Abstract
Background
Kyphosis in myelomeningocele is a rare and difficult problem. Many strategies have been used with no single procedure universally agreed on. Techniques involving anterior and posterior fixation may provide better fusion.
Questions/purposes
We describe a novel procedure for anteroposterior kyphectomy in patients with myelomeningocele. Apical posterior kyphectomy is followed by the insertion of two rods distally into the vertebral bodies and sacrum. Sublaminar wires are placed superiorly and the kyphosis is reduced by sequential tightening. We determined kyphosis correction and intraoperative blood loss for this new procedure
Methods
We retrospectively reviewed 22 patients (average age, 7.6 years [range, 2–17 years]) who underwent apical kyphectomy from 1982 to 2008. Charts were examined and radiographs measured preoperatively, immediately postoperatively, and at final followup. Followup averaged 6.4 years (range, 0–14 years) with 19 patients having at least 2 years of followup.
Results
Kyphosis decreased from a mean of 123° (range, 79°–163°) preoperatively to 40° (range, 13°–92°) immediately postoperatively and was a mean of 60° (range, 14°–126°) at final followup. Operating time was 248 minutes (range, 180–345 minutes), estimated blood loss was 765 mL (range, 140–2100 mL), and length of stay was 14 days (range, 1–57 days). Ten of the 22 patients had complications with eight requiring reoperation.
Conclusions
This anteroposterior kyphectomy provided a high level of kyphosis correction, which was largely maintained over the study period. Blood loss, surgical time, and complication rates were acceptable as compared with other techniques reported in the literature.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
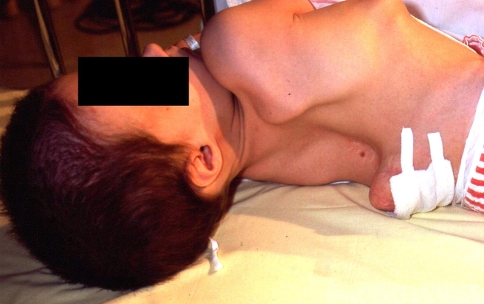
There are few conditions in the pediatric spine more challenging than kyphosis in patients with myelomeningocele (MMC) (Fig. 1). Although techniques for treatment of this condition have evolved, a definitive “gold standard” procedure, with uniformly predictable outcomes, remains elusive. The incidence of MMC is between 0.005% and 0.2% of all live births [6, 7]. Deformities in MMC occur in 50% of patients [7]. Congenital kyphosis and kyphoscoliosis are less common than congenital scoliosis [24, 25], occurring in 8% to 28% of these patients [4, 6, 12, 18, 22, 26, 28, 34].
Fig. 1.
A lateral radiograph of a patient with myelomeningocele and kyphosis (Patient 1) is shown.
The need to operate on kyphotic deformities mainly stems from the effect of the gibbus on posterior structures. Breakdown of the abnormal skin overlying the gibbus is a common indication, either to prevent breakdown or because braces would further compromise the skin [3, 5, 6, 8, 9, 12–14, 16, 17, 21, 22, 27–29, 31, 33]. Nonoperative treatment in the form of bracing, manual reduction, or observation to allow deformities to stabilize or the patient to reach skeletal maturity is often not successful [3, 4, 8, 12, 16, 17, 26, 28]. Other indications include sagittal imbalance (such that the patient needs to use the upper extremities for sitting) [3, 5, 6, 14, 16, 17, 26–29, 31] and progressive deformity [6, 10, 11, 13, 21, 31, 34].
Reported difficulties in maintaining fixation in this demanding patient population prompted the senior author (JCH) to explore alternative distal fixation techniques. His technique limits dissection over the compromised skin and allows anterior vertebral and sacral body fixation through a posterior approach.
The purposes of this article are to: (1) quantify the amount of kyphosis correction; (2) report the operative time and estimated blood loss; and (3) report the complications associated with our technique of kyphectomy for patients with myelomeningocele.
Patients and Methods
This is a retrospective review of a series of patients at a single institution (IWK Health Centre) treated with our kyphectomy technique from 1982 to 2008. An admission database was searched for the keyword “kyphectomy,” which identified 32 patients treated over this timeframe. Patients were included if they had myelomeningocele, kyphosis as their primary deformity, received a Halifax kyphectomy, and had the senior author (JCH) as the operator or primary assistant. Indications for this surgery included diagnosis of MMC, progressive kyphosis, or skin breakdown refractory to nonoperative treatment. Ten patients were excluded: One patient because the primary deformity was not kyphosis, five because they did not receive the Halifax kyphectomy, and four because JCH was not their surgeon. This left 22 patients eligible for inclusion in the study. Of these, the radiographic record of one patient was incomplete, and three patients had less than 2 years’ followup. Those patients are included for discussion of complications (n = 22) but not for radiographic assessment (n = 19). The average age of the patients was 7.6 years (range, 2–17 years) (Table 1) and average followup of 6.4 years (range, 0–14 years). No patients were recalled specifically for this study; all data were obtained from medical records and radiographs.
Table 1.
Patient results
| Patient number | Age (years) | Kyphosis (degrees) | Followup (years) | Length of stay (days) | Estimated blood loss (mL) | Operating room time (minutes) | Complications | ||
|---|---|---|---|---|---|---|---|---|---|
| Preoperative | Postoperative | Final | |||||||
| 1 | 2 | 133 | 46 | 59 | 14 | 57 | 800 | 235 | Rod cut out, fell out |
| 2 | 8 | 153 | 86 | 83 | 8 | 15 | 700 | 210 | None |
| 3 | 15 | — | — | — | — | 14 | 1250 | 285 | None |
| 4 | 14 | 127 | 22 | 30 | 7 | 19 | 1700 | 295 | None |
| 5 | 2 | 130 | 20 | 43 | 9 | 16 | 695 | 275 | None |
| 6 | 16 | 163 | 32 | 42 | 6 | 14 | 1124 | 235 | None |
| 7 | 13 | 147 | 54 | 87 | 6 | 19 | 2100 | 345 | Rod number, cut out |
| 8 | 10 | 88 | 31 | 34 | 5 | 16 | 1200 | 195 | None |
| 9 | 5 | 104 | 13 | 15 | 13 | 19 | 1000 | 255 | None |
| 10 | 7 | 159 | 92 | 121 | 1 | 11 | 200 | 210 | Removal, drainage |
| 11 | 2 | 89 | 50 | 126 | 11 | 12 | 400 | 250 | Cut out, removal |
| 12 | 2 | 89 | 36 | 44 | 6 | 11 | 170 | 240 | None |
| 13 | 4 | 110 | 37 | 45 | 13 | 13 | 1500 | 275 | Removal |
| 14 | 4 | 87 | 47 | 53 | 8 | 10 | 360 | 235 | Rod number |
| 15 | 7 | 176 | 23 | 14 | 7 | 8 | 300 | 230 | None |
| 16 | 3 | 161 | 59 | 82 | 4 | 15 | 140 | 205 | None |
| 17 | 12 | 79 | 24 | 50 | 5 | 9 | 442 | 305 | Cut out, pressure sore, |
| 18 | 2 | 147 | 24 | 62 | 3 | 4 | 150 | 245 | reoperation, cut out, 30° loss at 1 year |
| 19 | 7 | 95 | 13 | 61 | 2 | 5 | 200 | 255 | None |
| 20 | 3 | 117 | 21 | 71 | 4 | 5 | 500 | 180 | Skin breakdown, revision |
| 21 | 12 | 125 | 70 | 84 | 2 | 8 | 1349 | 288 | None |
| 22 | 17 | 108 | 49 | 59 | 0 | 1 | 550 | 205 | Death |
| Mean | 7.6 | 123.2 | 40.4 | 60.2 | 6.4 | 13.7 | 765.0 | 247.9 | |
The operative report and discharge summary were obtained from the inpatient charts. This gave a description of the procedure, the operating room (OR) time, estimated blood loss, and length of stay.
This single-stage surgery was performed with the patients in the prone position. The incision was made in the midline from the most proximal aspect of the planned instrumentation (upper thoracic) to just distal to the apex of the kyphosis, which avoided a skin incision distal to the kyphosis (Fig. 2). Deep dissection was started proximally over the normal spine and then gradually extended distally along the lateral aspects of the bifid segments onto the gibbus. The apex of the gibbus (generally upper lumbar) was isolated extraperiosteally using an elevator. Leksell rongeurs were used to create the osteotomy, which is most commonly at the apical 1.5 to two levels. It was preferred to have at least 2.5 to three levels distal to the kyphectomy to ensure adequate distal fixation. Cordectomy was performed in almost all cases because it improved anterior exposure. Blunt dissection was then used to remove the anterior tissues from the spine and the Leksell used to remove the proximal portion of the gibbus for a total of 1.5 to two levels. Blunt dissection was carried down distally and anteriorly. The tissue here fell away easily as an interval through areolar tissue was developed. Under fluoroscopic guidance, a drill was passed down through the anterior aspect of the spine distally through the vertebra and discs of the distal half of the spine and into the sacrum. A stainless steel rod was placed into this drill hole and then a second placed beside it, slightly converging (Fig. 3). The diameters of these rods varied as a result of patient size; however, ¼-inch stainless steel Luque rods were preferred. These rods were then reduced to the proximal, normal spine and fixed with sublaminar wires (Fig. 4). Resected local bone was used for apical fusion and no attempt was made for formal fusion cranially or caudally. This allowed for potential growth of the remainder of the spine. Postoperatively, these patients did not require casts, orthotics, or physiotherapy. They mobilized as tolerated and were back into their wheelchairs once their incisions healed. If wound healing was tenuous, patients were encouraged to spend time in the prone position. Patients followed up in the clinic with radiographs at 6 weeks, 3 months, 6 months, and 1 year postoperatively. They were then followed annually.
Fig. 2.
A preoperative photograph demonstrated the clinical deformity of kyphosis. There is skin breakdown distal to the apex of kyphosis (Patient 3).
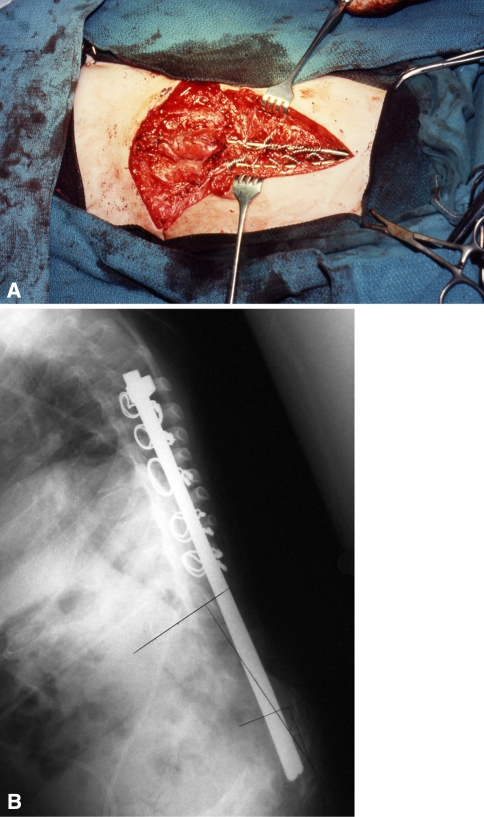
Fig. 3A–B.
(A) The insertion of a stainless steel rod distally into vertebral bodies is demonstrated through this posterior approach (Patient 5). (B) An intraoperative photograph demonstrates the two rods before reduction of kyphosis (Patient 5).
Fig. 4A–B.
(A) An intraoperative photograph demonstrates the two rods fixed distally and reduced proximally with sublaminar wires (Patient 5). (B) This postoperative radiograph demonstrates improvement in kyphosis and position of spinal implants.
Outpatient charts were examined to determine minor and major complications. Minor complications were defined as not requiring reoperation, whereas major complications were defined as requiring reoperation or resulting in perioperative mortality.
Lateral sitting films were measured preoperatively, immediately postoperatively, and at the last followup. Two observers (SAC, JLL) measured the radiographs using the Cobb method. Interobserver variability using this technique is approximately 6°; however, given the bone quality and small anatomy in these patients, the variability may have been higher in this study. The mean, standard deviation, and standard error were calculated for each variable.
Results
Kyphosis decreased from an average of 123° (range, 79°–163°) preoperatively to 40° (range, 13°–92°) postoperatively for an average correction of 82° (range, 39°–153°) (Table 1). The average kyphosis at final followup was 60° (range, 14°–126°) for an average final correction of 63° (range, −37°–162°). The average loss of correction was 19° (range, −9°–76°).
Mean operative time was 248 minutes (range, 180–345 minutes) with mean estimated blood loss of 765 mL (range, 140–2100 mL).
Twelve of the 22 patients had no complications. There were 18 complications in the remaining 10 patients. Major complications included one perioperative mortality, which occurred in a 17-year-old girl who weighed approximately 23 kg. She was brought to the hospital septic from an open decubitus overlying her kyphus. Attempts were made to treat with antibiotics and total parenteral nutrition. It was evident her infection was not clearing and she was brought to the OR. The case proceeded well, but as the deformity was being closed, she developed a coagulopathy and arrested secondary to massive blood loss. Eight patients required reoperation for implant removal, six for mechanical complications and two for skin/wound issues. Of these eight patients, six of them maintained their kyphosis correction after implant removal and did not require any further surgery. Nine minor complications (not requiring reoperation) included patients with superficial skin breakdown as well as one patient with an asymptomatic rod fracture. No deep infection was seen in these patients.
Discussion
Kyphosis in myelomeningocele is a rare and difficult problem. Many strategies have been used with no single procedure clearly better than any other. Techniques involving anterior and posterior fixation may provide better fixation. We describe a novel AP kyphectomy and report the amount of kyphosis correction, operative time, estimated blood loss, and complications associated with this technique.
We recognize certain limitations of our study. First, it is a retrospective review over a long period of time. Only limited data could be accurately obtained for this group of patients. The amount of spine growth and the effects on pulmonary function of this technique are areas of interest that should be studied in this patient population but were beyond the scope of this study given its retrospective nature. Second, the technique (ie, implant size) varied occasionally in small-statured patients. Rods varied from large-diameter Steinmann pins in young patients to ¼-inch Luque rods in older patients. Third, there is variability associated with measuring kyphosis, especially in patients with osteopenic bone and small anatomy. Fourth, it is difficult to compare results and complications for kyphectomy because there is inconsistent reporting in the literature. Despite these limitations, valuable information was gathered from this group of patients that can be used to assist surgeons when deciding how to treat this increasingly rare condition.
In our study, kyphosis improved from 123° preoperatively to 40° immediately postoperatively with a mean kyphosis of 60° at final followup. Five reports have described similar procedures to ours (Table 2). Heydemann and Gillespie [9] reported on 12 patients who underwent kyphectomy and posterior instrumented fusion with anterior fixation to the pelvis. Their preoperative kyphosis was 124°, and postoperative kyphosis was 33°, which was maintained at followup ranging from 6 to 57 months. Huang and Lubicky [13] reported a series of six kyphectomy patients fixed with rods contoured around the anterior aspect of the sacrum through the sacral foramina (Gillespie’s technique). The average preoperative curve was 126°, and the postoperative curve was 22° at a mean followup of 35 months. In 1995, Torode et al. [33] reported a series of four patients with average preoperative curve of 90° and, at mean followup of 2 years, the postoperative curve was 25°. McCall [22] published a series of 17 patients treated with Gillespie’s technique and a postoperative pantaloon spica for 3 months followed by a thoracolumbosacral orthosis. The average preoperative curve was 111° and the postoperative curve was 15°. He found that age at operation, level of paralysis, and severity of kyphosis were not predictors of long-term correction [22]. Kadic and Verbout [15] reported on three patients who underwent kyphectomy followed by a Luque-Galveston procedure. They achieved 55° correction and reported over an average of 39 months’ followup. Our study of 22 patients demonstrates comparable kyphosis correction with these five studies.
Table 2.
Comparison of kyphosis correction to the literature
| Author | Patients | Preoperative (degrees) | Immediate postoperative (degrees) | Loss of correction (degrees) | Followup (months, years) |
|---|---|---|---|---|---|
| Heydemann and Gillespie [9] | 12 | 124 | 33 | 1pt | 6–57 months |
| Huang and Lubicky [13] | 6 | 126 | 22 | 3 | 2 years 11 months |
| Torode and Godette [33] | 4 | 90 | 25 | 10 | 2 years |
| McCall [22] | 17 | 111 | 15 | 6 | 4 years 9 months |
| Kadic and Verbout [15] | 3 | 102 | 47 | 0 | 3 years 3 months |
| Comstock et al. [current study] | 22 | 124 | 40 | 19 | 6 |
Our technique resulted in a mean operative time of 248 minutes, which was less than almost all previously published operative times [4, 20, 22, 26–28, 31, 32] (Table 3). The lack of posterior exposure distal to the apex of our deformity in our technique is believed to save operative time as well as potential blood loss. This is especially important in the MMC population in which patients frequently have comorbid illnesses. The only exception to our lower operative time was Furderer’s study of 14 patients in which his operative time was 216 minutes; however, his blood loss was 1194 mL as compared with the mean of 765 mL in our study [6] . Estimated blood loss in other series of patients ranged from 155 mL to 3000 mL (Table 3). Despite a couple of outliers [4, 30], the remainder of studies reported blood loss greater than 800 mL [6, 20, 22, 27, 28, 32]. Longer operative times and the extent of distal exposure in their techniques likely resulted in greater blood loss. Epidural bleeding is sometimes encountered during these procedures and can result in substantial blood loss. We believe that, by performing cordectomy, exposure is improved and that bleeding can be more easily controlled.
Table 3.
Comparison of intraoperative time and blood loss
| Author | Number of patients | Operating room time (minutes) | Estimated blood loss (mL) |
|---|---|---|---|
| McCall [22] | 17 | — | 1121 |
| Furderer et al. [6] | 14 | 216 | 1194 |
| Nolden et al. [27] | 11 | — | 880 |
| Crawford et al. [4] | 12 | 307 | 155 |
| Niall et al. [26] | 24 | 285 | 115% |
| Odent et al. [28] | 9 | — | 3000 |
| Sharrard [30] | 6 | — | 185 |
| Sriram et al. [32] | 33 | 270 | 1400 |
| Lowe and Menelaus [22] | 11 | — | 1000 |
| Comstock et al. [current study] | 22 | 248 | 765 |
In our 22 patients, we encountered 18 complications in 10 patients. This included eight reoperations and one perioperative death. We acknowledge that it can be difficult to report complications and to compare complication rates between series. It is accepted that kyphectomy surgery has associated risks and a relatively high rate of complications (Table 4). Complications most frequently reported include loss of correction, revision surgery, and perioperative mortality (Table 4). Our immediate postoperative kyphosis was 40° with a final mean kyphosis of 60° at mean followup of 6.4 years. Nine of our 22 patients had loss of correction greater than 20°; however, only two of those patients required reoperation for this. This is similar to the 13 of 23 loss of correction rate published by Lindseth and Stelzer [18], although lower rates were reported in several other studies [3, 19, 21, 22]. It may be difficult to compare loss of correction between studies because a variety of definitions were used for “loss of correction.” We reoperated on eight of 22 patients, which is lower than that of the only other study that reported their rate of revision surgery (seven of 14 patients) [6]. Many studies reported the number of complications experienced but did not comment on whether these complications were minor or if they required revision surgery. Perioperative mortality was encountered in five other series in the literature [2, 5, 20, 22–25]. This is not unexpected given the extensive surgical exposures required to perform kyphectomy in this already compromised population of patients. Our one perioperative mortality was discussed in detail in the results section of this article but is thought to be attributed to the use of our kyphectomy as a “salvage” surgery in a very frail and sick patient. Mortality is a risk that should be discussed with all patients (and their families) who are undergoing kyphectomy.
Table 4.
Comparison of complications to the literature
| Author | Patients | Complications |
|---|---|---|
| Sharrard [30] | 6 | 5 |
| Sharrard and Drennan [31] | 18 | 6 |
| Sriram et al. [32] | 33 | 17 |
| Eckstein and Vora [5] | 16 | 8 (5 deaths) |
| Lowe and Menelaus [20] | 11 | 8 deaths |
| Lindseth and Stelzer [18] | 23 | 13 loss of correction |
| Christoferson and Brooks [3] | 9 | 3 loss of correction |
| Heydemann and Gillespie [9] | 12 | 1 # Rod |
| McMaster [23] | 10 | 3 (1 death) |
| Carstens et al. [2] | 33 | 3 deaths |
| Martin et al. [21] | 10 | 1 loss of correction |
| Huang and Lubicky [13] | 6 | 1 |
| Lintner and Lindseth [19] | 39 | 9 loss of correction |
| Torode and Godette [33] | 4 | 0 |
| McCall [22] | 17 | 1 death, 1 loss of correction |
| Furderer et al. [6] | 14 | 7 revisions |
| Nolden et al. [27] | 11 | 3 |
| Crawford et al. [4] | 12 | 4 |
| Niall et al. [26] | 24 | 20 |
| Odent et al. [28] | 9 | 3 |
| Comstock et al. [current study] | 22 | 8 reoperations, 1 death |
Spinal instrumentation for deformity should achieve three goals: (1) allow for correction forces; (2) start with a situation favorable to fusion; and (3) maintain correction until fusion [1, 11]. To accomplish these goals, most authors have advocated 360° fusion, but there are problems. For these often malnourished and poorly developed children, anterior and posterior surgeries may be staged, and the patients often take months to recover and require postoperative bracing. Furthermore, rigid fixation to the sacrum or pelvis does not allow for growth and has led some to reserve this surgery for older children who are close to skeletal maturity. However, some of these patients require surgery when they are very young, and waiting only leads to further progression and a more difficult problem [16, 17]. However, in certain situations, it may be advantageous to wait until children are school aged to use larger diameter stainless steel rods.
The AP kyphectomy described here provides for solid fixation without the need for extensive posterior dissection. By placing the rods in the anterior part of the vertebral body distally, it effects “three-point fixation” and a very rigid construct. It also theoretically allows for growth. The rods are free in the distal segment and may allow the children to “grow off” the fixation.
In conclusion, the described AP kyphectomy provided a high level of correction, which was largely maintained over the study period. Operative time and estimated blood loss were lower than the majority of studies in the literature. The types of complications encountered in our series were similar to those in the literature and 10 of our 22 patients experienced complications. This technique of posterior kyphectomy with anterior placement of fixation is a procedure that should be considered for patients with myelomeningocele.
Acknowledgments
We thank Ms Maureen Marriott for her tireless efforts without which this study could not have been presented.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved or waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the Isaac Walton Killam Children’s Hospital, Dalhousie University, Halifax, Nova Scotia, Canada.
References
- 1.Ashman RB, Bechtold JE, Edwards WT, Johnston CE, 2nd, McAfee PC, Tencer AF. In vitro spinal arthrodesis implant mechanical testing protocols. J Spinal Disord. 1989;2:274–281. doi: 10.1097/00002517-198912000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Carstens C, Vetter J, Niethard FU. Development of paralytic scoliosis in myelomeningocele. Z Orthop Ihre Grenzgeb. 1990;2:174–182. doi: 10.1055/s-2008-1039496. [DOI] [PubMed] [Google Scholar]
- 3.Christofersen MR, Brooks AL. Excision and wire fixation of rigid myelomeningocele kyphosis. J Pediatr Orthop. 1985;6:691–696. doi: 10.1097/01241398-198511000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Crawford AH, Strub WM, Lewis R, Gabriel KR, Billmire DA, Berger T, Crone K. Neonatal kyphectomy in the patient with myelomeningocele. Spine (Phila Pa 1976) 2003;3:260–266. doi: 10.1097/01.BRS.0000042234.98512.BE. [DOI] [PubMed] [Google Scholar]
- 5.Eckstein HB, Vora RM. Spinal osteotomy for severe kyphosis in children with myelomeningocele. J Bone Joint Surg Br. 1972;54:328–333. [PubMed] [Google Scholar]
- 6.Furderer S, Eysel P, Hopf C, Heine J. Sagittal static imbalance in myelomeningocele patients: Improvement in sitting ability by partial and total gibbus resection. Eur Spine J. 1999;8:451–457. doi: 10.1007/s005860050204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furderer S, Hopf C, Schwarz M, Voth D. Orthopedic and neurosurgical treatment of severe kyphosis in myelomeningocele. Neurosurg Rev. 1999;22:45–49. doi: 10.1007/s101430050008. [DOI] [PubMed] [Google Scholar]
- 8.Hall JE, Poitras B. The management of kyphosis in patients with myelomeningocele. Clin Orthop Relat Res. 1977;128:33–40. [PubMed] [Google Scholar]
- 9.Heydemann JS, Gillespie R. Management of myelomeningocele kyphosis in the older child by kyphectomy and segmental spinal instrumentation. Spine (Phila Pa 1976) 1987;1:37–41. doi: 10.1097/00007632-198701000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Hopf C, Schwarz M, Wackerhagen A, Voth D. The operative treatment of spinal deformities in MMC. Neurosurg Rev. 1993;1:45–52. doi: 10.1007/BF00308613. [DOI] [PubMed] [Google Scholar]
- 11.Hopf CG, Eysel P, Dubousset J. Operative treatment of scoliosis with Cotrel-Dubousset-Hopf instrumentation. new anterior spinal device. Spine (Phila Pa 1976) 1997;6:618–627. doi: 10.1097/00007632-199703150-00008. [DOI] [PubMed] [Google Scholar]
- 12.Hoppenfeld S. Congenital kyphosis in myelomeningocele. J Bone Joint Surg Br. 1967;49:276–280. [PubMed] [Google Scholar]
- 13.Huang TJ, Lubicky JP. Kyphectomy and segmental spinal instrumentation in young children with myelomeningocele kyphosis. J Formos Med Assoc. 1994;93:503–508. [PubMed] [Google Scholar]
- 14.James JI. Spinal deformities in myelomeningocele. J Bone Joint Surg Br. 1978;60:3–4. doi: 10.1302/0301-620X.60B1.564351. [DOI] [PubMed] [Google Scholar]
- 15.Kadic MA, Verbout AJ. Treatment of severe kyphosis in myelomeningocele by segmental spinal instrumentation with Luque rods. Acta Orthop Belg. 1991;57:45–51. [PubMed] [Google Scholar]
- 16.Lindseth RE. Myelomeningocele spine. In: Weinstein SL, ed. The Pediatric Spine: Principles and Practice. New York, NY: Raven Press; 1994:1043.
- 17.Lindseth RE, Dias LS, Drennan JC. Myelomeningocele. Instr Course Lect. 1991;40:271. [PubMed] [Google Scholar]
- 18.Lindseth RE, Stelzer L., Jr Vertebral excision for kyphosis in children with myelomeningocele. J Bone Joint Surg Am. 1979;61:699–704. [PubMed] [Google Scholar]
- 19.Lintner SA, Lindseth RE. Kyphotic deformity in patients who have a myelomeningocele. operative treatment and long-term follow-up. J Bone Joint Surg Am. 1994;76:1301–1307. doi: 10.2106/00004623-199409000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Lowe GP, Menelaus MB. The surgical management of kyphosis in older children with myelomeningocele. J Bone Joint Surg Br. 1978;60:40–45. doi: 10.1302/0301-620X.60B1.627577. [DOI] [PubMed] [Google Scholar]
- 21.Martin J, Jr, Kumar SJ, Guille JT, Ger D, Gibbs M. Congenital kyphosis in myelomeningocele: results following operative and nonoperative treatment. J Pediatr Orthop. 1994;3:323–328. doi: 10.1097/01241398-199405000-00009. [DOI] [PubMed] [Google Scholar]
- 22.McCall RE. Modified Luque instrumentation after myelomeningocele kyphectomy. Spine (Phila Pa 1976) 1998;12:1406–1411. doi: 10.1097/00007632-199806150-00020. [DOI] [PubMed] [Google Scholar]
- 23.McMaster MJ. The long-term results of kyphectomy and spinal stabilization in children with myelomeningocele. Spine (Phila Pa 1976) 1988;4:417–424. doi: 10.1097/00007632-198804000-00009. [DOI] [PubMed] [Google Scholar]
- 24.McMaster MJ, Singh H. Natural history of congenital kyphosis and kyphoscoliosis. A study of one hundred and twelve patients. J Bone Joint Surg Am. 1999;81:1367–1383. doi: 10.2106/00004623-199910000-00002. [DOI] [PubMed] [Google Scholar]
- 25.McMaster MJ, Singh H. The surgical management of congenital kyphosis and kyphoscoliosis. Spine (Phila Pa 1976) 2001;19:2146–2154. doi: 10.1097/00007632-200110010-00021. [DOI] [PubMed] [Google Scholar]
- 26.Niall DM, Dowling FE, Fogarty EE, Moore DP, Goldberg C. Kyphectomy in children with myelomeningocele: a long-term outcome study. J Pediatr Orthop. 2004;1:37–44. doi: 10.1097/00004694-200401000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Nolden MT, Sarwark JF, Vora A, Grayhack JJ. A kyphectomy technique with reduced perioperative morbidity for myelomeningocele kyphosis. Spine (Phila Pa 1976) 2002;16:1807–1813. doi: 10.1097/00007632-200208150-00022. [DOI] [PubMed] [Google Scholar]
- 28.Odent T, Arlet V, Ouellet J, Bitan F. Kyphectomy in myelomeningocele with a modified Dunn-McCarthy technique followed by an anterior inlayed strut graft. Eur Spine J. 2004;3:206–212. doi: 10.1007/s00586-003-0662-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarwark JF. Kyphosis deformity in myelomeningocele. Orthop Clin North Am. 1999;3:451–455, viii–ix. [DOI] [PubMed]
- 30.Sharrard WJ. Spinal osteotomy for congenital kyphosis in myelomeningocele. J Bone Joint Surg Br. 1968;50:466–471. [PubMed] [Google Scholar]
- 31.Sharrard WJ, Drennan JC. Osteotomy-excision of the spine for lumbar kyphosis in older children with myelomeningocele. J Bone Joint Surg Br. 1972;54:50–60. [PubMed] [Google Scholar]
- 32.Sriram K, Bobechko WP, Hall JE. Surgical management of spinal deformities in spina bifida. J Bone Joint Surg Br. 1972;54:666–676. [PubMed] [Google Scholar]
- 33.Torode I, Godette G. Surgical correction of congenital kyphosis in myelomeningocele. J Pediatr Orthop. 1995;15:202–205. [PubMed] [Google Scholar]
- 34.Widmann RF, Hresko MT, Hall JE. Lumbosacral fusion in children and adolescents using the modified sacral bar technique. Clin Orthop Relat Res. 1999;364:85–91. doi: 10.1097/00003086-199907000-00012. [DOI] [PubMed] [Google Scholar]