Abstract
Background
The challenge when managing early-onset scoliosis (EOS) is to prevent curve progression while maintaining spinal growth. Current surgical treatments (growing rods, VEPTR) require repetitive interventions to lengthen the implants.
Questions/purposes
We asked whether a modern Luqué trolley construct could achieve and maintain scoliosis correction in EOS during spinal growth without the need for repetitive surgeries, thus decreasing the morbidity of the surgical treatment.
Methods
We retrospectively reviewed five patients who underwent a modern Luqué trolley construct between 2003 and 2008. This construct consists of inserting apical gliding spinal anchors using muscle-sparing techniques to the proximal and distal fixed anchors found in the dual growing rod construct. We documented age at surgery, correction and maintenance of deformity, spinal growth, number of procedures, and complications.
Results
The primary curve was corrected from 60° to 21° and was maintained at 21° at a minimum followup of 2 years (mean, 4 years; range, 2–5.5 years). An average of 10 vertebrae was spanned allowing the spine to grow a mean of 3 cm over 4 years, representing a mean of 77% of the expected growth. Two of the five cases outgrew the construct requiring lengthening of rods. One patient had gradual recurrence of deformity without substantial axial growth that required revision surgery after 4 years.
Conclusion
Modifications of the Luqué trolley may be useful for managing EOS without the morbidity of repetitive surgery. However, questions such as the effect of wear debris and the risk of spontaneous fusions still remain.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Early-onset scoliosis (EOS) can lead to severe spinal deformities that hinder lung development, which is the primary morbidity associated with EOS [4, 10, 17]. Spinal fusions performed before the age of 8 can lead to a decrease in lung volume, a common limiting factor in the life expectancy of children with EOS [5]. To avoid these early fusions, new surgical techniques are emerging to address the core challenge when managing EOS: the ability to prevent curve progression while maintaining longitudinal growth of the spine [6]. Because the etiology of EOS varies, the treatment options also vary. With the exception of definitive spinal fusion, all current treatments (serial casting [7], dual growing rods (DGR) [2], and vertical expandable prosthetic titanium ribs (VEPTR) [9]) require repetitive interventions as the spine grows.
The initial management of EOS is to proceed with serial casting followed by bracing [1]. If such treatment is not feasible or not successful (malignant curve progression reaching a critical curve > 50°), then surgical management is warranted [1]. Conceptually rods are anchors to the spine, the rib, or the pelvis and are lengthened every 6 months.
To offset these recurrent interventions, we explored a new surgical technique that allows for spinal growth without repetitive surgical interventions. Luqué and Cardoso [12] described the first growing rod construct consisting of two L- or U-shaped rods fixed to the spine in a segmental fashion using sublaminar wires [11]. As the spine grew, these rods were able to “guide” the long axis of the spine, allowing for growth while maintaining spinal correction. However, the use of the Luqué trolley has been abandoned as a result of poor spinal growth maintenance (32%–49%) [13, 18], high spontaneous fusion (4%–100%) [13], and high implant failure (32%) [18]. We modified Luqué’s original idea of an internal tutor for the scoliotic spine [15, 20] by using minimally invasive approaches and newer implants in the hope of addressing some of its shortfalls.
Our purposes were to determine whether a modern Luqué construct could (1) correct and maintain spinal deformity in EOS; (2) allow for spinal growth; (3) lead to lower complication rates; and (4) whether patients require additional surgeries.
Patients and Methods
We reviewed all radiographs and charts of 17 patients with EOS who had been treated at our institution between 2003 and 2008. Twelve of these 17 patients had conventional treatment consisting of three successful Risser casts; four failed casting requiring DGR; one DGR as an initial treatment; and four VEPTRs as the initial treatment. The diagnoses were: four infantile scoliosis; three congenital scoliosis; one neuromuscular scoliosis; and four syndromic scoliosis. Their average age at the time of initiation of treatment was 4.5 years (range, 11 months to 8.5 years) with a minimum 2.5-year followup (average, 3.6 years; range, 2.5–5 years). Average Cobb angle of the major curve was 61° (range, 38°–94°), which at last followup was 35° (range, 23°–46°). In total, these 12 patients underwent 73 procedures representing on average six procedures over 3.5 years. None of these patients had thoracic insufficiency syndrome.
The remaining five patients had the modern Luqué trolley construct with the first surgery performed in 2003. Surgical indications for this procedure included: (1) large spinal deformity (Cobb greater than 40°–50°); (2) failed nonoperative treatment (curve progression greater than 10° over a 6-month interval); and (3) considerable growth potential (open triradiate, prepeak growth velocity). Associated factors that influenced the choice of this technique over the conventional techniques (DGR, VEPTR) were: unfavorable social environments for repetitive surgery, families’ reluctance to impose repetitive surgery on their child, and comorbid factors leading to a higher risk of repetitive surgery (eg, difficult airway). Contraindications for this surgery were patients with chest wall abnormalities contributing to thoracic insufficiency syndrome or patients with rigid kyphoscoliosis. The etiology of the deformities in these five patients were: two idiopathic EOS, one patient with cerebral palsy, and two with syndromes (Prader-Willi syndrome and global hypotonia of unknown etiology). With the exception of the child with cerebral palsy, all were ambulators. The average age at surgery was 6.5 years (range, 3 years 10 months to 8 years 6 months). The minimum followup was 2 years with an average followup of 4 years (range, 2–6 years). No patients were lost to followup and no patients were recalled specifically for this study.
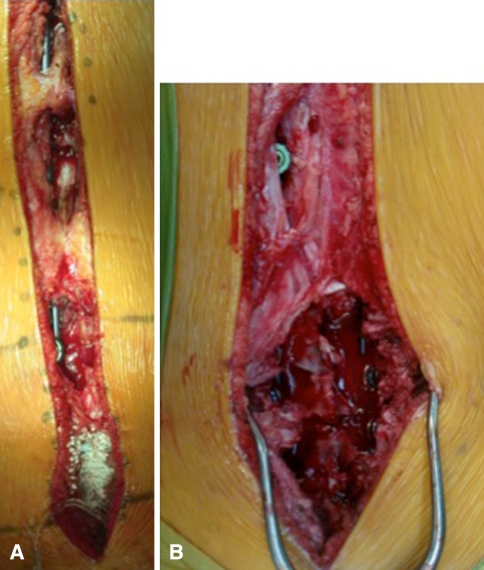
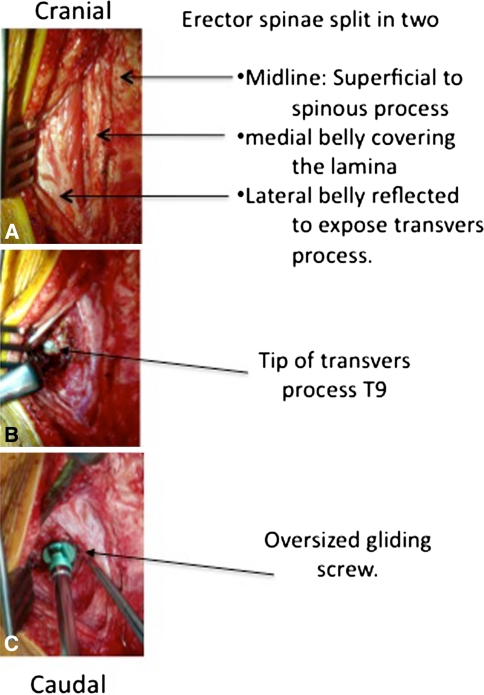
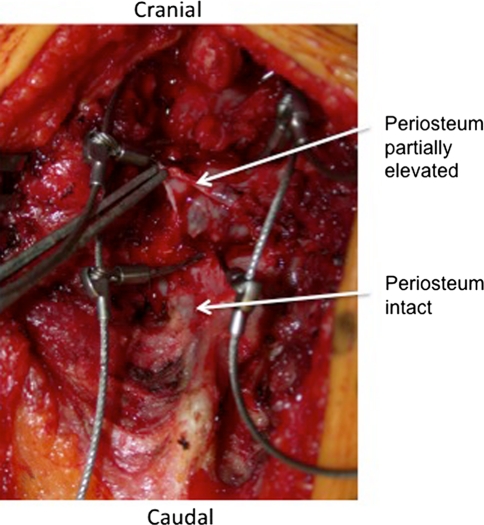
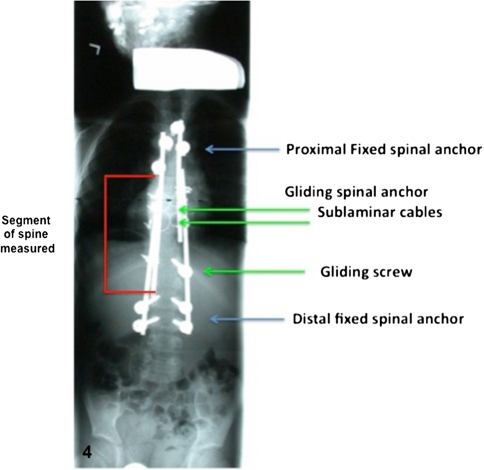
In the prone position, the modern Luqué construct involved a midline incision spanning the segment of the spine to be instrumented (we note that the instrumentation used to develop this growing rod construct was used off-label). Fixed spinal anchors (screws or hooks locked to the rods) and gliding spinal anchors (screws or sublaminar wire free to travel along the rods) were inserted through multiple “keyhole” dissections (Fig. 1A). At the fixed proximal and/or distal anchorage points, a classic subperiosteal dissection (Fig. 1B) was performed because these segments were to be fused. At the apex of the deformity, gliding anchors (either screws or sublaminar cables) were placed for maximal apical translation and deformity correction. The dissection at the gliding anchors was kept to a minimum using extraperiosteal and muscle-sparing techniques to avoid spontaneous fusion. In the lumbar spine, the gliding pedicle screws were inserted through a Wiltse approach sparing the joints and minimizing bony exposure. In the thoracic spine, the gliding pedicle screws were inserted lateral to the midline erector spinae, dissecting directly onto the transverse process and avoiding exposure of the lamina (Fig. 2A–C). Fluoroscopic guidance confirmed the pedicle entry point, and using a freehand technique, the gliding screws were inserted at strategic points allowing maximal apical translation. These gliding screws captured a 5-mm rod with a locking cap and nut designed for 6-mm rods, thus permitting motion. At segments where sublaminar titanium cables were to be passed, the dissection was carried from midline to the medial border of the facet. We were careful to leave the periosteum on the bone even with some muscle still attached. Dissection was performed with bipolar cautery and forceps at hand to control blood loss and minimize disruption of the periosteum off the bone. We carefully removed the spinous processes to avoid stripping the periosteum off the lamina while giving access to the ligamentum flavum and permitting the passage of sublaminar cables (Fig. 3). Two pairs of 5-mm titanium rods were tunneled in a subfascial/intramuscular fashion (below the fascia, above the periosteum) from the opened proximal and distal ends. Each rod had only one end rigidly anchored to the spine. In the intermediate segments, a series of gliding spinal anchors maintain the correction by keeping the rods parallel and engaged. As the spine grows, the rigidly proximal-fixed rods move away from the distally fixed rods (Fig. 4). A classic apical translation reduction maneuver was performed to correct the deformity. The number of gliding anchorage points was kept to a minimum to minimize the risk of spontaneous fusion, yet an adequate number of gliding anchors was necessary to translate the apex of the deformity toward the midline to control and correct the spinal deformity. An average of 10 vertebrae (range, 8–13) was between the fixed anchors.
Fig. 1A–B.
(A) A single midline incision is made with multiple keyhole transmuscular dissections for the gliding anchors and a subperiosteal dissection for distal and/or proximal anchor fusions. (B) Two types of dissections are demonstrated, a muscle-sparing minimally invasive approach versus a standard subperiosteal approach.
Fig. 2A–C.
A technique to insert a thoracic gliding anchor is shown. (A) To avoid spontaneous fusion, dissection is taken lateral to the midline erector spinae. (B) The transverse process is exposed. (C) Insertion of pedicle screws is shown. Gliding screws must be a low-profile implant and must be left slightly proud to keep rods of bony elements.
Fig. 3.
A technique to insert a sublaminar gliding wire is shown. Sublaminar cables are inserted through an extraperiosteal dissection. The dissection is done leaving some muscle and periosteum on the lamina. When removing the tips of the spinous process, great care must be taken not to strip the periosteum off the lamina as demonstrated with the forceps.
Fig. 4.
Classic construct of a modern Luqué trolley consisting of two proximal claws (or four proximal screws) and four distal pedicle screws as fixed anchors are shown. Two intercalated sublaminar cables are placed across the thoracic curve and one gliding screw at the apex of the lumbar curve.
Postoperatively, patients were mobilized on Day 2 to 3 and discharged home with a soft Boston brace to be worn for 12 weeks. No specific physiotherapy was initiated and return to regular activities was recommended by 6 months after surgery.
All patients were seen at a regular postoperative visits, 6 weeks, 12 weeks, and then at 6-month intervals until they reached skeletal maturity. Parents and patients were questioned about pain and limitation of activities. A detailed neurologic examination was performed at each visit. All the data were collected from the medical records and patients’ radiographs. Complications and additional procedures were recorded. As part of our standard perioperative scoliosis imaging protocol, posteroanterior and lateral radiographs of the entire spine were done with an embedded radio-opaque ruler allowing us to calibrate actual lengths.
One independent reviewer (FA) measured the Cobb angles and the spinal heights from plain radiographs, posteroanterior, and lateral scoliosis films. The interobserver error on Cobb measurement is 7.2°, whereas the intraobserver is 4.9° [18]. Spinal growth was measured from the inferior end plate of the proximal anchor to the superior end plate of the distal anchor (Fig. 4) and compared with the expected growth. Dimeglio’s maximum growth estimate of 0.1 cm per year per vertebra was used as the expected growth reference [8]. The expected growth was calculated by multiplying the number of vertebra between the proximal and distal anchors times the number of years since the initial surgery times. This 0.1 cm per vertebra per year represents the vertebral body growth during the initial peak growth of infancy from 0 to 5 years. Despite knowing that spinal growth has a bimodal peak, we decided empirically to apply it across all ages. We expect that this estimate may be inflated during the growth between 5 and 10 years but should be valid again during the second peak growth.
Results
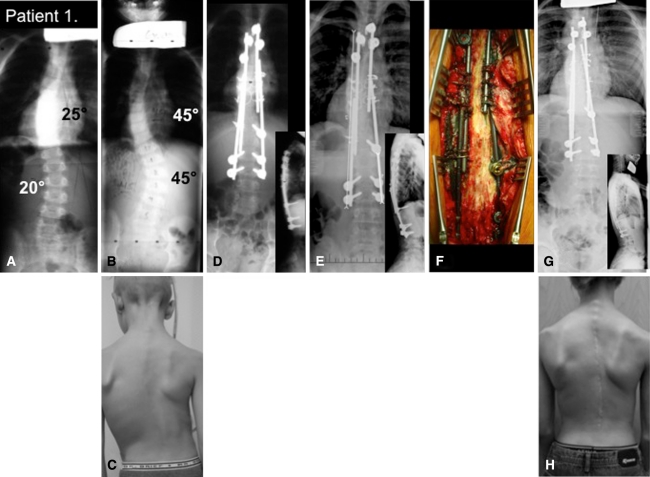
The mean preoperative Cobb angle of the primary curve was corrected from 60° (range, 45°–70°) to 21° (range, 15°–32°). Over the course of the next 4.5 years, the mean Cobb angle had gradually increased back to 31° (range, 14°–54°) as Patients 1 and 2 outgrew their initial constructs and required revision surgery. Patient 3’s Cobb angle regressed close to its initial value and demonstrated little spinal growth (Table 1). All three underwent revision surgery at 5.5, 4, and 4.5 years, respectively, after their initial surgery (Figs. 5, 6). The revision surgery resulted in improvements in the Cobb angle for all cases. Patients 4 and 5 continued to grow with no evidence of curve regression. The average Cobb angle at the last followup was back to the initial correction of 21° (range, 5°–33°) after Patients 1 through 3 underwent revision surgery (Table 2). The mean preoperative Cobb angle of the secondary curve decreased from 33° to 15° and back up to 20° before any revision surgery. At the last followup, the mean Cobb of the secondary curve also returned to the initial correction of 15°.
Table 1.
Clinical results before any revision surgery
| Patient number | Gender | Diagnosis of scoliosis | Date of surgery | Age at surgery | Age at followup | Instrumented segments | Number growing vertebrae | Curve type Lenke | 1° Cobb | 2° Cobb |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | Infantile | 10/27/2003 | 6 years 3 months | 11 years 10 months | T4-L3 | 8 (T6-L1) | 3B n | 45° | 46° |
| 2 | M | Prader Willi | 4/13/2005 | 3 years 10 months | 7 years 9 months | T4-L5 | 10 (T6-L3) | 5C + | 70° | N/A |
| 3 | F | Syndromic hypotonia | 3/28/2005 | 4 years 6 months | 8 years 9 months | T1-L5 | 13 (T3-L3) | 6C + | 70° | 49° |
| 4 | F | Cerebral palsy | 1/4/2008 | 8 years 6 months | 11 years 1 month | T2-S1 | 12 (T4-L3) | 6C + | 59° | 36° |
| 5 | F | Infantile | 3/11/2008 | 7 years 10 months | 9 years 8 months | T3-L1 | 7 (T5-T11) | 1A − | 55° | 35° |
| Average | 6.5 years | 10 | 60° | 42° |
| Patient number | Postoperative 1° Cobb | Postoperative 2° Cobb | Last followup 1° Cobb | Last followup 2° Cobb | Spinal height postoperatively | Spinal height last followup | Measure growth (cm) | Expected growth: 1 mm × number segments spanned × years | Percent of expected growth |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 17° | 7° | 23° | 17° | 16.3 | 20.4 | 4.1 | 1 mm × 8 levels × 5.5 years = 4.6 cm | 89% |
| 2 | 15° | N/A | 37° | N/A | 20.2 | 23.0 | 2.8 | 1 mm × 10 levels × 4 years = 4.0 cm | 70% |
| 3 | 32° | 25° | 54° | 38° | 26.8 | 27.5 | 0.7 | 1 mm × 13 levels × 4.3 years = 5.6 cm | 12% |
| 4 | 26° | 13° | 30° | 18° | 32.4 | 34.4 | 2.2 | 1 mm × 12 levels × 2.3 years = 2.7 cm | 81% |
| 5 | 16° | 16° | 22° | 11° | 14.6 | 16.3 | 1.7 | 1 mm × 7 levels × 2 years = 1.4 cm | > 100% |
| Average | 21° | 15° | 33° | 21° | 2.3 cm | 3.6 cm over 3.5 years | 63% |
M = male; F = female; N/A = not available.
Fig. 5A–D.
Clinical case, Patient 1. (A) The presenting radiograph shows the patient at 3 years of age. (B) Curves progress despite serial casting. A radiograph was performed when the patient was 6 years old. (C) Preoperative clinical pictures are shown. (D) Immediate postoperative radiograph is shown. (E) Five years postoperatively the rod is starting to disengage warranting revision surgery. (F) Intraoperative finding demonstrates particle debris adjacent to rods, no evidence of fusion, and no implant loosening. (G) Postoperative revision surgery with longer rods is shown. (H) A clinical picture is shown of the patient at the age of 12 years.
Fig. 6A–D.
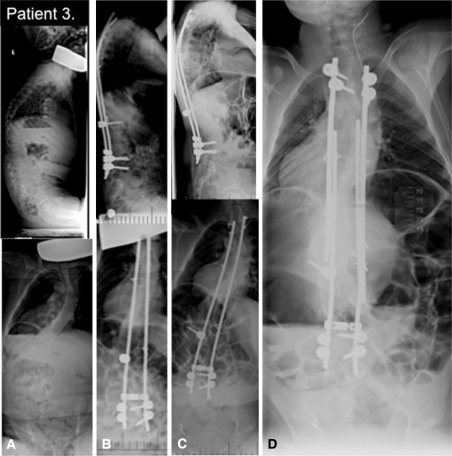
The radiographic evolution of Case 3 is shown. (A) A preoperative posteroanterior and lateral radiograph of the spine of a 4.5 year old with global hypotonia is shown. (B) The 6-month postoperative posteroanterior and lateral radiograph is shown. (C) This 4-year postoperative posteroanterior and lateral radiograph demonstrates recurrence of deformity and worsening apical rotation. (D) The 6-month postrevision growing rod surgery shows some correction and good balance in this image.
Table 2.
Results including postrevision surgery
| Patient number | Gender | Diagnosis of scoliosis | Reason for revision | Number of years after initial procedure | Current age | Instrumented segments | Number of growing vertebrae | Initial 1° Cobb | Initial 2° Cobb |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | Infantile | Outgrew construct | 6 years 0 month | 12 years 6 months | T4-L3 | 8 (T6-L1) | 45° | 46° |
| 2 | M | Prader Willi | Outgrew construct | 5 years 0 month | 8 years 6 months | T2-L5 | 10 (T6-L3) | 70° | N/A |
| 3 | F | Syndromic hypotonia | Curve progression | 5 years 0 month | 9 years 5 months | T1-L5 | 13 (T3-L3) | 70° | 49° |
| 4 | F | Cerebral palsy | None | 2 years 3 months | 10 years 6 months | T2-S1 | 12 (T4-L3) | 59° | 36° |
| 5 | F | Infantile | None | 2 years 0 month | 9 years 8 months | T3-L1 | 7 (T5-T11) | 55° | 35° |
| Average | 4 years | 10 | 60° | 42° |
| Patient number | 1° Cobb before revision | 2° Cobb before revision | Postrevision 1° Cobb | Postrevision 2° Cobb | Initial spinal height (cm) | Last followup spinal height (cm) | Measure growth (cm) | Expected growth: 1 mm × number of segments spanned × years | Percent of growth |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 23° | 17° | 5° | 2° | 16.3 | 20.8 | 4.5 | 1 mm × 8 levels × 6.3 years = 5.0 cm | 93% |
| 2 | 37° | N/A | 12° | N/A | 20.2 | 25.0 | 4.8 | 1 mm × 10 levels × 5 years = 5.0 cm | 96% |
| 3 | 54° | 38° | 33° | 29° | 26.8 | 28.5 | 1.7 | 1 mm × 13 levels × 5 years = 6.5 cm | 26% |
| 4 | N/A | N/A | 30° last followup | 18° last followup | 32.4 | 34.4 | 2.2 | 1 mm × 12 levels × 2.3 years = 2.7 cm | 81% |
| 5 | N/A | N/A | 22° last followup | 11° last followup | 14.6 | 16.3 | 1.7 | 1 mm × 7 levels × 2 years = 1.4 cm | > 100% |
| Average | 21° | 15° | 3 cm | 4.1 cm | 73% |
M = male; F = female; N/A = not available.
The average total growth across the average 10 vertebrae before any revision surgery was 2.3 cm (range, 0.7–4.1 cm) over an average of 3.6 years, or 63% of the expected growth (range, 12%–100%). Patient 1 (Fig. 5) and Patient 2 outgrew their constructs, but Patient 3 lost the initial gain in spinal height over a 4-year period as his deformity recurred (Fig. 6). At the last followup, subsequent to the revision surgery, the average total growth was 3 cm (range, 1.7–4.8 cm) over a 4-year period, representing 73% (range, 26%–100%) of the expected growth. Intraoperative findings demonstrated persistent gliding motion across the three gliding screws and at the sublaminar cables. In keeping with motion, particle debris was noted at the rod-screw interface as well as at the cable-rod interface (Fig. 5F). The last two patients continued to grow with their deformity remaining stable.
During the ongoing treatment, one patient had a major complication. Patient 3 required an unplanned surgery 4.5 years after the initial surgery as a result of recurrence of the deformity and lack of growth (Fig. 6). It was also noted that spontaneous fusion had occurred across the convexity just distal to the proximal anchor. The rod was pressing down onto the spine correcting a proximal kyphosis. All five patients’ postoperative courses were uneventful; all four ambulatory patients remained active with no undue pain. There were no superficial or deep wound infections or rod breakage. However, radiographs did reveal that three of the 22 sublaminar cables ruptured in two patients but without any clinical consequences.
None of these patients underwent repetitive surgery. Three additional surgeries were performed to extend the constructs; Patients 1 and 2 had outgrown them and one unplanned surgery was performed on Patient 3. In all three cases, the constructs were revised to new self-growing constructs, the spinal deformities were further corrected, and the spines continued to grow (Table 2).
Discussion
Despite major advances in the management of EOS, many questions remain [16]. The fundamental challenge of preventing curve progression while maintaining longitudinal spinal growth in EOS has resulted in novel treatment modalities that unfortunately are also associated with multiple subsequent procedures and relative high complication rates [2, 5, 9, 13, 15, 18]. Our purposes were to determine whether a modern Luqué construct would (1) correct and maintain the spinal deformity in EOS; (2) allow for spinal growth; (3) lead to higher complication rates; and (4) require patients to undergo additional surgeries.
Our case series is subject to several limitations. First, our small numbers cannot generate any relevant statistical values and can only be looked at as an initial evaluation to determine if this technique should be further explored. Second, having small numbers with different etiologies makes it difficult to draw any clinical recommendations. Third, our lack of long-term followup is of concern, particularly considering the nature of complications associated with EOS. Lastly, the lack of additional systematic spinal imaging (CT, MRI) makes it difficult to confirm or exclude the presence of spontaneous fusion but can be inferred from worsening Cobb angles or lack of growth (as seen in Patient 3).
Our modern Luqué trolley construct achieved 60% of Cobb correction, leading us to believe that it can correct and maintain spinal deformities in EOS and other techniques when compared with the literature (Table 3). Our observations are similar to those in the literature with the exception of the derotational casting technique in which 25% of patients had complete resolution of their scoliosis and another 50% had residual curves below 40° [19]. DGR reportedly initially decreases deformities by 60% with a residual correction of 50% [3]. The use of VEPTR for noncongenital scoliosis as a purely growing rod technique does not control spinal deformities well (final Cobb correction of only 20%) [9] but controls spinal deformity in patients with congenital scoliosis and chest wall abnormalities [5]. The self-lengthening constructs with apical epiphysiodesis or fusion (ie, conventional Luqué trolley and Shilla technique, respectively) have a similar initial correction of 60% with a latest followup correction of 50% [14, 18].
Table 3.
Comparative results of current treatments for early-onset scoliosis
| Author | Sanders et al., 2009 [19] | Pratt et al., 1999 [18] | Akbarnia et al., 2005 [3] | Campbell et al., 2004 [5] | Hasler et al., 2010 [9] | McCarthy et al., 2009 [14] | Ouellet, 2010 (current study) |
|---|---|---|---|---|---|---|---|
| Techniques Time span |
Derotation casting 2003–2008 | Luqué Trolley and epiphysiodesis 1983–1990 | Dual growing rod 1993–2001 | VEPTR | VEPTR | Shilla Apical fusion | Modern trolley |
| Indication | 38 EOS 15 syndesmosis; 3 NM |
25 EOS | 7 EOS; 3 Cong; 2 NM; 11 Synd | 21 Cong et chest abnormality | 1 EOS; 12 NM; 0 Cong; 5 syndesmosis; 5 chest |
3 EOS; 1 Cong; 2 Synd 4 | 2 EOS; 1 NM; 2 syndesmosis |
| No. | 55 | 25 | 23 | 21 | 23 | 10 | 5 |
| Minimum and average followup | Minimum 1 year Average 2.1 years |
Minimum 5 years | Minimum 2 years Average 4.75 (2–9.25) |
Minimum 2 years Average 4.2 |
Minimum 2 years Average 3.6 years (2–5.8) |
Minimum 2 years | Minimum 2 years Average 3.6 (2–5.5) |
| Average age at initiation of treatment | 2.2 years (1.1–6) | 3.1 years (1.5–7.4) | 5.4 years (1.9–12) | 3.3 years | 6.5 years (1.1–10.5) | 7.6 years (2–10) | 6.5 years (3.8–8.5) |
| Average time of treatment | 1 year | 5 years | 4 years | Not mentioned | Not mentioned | Not mentioned | 4 years |
| Number of additional procedures (planned lengthening) | < 2 years: 2/month < 3 years: 3/month > 4 years: 4/month Cast change under GA |
13 additional procedures Average 0.5 per patient |
166 additional procedures 15 unplanned Average 6.6 per patient |
Not mentioned | 149 additional procedures 15 unplanned Average 7.1 per patient |
5 additional procedure 1 rod exchange do to overgrowth 1 prominent implants 1 rod fracture 2 I&D for infection Average 0.5 per patient |
3 add procedures 2 rod exchange due to overgrowth 1 rod revision resulting from recurrence deficiency Average 0.6 per patient |
| Number of vertebrae spanned | All | 10 (9–12) | 13 (11–17) | Not mentioned | Not mentioned | Not mentioned | 10 |
| Growth across the spine | No data | Average 2.9 cm 49% expected With epiphysiodesis Average 2.0 cm 32% expected |
Average of 4.8 cm average 1.2 cm/year |
Average 3.3 cm Average 0.8 cm/year Prior fusion Average 0.3 cm/year |
Not mentioned | Addition of 12% | Average 3 cm average per year of 0.75 cm |
| Complications | No data transient Skin irritation |
32% 8 patients with implant failure 1 infection 1 fusion 1 kyphosis |
47% 11 patients had total of 13 complications |
Not mentioned | 100% 23 complications 10 skin sloughs, 7 implant failure, 6 infection |
50% 1 prominent implants 1 rod fracture 2 I&D for infection |
40% 1 patient loss of correction, unilateral convex 4-segment fusion |
| Percent correction primary curve | ¼ > 80% ½ = 50% ¼ < 40% |
Initial correction 60% Latest followup 51% |
Initial correction 54% Latest followup 53% |
Initial correction 30% Latest followup 25% |
Initial correction 61% Latest followup 51% |
Initial correction 65% Latest followup 61% |
VEPTR = vertical expandable prosthetic titanium ribs; EOS = early-onset scoliosis; Cong = congenital scoliosis; NM = neuromuscular scoliosis.
From a growth perspective, the modern Luqué trolley allowed on average 0.75 cm of spinal growth per year in four of the five patients representing 90% of their expected growth occurring across the instrumented spine. However, one of five patients (20%) only grew 25% (Patient 3). We believe the overall growing construct was less than ideal in this patient; a combination of the inadequate amounts of gliding anchors, the residual coronal deformity, and the sagittal deformity that led the rod to rest directly on the spine inducing a spontaneous fusion contributed to this poor growth outcome. It is difficult to compare our technique’s spinal growth with the other techniques because the reporting on growth is not standardized in the literature. At best, we can state that on average the DGR technique appears to provide 1.2 cm of growth per year, whereas the VEPTR technique, in the face of congenital scoliosis and chest wall abnormality, results in 0.8 cm of growth per year. The conventional Luqué trolley with epiphysiodesis resulted in marked growth inhibition (30% of expected growth). It is important to emphasize that the conventional Luqué trolley and the Shilla techniques intentionally inhibit growth by performing either a hemiepiphysiodesis or a frank fusion across a segment of the spine. The current comparison of growth is specific to spinal growth and not growth in relation to space available for the lung (SAL). We did not analyze the SAL because this technique is not used for thoracic insufficiency syndrome.
The complication rate of the modern Luqué trolley is similar in type and rate of the complications seen with the other growing rod techniques. When comparing it with the original trolley results, it has less implant failures leading to revision surgeries. Spontaneous fusions, as previously discussed, remain a main concern with this technique and as we move forward, a protocol for investigating the presence of fusions will be required.
The apparent benefits for growth using the DGR and the VEPTR are achieved at the expense of additional multiple surgeries. In comparison, the self-lengthening techniques have a substantially smaller number of additional surgeries, but possibly at the cost of less achievable growth. The DGR and the VEPTR have on average an additional 6.6 and 7.1 surgeries per patient, respectively, in contrast to the self-lengthening techniques such as the Shilla and the modern Luqué trolley that have less than one (average of 0.5 and 0.6 additional procedures, respectively) per patient.
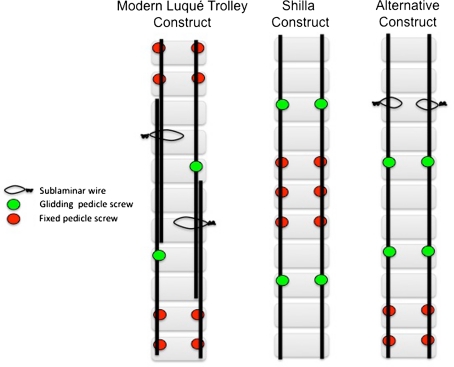
It is apparent that the next generation of surgical techniques for the management of EOS will attempt to avoid the need for recurrent lengthening. Gliding anchors will allow us to apply Luqué’s concept of self-growth to different construct (Fig. 7), and each construct will bear specific risks and benefits. For example, the patient is at risk of developing proximal or distal junctional kyphosis when the proximal and possibly the distal segment grow off the gliding anchors. Despite the apparent advantages of these self-growing constructs, there still remain unresolved issues. The risk of spontaneous fusion must be balanced with the inadequate control of the spine to determine the number of gliding anchors needed. All these constructs have motion across many interfaces where debris particles are generated with little understanding of their possible local and systemic repercussions [15]. Finally, a persistent shortfall of all these growing rod constructs is our inability to control rotational deformity in the presence of ongoing spinal growth.
Fig. 7A–D.
Illustrations show the different self-lengthening growing constructs.
In conclusion, the modern Luqué trolley technique combines stable base (end vertebrae) fixation with apical control and gliding anchors, permitting self-lengthening of the construct by guided growth and avoiding repetitive and scheduled surgeries. In this small series of five patients, only one was an outright failure, suggesting the initial experience was favorable enough to justify this preliminary report and continue evaluating this procedure.
Acknowledgment
We thank Dr Farid Alfaya for reviewing the radiographs and medical records.
Footnotes
The institution of the author receives fellowship research funding from AO Spine North America unrelated to this work. The author has had commercial associations in the form of contractual consultancies work with Synthes Europe.
References
- 1.Akbarnia BA. Management themes in early onset scoliosis. J Bone Joint Surg Am. 2007;89(Suppl 1):42–54. doi: 10.2106/JBJS.F.01256. [DOI] [PubMed] [Google Scholar]
- 2.Akbarnia BA, Breakwell LM, Marks DS, McCarthy RE, Thompson AG, Canale SK, Kostial PN, Tambe A, Asher MA. Dual growing rod technique followed for three to eleven years until final fusion: the effect of frequency of lengthening. Spine (Phila Pa 1976) 2008;33:984–990. doi: 10.1097/BRS.0b013e31816c8b4e. [DOI] [PubMed] [Google Scholar]
- 3.Akbarnia BA, Marks DS, Boachie-Adjei O, Thompson AG, Asher MA. Dual growing rod technique for the treatment of progressive early-onset scoliosis: a multicenter study. Spine (Phila Pa 1976) 2005;30(Suppl):S46–S57. doi: 10.1097/01.brs.0000175190.08134.73. [DOI] [PubMed] [Google Scholar]
- 4.Branthwaite MA. Cardiorespiratory consequences of unfused idiopathic scoliosis. Br J Dis Chest. 1986;80:360–369. doi: 10.1016/0007-0971(86)90089-6. [DOI] [PubMed] [Google Scholar]
- 5.Campbell RM, Jr, Smith MD, Mayes TC, Mangos JA, Willey-Courand DB, Kose N, Pinero RF, Alder ME, Duong HL, Surber JL. The effect of opening wedge thoracostomy on thoracic insufficiency syndrome associated with fused ribs and congenital scoliosis. J Bone Joint Surg Am. 2004;86:1659–1674. doi: 10.2106/00004623-200408000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham ME, Frelinghuysen PH, Roh JS, Boachie-Adjei O, Green DW. Fusionless scoliosis surgery. Curr Opin Pediatr. 2005;17:48–53. doi: 10.1097/01.mop.0000149603.33508.8d. [DOI] [PubMed] [Google Scholar]
- 7.D’Astous JL, Sanders JO. Casting and traction treatment methods for scoliosis. Orthop Clin North Am. 2007;38:477–484. doi: 10.1016/j.ocl.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Dimeglio A. Growth of the spine before age 5 years. J Pediatr Orthop Br. 1992;1:102–107. doi: 10.1097/01202412-199201020-00003. [DOI] [Google Scholar]
- 9.Hasler CC, Mehrkens A, Hefti F. Efficacy and safety of VEPTR instrumentation for progressive spine deformities in young children without rib fusions. Eur Spine J. 2010;19:400–408. doi: 10.1007/s00586-009-1253-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.James JI, Lloyd-Roberts GC, Pilcher MF. Infantile structural scoliosis. J Bone Joint Surg Br. 1959;41:719–735. doi: 10.1302/0301-620X.41B4.719. [DOI] [PubMed] [Google Scholar]
- 11.Luqué ER. Paralytic scoliosis in growing children. Clin Orthop Relat Res. 1982;163:202–209. [PubMed] [Google Scholar]
- 12.Luqué ER, Cardoso A. Treatment of scoliosis without arthrodesis or external support, preliminary report. Orthop Trans. 1977;1:37–38. [Google Scholar]
- 13.Mardjetko SM, Hammerberg KW, Lubicky JP, Fister JS. The Luqué trolley revisited Review of nine cases requiring revision. Spine (Phila Pa 1976) 1992;17:582–589. doi: 10.1097/00007632-199205000-00018. [DOI] [PubMed] [Google Scholar]
- 14.McCarthy RE, Luhmann S, Lenke L. Greater than two year follow-up Shilla growth enhancing system for the treatment of scoliosis in children. 2nd International Congress on Early Onset Scoliosis and Growing Spine (Montreal 2008) J Child Orthop. 2009;3:145–168. doi: 10.1007/s11832-008-0152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCarthy RE, Sucato D, Turner JL, Zhang H, Henson MA, McCarthy K. Shilla growing rods in a caprine animal model: a pilot study. Clin Orthop Relat Res. 2010;468:705–710. doi: 10.1007/s11999-009-1028-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrissy RT, Goldsmith GS, Hall EC, Kehl D, Cowie GH. Measurement of the Cobb angle on radiographs of patients who have scoliosis Evaluation of intrinsic error. J Bone Joint Surg Am. 1990;72:320–327. [PubMed] [Google Scholar]
- 17.Pehrsson K, Larsson S, Oden A, Nachemson A. Long-term follow-up of patients with untreated scoliosis A study of mortality, causes of death, and symptoms. Spine (Phila Pa 1976) 1992;17:1091–1096. doi: 10.1097/00007632-199209000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Pratt RK, Webb JK, Burwell RG, Cummings SL. Luqué trolley and convex epiphysiodesis in the management of infantile and juvenile idiopathic scoliosis. Spine (Phila Pa 1976) 1999;24:1538–1547. doi: 10.1097/00007632-199908010-00007. [DOI] [PubMed] [Google Scholar]
- 19.Sanders JO, D’Astous J, Fitzgerald M, Khoury JG, Kishan S, Sturm PF. Derotational casting for progressive infantile scoliosis. J Pediatr Orthop. 2009;29:581–587. doi: 10.1097/BPO.0b013e3181b2f8df. [DOI] [PubMed] [Google Scholar]
- 20.Wild A, Jager M, Kramer R, Werner A, Krauspe R. A new technique for the surgical management of deformities in the growing spine. Biomed Tech (Berl) 2002;47:270–271. doi: 10.1515/bmte.2002.47.11.270. [DOI] [PubMed] [Google Scholar]