Abstract
Background
Patients with myelomeningocele and rigid lumbar and thoracolumbar kyphosis face substantial functional difficulties with sitting and lying supine and are prone to skin breakdown over the gibbus and risk of infection. Kyphectomy, along with cordotomy and segmental spinal instrumentation down to the pelvis, is one alternative that can provide reliable correction of the deformity but also can maintain that correction over a period of time.
Questions/purposes
We determined the fusion rates, deformity correction and maintenance, and perioperative complications of kyphectomy with long segmental spinal instrumentation using the Warner and Fackler technique.
Methods
We retrospectively reviewed the charts and radiographs of 33 patients with myelomeningocele who had kyphectomy with segmental spinal instrumentation down to the pelvis between 1991 and 2006. The average age at surgery was 7.6 years (range, 3–19 years). Twenty-one patients had a minimum 2-year followup (average, 7.0 years; range, 2.4–15.7 years).
Results
The average preoperative kyphosis of 124° (range, 75°–210°) improved at last followup to 22° (range, 3°–55°) with an average correction of 81% (range, 59%–98%). We identified 17 postoperative complications. Wound and skin complications were most common; 11 secondary surgeries were performed in 10 patients.
Conclusions
Surgery for myelomeningocele kyphosis is technically demanding and carries substantial risk. Kyphectomy and posterior spinal fusion and instrumentation with the Warner and Fackler technique allow correction and maintenance of sagittal alignment.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Spinal deformity is a common problem in myelomeningocele. Although it is less common than scoliosis, rigid kyphosis reportedly occurs in 8% to 20% of patients with myelomeningocele [1–3, 6, 9, 21, 25]. Rigid lumbar and thoracolumbar kyphosis potentially presents a substantial functional impediment with regard to sitting and use of the upper extremities other than for balance and predisposes these patients to chronic skin breakdown over the gibbus deformity with a potential risk of infection. Kyphotic curves are often present at birth and are resistant to nonoperative management. Progression of the kyphotic deformity ranges from 4° to 13° per year and accelerates once the patient begins sitting upright and as the curve grows in magnitude [17, 18, 25]. Bracing may be used to improve sitting posture but does not change the natural history of the paralytic kyphosis. Surgery is required [21, 25] to prevent progressive kyphotic deformity and to correct the sagittal spine balance.
Since Sharrard [29] first described kyphectomy in the neonate, several surgical methods have been described [8, 11, 14, 20, 23, 24, 27, 31], but the optimal age for surgery, the type of instrumentation, and the method of distal fixation down to the pelvis remain controversial [8, 11, 12, 15, 16, 18, 21–24, 26–30, 33–35]; whereas the idea of performing the kyphectomy when patients are young as possible may be appealing because the kyphotic deformity is smaller, surgery in a skeletally immature patient may impair the trunk growth and lead to a short torso with small chest cavity underdevelopment. On the other hand, kyphectomy with short fusion and fixation with wires, plates, and staples may prevent a short torso by preserving growth through the rest of the spine. This method traditionally requires long-term immobilization and the extent of the loss of correction over a period of time and recurrence of the deformity appear to be a far greater problem [8, 18, 19, 21, 29, 30] compared with the series in which long spinal fixation is used (Table 1). Christofersen and Brooks [8] reported more than 50% correction loss in three of their nine patients with fixation achieved with wires only. Sharrard and Drennan [29, 30] reported their series on newborns and older children with a statement that there is a tendency to recurrence of the deformity without giving any specifics. Lindseth and Stelzer [18] and Lintner and Lindseth [19] reported a 58% to 100% loss of correction at the final followup in their series with fixation achieved with wires only. Segmental long fixation with limited fusion at the kyphectomy level may provide stable fixation while potentially providing some trunk growth. The method of distal pelvic fixation [11, 23, 35] plays a pivotal role in treatment of the myelomeningocele kyphosis in which the structural geometry and quality of the bone demand a mechanically better and longer lever arm. When the distal end of the rods are placed in front of the sacrum, either over the top of sacral ala (Dunn-McCarthy) [23] or through sacral foramina (Warner-Fackler) [35], coupling of the pelvis with the rest of the spine leads to better alignment of the spine and correction of the deformity. These reports [23, 35] and similar series [1, 11, 12, 22, 33] had relatively small numbers of patients (six to 24) and short-term followup (range, 0.5–7.8 years) (Table 1).
Table 1.
Previous literature reporting postoperative results of kyphectomy with long segmental spinal fixation to pelvis
| Study | Surgical technique | Number of patients | Age at surgery (years)* | Followup (years)* | Preoperative kyphosis* | Immediate postoperative kyphosis* | Final followup kyphosis* |
|---|---|---|---|---|---|---|---|
| Altiok et al. [current study] | Warner-Fackler | 22 | 7.6 (3–19) | 7 (2.4–15.7) | 123° (75°–210°) | 22° (4°–70°) | 22° (3°–55°) |
| Akbar et al. [1] (2006) | Warner-Fackler | 24 | 7.6 (1.6–16.2) | 3 (1–6) | 124° (90°–170°) | 43° (15°–90°) | 48° (15°–100°) |
| McCall [22] (1998) | Warner-Fackler | 17 | 8 (2.3–12.8) | 4.7 (3–7.8) | 111° (75°–157°) | 15° (−18°–36°) | 20° (−17°–83°) |
| Thomsen et al. [33] (2000) | Warner-Fackler | 9 | 8 (5–16) | 2.3 (0.5–4) | 152° (90°–180°) | 44° (22°–70°) | 49° (30°–85°) |
| Warner and Fackler [35] (1993) | Warner-Fackler | 12 | 6.5 (3–16) | 2.5 (NA) | 101° (68°–150°) | 4° (−12°–30°) | 7° (−7°–25°) |
| Huang and Lubicky [12] (1994) | Warner-Fackler | 6 | 5 (3.4–5.1) | 3 (0.8–4) | 126° (98°–165°) | 22° (5°–40°) | 23° (10°–41°) |
| Heydemann and Gillespie [11] (1987) | Dunn/Galveston | 12 | 11 (7–14) | 2.2 (0.5–4.7) | 124° (95°–172°) | NA | 33° (−20°–48°) |
| McCarthy et al. [23] (1989) | Dunn | 24 | 12.9 (9–17) | 2.1 (0.5–5) | NA | NA | NA |
| Banta [2] (1990) | Galveston | 16 | 7.2 | NA | 113° (80°–180°) | NA | 35° (0°–80°) |
| Torode and Godette [34] (1995) | Rods driven into sacrum | 4 | 10.7 (9–13) | 2 (0.8–4.5) | 90° (74°–102°) | 25° (11°–38°) | 36° (17°–48°) |
| Nolden et al. [27] (2002) | Subtraction (decancellation) vertebrectomy with rods driven into sacrum | 11 | 6 (3–12) | 2 | 88° (50°–149°) | −3° (−50°–50°) | 20° (−34°–80°) |
| Study | Blood loss (mL)* | Number of deaths | Number of shunt failures | Number of infections | Number of wound breakdowns | Number of implant failures | Number of pseudarthroses | Number of pleural tears |
|---|---|---|---|---|---|---|---|---|
| Altiok et al. [current study] | 1358 (400–4000) | 1 | 1 | 1 | 6 | 7 | 5 | 2 |
| Akbar et al. [1] (2006) | 1240 (300–3000) | 1 | 1 | 3 | NA | 7 | NA | 0 |
| McCall [22] (1998) | 1121 (450–2580) | 1 | 0 | 1 | 2 | 0 | 0 | NA |
| Thomsen et al. [33] (2000) | 635 (260–1020) | 0 | 0 | 0 | 0 | 2 | 0 | NA |
| Warner and Fackler [35] (1993) | NA | 0 | 0 | 1 | 2 | 1 | 0 | NA |
| Huang and Lubicky [12] (1994) | NA | 0 | 0 | 0 | 2 | 0 | 1 | 0 |
| Heydemann and Gillespie [11] (1987) | NA | 0 | 0 | 1 | 0 | 2 | 1 | NA |
| McCarthy et al. [23] (1989) | NA | 0 | NA | NA | NA | 0 | 0 | NA |
| Banta [2] (1990) | NA | 1 | 0 | 0 | 0 | 0 | 0 | NA |
| Torode and Godette [34] (1995) | NA | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nolden et al. [27] (2002) | 969 (125–1700) | 0 | 1 | 2 | 1 | 0 | NA | NA |
* Values are expressed as averages with ranges in parentheses; NA = not available.
We therefore determined the fusion rates, deformity correction and its maintenance, and perioperative complications of a specific spinal pelvic fixation along with kyphectomy and cordotomy in patients with myelomeningocele over a longer term of followup.
Patients and Methods
Between 1991 and 2006, 33 patients with myelomeningocele and kyphosis underwent kyphectomy and cordotomy with long segmental spinal instrumentation at Shriners Hospital for Children–Chicago. All 33 patients had distal pelvic fixation through the Warner and Fackler [35] technique. One patient died from intraoperative complications. Of the 32 patients, 21 had a minimum of 2 years followup. We retrospectively reviewed the charts and radiographs of these 21 patients. These 21 patients formed the study group.
Nineteen of these 21 patients had thoracic-level motor and sensory levels; two patients had upper lumbar motor and sensory levels. All patients had ventriculoperitoneal shunts and were wheelchair-dependent. Skin breakdown or impending breakdown was the most common indication for surgery and was noted in 15 of 21 patients. Difficulty with sitting balance and posture was the primary indication for surgery in the remaining six patients and was noted in 12 of 21 patients. Two patients had failed previous attempts at kyphectomy and spinal fusion performed at outside institutions. Of these, one patient had a one-level vertebrectomy with attempted fusion in situ. The average age at the time of surgery was 7.6 years (range, 3–19 years). The minimum followup was 2.4 years (average, 7.0 years; range, 2.4–15.7 years). Prior approval for the study was obtained from the Institutional Review Board in conjunction with Shriners Hospital for Children–Chicago and Rush University Medical Center.
The average preoperative kyphosis in the study group was 124° (range, 75°–210°). Six patients presented with a scoliosis of greater than 25°, averaging 45° (range, 32°–93°). Of 21 patients, four had additional underlying congenital spinal anomalies associated with their kyphosis.
All surgeries were performed at the Shriners Hospital for Children–Chicago and performed by two senior surgeons (PS, JPL). The preoperative examination included evaluation of hip ROM. Flexion of less than 90° impairs postoperative sitting ability [12]. We obtained plastic surgery consultation in patients when we anticipated problems with soft tissue coverage.
The technique of instrumentation was a modification of the original Dunn-McCarthy technique in which the rods are contoured through their lower ends and passed through the first sacral foramina to lie against the anterior cortex of the sacrum as described by Warner and Fackler [35]. The kyphectomy was performed by excision of the apical segments through a posterior approach for 19 patients, whereas two had pedicle subtraction osteotomies. The posterior elements were exposed subperiosteally. The dura was opened at the level of the resection, the spinal cord was divided (one patient did not have spinal cord transection) and allowed to retract proximally, hemostasis was obtained, and the dura was repaired. Cord transection facilitates bony work, provides greater area for bone grafting, and removes the sac from danger of perforation. However, substantial blood loss resulting from epidural bleeding during cord resection should be anticipated [12]. We then performed circumferential subperiosteal dissection of the spine. The spine was osteotomized at the apex of the deformity, and the amount of bone to be resected was gauged by overlapping the proximal and distal segments. Rods were then contoured appropriately and passed through the S1 foramina to lie along the anterior aspect of the sacrum as described by Warner and Fackler [35]. The spine was then extended by levering the proximal end of the contoured rods anteriorly to obtain correction of the kyphosis and compression between the proximal and distal segments. Further segmental fixation to the spine along the rod was achieved with sublaminar wires, hooks, and pedicle screws (DePuy Spine, Raynham, MA) as needed. Close attention was paid to wound closure with muscle closed over the spine and instrumentation in all cases. A variety of techniques were used in this series, including mobilization of the paraspinal muscles (eight), latissimus flap (seven), primary closure (three), thoracolumbar rotational flap (two), and gluteus flap (one). Autogenous bone graft was used in all patients and supplemented with allograft bone in 15 patients (71%). The average estimated blood loss was 1358 mL (range, 400–4000 mL). One surgery was aborted as a result of hemodynamic and respiratory compromise. The surgery was completed 3 months later without complication.
All patients were fitted for thoracolumbosacral orthoses postoperatively, and resumption of activities was allowed as tolerated. Patients were allowed to be bed to chair with close supervision by physical therapy. Appropriate instructions were given in regard to proper techniques of patient transfer. Wheelchair modification and pressure mapping were performed postoperatively when the patients were able to sit upright. Close attention was given to the patient’s nutrition.
After their discharge to home, patients were subsequently seen in the outpatient clinic within 2 weeks to check their incision healing. After this visit, they were seen at 6 weeks and at 3, 6, and 12 months with radiographs of the spine.
One surgeon (CF) measured the kyphosis using the Cobb angle on sitting lateral radiographs taken with the patient out of postoperative braces when possible. Measurements were made using the immediate pre- and postoperative radiographs and the most recent radiographs available. Measurement of the kyphosis can be difficult as a result of variable factors [32]. Shoulder girdle, chest, ribs, and intraabdominal contents can all overshadow the outline of the individual vertebrae and make it difficult to select the landmarks. The interobserver and intraobserver variability values of 3.3º (interobserver; 95% confidence interval, ± 7º) and 4.3º (intraobserver; 95% confidence interval, ± 9.6º) have been reported [5, 32]. However, neither the absolute values of the kyphotic deformity nor these levels of variability in the radiographic measurement of the kyphosis through clinic followups were used as major indicators for the surgery. The major indications for surgery were skin breakdown and/or difficulties in sitting and posture.
The achievement of spinal fusion in our study was based on the assessment of the plain radiographs and the presence of solid trabecular bony bridging. We acknowledge plain radiographs have some shortcomings for spine fusion determination although they are the most widely used technique in the daily clinic. The study of Blumenthal and Gill [4] demonstrated the overall agreement between the radiographic assessment of lumbar spine fusion and the actual surgical findings was 69% with an overall false-positive rate of 42% and false-negative rate of 29%. Hamill and Simmons [10] reported only fair reliability in terms of interobserver agreement to grading of lumbar fusion status based on plain radiographs. Christensen et al. [7] studied the influence of the instrumentation on the assessment of spine fusion on plain radiographs and showed the instrumentation did not influence the reproducibility but led to a slight underestimation of fusion rates. Clinical assessment of the patients, the presence of complaints such as pain, increased deformity, and radiographic evidence of implant failure are all important factors, in addition to plain radiographs, that help to determine the presence of a successful spine fusion versus pseudarthrosis. All patients with an established or impending pseudarthrosis were worked up further with tomograms.
Results
Presumed spinal fusion was achieved at final followup in all 21 patients. Five patients developed a pseudarthrosis, and all five needed revision surgery. Three patients had implant failure, progression of the kyphotic deformity, and pain on clinical examination. Pseudarthrosis occurred at the thoracolumbar junction in two of the patients and at the lumbosacral junction in one; fusion was achieved with one revision surgery in each patient. In addition to bone grafting, additional procedures were performed, including revision of the rods and plating and anterior spinal fusion. On average, there was no loss of correction in these three patients at final followup (range, −6° to +6°). At 44 months and 30 months after the initial surgery, two additional patients needed supplemental anterior interbody fusion for worsening of the kyphotic deformity in the absence of hardware failure. Their radiographs and tomograms showed lack of anterior fusion despite the presence of posterior fusion. The goals of the procedure were prevention of implant failure and collapse and worsening of the deformity. Both of these patients went on to a solid circumferential fusion. One patient, however, lost 20° of correction at final followup, whereas the other patient had no further loss of correction. There were four more patients with implant failure. They all remained asymptomatic, and no intervention was performed. These patients did not show any loss of correction through their last followup (Fig. 1).
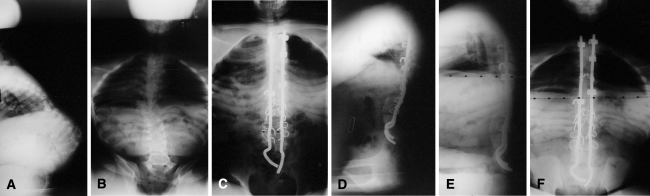
Fig. 1A–F.
A series of radiographs of a 13-year-old girl is shown. (A) The radiograph shows the preoperative sitting thoracolumbar spine. (B) The preoperative sitting posteroanterior thoracolumbar spine radiograph is shown. (C) The postoperative sitting posteroanterior thoracolumbar spine radiograph is shown. (D) The postoperative sitting lateral thoracolumbar spine radiograph is shown. (E) This is the same patient at the 7-year followup. The lateral thoracolumbar spine radiograph is shown with one rod broken, but correction is maintained. (F) A posteroanterior sitting radiograph is shown.
Kyphosis in the initial postoperative sitting radiographs measured 22° (range, 4°–70°), and the average correction was 82% (range, 61%–98%). At final followup, kyphosis measured 22° (range, 3°–55°) with an average correction of 81% (range, 59%–98%). The six patients with kyphoscoliosis obtained an average final correction of 82% (range, 59%–98%) compared with 81% in the patients with only kyphosis (range, 52%–98%). In the five patients who had secondary surgery for established pseudarthrosis and/or potential pseudarthrosis, the average progression from initial postoperative kyphosis to kyphosis at final followup was 3° (range, −6°–21°).
We identified 17 perioperative complications (Table 2), and 11 secondary surgeries were performed in 10 patients. One death occurred secondary to intraoperative complications of hemodynamic instability and excessive bleeding. Wound and skin complications were the most common. Superficial wound breakdown that healed with local wound care occurred in five patients. One patient who had a surgical wound breakdown had been treated with a latissimus flap 11 days after the index procedure. Five of the six patients with postoperative wound breakdown had a history of skin problems over the kyphotic deformity preoperatively. One deep infection was diagnosed. The patient presented with a draining sinus, and débridement without hardware removal was performed at 10 months postoperatively. The sinus recurred, and repeat débridement with removal of hardware was performed at 4 years postoperatively. Spinal fusion had been obtained, and sagittal alignment was maintained at final followup 18 months after removal of all hardware.
Table 2.
Perioperative complications
| Complication | Frequency | Resulting care |
|---|---|---|
| Death | 1 | Intraoperative hemodynamic complications |
| Shunt failure | 1 | Shunt revision |
| Infection | 1 | Irrigation and débridement with subsequent removal of implant |
| Wound breakdown | 6 | Local wound care with subsequent healing; gluteal flap (1) |
| Implant failure | 7 | Revision (3) |
| Pseudarthrosis | 5 | Repeat surgery |
| Pleural tear | 2 | Chest tube placement |
| Seroma | 1 | Surgical drainage |
| Excessive blood loss | 1 | Surgery abandoned; repeat surgery required |
| Deep vein thrombosis | 1 | Anticoagulant therapy |
Discussion
Kyphosis associated with myelomeningocele presents a complex treatment problem for the orthopaedic surgeon. The kyphosis is universally progressive, compromising patient sitting balance causing compensatory spinal deformities and increasing the risk of skin breakdown overlying the deformity. Surgical treatment is not without substantial risk; however, surgical technique and its execution play a major role in the outcome of the surgery [8, 11, 12, 15, 16, 18, 21–24, 27–30, 33–35]. In this study, we determined the fusion rates, deformity correction and its maintenance, and perioperative complications after a long segmental spinal fixation down to the pelvis using the method of Warner and Fackler [35] along with kyphectomy.
We acknowledge limitations to our study. Although average followup in our study was longer compared with previous reports, it would be ideal to follow up these patients into their skeletal maturity and young adulthood. The detail and depth of information available for each patient were dependent on the circumstances at the time it was collected given the retrospective nature of our study. The statement of the presence of successful fusion was based on plain radiographs and was not verified by other imaging studies. Spinal fusion assessment based on plain radiographs may have high false-positive and false-negative rates [4, 10] and the presence of the implant may further complicate the assessment process with underestimation of fusion rates [7]. Despite this, plain radiographs are still the most widely used technique in the daily clinic setting and in the literature in regard to the assessment of spinal fusion. Radiographic measurements of the deformity and its values were never used as the absolute indication for the surgery in our study. Clinical assessment played a vital role in addition to plain radiographs in the decision-making process.
Presumed fusion was achieved at final followup in all 21 patients. This is consistent with the literature (Table 1). We had more pseudarthrosis (five cases) compared with the literature. Three of these patients had implant failure, whereas two did not. The average time from index surgery to revision was 3.9 years. One possible explanation for the high incidence could be relatively longer followup. In addition to our patients with pseudarthrosis and implant failure, we had four additional patients with implant failure that did not require any further intervention and remained stable with no loss of correction. It is possible these patients had undetected radiographic subtle pseudarthrosis [13], but none of these patients were further worked up with other imaging studies to verify this. We do not believe asymptomatic patients with no loss of correction require revision. Akbar et al. [1] reported seven implant failures. Four patients had rod breakage, whereas three patients had their rods penetrated through the sacrum. Four of the seven patients required revisions. They concluded the residual deformity after the index surgery was the reason for these implant failures. They did not make any comments on the presence of pseudarthrosis. Warner and Fackler [35] suggested the improved amount of correction achieved with segmental fixation and distal pelvic fixation could be a reason for the higher fusion rate and the decrease in recurrence rate. They also suggested the kyphectomy should be performed before the compensatory lordosis progresses.
Our results compare favorably to previously published studies in terms of overall correction and maintenance of correction (Table 1). The long-term study of Lintner and Lindseth [19] on kyphectomy with sublaminar wiring demonstrates how limited fixation may lead to loss of correction over a period of time. Thirty-four of 39 patients had partial loss of correction, with 25 patients maintaining at least 50% of the initial correction, whereas four patients went on to have a second surgery because of the progression of the kyphosis. The reports of segmental spinal fixation and distal pelvic anchoring anterior to the sacrum proved excellent correction and maintenance of the kyphotic deformity are possible [1, 11, 12, 22, 23, 35]. The distal fixation anterior to the sacrum improves the moment arm of the construct, providing more resistance against progressive kyphosis. In our study, we had one patient with rod breakage proximally and the lower end of the rod penetrating through the sacrum posteriorly without any loss of correction. Akbar et al. [1] had three patients with rod penetration through the sacrum, all requiring revision. They questioned the reliability of Warner and Fackler pelvic fixation and brought up the consideration of different sacral anchoring techniques.
Overall, the nature of our perioperative complications was consistent with the literature. One death occurred secondary to intraoperative complications of excessive bleeding and hemodynamic instability [1, 22]. Wound complications were the most frequent in our series, yet very few major sequelae developed from the skin problems. One patient had a latissimus flap 11 days after the index procedure because of wound dehiscence and risk of implant exposure. Overall, our rate of skin complications was consistent with previous reports using similar techniques [1, 22, 33–35]. The deep infection rate is surprisingly low not only in our series, but also in the literature. Soft tissue coverage over the implant plays a crucial role in the overall success of the procedure and well-being of the patient.
Kyphosis is a major problem in myelomeningocele with regard to the integrity of the soft tissues of the spine and the ability of the patient to maintain a functional sitting position without the use of the upper extremities for balance. Surgical treatment of myelomeningocele kyphosis is technically demanding and carries substantial risk. Kyphectomy and posterior spinal fusion and instrumentation with sacral fixation using the Warner and Fackler [35] technique can provide excellent ability to correct and maintain sagittal alignment.
Acknowledgment
We thank John P. Lubicky, MD, former chief of staff at Shriners Hospital for Children–Chicago and one of the two senior surgeons.
Footnotes
One of the authors (PS) is a consultant for DePuy Spine (Raynham, MA) and Pioneer Surgical Technology (Marquette, MI) and a stockholder/shareholder for Pioneer Surgical Technology.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Akbar M, Bremer R, Thomsen M, Carstens C, Abel R. Kyphectomy in children with myelodysplasia: results 1994–2004. Spine (Phila Pa 1976) 2006;31:1007–1013. doi: 10.1097/01.brs.0000215018.14774.0f. [DOI] [PubMed] [Google Scholar]
- 2.Banta JV. Combined anterior and posterior fusion for spinal deformity in myelomeningocele. Spine (Phila Pa 1976) 1990;15:946–952. doi: 10.1097/00007632-199009000-00020. [DOI] [PubMed] [Google Scholar]
- 3.Banta JV, Hama JS. Natural history of the kyphotic deformity in myelomeningocele. J Bone Joint Surg Am. 1976;55:279. [Google Scholar]
- 4.Blumenthal SL, Gill K. Can lumbar spine radiographs accurately determine fusion in postoperative patients? Correlation of routine radiographs with a second look at lumbar fusions. Spine (Phila Pa 1976) 1993;18:1186–1189. doi: 10.1097/00007632-199307000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Carman DL, Browne RH, Birch JG. Measurements of scoliosis and kyphosis radiographs: interobserver and intraobserver variation. J Bone Joint Surg Am. 1990;72:328–333. [PubMed] [Google Scholar]
- 6.Carstens C, Koch H, Brocal DR, Niethard FU. Development of pathological lumbar kyphosis in myelomeningocele. J Bone Joint Surg Br. 1996;78:945–950. doi: 10.1302/0301-620X78B6.1272. [DOI] [PubMed] [Google Scholar]
- 7.Christensen FB, Laursen M, Gelineck J, Eiskjaer SP, Thomsen K, Bunger CE. Interobserver and intraobserver agreement of radiograph interpretation with and without pedicle screw implants: the need for a detailed classification system in posterolateral spinal fusion. Spine (Phila Pa 1976) 2001;26:538–544. doi: 10.1097/00007632-200103010-00018. [DOI] [PubMed] [Google Scholar]
- 8.Christofersen MR, Brooks AL. Excision and wire fixation of rigid myelomeningocele kyphosis. J Pediatr Orthop. 1985;5:691–696. doi: 10.1097/01241398-198511000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Doers T, Walker JL, Brink KD, Stevens DB, Heavilon J. The progression of untreated lumbar kyphosis and the compensatory thoracic lordosis in myelomeningocele. Dev Med Child Neurol. 1997;39:326–330. [PubMed] [Google Scholar]
- 10.Hamill CL, Simmons ED. Interobserver variability in grading lumbar fusions. J Spinal Disord. 1997;10:387–390. doi: 10.1097/00002517-199710000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Heydemann JS, Gillespie R. Management of myelomeningocele kyphosis in the older child by kyphectomy and segmental spinal instrumentation. Spine (Phila Pa 1976) 1987;12:37–41. doi: 10.1097/00007632-198701000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Huang TJ, Lubicky JP. Kyphectomy and segmental instrumentation in young children with myelomeningocele kyphosis. J Formos Med Assoc. 1994;93:503–508. [PubMed] [Google Scholar]
- 13.Johnson JTH, Southwick WO. Bone growth after spine fusion: A clinical survey. J Bone Joint Surg Am. 1960;42:1396–1412. [Google Scholar]
- 14.Kadic MA, Verbout AJ. Treatment of severe kyphosis in myelomeningocele by segmental spinal instrumentation with Luque rods. Acta Orthop Belg. 1991;57:45–51. [PubMed] [Google Scholar]
- 15.Ko AL, Song K, Ellenbogen RG, Avellino AM. Retrospective review of multilevel spinal fusion combined with spinal cord transection for treatment of kyphoscoliosis in pediatric myelomeningocele patients. Spine (Phila Pa 1976) 2007;32:2493–2501. doi: 10.1097/BRS.0b013e3181573b11. [DOI] [PubMed] [Google Scholar]
- 16.Leatherman KD, Dickson RA. Congenital kyphosis in myelomeningocele: vertebral body resection and posterior spine fusion. Spine (Phila Pa 1976) 1978;3:222–226. [PubMed] [Google Scholar]
- 17.Lindseth RE. Spine deformity in myelomeningocele. Instr Course Lect. 1991;40:273–279. [PubMed] [Google Scholar]
- 18.Lindseth RE, Stelzer L. Vertebral excision for kyphosis in children with myelomeningocele. J Bone Joint Surg Am. 1979;61:699–704. [PubMed] [Google Scholar]
- 19.Lintner SA, Lindseth RE. Kyphotic deformity in patients who have myelomeningocele: operative treatment and long-term follow-up. J Bone Joint Surg Am. 1994;76:1301–1307. doi: 10.2106/00004623-199409000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Majd ME, Richey JL, Holt RT. Treatment of spinal deformities in myelodysplasia: lumbar kyphectomy. Spine: State of the Art Reviews. 1998;12:91–102. [Google Scholar]
- 21.Martin J, Jr, Kumar SJ, Guille JT, Ger D, Gibbs M. Congenital kyphosis in myelomeningocele: results following operative and nonoperative treatment. J Pediatr Orthop. 1994;14:323–328. doi: 10.1097/01241398-199405000-00009. [DOI] [PubMed] [Google Scholar]
- 22.McCall R. Modified Luque instrumentation after myelomeningocele kyphectomy. Spine (Phila Pa 1976) 1998;23:1406–1411. doi: 10.1097/00007632-199806150-00020. [DOI] [PubMed] [Google Scholar]
- 23.McCarthy RE, Dunn H, McCullough FL. Luque fixation to the sacral ala using the Dunn-McCarthy modification. Spine (Phila Pa 1976) 1989;14:281–283. doi: 10.1097/00007632-198903000-00007. [DOI] [PubMed] [Google Scholar]
- 24.McMaster MJ. The long-term results of kyphectomy and spinal stabilization in children with myelomeningocele. Spine (Phila Pa 1976) 1988;13:417–424. doi: 10.1097/00007632-198804000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Mintz LJ, Sarwark JF, Dias LS, Schafer MF. The natural history of congenital kyphosis in myelomeningocele: a review of 51 children. Spine (Phila Pa 1976) 1991;16:S348–S350. [PubMed] [Google Scholar]
- 26.Niall DM, Dowling FE, Fogarty EE, Moore DP, Goldberg C. Kyphectomy in children with myelomeningocele: a long-term outcome study. J Pediatr Orthop. 2004;24:37–44. doi: 10.1097/01241398-200401000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Nolden MT, Sarwark JF, Vora A, Grayhack JJ. A kyphectomy technique with reduced perioperative morbidity for myelomeningocele kyphosis. Spine (Phila Pa 1976) 2002;27:1807–1813. doi: 10.1097/00007632-200208150-00022. [DOI] [PubMed] [Google Scholar]
- 28.Sarwark JF. Kyphosis deformity in myelomeningocele. Orthop Clin North Am. 1999;30:451–455. doi: 10.1016/S0030-5898(05)70097-3. [DOI] [PubMed] [Google Scholar]
- 29.Sharrard WJ. Spinal osteotomy for congenital kyphosis in myelomeningocele. J Bone Joint Surg Br. 1968;50:466–471. [PubMed] [Google Scholar]
- 30.Sharrard WJ, Drennan JC. Osteotomy-excision of the spine for lumbar kyphosis in older children with myelomeningocele. J Bone Joint Surg Br. 1972;54:50–60. [PubMed] [Google Scholar]
- 31.Sriram K, Babchenko WP, Hall JE. Surgical management of spinal deformities in spina bifida. J Bone Joint Surg Br. 1972;54:666–676. [PubMed] [Google Scholar]
- 32.Stotts AK, Smith JT, Santora SD, Roach JW, D’Astous JL. Measurement of spinal kyphosis: implications for the management of Scheuermann`s kyphosis. Spine (Phila Pa 1976) 2002;27:2143–2146. doi: 10.1097/00007632-200210010-00013. [DOI] [PubMed] [Google Scholar]
- 33.Thomsen MD, Lang RD, Carstens C. Results of kyphectomy with the technique of Warner and Fackler in children with myelodysplasia. J Pediatr Orthop B. 2000;9:143–147. doi: 10.1097/01202412-200006000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Torode I, Godette G. Surgical correction of congenital kyphosis in myelomeningocele. J Pediatr Orthop. 1995;15:202–205. [PubMed] [Google Scholar]
- 35.Warner WC, Fackler CD. Comparison of two instrumentation techniques in treatment of lumbar kyphosis in myelodysplasia. J Pediatr Orthop. 1993;13:704–708. doi: 10.1097/01241398-199311000-00002. [DOI] [PubMed] [Google Scholar]