Abstract
Background
Spine and chest wall deformities in children with early onset scoliosis (EOS) frequently impair respiratory function and postnatal growth of the lung. While a relationship between deformity and such impairment has been reported in children with adolescent idiopathic scoliosis it is not well understood in children with early-onset scoliosis (EOS).
Questions/purposes
We therefore describe (1) the preoperative relation between Cobb angle and forced vital capacity (FVC) in infants with EOS; (2) how changes in Cobb angle before and after surgery relate to changes in lung ventilation and perfusion in the right and left lungs.
Methods
We measured FVC in 10 children with EOS < 3 years old using the raised volume rapid thoracic compression (RVRTC) technique and correlated them with Cobb angles. We then measured right lung contributions to total lung ventilation and perfusion using lung scans before and 4 to 57 months after placement of vertical expandable prosthetic titanium ribs (VEPTRs) in 15 children with EOS and correlated changes in right lung function with postoperative changes in Cobb angles.
Results
In children 4 to 57 months of age, preoperative FVC (mean value, 83%; range, 63%–109% of predicted values) did not correlate with Cobb angles (mean value, 56º; range, 14°–120º). In children 1.8 to 11.5 years old, right lung ventilation and perfusion were abnormal in eight and seven children, respectively, but neither ventilation nor perfusion predictably normalized despite reductions in Cobb angle postoperatively.
Conclusions
The data extend the age range of children with EOS whose Cobb angles correlate poorly with FVC preoperatively. The data are also consistent with reports that reduced Cobb angles after VEPTR insertion do not correlate with postoperative changes in respiratory function.
Introduction
Thoracic insufficiency syndrome (TIS) arises from spine and chest wall deformities that impair respiratory function and postnatal growth of the lung [1]. Early-onset scoliosis (EOS) with or without underlying neuromuscular weakness is one form of TIS that occurs in infants and young children. The structural changes of the spine and chest wall associated with EOS include scoliosis, kyphosis, lordosis, spine rotation, spine intrusion into the thorax, and chest wall distortion, eg, rib hump. Each of these features can vary regarding site, length of the deformity, and the rate of progression. Abnormal rib configuration such as fused ribs may also influence thoracic size and rigidity [3]. In addition, loss of flexibility of the spine and costovertebral joints can further impair respiratory mechanics. These structural changes are often quantified individually, but they have not been integrated together in a way that accurately reflects loss of respiratory function.
The concept of TIS prompted the development of surgical constructs that can be implanted in very young children and serially expanded to stabilize the spine and chest wall and promote spine and thoracic growth over time [2]. The device initially approved by the Food and Drug Administration in 2005 for use in TIS was the vertical expandable prosthetic titanium rib (VEPTR) [3]. Additional surgical techniques such as spine-based “growing” rods and growth guidance methods are also used for EOS [12]. New surgical options for young children have generated interest in how and when EOS affects lung function in early childhood and how these new procedures subsequently impact respiratory function. Respiratory outcomes up to 3 years after spine and chest wall surgery have been reported in two case series [6, 7]. These studies suggest absolute lung volume in milliliters improved over time but that lung volume as a percent of predicted values was unchanged or slightly worse than preoperative values [6, 7].
The lung function most often measured in patients with adolescent idiopathic scoliosis (AIS) is the forced vital capacity (FVC), the volume of air a patient can exhale after a full inspiratory effort. FVC among adolescents with AIS correlates moderately well with the curvature of the spine measured by the Cobb angle [8]. Loss of FVC is also a feature associated with EOS among children who are able to perform spirometry [4]. However, the relationship between Cobb angle and vital capacity in children with EOS has not been described as well as in AIS.
We describe (1) the preoperative relation between Cobb angle and FVC in infants with EOS using a validated lung function testing procedure for infants; (2) how the changes in Cobb angle before and after surgical correction of scoliosis relate to changes in distribution of lung ventilation and perfusion in the right and left lungs; and then (3) place these findings in the context of previously reported structure-respiratory function relations in children with EOS.
Patients and Methods
Study 1: Infant Lung Function Tests and Cobb Angle—Ten infants and young children (ages 4–57 months; mean age, 25 months) were referred to Children’s Hospital of Philadelphia and enrolled in this study as part of their preoperative evaluation of EOS. There were three girls and seven boys. There were four children with congenital scoliosis, three with infantile scoliosis, and one each with neuromuscular weakness, flail chest resulting from absent ribs, and Goldenhar syndrome, respectively.
All subjects were clinically well and none had a history of a respiratory infection within the 4 weeks before testing. Informed consent was obtained from parents of all participants using Institutional Review Board-approved forms. All infants were sedated with oral chloral hydrate and placed in an infant body plethysmograph (Nspire® Infant Pulmonary Lab, Longmont, CO) wearing an inflatable jacket surrounding their chest and abdomen. A tight-fitting face mask was placed on the nose and mouth to inflate the lungs with 25 cm H2O pressure. After passive lung inflation, the body jacket was rapidly inflated with air using pressures up to 50 cm H2O to produce maximal passive exhalation. Exhaled flow and volume were quantified using a pneumotachograph attached to the mask. The curve with the best FVC and forced expiratory volume at 1 second was recorded for correlation with each child’s Cobb angle measured from spine radiographs obtained within 2 months of the lung function measurements.
FVC values are described by z scores from predicted normal values to account for variations in confidence ranges that change with age for normal values. Correlations were calculated using Pearson’s correlation method.
Study 2: Changes in Regional Lung Function and Cobb Angles After Surgical Insertion of VEPTRs for EOS—We retrospectively reviewed the medical records of 15 children with EOS who underwent ventilation and lung perfusion scans before and an average of 13 ± 5 months after VETPR insertion at Seattle Children’s Hospital. The indication for surgery was progressive scoliosis based on serial physical examinations and spine radiographs; we had no specific contraindications for these patients. Six children had congenital scoliosis, two had infantile idiopathic scoliosis, two had arthrogryposis, four had undefined multiorgan syndromes with associated neuromuscular weakness, and one had a chest wall deformity after fibrosarcoma resection. The average age was 20 to 125 months (mean, 67 months) at the time of surgery. The time of followup reassessment postoperatively ranged from 4 to 57 months (mean, 13 months). No patients were lost to followup. Studies used to evaluate VETPR therapy were approved through the Seattle Children’s Hospital Institutional Review Board and informed consent for studies was provided by all families.
Ten children underwent bilateral VETPRS insertions; five children had unilateral VETPR placement. VETPRs were placed rib to spine in 11 children, rib to rib in three patients, and rib to pelvis in one child. Only one child who had multiple rib fusions had an opening wedge thoracostomy. Spine anchors were two- or three-level pedicle screw constructs used in an off-label non-Food and Drug Administration-approved manner. Proximal rib anchors were to two or three ribs. All were completed through a midline approach. No chest tubes nor bracing were used postoperatively. Passage of the preassembled hybrid VEPTR device was done using a ventriculoperitoneal shunt passer and chest tube in the subfascial plane. Three children had tracheostomies and were receiving positive pressure ventilation pre- and postoperatively.
Followup care included followup outpatient assessments on average every 3 months for the first year and expansions were performed every 6 months. Spirometry is measured in children older than 5 years of age every other visit. Lung scans are performed 1 year after surgery unless clinical changes prompt earlier assessments.
Lung perfusion scans were performed in all 15 patients using intravenous technetium-labeled albumin microaggregates and ventilation scans were performed in 14 patients using inhaled radiolabeled xenon. Both scans were conducted in children lying supine and breathing normally at rest, similar to the position and breathing pattern during the CT scans.
Changes in the relative contributions of the right lung to total ventilation and total lung perfusion before and after surgery were correlated with changes in Cobb angles before and after surgery using Pearson’s correlation coefficients.
Results
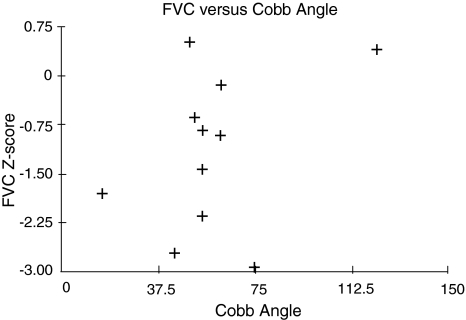
Study 1: FVC values ranged between 63% and 109% of predicted values. Five of 10 had FVC values greater than 1 Z score below predicted normal values reflecting underlying restrictive lung disease as a result of chest wall deformities (Fig. 1). Cobb angles ranged between 25° and 113º (mean value, 62º). The correlation coefficient (r) between vital capacity and Cobb angle for the groups was 0.4 (p = 0.91) (Fig. 1).
Fig. 1.
Correlations are shown between forced vital capacity (FVC) measured by the raised volume rapid thoracic compression (RVRTC) method in young children and Cobb angles before surgery. FVC is described by Z scores to account for variable confidence intervals in data of normal children in this age range.
Study 2: Right lung ventilation ranged between 21% and 76% of total ventilation and right lung perfusion ranged between 48% and 75% of total lung perfusion preoperatively. Eight and seven children had more than 5% deviation from the normal 55% right/45% left lung distribution of ventilation and perfusion, respectively, before surgery. The directional changes in lung function toward normal right/left lung distributions postoperatively were inconsistent for the group. Of the eight children with preoperative asymmetric ventilation, three had more symmetric ventilation and five had the same degree of lung function asymmetry or worse postoperatively. Of the seven with normal regional ventilation preoperatively, three remained normal but four children developed asymmetric ventilation postoperatively. In contrast, Cobb angles were reduced in 14 of 15 children from 59° to 130º (mean, 84º) preoperatively to a mean of −64º (range, 51°–82º) postoperatively. There was no correlation between changes in Cobb angle and changes in lung function asymmetry after surgery (r value for directional change = 0.18, p = 0.45).
Discussion
Children with EOS can experience progression of the spine and chest wall deformity as they grow. One goal of inserting the VEPTR and other devices is to halt the progressive thoracic and spine deformity to halt respiratory decline and perhaps improve respiratory function. Therefore, measuring lung functions in children with EOS before and after surgical therapy is an integral part of the overall evaluation process. However, when lacking pulmonary diagnostic services, structural features alone are used to make decisions about surgery. The key question is whether Cobb angle, as the most commonly used index of scoliosis severity, can suffice as a surrogate for respiratory function abnormalities as it has in patients with AIS [8]. We describe (1) the preoperative relation between Cobb angle and FVC in infants with EOS using a validated lung function testing procedure for infants; (2) how the changes in Cobb angle before and after surgical correction of scoliosis relate to changes in distribution of lung ventilation and perfusion in the right and left lungs; and then (3) place these findings in the context of previously reported structure-respiratory function relations in children with EOS.
There are several limitations to the data presented in this report. First, most studies of structure and function, including ours, have been conducted on small numbers of patients and changes in both structure and function after surgery have been derived from only two time points. There are no published data on serial changes in structure and respiratory function over time during the preoperative period to determine how much change occurs in both domains as spine deformities worsen. This is important because halting progression of respiratory decline may be the primary outcome that is achieved with current surgical methods. Serial measurements both pre- and postoperatively are limited to small case series that do not describe both structure and functional features [6, 7]. The patient population is sufficiently small that multicenter studies are needed to address these knowledge gaps. Second, there are multiple ways to assess respiratory function. There are little data on the relationships among functional respiratory outcomes to one another. There is a need to standardize the ways that children with EOS are assessed using the most specific, sensitive, and predictive methods available for all age groups. This will require multidisciplinary collaboration among centers that care for this group of children.
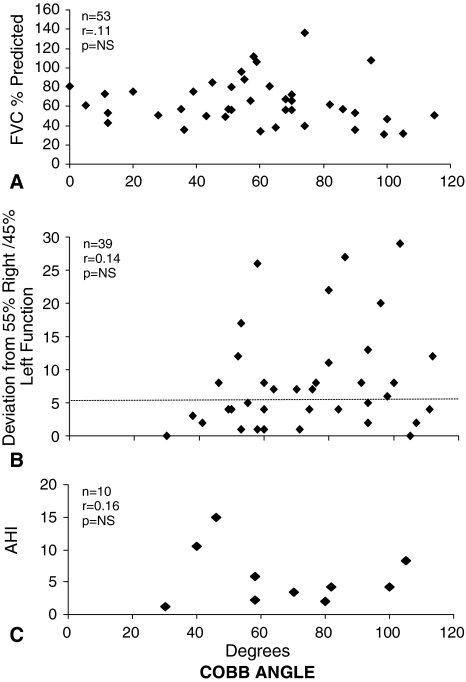
With these limitations in mind, the data are consistent with previously published reports correlating structure and respiratory function in children with EOS. In a previous report of 24 children with congenital scoliosis studied preoperatively, their vital capacity values (ranging from 13% to 68% of predicted normal values) and their Cobb angles (range, 27º–150º) correlated poorly with an r value of 0.5 [9]. More recently, Mayer and Redding reported a poor correlation between the preoperative vital capacities and the Cobb angles of 53 children 5 to 15 years of age with EOS identified from seven collaborating spine centers (r = 0.11) [4] (Fig. 2A). Using lung scans, Redding et al. reported a poor correlation (r = −0.14) between lung function asymmetry (right versus left lung contributions to total ventilation and perfusion) and preoperative Cobb angles (range, 30°–112º) in 39 children with EOS, ages 20 to 186 months of age, with congenital or infantile scoliosis [10] (Fig. 2B).
Fig. 2A–C.
Correlations are poor among (A) forced vital capacity (FVC), (B) lung function asymmetry based on lung perfusion scans, and (C) apnea-hypopnea index (AHI) during sleep with Cobb angle in children with early-onset scoliosis [4, 10, 11]. Reprinted with permission from Redding G, Song K, Inscore S, Effmann E, Campbell R. Lung function asymmetry in children with congenital and infantile scoliosis. Spine. 2008;8:639–644. Copyright © Elsevier.
Lung function has also been assessed by evaluating breathing during sleep in children with EOS. The most common abnormality that was described in children with EOS during sleep is an elevated apnea-hypopnea index (AHI), which includes the number of apneas or hypopneas per hour that occur during sleep. Normally the AHI is higher in rapid eye movement (REM) sleep compared with other sleep stages [5]. Among 13 children with EOS studied preoperatively, Cobb angles ranged from 30º to 105º; the overall AHI (normal less than one event/hour) was elevated to four/hour throughout the night but 17/hour specifically during REM sleep [11]. The AHI did not correlate with Cobb angle (r = 0.16) (Fig. 2C).
The data from our second study are consistent with those in a previous report suggesting changes in lung function after surgery do not correlate with changes in Cobb angles postoperatively. In a series of 40 children who underwent VEPTR insertion for EOS, the mean Cobb angle was reduced from 58° ± 4.5° to 46° ± 4°, whereas FVC, expressed as a percent predicted, fell from 61% ± 4% to 54% ± 3% [4]. The correlation coefficient between change in Cobb angle and postoperative change in FVC was poor (r = −0.11). Of interest, measures of lung volumes before and after VEPTR treatment demonstrated an increase in residual volume, ie, the volume residing in the chest after a vital capacity maneuver, but no increase in vital capacity [4]. The pre- to postoperative comparisons were limited to older children who could perform spirometry and postoperative lung function measurements were conducted before the first VEPTR expansion and therefore represent short-term results.
Our observations demonstrate the Cobb angle, however convenient, does not correlate with respiratory functions awake or asleep in children with EOS [4, 10, 11]. Although one study reported a relationship between vital capacity and Cobb angles in AIS [8], the Cobb angle alone does not accurately reflect the three-dimensional deformities of the spine and chest wall in EOS and hence the respiratory consequences. It is therefore logical to measure respiratory functions in conjunction with structural assessments and use them as complementary data in clinical decision-making and assessment of new surgical approaches.
More importantly, the respiratory impact of VETPR treatment is not reflected by the improvements in Cobb angle postoperatively [4]. FVC, for example, is dependent on both the size of the thorax and also its ability to move with respiration. An increase in intrathoracic volume and a straighter spine postoperatively do not necessarily lead to more chest wall mobility or excursion during inspiration. The increase in residual volume rather than FVC in older children undergoing VEPTR implantation suggests the lung may be initially stretched after surgery rather than immediately growing into the new intrathoracic space [4]. Whether it promotes long-term postnatal lung growth remains to be determined.
There have been apparent recent advances in the treatment options available for children with severe or progressive EOS. The respiratory features of this high-risk patient population have been described, but the prognostic importance of these findings is unknown. However, there are now fewer opportunities to study the natural history of EOS because approved procedures are available for use. There is a pressing need to assess the relative short- and long-term impact of various growth modulation and growth guidance constructs from a respiratory perspective. Standardization of preoperative and postoperative assessments using both structural and functional measures would represent an important first step in addressing these issues.
Acknowledgment
We thank Ms Holly Kaopuiki for her assistance with preparation of the manuscript.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Seattle Children’s Hospital, Seattle, WA, and Children’s Hospital at Philadelphia, Philadelphia, PA, USA.
References
- 1.Campbell R, Smith MD. Thoracic insufficiency syndrome and exotic scoliosis. J Bone Joint Surg Am. 2007;89(Suppl 1):108–122. doi: 10.2106/JBJS.F.00270. [DOI] [PubMed] [Google Scholar]
- 2.Campbell RM, Hell-Vocke AK. Growth of the thoracic spine in congenital scoliosis after expansion thoracoplasty. J Bone Joint Surg Am. 2003;85:409–420. doi: 10.2106/00004623-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Campbell RM, Jr, Smith MD, Mayes TC, Mangos JA, Willey-Courand DB, Kose N, Pinero RF, Adler ME, Duong HL, Surber JL. The effect of opening wedge thoracostomy on thoracic insufficiency syndrome associated with fused ribs and congenital scoliosis. J Bone Joint Surg Am. 2004;86:1659–1674. doi: 10.2106/00004623-200408000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Mayer OH, Redding G. Early changes in pulmonary function after vertical expandable prosthetic titanium rib insertion in children with thoracic insufficiency syndrome. J Pediatr Orthop. 2009;29:35–38. doi: 10.1097/BPO.0b013e3181929c8b. [DOI] [PubMed] [Google Scholar]
- 5.Montgomery-Downs HE, O’Brien LM, Gulliver TE, Gozal D. Polysomnographic characteristics in normal preschool and early school-aged children. Pediatrics. 2006;117:741–753. doi: 10.1542/peds.2005-1067. [DOI] [PubMed] [Google Scholar]
- 6.Motoyama EK, Deeney VF, Fine GF, Yang CI, Mutich RL, Walczak SA, Moreland MS. Effects of lung function of multiple expansion thoracoplasty in children with thoracic insufficiency syndrome: a longitudinal study. Spine. 2006;31:284–290. doi: 10.1097/01.brs.0000197203.76653.d0. [DOI] [PubMed] [Google Scholar]
- 7.Motoyama EK, Yang CI, Deeney VF. Thoracic malformation with early-onset scoliosis: effect of serial VEPTR expansion thoracoplasty on lung growth and function in children. Paediatr Respir Rev. 2009;10:12–17. doi: 10.1016/j.prrv.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Newton PO, Faro FD, Gollogly S, Betz RR, Lenke LG, Lowe TG. Results of preoperative pulmonary function testing of adolescents with idiopathic scoliosis A study of six hundred and thirty-one patients. J Bone Joint Surg Am. 2005;87:1937–1946. doi: 10.2106/JBJS.D.02209. [DOI] [PubMed] [Google Scholar]
- 9.Owange-Iraka JW, Harrison A, Warner JO. Lung function in congenital and idiopathic scoliosis. Eur J Pediatr. 1984;142:198–200. doi: 10.1007/BF00442448. [DOI] [PubMed] [Google Scholar]
- 10.Redding G, Song K, Inscore S, Effmann E, Campbell R. Lung function asymmetry in children with congenital and infantile scoliosis. Spine J. 2008;8:639–644. doi: 10.1016/j.spinee.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 11.Striegl A, Chen ML, Kifle Y, Song K, Redding G. Sleep-disordered breathing in children with thoracic insufficiency syndrome. Pediatr Pulmonol. 2010;45:469–474. doi: 10.1002/ppul.21197. [DOI] [PubMed] [Google Scholar]
- 12.Thompson GH, Akbarnia BA, Campbell R. Growing rod techniques in early-onset scoliosis. J Pediatr Orthop. 2007;27:354–361. doi: 10.1097/BPO.0b013e3180333eea. [DOI] [PubMed] [Google Scholar]