercalary allografts after resection of tumors: a long-term follow-up study. J Bone Joint Surg Am. 1997;79:97–106. doi: 10.2106/00004623-199701000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Reikerås Impact of freezing on bone graft incorporation: biomechanical evaluations in rats. Clin Biomech. 2010;25:177–180. doi: 10.1016/j.clinbiomech.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 24.Reikerås O, Shegarfi H, Naper C, Reinholt FP, Rolstad B. Impact of MHC mismatch and freezing on bone graft incorporation: an experimental study in rats. J Orthop Res. 2008;26:925–931. doi: 10.1002/jor.20595. [DOI] [PubMed] [Google Scholar]
- 25.Sayegh MH, Watschinger B, Carpenter CB. Mechanisms of T cell recognition of alloantigen: the role of peptides. Transplantation. 1994;57:1295–1302. doi: 10.1097/00007890-199405150-00001. [DOI] [PubMed] [Google Scholar]
- 26.Skjodt H, Hughes DE, Dobson PR, Russell RG. Constitutive and inducible expression of HLA class II determinants by human osteoblast-like cells in vitro. J Clin Invest. 1990;85:1421–1426. doi: 10.1172/JCI114586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stevenson S, Li XQ, Davy DT, Klein L, Goldberg VM. Critical biological determinants of incorporation of non-vascularized cortical bone grafts: quantification of a complex process and structure. J Bone Joint Surg Am. 1997;79:1–16. doi: 10.1302/0301-620X.79B1.7020. [DOI] [PubMed] [Google Scholar]
- 28.Stevenson S, Li XQ, Martin B. The fate of cancellous and cortical bone after transplantation of fresh and frozen tissue-antigen-matched and mismatched osteochondral allografts in dogs. J Bone Joint Surg Am. 1991;73:1143–1156. [PubMed] [Google Scholar]
- 29.Stevenson S, Shaffer JW, Goldberg VM. The humoral response to vascular and nonvascular allografts of bone. Clin Orthop Relat Res. 1996;326:86–95. doi: 10.1097/00003086-199605000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Strong DM, Friedlaender GE, Tomford WW, Springfield DS, Shives TC, Burchardt H, Enneking WF, Mankin HJ. Immunologic responses in human recipients of osseous and osteochondral allografts. Clin Orthop Relat Res. 1996;326:107–114. doi: 10.1097/00003086-199605000-00013. [DOI] [PubMed] [Google Scholar]
- 31.VandeVord P, Nasser S, Wooley PH. Immunological responses to bone soluble proteins in recipients of bone allografts. J Orthop Res. 2005;23:1059–1064. doi: 10.1016/j.orthres.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Ward WG, Gautreaux MD, Lippert DC, 2nd, Boles C. HLA sensitization and allograft bone graft incorporation. Clin Orthop Relat Res. 2008;466:1837–1848. doi: 10.1007/s11999-008-0294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]