Abstract
Polycomb group (PcG) proteins maintain transcriptional repression of hundreds of genes involved in development, signaling or cancer using chromatin-based epigenetic mechanisms. Biochemical studies in Drosophila have revealed that PcG proteins associate in at least two classes of protein complexes known as Polycomb repressive complexes 1 and 2 (PRC1 and PRC2). Drosophila core PRC1 is composed of four subunits, Polycomb (Pc), Sex combs extra (Sce), Polyhomeotic (Ph), and Posterior sex combs (Psc). Each of these proteins has multiple orthologs in vertebrates classified respectively as the CBX, RING1/RNF2, PHC, and BMI1/PCGF families. Mammalian genomes encode five CBX family members (CBX2, CBX4, CBX6, CBX7, and CBX8) that are believed to have distinct biological functions. Here, we applied a tandem affinity purification (TAP) approach coupled with tandem mass spectrometry (MS/MS) methodologies in order to identify interacting partners of CBX family proteins under the same experimental conditions. Our analysis identified with high confidence about 20 proteins co-eluted with CBX2 and CBX7 tagged proteins, about 40 with CBX4, and around 60 with CBX6 and CBX8. We provide evidences that the CBX family proteins are mutually exclusive and define distinct PRC1-like protein complexes. CBX proteins also interact with different efficiencies with the other PRC1 components. Among the novel CBX interacting partners, protein kinase 2 associates with all CBX-PRC1 protein complexes, whereas 14-3-3 proteins specifically bind to CBX4. 14-3-3 protein binding to CBX4 appears to modulate the interaction between CBX4 and the BMI1/PCGF components of PRC1, but has no effect on CBX4-RING1/RNF2 interaction. Finally, we suggest that differences in CBX protein interactions would account, at least in part, for distinct subnuclear localization of the CBX family members.
During embryogenesis, the fertilized egg develops into a complex organism composed of many differentiated cell types. The maintenance of the differentiation status of each cell requires a cellular memory system that is responsible for stable maintenance of gene expression programs. The Polycomb group (PcG)1 proteins, together with the counteracting trithorax group (trxG) proteins, were discovered in Drosophila melanogaster as part of such a memory system maintaining transcription states, that have been initiated by transiently expressed regulatory factors (1). In particular, although homeotic gene expression patterns are properly initiated by segmentation genes in the embryo, Drosophila PcG mutants show severe segmental transformations along the head-to-tail axis because of derepression of homeotic genes outside of their normal expression territories (2). In vertebrates, the PcG and trxG proteins have similar roles in the maintenance of homeotic gene expression patterns. Indeed, changes in the body plan are observed in PcG and trxG gene homolog mouse mutants (3–6). However mutations in some vertebrate PcG genes also result in very early defects in development (7). Moreover, genome-wide chromatin immunoprecipitation studies and other approaches in Drosophila and mammalian cells have identified hundreds of PcG target genes including transcription factors and signaling components for most major developmental pathways (8–14).
PcG proteins have been found to interact with each other to form multimeric, chromatin-associated protein complexes of two general types: the Polycomb Repressive Complex 1 (PRC1) and PRC2 (15–19). These complexes post-translationally modify histone residues and are believed to cooperate in transcriptional repression of target genes by altering local, higher-order chromatin structure. Drosophila PRC2 contains four core proteins: Enhancer of zeste [E(z)], Extra sex combs (Esc), Suppressor of zeste 12 [Su(z)12], and Nucleosome remodeling factor 55-kDa subunit (Nurf55). E(z), a histone methyltransferase, catalyzes the trimethylation of histone H3 at lysine 27 (H3K27me3) via its SET domain (20, 21). Core PRC1 is composed of Polycomb (Pc), Sex combs extra (Sce), Posterior sex combs (Psc), and Polyhomeotic (Ph) (22). Pc has an N-terminal chromodomain that binds strongly to H3K27me3 (23, 24), the modification generated by PRC2, whereas Sce, the catalytically active subunit of PRC1, is an E3 ubiquitin ligase that monoubiquitylates histone H2A at lysine 119 (H2AK119ub1) (25, 26). PcG proteins bound at chromatin, as well as modified histones might then repress transcription through different mechanisms involving reorganization of chromatin and DNA structures (15, 27), interference with nucleosome remodeling (28), inhibition of transcriptional initiation and/or transcriptional elongation (29–31).
In mammals, Polycomb-mediated gene silencing is more complex than in Drosophila because the number of PcG ortholog is higher. In the case of PRC1, mammalian genomes encode at least five orthologs for Pc (CBX2, 4, 6, 7, and 8), six Psc orthologs (BMI1, PCGF1, 2, 3, 5, and 6), three Ph orthologs (PHC1, 2, and 3), and two Sce orthologs (RING1 and RNF2). There are evidences that different PRC complexes exist in cells (32). In particular, mice deficient for individual PRC1 components share homeotic defects, but harbor distinct phenotypes (4, 6, 33–38), suggesting that different PRC1 subcomplexes have a least some nonredundant target genes. Furthermore, null mutations of Eed and Suz12 PRC2 subunits abolish H3K27 trimethylation, but do not prevent the recruitment of PRC1 proteins to either the inactive X chromosome or to many of their target genes (39, 40). Thus, it is unclear whether H3K27me3 recognition is necessary or sufficient for stable chromatin association by CBX proteins or whether other interactions, potentially mediated by additional components of the PRC1 complex, are involved.
To gain insights into the PRC1 complex composition and into the CBX protein function in vertebrates, we generated stable cell lines expressing tagged versions of these proteins. A tandem affinity purification (TAP)-liquid chromatography-tandem mass spectrometry approach (LC-MS/MS) (41, 42) was used to purify and characterize PRC1-like protein complexes associated to human chromodomain-containing Polycomb proteins CBX2, CBX4, CBX6, CBX7, and CBX8 under the same experimental conditions. Here, we provide evidences that the CBX family proteins define distinct protein complexes. CBX proteins interact with different efficiencies with the other PRC1 components and bind specifically additional proteins. Differences in CBX protein interactions and the existence of distinct CBX-PRC1 complexes could account for the distinct subnuclear localization of the CBX family proteins as well as for their different transcriptional repression activities. We also show that protein kinase 2 binds to PRC1 protein complexes conferring to Polycomb complexes an additional kinase activity.
EXPERIMENTAL PROCEDURES
Constructs and Vectors Engineering
Expression vectors were generated by site-specific recombination using the GATEWAY system (Invitrogen) of PCR-amplified ORFs into TAP-, green fluorescence protein (GFP)-, or hemagglutinin (HA) epitope-tagged Moloney murine leukemia virus-based vectors previously described (43, 44). FLAG epitope-tagged expression vectors were engineered by inserting GATEWAY and FLAG epitope cassettes into pSG-5 (Stratagene, LaJolla, CA). GAL4 DNA-binding domain (DBD) fusion expression vectors were generated by inserting the yeast GAL4 DBD (amino-acids 1 to 147) in place of the HA epitope tag in our HA epitope-tagged Moloney murine leukemia virus-based vector. Glutathione S-transferase (GST)-tagged E. coli expression vectors were derived from pGEX-4T1 (Amersham Biosciences) (44). Full-length open reading frames of all cDNAs were PCR amplified from IMAGE cDNA clones purchased from imaGenes GmbH, cloned into the GATEWAY entry vector pDONR201 (Invitrogen) and sequence verified. Entry clones were then recombined into suitable expression vectors by GATEWAY LR reactions.
The inserted open reading frames (ORFs) were subcloned from the following human Mammalian Gene Collection (MGC) cDNA clones: CBX4, IMAGE:4856728; CBX6, IMAGE:4554483; CBX8, IMAGE:2821280; BMI1, IMAGE:4138748; PCGF1, IMAGE:3621400; PCGF2, IMAGE:3841545; PCGF3, IMAGE:40006331; PCGF5, IMAGE:5199133; PCGF6, IMAGE:3461513; PHC1, IMAGE:5788132; PHC2, IMAGE:40146661; PHC3, IMAGE:40123356; RING1, IMAGE:3841545; RNF2, IMAGE:4285715; CSNK2A1, IMAGE:3908058; CSNK2B, IMAGE:8327485; 14-3-3ζ, IMAGE:2988020; 14-3-3ε, IMAGE:2900956. CBX2 full length cDNA was reconstructed from the clone IMAGE:3626683 and genomic DNA covering the 3′ cDNA part. cDNA for CBX7 was kindly provided by David Bernard and Jesus Gil.
The S164A mutant version of CBX4 was generated by PCR-base site-specific mutagenesis of CBX4 cloned into the pDONR201 vector using the primers TA112_mut5: GCGCAGCATCGCCACCCCCACC and TA112_mut3: GGTGGGGGTGGCGATGCTGCGC.
Cell Culture and Stable Cell Lines
HeLa (Human cervix epitheloid carcinoma - ECACC), U2–0S (Human osteosarcoma - ECACC), and HEK293 (ECACC) cell lines were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (Invitrogen), 100 U/ml penicillin (Invitrogen), and 100 μg/ml streptomycin (Invitrogen) at 37 °C in humidity-saturated 5% CO2 atmosphere.
Retroviral stable cell lines were generated according to the following procedure. Phoenix amphotropic packaging cells (3 × 105 cells/well) were seeded in a 6-well plate and transfected 24 h later with 0.8 μg of retroviral plasmid using Exgen 500 (Euromedex) following the instructions of the manufacturer. Following 48 h virus-containing supernatant was filtered through a 0.45-μm-pore-size filter. HeLa or U2-OS cells (105 each) were seeded in a 6-well plate and transduced with 3 ml filtered virus supernatant in the presence of 8 μg/ml of Polybrene for an infectious round of 24 h. Cells were then incubated for 24 h in normal medium. The polyclonal population of cells was then selected with 1 μg/ml of puromycin. Growing cells were then tested for recombinant protein expression using immunocytochemistry for TAP-tagged protein expression or immunofluorescence for GFP-tagged protein expression.
Antibodies and Western Blotting
The following primary antibodies were used for Western blotting: rabbit anti-PCGF3 antibody (ARP34416, Euromedex (France); used at a dilution of 1:8,000), rabbit anti-RING1 antibody (ab32807, Abcam (Cambridge, MA); used at a dilution of 1:300), rabbit anti-FLAG antibody (F7425, Sigma-Aldrich; used at a dilution of 1:2,000), rabbit anti-HA antibody (HA.11, PRB-101C-0200, Eurogentec (San Diego,CA); used at a dilution of 1:5,000), mouse anti-GFP antibody (JL-8, 632381, Clontech (Mountain View, CA); used at a dilution of 1:6,000). Secondary antibodies were: horseradish peroxidase-congugated goat anti-mouse antibody (115-035-003, Jackson ImmunoResearch (West Grove, PA); used at a dilution of 1:10,000), horseradish peroxidase-conjugated donkey anti-rabbit antibody (111-035-003, Jackson ImmunoResearch; used at a dilution of 1:10,000). The protein A moiety of the TAP tag was revealed with rabbit peroxidase anti-peroxidase antibody (P1291, Sigma; used at a dilution of 1:10,000).
For Western blotting, protein samples (50 μg) in SDS loading buffer were electrophoresed on 4–12% Bis-Tris gels (Invitrogen) and transferred to nitrocellulose membranes (Schleicher and Schuell, Dassel, Germany). The membranes were blocked in 10% milk powder in phosphate-buffered saline (PBS)-T (1X PBS with 0.1% Tween20) for 1 h at room temperature, incubated for the same time with the primary antibody in PBS-T, and washed three times 10 min in PBS-T. The membranes were then incubated with the peroxidase-conjugated secondary antibody in PBS-T for 1 h and afterward washed three times 10 min in PBS-T. Signal was detected using chemiluminescence reagent (ECL, Amersham Biosciences) on imaging film (GE Healthcare).
Tandem Affinity Purification
For tandem affinity purification (TAP), cells expressing a TAP-tagged protein were expanded into forty 15-cm dishes. At confluence (about 100 mg total protein), cells were harvested, washed with PBS, resuspended in cell lysis buffer (10 mm Tris-HCl pH 7.4, 1.5 mm MgCl2, 10 mm KCl, 25 mm NaF, 1 mm Na3VO4, 1 mm dithiothreitol [DTT] and complete protease inhibitors [Roche]) and homogenized by 20 strokes with a type B pestle. Nuclei were recovered by centrifugation 10 min at 2000 × g, resuspended in nuclear lysis buffer (50 mm Tris-HCl pH 7.4, 1.5 mm MgCl2, 420 mm NaCl, 20% glycerol, 25 mm NaF, 1 mm Na3VO4, 1 mm DTT and complete protease inhibitors [Roche]), and then incubated for 1 h in a rotation wheel at 4 °C to extract nuclear proteins. Lysates were subsequently clarified by ultracentrifugation at 100,000 × g, 1 h.
Nuclear lysates were incubated with IgG agarose beads (Sigma) for 2 h at 4 °C in a rotation wheel. Bound proteins were washed with 10 ml of lysis buffer, then with 5 ml of TEV-protease cleavage buffer (10 mm Tris-HCl pH 7.5, 100 mm NaCl, and 0.2% Nonidet P-40), and eluted by addition of 30 mg TEV protease (Invitrogen) for 2 h at 16 °C. The TEV-protease cleavage product was incubated with calmodulin Sepharose (Amersham Biosciences) in the presence of 2 mm CaCl2 for 30 min at 4 °C in a rotation wheel. Bound material was washed with 10 ml of 100 mm Tris-HCl pH8, 100 mm NaCl, 0.5 mm EDTA, 2 mm CaCl2, and calmodulin-bound proteins were then eluted by boiling in SDS loading buffer.
Mass Spectrometric Analysis
Protein eluate was separated on a 4–12% NuPAGE Novex gel (Invitrogen) and stained with Imperial Protein Stain (Pierce). The gel was sliced into 19 to 46 bands, depending on Coomassie-staining intensity, across the entire separation range of the lane. Cut bands were reduced, alkylated with iodoacetamide, and in-gel digested with trypsin (Promega) as described previously (44). In brief, gel bands were destained overnight at 4 °C in a solution containing 50 mm NH4HCO3 and 50% acetonitrile, dehydrated in acetonitrile, and dried in a vacuum centrifuge. Gel pieces were then rehydrated at 4 °C for 45 min in a digestion buffer (25 mm NH4HCO3 and 12.5 ng/μl trypsin). The supernatant was replaced by 50 μl of 25 mm NH4HCO3, and the samples were incubated overnight at 37 °C. The tryptic peptides were recovered by 10-min incubations, twice in 45% acetonitrile, 10% HCOOH and once in 95% acetonitrile, 5% HCOOH. All supernatants were pooled and dried in a vacuum centrifuge.
Each tryptic digest sample was subjected to nano-LC-nano-electrospray ionization (ESI)-MS/MS analysis on an ion trap mass spectrometer (LCQ Deca XP+, Thermo Electron Corp.), equipped with a nanoelectrospray ion source, coupled with a nano-high pressure liquid chromatography system (LC Packings Dionex). Samples were resuspended in 3 μl of 0.1% HCOOH, and 1.4 μl were injected into the mass spectrometer using a Famos autosampler (LC Packings Dionex). The samples were first desalted and then concentrated on a reverse phase precolumn of 5 mm × 0.3 mm inner diameter (Dionex) by solvent A (95% H2O, 5% acetonitrile, 0.1% HCOOH) delivered by the Switchos pumping device (LC Packings Dionex) at a flow rate of 10 μl/min for 3 min. Peptides were separated on a 15 cm × 75 μm-inner diameter C18 PepMap column (Dionex). The flow rate was set at 200 nl/min. Peptides were eluted using a 5–70% linear gradient of solvent B (20% H2O, 80% acetonitrile, 0.08% HCOOH) in 45 min. Coated nanoelectrospray needles (360 μm outer diameter, 20 μm inner diameter, 10 μm tip inner diameter, standard coating) were obtained from New Objective (Woburn, MA). Spray voltage was set at 1.5 kV, and capillary temperature was set at 170 °C. The mass spectrometer was operated in positive ionization mode.
Data acquisition was performed in a data-dependent mode consisting of, alternatively in a single run, a full-scan MS over the range m/z 500–2000 and a full MS/MS of the ion selected in an exclusion dynamic mode (the most intense ion is selected and excluded for further selection for a duration of 3 min). MS/MS data were acquired using a 2 m/z unit ion isolation window and 35% relative collision energy. MS/MS .raw data files were transformed to .dta files with the Bioworks 3.1 software (Thermo Electron Corp.). The .dta files generated were next concatenated with merge.bat (a DOS batch file for Windows) to be uploaded in Mascot public interface version 2.2.03 (www.matrixscience.com) for database searches in Swiss-Prot 55.1 (359,942 sequences; 129,199,355 residues).
Search parameters in human sequences were: three allowed missed cleavages, methionine oxidation and cysteine carbamidomethylation as variable modifications, 2 Da for peptide tolerance, and 0.8 Da for MS/MS tolerance. Results were scored using the probability-based Mowse score [the protein score is −10 × log(p), where p is the probability that the observed match is a random event]. Most proteins were unambiguously identified by the sequencing of several independent peptides. Identifications with Mascot individual ion score < 38 or with the significance threshold p > 5% (indicate identity or extensive homology) were categorically rejected. In addition, because a shared sequence may represent a problem, for single peptide identification (supplemental Table S3) all sequences obtained by MS/MS analysis were checked using the Basic Local Alignment Search Tool (BLAST) public interface (version 2.2.18) to exclude that sequence sharing with other proteins could interfere with the reliability of the identification.
In Vitro GST Protein Binding Assays
GST fusion proteins, were expressed in E. coli BL21 (DE3) and purified on glutathione-Sepharose 4B (GE Healthcare) according to the manufacturer's instructions. GST-proteins were then fixed on glutathione-Sepharose 4B and stored in STE buffer (10 mm Tris-HCl pH8, 150 mm NaCl, 1 mm EDTA and complete protease inhibitors [Roche]). Following preclearing with empty beads, nuclear extracts (∼ 500 μg proteins) were incubated with immobilized GST-fusion proteins overnight at 4 °C. Beads were washed four times with 500 μl of E1A Buffer (50 mm Hepes, pH 7.9, 1 mm EGTA, 250 mm NaCl, 1 mm DTT, 1 mm EDTA, and complete protease inhibitors [Roche]) and bound proteins were recovered with Elution Buffer (10 mm glutathione in 50 mm Tris-HCl pH 8.0), resolved by 4–12% gradient SDS-PAGE (Invitrogen) and visualized by Western blotting using the peroxidase-anti-peroxidase (PAP) antibody (Sigma) which recognizes the protein A moiety of the TAP tag. Input material corresponds to 2% of the material used in the binding assays.
Nuclear extracts used for GST-pull downs were prepared from HEK293 cells transiently expressing TAP-tagged proteins. Transfections were performed using Exgen 500 (Euromedex) following the instructions of the manufacturer. Forty eight hours following transfection, cells were lysed in low salt buffer (10 mm Tris-HCl pH 7.4, 25 mm NaCl, 2 mm MgOAc, 1 mm DTT, 1 mm EDTA, 0.05% Nonidet P-40, and complete protease inhibitors [Roche]) for 15 min at 4 °C. Nuclei were pelleted by centrifugation at 4 °C for 10 min at 800 g, and lysed in E1A buffer during 2 h by gently shaking at 4 °C. Nuclear proteins were recovered by centrifugation at 2500 × g, 10 min at 4 °C.
In vitro translated proteins used for GST-pull downs were produced with the TnT7 Quick Coupled Transcription/Translation System (Promega). Immobilized GST-fusion proteins were incubated with in vitro translated proteins overnight at 4 °C in IP buffer (50 mm Tris-HCl pH7.5, 500 mm NaCl, 1 mm EDTA, 0.5% Nonidet P-40, 10% glycerol, and complete protease inhibitors [Roche]). The beads were washed four times with IP buffer and resuspended in loading buffer (12 mm Tris-HCl pH 6.8, 10% glycerol, 0.4% SDS, 80 mm DTT, and 0.025% bromphenol blue). Bound proteins were resolved by SDS-PAGE and visualized by Western blotting.
Analytical Gel Filtration Chromatography
Cells were lysed in low salt buffer (10 mm Tris-HCl pH 7.4, 25 mm NaCl, 2 mm MgOAc, 1 mm DTT, 1 mm EDTA, 0.05% Nonidet P-40, and complete protease inhibitors [Roche]) for 15 min at 4 °C. Nuclei were pelleted by centrifugation at 4 °C for 10 min at 800 g, and lysed in E1A buffer during 2 h by gently shaking at 4 °C. Nuclear extracts were recovered by centrifugation at 2500 × g, 10 min at 4 °C and clarified by two centrifugations at 10,000 × g for 10 min. One mg of fresh nuclear extract were fractionated on a Superose 6 HR 10/300 GL column (GE Healthcare) equilibrated in 50 mm Tris-HCl pH 7.4, 100 mm KCl. Size exclusion chromatography was performed on a fast protein liquid chromatography (FPLC) system and an ÄKTATM purifier (GE Healthcare). Elution profiles of blue dextran (V0), thyroglobulin (669 kDa), apoferritin (443 kDa), β-amylase (200 kDa), alcohol dehydrogenase (150 kDa), bovine serum albumin (66 kDa), and carbonic anhydrase (29 kDa, all from Sigma) were used for calibration. All experiments were run at room temperature. Fractions of 0.5 ml were collected and precipitated with 25% trichloroacetic acid and then centrifuged at 14,000 × g for 10 min at 4 °C. Pellets were washed two times in cold acetone, air dried, and resuspended in loading buffer for Western blot analysis.
Co-immunoprecipitations
HEK293 cells (6 × 105 cells) were transfected with 0.5 μg of each expression vectors using Exgen 500 (Euromedex) following the instructions of the manufacturer. Forty height hours later, transfected cells were washed twice with cold PBS and lyzed in Co-IP buffer (50 mm Tris-HCl pH 8.0, 5% glycerol, 1.5 mm MgCl2, 100 mm NaCl, 25 mm NaF, 1 mm Na3VO4, 1 mm DTT, 0.2% Nonidet P-40) for 10 min at 4 °C. Protein extracts were recovered by centrifugation at 17,500 × g for 30 min at 4 °C and incubated with 20 μl of anti-HA antibodies linked to agarose beads (E6779, Sigma-Aldrich) or anti-FLAG agarose bead-linked antibodies (A2220, Sigma-Aldrich) for 4 h in a rotation wheel at 4 °C. Bound material was washed four times with 1 ml Co-IP buffer before Western blotting.
Fluorescence Imaging
GFP-expressing cells were seeded on coverslips and fixed with 4% paraformaldehyde for 20 min at room temperature and washed twice with PBS. Nuclei were stained with 10 μg/ml Hoechst 33342 for 1 min before being mounted for microscopy. Images were acquired using a Nikon Eclipse 80i epifluorescence microscope.
Repression Assays
GAL4(DBD)-CBX expression vectors were generated by GATEWAY-based site-specific recombination of CBX cDNAs from pDONR201 entry clones into the pNGAL4-GW expression plasmid. The firefly luciferase pGL3-derived reporters were kindly provided by Didier Monté (Interdisciplinary Research Institute, Villeneuve d'Ascq, France); pUAS-bax(337)-luc contains five UAS binding sites for GAL4 DBD upstream of the 337 pb bax promoter driving the expression of the firefly reporter gene. pUAS-p21(2278)-luc possesses five UAS (GAL4) sites upstream of 2278 pb p21 promoter driving the luciferase gene. HEK293 cells (3 × 105 cells) were plated in a 6-cm Petri dish the day before transfection. All transfections were done using Exgen 500 (Euromedex) following the instructions of the manufacturer with 100 ng of GAL4(DBD) expression vector, 100 ng of luciferase reporter plasmid, and 5 ng of reference pUbi-Rluc plasmid. pUbi-Rluc contains the ubiquitin C promoter driving the expression of the Renilla luciferase. Cells were harvested 48 h following transfection and both firefly luciferase and Renilla luciferase expression assayed with the Dual Luciferase Assay Kit (Promega) and measured using a luminometer (Centro LB 960, Berthold). Firefly luciferase expression values were standardized against Renilla luciferase expression from pUbi-Rluc reference plasmid. Luciferase expression values obtained with the reporter plasmid in the presence of GAL-GFP effector plasmid were set up to 100 arbitrary units, and luciferase levels in cells transfected with GAL4-CBX are expressed relative to those obtained with the GAL4-GFP vector. The results represent the mean values of at least three independent experiments.
RESULTS
Purification of TAP-tagged Chromodomain-containing Polycomb Proteins
To gain insights into the organization and the function of the PRC1 protein complexes and in order to identify the interacting partners of the different Pc orthologs, we applied a tandem affinity purification (TAP) coupled to tandem mass spectrometry (MS/MS) technology to the five Polycomb CBX proteins. This approach has indeed proven to efficiently allow the characterization of protein complexes from different cells in culture or organisms (45, 46). TAP-MS/MS analyses of protein associations around human CBX proteins were performed under the same experimental conditions from HeLa cells. HeLa cells were chosen because we have assembled a database of more than 30 TAP-MS/MS experiments from proteins involved in chromatin regulation in this cell line. This dataset allows reliable assessment of a given protein interaction (data not shown).
A retrovirus gene transfer strategy was used to generate cell pools stably expressing N-terminally TAP-tagged CBX proteins. To assess whether TAP-tagged CBX proteins are able to associate into protein complexes, size-exclusion chromatography experiments were performed. First, antibodies directed against two components of the PRC1, RING1, and PCGF3 were used to identify PRC1-like-containing fractions. RING1 elutes in fractions of ∼200 to 1000 kDa, whereas PCGF3 is associated to fractions of molecular weight higher than 1 MDa (Fig. 1A). Thus, PRC1 components are associated to different protein complexes eluting in fractions of ∼200 to 1000 kDa and of molecular weight higher than 1 MDa. Gel filtration analyses of nuclear-cell extracts derived from TAP-tagged cell lines showed that most CBX proteins are present in fractions of molecular weight higher than 1 MDa, and smaller protein amounts are also present in fractions of ∼200 to 500 kDa (Fig. 1A). This shows that N-terminal extension of the CBX proteins with the TAP-tag does not prevent the formation of high-molecular-weight protein complexes. The size-exclusion chromatography patterns of CBX TAP-tagged proteins correspond to patterns containing PRC1-like protein complexes and are in agreement with previous studies (28, 47). In addition, overexpression of the TAP-tagged CBX proteins does not lead to accumulation of free, unbound, proteins.
Fig. 1.
Tandem affinity purification of CBX family proteins and identification of associated polypeptides. A, Superose 6 gel filtration of nuclear extracts derived from HeLa cell lines stably expressing TAP-CBX2, TAP-CBX4, TAP-CBX6, TAP-CBX7, or TAP-CBX8. Fractions were analyzed by Western blotting using a peroxidase anti-peroxidase (αPAP) antibody to reveal the protein A moiety of the TAP tag. Superose 6 gel filtration profiles obtained from HeLa nuclear cell extracts for the endogenous PRC1 components RING1 and PCGF3 are shown. B, TAP-CBX2, TAP-CBX4, TAP-CBX6, TAP-CBX7, and TAP-CBX8 purifications from HeLa cells. Protein complexes were tandem affinity purified from stable cell lines, separated by SDS-PAGE, and stained with colloidal Coomassie. Some of the co-eluted proteins identified by LC-MS/MS are indicated. Position in the TAP gel of the bait CBX proteins is shown with an arrow and PRC1 known components are indicated in italic.
Expended cell pools were then subjected to tandem affinity protein complex purification using two specific binding and two specific elution steps under mild conditions, which preserve the integrity of nontransient protein-protein interactions (42). The affinity purified protein complexes were resolved on SDS-PAGE and Coomassie stained (Fig. 1B). The bands were excised from the gel, subjected to trypsin digestion and proteins were identified by peptide sequence determination using tandem mass spectrometry. Protein purifications were performed twice and most proteins were characterized by the identification of several peptides (supplemental Table S1). Details on protein identification procedures are described in the Experimental Procedures section. Background proteins such as ribosomal proteins, cytoskeletal proteins like tubulins, actins, and keratins, as well as proteins identified in similar unrelated TAP analyses from HeLa cells were excluded from the list of CBX interacting partners (See supplemental Table S2). Based on these criteria, around 20 proteins were identified in the CBX2 and CBX7 purifications, around 40 in the CBX4 purification, whereas about 60 proteins were co-eluted with CBX6 and CBX8 (Table I).
Table I. Proteins recovered by CBX-TAP from HeLa cells.
| Bait | Experiment | Accessiona | Protein description | Mass (Da) | Scoreb | Petidesc |
|---|---|---|---|---|---|---|
| CBX2 | Exp. 1 | ANR26_HUMAN | Ankyrin repeat domain-containing protein 26 | 196202 | 39 | 2 |
| CBX2 | Exp. 2 | BMI1_HUMAN | Polycomb complex protein BMI-1 | 36925 | 201 | 4 |
| CBX2 | Exp. 1/Exp. 2 | CBX2_HUMAN | Chromobox protein homolog 2 | 56046 | 583/379 | 43/26 |
| CBX2 | Exp. 2 | CENPE_HUMAN | Centromeric protein E | 316219 | 62 | 6 |
| CBX2 | Exp. 1/Exp. 2 | CSK21_HUMAN | Casein kinase II subunit α | 45115 | 136/66 | 2/2 |
| CBX2 | Exp. 2 | CSK22_HUMAN | Casein kinase II subunit α | 41187 | 94 | 1 |
| CBX2 | Exp. 2 | CSK2B_HUMAN | Casein kinase II subunit β | 24926 | 136 | 3 |
| CBX2 | Exp. 2 | DI3L2_HUMAN | DIS3-like exonuclease 2 | 99147 | 43 | 2 |
| CBX2 | Exp. 2 | GTF2I_HUMAN | General transcription factor II-I | 112346 | 211 | 4 |
| CBX2 | Exp. 2 | H2A1B_HUMAN | Histone H2A type 1-B | 14127 | 80 | 6 |
| CBX2 | Exp. 2 | H2B1C_HUMAN | Histone H2B type 1-C/E/F/G/I | 13811 | 98 | 4 |
| CBX2 | Exp. 2 | H4_HUMAN | Histone H4 | 11360 | 57 | 1 |
| CBX2 | Exp. 1 | IQWD1_HUMAN | Nuclear receptor interaction protein | 96292 | 48 | 2 |
| CBX2 | Exp. 1 | JHD2A_HUMAN | JmjC domain-containing histone demethylation protein 2A | 147234 | 49 | 3 |
| CBX2 | Exp. 1 | LBA1_HUMAN | Lupus brain antigen 1 homolog | 336007 | 64 | 3 |
| CBX2 | Exp. 1 | MYCB2_HUMAN | Probable E3 ubiquitin-protein ligase MYCBP2 | 509759 | 39 | 5 |
| CBX2 | Exp. 1 | NPM_HUMAN | Nucleophosmin | 32555 | 51 | 1 |
| CBX2 | Exp. 1 | PAR12_HUMAN | Poly [ADP-ribose] polymerase 12 | 79013 | 44 | 2 |
| CBX2 | Exp. 2 | PARP1_HUMAN | Poly [ADP-ribose] polymerase 1 | 113012 | 113 | 2 |
| CBX2 | Exp. 2 | PHC2_HUMAN | Polyhomeotic-like protein 2 | 90657 | 350 | 7 |
| CBX2 | Exp. 1/Exp. 2 | PSME3_HUMAN | Proteasome activator complex subunit 3 | 29488 | 280/195 | 5/8 |
| CBX2 | Exp. 1/Exp. 2 | RING2_HUMAN | E3 ubiquitin-protein ligase RING2 | 37632 | 281/429 | 7/8 |
| CBX2 | Exp. 1 | RN213_HUMAN | RING finger protein 213 | 373742 | 57 | 5 |
| CBX2 | Exp. 1 | YD007_HUMAN | Coiled-coil domain-containing protein FLJ25770 | 127062 | 41 | 6 |
| CBX2 | Exp. 1 | ZN167_HUMAN | Zinc finger protein 167 | 84979 | 48 | 2 |
| CBX4 | Exp. 1/Exp. 2 | 1433B_HUMAN | 14-3-3 protein β/α | 28065 | 154/329 | 8/21 |
| CBX4 | Exp. 1/Exp. 2 | 1433E_HUMAN | 14-3-3 protein ϵ | 29155 | 566/1151 | 25/97 |
| CBX4 | Exp. 1/Exp. 2 | 1433F_HUMAN | 14-3-3 protein η | 28201 | 79/670 | 2/42 |
| CBX4 | Exp. 1/Exp. 2 | 1433G_HUMAN | 14-3-3 protein γ | 28285 | 188/467 | 8/36 |
| CBX4 | Exp. 2 | 1433S_HUMAN | 14-3-3 protein σ | 27757 | 151 | 7 |
| CBX4 | Exp. 1/Exp. 2 | 1433T_HUMAN | 14-3-3 protein θ | 27747 | 182/600 | 9/20 |
| CBX4 | Exp. 1/Exp. 2 | 1433Z_HUMAN | 14-3-3 protein ζ/Δ | 27728 | 254/572 | 10/44 |
| CBX4 | Exp. 1 | ADT2_HUMAN | ADP/ATP translocase 2 | 32874 | 143 | 3 |
| CBX4 | Exp. 1/Exp. 2 | BMI1_HUMAN | Polycomb complex protein BMI-1 | 36925 | 212/337 | 7/18 |
| CBX4 | Exp. 1/Exp. 2 | CBX4_HUMAN | E3 SUMO-protein ligase CBX4 | 61190 | 266/641 | 66/137 |
| CBX4 | Exp. 2 | CENPF_HUMAN | Centromere protein F | 367537 | 46 | 4 |
| CBX4 | Exp. 2 | CHD7_HUMAN | Chromodomain-helicase-DNA-binding protein 7 | 335717 | 42 | 4 |
| CBX4 | Exp. 1 | CSK21_HUMAN | Casein kinase II subunit α | 45115 | 169 | 3 |
| CBX4 | Exp. 1 | CSK22_HUMAN | Casein kinase II subunit α | 41187 | 46 | 1 |
| CBX4 | Exp. 1/Exp. 2 | CSK2B_HUMAN | Casein kinase II subunit β | 24926 | 59/107 | 1/2 |
| CBX4 | Exp. 1 | DDEF1_HUMAN | 130 kDa phosphatidylinositol 4,5-biphosphate-dependent ARF1 GTPase-activating protein | 125393 | 49 | 2 |
| CBX4 | Exp. 2 | ERCC6_HUMAN | DNA excision repair protein ERCC-6 | 168416 | 43 | 3 |
| CBX4 | Exp. 2 | FER_HUMAN | Proto-oncogene tyrosine-protein kinase FER | 94638 | 64 | 2 |
| CBX4 | Exp. 1 | H11_HUMAN | Histone H1.1 | 21829 | 41 | 4 |
| CBX4 | Exp. 2 | KHDR2_HUMAN | KH domain-containing, RNA-binding, signal transduction-associated protein 2 | 38927 | 46 | 2 |
| CBX4 | Exp. 2 | KI67_HUMAN | Antigen KI-67 | 358474 | 60 | 4 |
| CBX4 | Exp. 2 | MDM1_HUMAN | Nuclear protein MDM1 | 80686 | 41 | 2 |
| CBX4 | Exp. 1 | MOV10_HUMAN | Putative helicase MOV-10 | 113599 | 44 | 1 |
| CBX4 | Exp. 1/Exp. 2 | PCGF2_HUMAN | Polycomb group RING finger protein 2 | 37764 | 104/143 | 3/12 |
| CBX4 | Exp. 1/Exp. 2 | PCGF6_HUMAN | Polycomb group RING finger protein 6 | 39023 | 63/78 | 1/1 |
| CBX4 | Exp. 1 | PHC1_HUMAN | Polyhomeotic-like protein 1 | 105353 | 40 | 1 |
| CBX4 | Exp. 1/Exp. 2 | PHC2_HUMAN | Polyhomeotic-like protein 2 | 90657 | 141/628 | 4/19 |
| CBX4 | Exp. 1 | PHC3_HUMAN | Polyhomeotic-like protein 3 | 106096 | 103 | 4 |
| CBX4 | Exp. 1/Exp. 2 | RING1_HUMAN | E3 ubiquitin-protein ligase RING1 | 42403 | 345/379 | 12/8 |
| CBX4 | Exp. 1/Exp. 2 | RING2_HUMAN | E3 ubiquitin-protein ligase RING2 | 37632 | 284/598 | 7/19 |
| CBX4 | Exp. 2 | RNF17_HUMAN | RING finger protein 17 | 184643 | 43 | 2 |
| CBX4 | Exp. 2 | RUVB2_HUMAN | RuvB-like 2 | 51125 | 84 | 2 |
| CBX4 | Exp. 1 | SMC3_HUMAN | Structural maintenance of chromosomes protein 3 | 141454 | 61 | 5 |
| CBX4 | Exp. 1 | SMG1_HUMAN | Serine/threonine-protein kinase SMG1 | 410261 | 46 | 3 |
| CBX4 | Exp. 2 | SSX10_HUMAN | Protein SSX10 | 17575 | 42 | 2 |
| CBX4 | Exp. 1 | STALP_HUMAN | AMSH-like protease | 49783 | 38 | 2 |
| CBX4 | Exp. 2 | WDHD1_HUMAN | WD repeat and HMG-box DNA-binding protein 1 | 125888 | 41 | 4 |
| CBX4 | Exp. 2 | YD007_HUMAN | Coiled-coil domain-containing protein FLJ25770 | 127062 | 38 | 3 |
| CBX4 | Exp. 1 | YR010_HUMAN | Putative uncharacterized protein ENSP00000344348 | 46232 | 42 | 3 |
| CBX4 | Exp. 2 | ZN546_HUMAN | Zinc finger protein 546 | 98369 | 40 | 2 |
| CBX6 | Exp. 2 | AHDC1_HUMAN | A.T hook DNA-binding motif-containing protein 1 | 168245 | 41 | 3 |
| CBX6 | Exp. 2 | BPTF_HUMAN | Nucleosome-remodeling factor subunit BPTF | 324019 | 49 | 3 |
| CBX6 | Exp. 2 | BRWD1_HUMAN | Bromodomain and WD repeat-containing protein 1 | 262756 | 40 | 3 |
| CBX6 | Exp. 2 | CAF1A_HUMAN | Chromatin assembly factor 1 subunit A | 105223 | 47 | 2 |
| CBX6 | Exp. 2 | CASC5_HUMAN | Protein CASC5 | 265193 | 43 | 5 |
| CBX6 | Exp. 1/Exp. 2 | CBX6_HUMAN | Chromobox protein homolog 2 | 43871 | 375/572 | 72/96 |
| CBX6 | Exp. 2 | CD014_HUMAN | Uncharacterized protein C4orf14 | 78409 | 64 | 1 |
| CBX6 | Exp. 2 | CE350_HUMAN | Centrosome-associated protein 350 | 350716 | 46 | 3 |
| CBX6 | Exp. 1 | CP135_HUMAN | Centrosomal protein of 135 kDa | 133422 | 43 | 2 |
| CBX6 | Exp. 2 | DACT1_HUMAN | Dapper homolog 1 | 90119 | 48 | 2 |
| CBX6 | Exp. 2 | DNMT1_HUMAN | DNA (cytosine-5)-methyltransferase 1 | 183050 | 45 | 3 |
| CBX6 | Exp. 2 | ELP4_HUMAN | Elongator complex protein 4 | 46588 | 59 | 3 |
| CBX6 | Exp. 2 | ESF1_HUMAN | ESF1 homolog | 98735 | 69 | 1 |
| CBX6 | Exp. 2 | GNL3_HUMAN | Guanine nucleotide-binding protein-like 3 | 61958 | 107 | 3 |
| CBX6 | Exp. 2 | H12_HUMAN | Histone H1.2 | 21352 | 45 | 1 |
| CBX6 | Exp. 2 | H14_HUMAN | Histone H1.4 | 21852 | 44 | 2 |
| CBX6 | Exp. 2 | H1X_HUMAN | Histone H1x | 22474 | 50 | 1 |
| CBX6 | Exp. 2 | H2A1B_HUMAN | Histone H2A type 1-B | 14127 | 43 | 1 |
| CBX6 | Exp. 2 | H2B1B_HUMAN | Histone H2B type 1-B | 13942 | 118 | 2 |
| CBX6 | Exp. 2 | H31T_HUMAN | Histone H3.1t | 15499 | 47 | 3 |
| CBX6 | Exp. 1/Exp. 2 | H4_HUMAN | Histone H4 | 11360 | 68/54 | 1/1 |
| CBX6 | Exp. 2 | IF2B1_HUMAN | Insulin-like growth factor 2 mRNA-binding protein 1 | 63417 | 576 | 15 |
| CBX6 | Exp. 2 | IF2B2_HUMAN | Insulin-like growth factor 2 mRNA-binding protein 1 | 61804 | 89 | 3 |
| CBX6 | Exp. 1/Exp. 2 | IF2B3_HUMAN | Insulin-like growth factor 2 mRNA-binding protein 1 | 63681 | 322/39 | 8/1 |
| CBX6 | Exp. 2 | ILF2_HUMAN | Interleukin enhancer-binding factor 2 | 43035 | 284 | 4 |
| CBX6 | Exp. 2 | ILF3_HUMAN | Interleukin enhancer-binding factor 3 | 95279 | 162 | 4 |
| CBX6 | Exp. 2 | KHDR1_HUMAN | KH domain-containing, RNA-binding, signal transduction-associated protein 1 | 48197 | 49 | 3 |
| CBX6 | Exp. 2 | KRI1_HUMAN | Protein KRI1 homolog | 83201 | 224 | 4 |
| CBX6 | Exp. 2 | LA_HUMAN | Lupus La protein | 46808 | 88 | 1 |
| CBX6 | Exp. 2 | LAS1L_HUMAN | LAS1-like protein | 83013 | 126 | 2 |
| CBX6 | Exp. 2 | LTV1_HUMAN | Protein LTV1 homolog | 54821 | 93 | 3 |
| CBX6 | Exp. 2 | MOV10_HUMAN | Putative helicase MOV-10 | 113599 | 254 | 6 |
| CBX6 | Exp. 2 | MPPH1_HUMAN | M-phase phosphoprotein 1 | 210553 | 49 | 7 |
| CBX6 | Exp. 2 | NCOR2_HUMAN | Nuclear receptor corepressor 2 | 274034 | 49 | 4 |
| CBX6 | Exp. 2 | NUCL_HUMAN | Nucleolin | 76568 | 238 | 4 |
| CBX6 | Exp. 2 | NUMA1_HUMAN | Nuclear mitotic apparatus protein 1 | 238260 | 45 | 2 |
| CBX6 | Exp. 2 | PABP1_HUMAN | Polyadenylate-binding protein 1 | 70626 | 155 | 3 |
| CBX6 | Exp. 2 | PHC2_HUMAN | Polyhomeotic-like protein 2 | 90657 | 61 | 1 |
| CBX6 | Exp. 2 | PPR3A_HUMAN | Protein phosphatase 1 regulatory subunit 3A | 125759 | 52 | 3 |
| CBX6 | Exp. 2 | RAD17_HUMAN | Cell cycle checkpoint protein RAD17 | 77006 | 41 | 2 |
| CBX6 | Exp. 2 | RAD26_HUMAN | Putative DNA repair and recombination protein RAD26-like | 81094 | 42 | 2 |
| CBX6 | Exp. 2 | RGAG1_HUMAN | Retrotransposon gag domain-containing protein 1 | 144280 | 58 | 2 |
| CBX6 | Exp. 1 | RING1_HUMAN | E3 ubiquitin-protein ligase RING1 | 42403 | 73 | 1 |
| CBX6 | Exp. 2 | RING2_HUMAN | E3 ubiquitin-protein ligase RING2 | 37632 | 91 | 2 |
| CBX6 | Exp. 2 | SCML2_HUMAN | Sex comb on midleg-like protein 2 | 77257 | 40 | 3 |
| CBX6 | Exp. 2 | THOC4_HUMAN | THO complex subunit 4 | 26872 | 64 | 1 |
| CBX6 | Exp. 2 | UBE2O_HUMAN | Ubiquitin-conjugating enzyme E2 O | 141265 | 221 | 5 |
| CBX6 | Exp. 2 | ULK2_HUMAN | Serine/threonine-protein kinase ULK2 | 112654 | 41 | 3 |
| CBX6 | Exp. 2 | WD40C_HUMAN | WD repeat-containing protein 40C | 50803 | 45 | 4 |
| CBX6 | Exp. 1/Exp. 2 | YBOX1_HUMAN | Nuclease-sensitive element-binding protein 1 | 35903 | 237/127 | 8/3 |
| CBX6 | Exp. 2 | YC009_HUMAN | Uncharacterized protein FLJ36157 | 190890 | 52 | 3 |
| CBX6 | Exp. 2 | ZMYM4_HUMAN | Zinc finger MYM-type protein 4 | 172677 | 43 | 3 |
| CBX6 | Exp. 1/Exp. 2 | ZN33A_HUMAN | Zinc finger protein 33A | 94384 | 45/42 | 3/3 |
| CBX6 | Exp. 2 | ZN33B_HUMAN | Zinc finger protein 33B | 90683 | 60 | 3 |
| CBX7 | Exp. 1/Exp. 2 | BMI1_HUMAN | Polycomb complex protein BMI-1 | 36925 | 199/100 | 4/3 |
| CBX7 | Exp. 1/Exp. 2 | CBX7_HUMAN | Chromobox protein homolog 7 | 28323 | 232/55 | 10/1 |
| CBX7 | Exp. 2 | CSK21_HUMAN | Casein kinase II subunit α | 45115 | 255 | 3 |
| CBX7 | Exp. 2 | CSK2B_HUMAN | Casein kinase II subunit β | 24926 | 103 | 2 |
| CBX7 | Exp. 1/Exp. 2 | PCGF2_HUMAN | Polycomb group RING finger protein 2 | 37764 | 75/47 | 4/2 |
| CBX7 | Exp. 1/Exp. 2 | PCGF6_HUMAN | Polycomb group RING finger protein 6 | 39023 | 95/56 | 2/3 |
| CBX7 | Exp. 1/Exp. 2 | PHC2_HUMAN | Polyhomeotic-like protein 2 | 90657 | 111/66 | 3/2 |
| CBX7 | Exp. 1 | RAD17_HUMAN | Cell cycle checkpoint protein RAD17 | 77055 | 45 | 2 |
| CBX7 | Exp. 1/Exp. 2 | RING1_HUMAN | E3 ubiquitin-protein ligase RING1 | 42403 | 384/334 | 8/7 |
| CBX7 | Exp. 1/Exp. 2 | RING2_HUMAN | E3 ubiquitin-protein ligase RING2 | 37632 | 627/571 | 15/12 |
| CBX7 | Exp. 1 | SPEC1_HUMAN | Sperm antigen with calponin homology and coiled-coil domains 1 | 118512 | 48 | 3 |
| CBX7 | Exp. 2 | SRBD1_HUMAN | S1 RNA-binding domain-containing protein 1 | 111705 | 39 | 3 |
| CBX7 | Exp. 1 | TPR_HUMAN | Nucleoprotein TPR | 267293 | 45 | 3 |
| CBX7 | Exp. 2 | UBE4A_HUMAN | Ubiquitin conjugation factor E4 A | 122561 | 41 | 2 |
| CBX7 | Exp. 2 | UBP20_HUMAN | Ubiquitin carboxyl-terminal hydrolase 20 | 101938 | 46 | 1 |
| CBX7 | Exp. 2 | WDR68_HUMAN | WD repeat-containing protein 68 | 38901 | 41 | 2 |
| CBX8 | Exp. 2 | BCLF1_HUMAN | Bcl-2-associated transcription factor 1 | 106059 | 84 | 2 |
| CBX8 | Exp. 1/Exp. 2 | BMI1_HUMAN | Polycomb complex protein BMI-1 | 36925 | 154/122 | 9/13 |
| CBX8 | Exp. 1/Exp. 2 | CBX8_HUMAN | Chromobox protein homolog 8 | 43369 | 866/803 | 217/143 |
| CBX8 | Exp. 1/Exp. 2 | CSK21_HUMAN | Casein kinase II subunit α | 45115 | 63/236 | 1/6 |
| CBX8 | Exp. 1/Exp. 2 | CSK2B_HUMAN | Casein kinase II subunit β | 24926 | 76/111 | 2/2 |
| CBX8 | Exp. 1/Exp. 2 | DBPA_HUMAN | DNA-binding protein A | 40066 | 213/218 | 8/4 |
| CBX8 | Exp. 2 | DGKZ_HUMAN | Diacylglycerol kinase ζ | 124044 | 67 | 5 |
| CBX8 | Exp. 1 | DIMT1_HUMAN | Probable dimethyladenosine transferase | 35214 | 253 | 4 |
| CBX8 | Exp. 2 | DNL3_HUMAN | DNA ligase 3 | 102625 | 44 | 6 |
| CBX8 | Exp. 2 | EP300_HUMAN | Histone acetyltransferase p300 | 263973 | 43 | 2 |
| CBX8 | Exp. 2 | ESCO2_HUMAN | N-acetyltransferase ESCO2 | 68264 | 58 | 2 |
| CBX8 | Exp. 2 | FBRL_HUMAN | rRNA 2′-O-methyltransferase fibrillarin | 33763 | 94 | 2 |
| CBX8 | Exp. 1 | GBLP_HUMAN | Guanine nucleotide-binding protein subunit beta-2-like 1 | 35055 | 110 | 2 |
| CBX8 | Exp. 1/Exp. 2 | H2A1B_HUMAN | Histone H2A type 1-B | 14127 | 62/42 | 2/1 |
| CBX8 | Exp. 2 | H2B1A_HUMAN | Histone H2B type 1-A | 14159 | 40 | 2 |
| CBX8 | Exp. 1/Exp. 2 | H2B1B_HUMAN | Histone H2B type 1-B | 13942 | 116/85 | 2/2 |
| CBX8 | Exp. 1/Exp. 2 | H4_HUMAN | Histone H4 | 11360 | 143/66 | 6/4 |
| CBX8 | Exp. 1/Exp. 2 | HLF_HUMAN | Hepatic leukemia factor | 33199 | 59/40 | 3/3 |
| CBX8 | Exp. 1/Exp. 2 | IF2B1_HUMAN | Insulin-like growth factor 2 mRNA-binding protein 1 | 63417 | 365/169 | 6/6 |
| CBX8 | Exp. 1/Exp. 2 | IF2B2_HUMAN | Insulin-like growth factor 2 mRNA-binding protein 2 | 61804 | 65/46 | 2/1 |
| CBX8 | Exp. 2 | IF2B3_HUMAN | Insulin-like growth factor 2 mRNA-binding protein 3 | 63681 | 49 | 2 |
| CBX8 | Exp. 1/Exp. 2 | ILF2_HUMAN | Interleukin enhancer-binding factor 2 | 43035 | 381/65 | 7/2 |
| CBX8 | Exp. 1/Exp. 2 | ILF3_HUMAN | Interleukin enhancer-binding factor 3 | 95279 | 146/76 | 2/2 |
| CBX8 | Exp. 2 | JIP4_HUMAN | C-jun-amino-terminal kinase-interacting protein 4 | 146115 | 48 | 2 |
| CBX8 | Exp. 2 | K0020_HUMAN | Pumilio domain-containing protein KIAA0020 | 73538 | 47 | 1 |
| CBX8 | Exp. 1/Exp. 2 | KRI1_HUMAN | Protein KRI1 homolog | 83201 | 241/100 | 4/1 |
| CBX8 | Exp. 2 | KRR1_HUMAN | KRR1 small subunit processome component homolog | 43638 | 91 | 2 |
| CBX8 | Exp. 2 | LTV1_HUMAN | Protein LTV1 homolog | 54821 | 127 | 3 |
| CBX8 | Exp. 1 | MK67I_HUMAN | MKI67 FHA domain-interacting nucleolar phosphoprotein | 34201 | 67 | 1 |
| CBX8 | Exp. 1/Exp. 2 | MOV10_HUMAN | Putative helicase MOV-10 | 113599 | 364/268 | 9/6 |
| CBX8 | Exp. 2 | MPPH1_HUMAN | M-phase phosphoprotein 1 | 210553 | 42 | 3 |
| CBX8 | Exp. 2 | NBN_HUMAN | Nibrin | 84906 | 48 | 2 |
| CBX8 | Exp. 2 | NCOAT_HUMAN | Bifunctional protein NCOAT | 102849 | 59 | 4 |
| CBX8 | Exp. 2 | NFIL3_HUMAN | Nuclear factor interleukin-3-regulated protein | 51472 | 53 | 2 |
| CBX8 | Exp. 1 | NOG1_HUMAN | Nucleolar GTP-binding protein 1 | 73918 | 71 | 2 |
| CBX8 | Exp. 1/Exp. 2 | NOL1_HUMAN | Putative RNA methyltransferase NOL1 | 89247 | 105/62 | 2/1 |
| CBX8 | Exp. 2 | NPM_HUMAN | Nucleophosmin | 32555 | 165 | 4 |
| CBX8 | Exp. 1 | NSBP1_HUMAN | Nucleosome-binding protein 1 | 31525 | 47 | 2 |
| CBX8 | Exp. 1/Exp. 2 | NUCL_HUMAN | Nucleolin | 76568 | 376/287 | 10/10 |
| CBX8 | Exp. 2 | PCGF1_HUMAN | Polycomb group RING finger protein 1 | 29202 | 54 | 3 |
| CBX8 | Exp. 1/Exp. 2 | PCGF2_HUMAN | Polycomb group RING finger protein 2 | 37764 | 110/59 | 4/2 |
| CBX8 | Exp. 2 | PCGF3_HUMAN | Polycomb group RING finger protein 3 | 28097 | 58 | 1 |
| CBX8 | Exp. 2 | PCGF5_HUMAN | Polycomb group RING finger protein 5 | 29694 | 50 | 2 |
| CBX8 | Exp. 1/Exp. 2 | PCGF6_HUMAN | Polycomb group RING finger protein 6 | 39023 | 136/42 | 3/1 |
| CBX8 | Exp. 1/Exp. 2 | PHC2_HUMAN | Polyhomeotic-like protein 2 | 90657 | 192/53 | 3/1 |
| CBX8 | Exp. 1 | RING1_HUMAN | E3 ubiquitin-protein ligase RING1 | 42403 | 124 | 3 |
| CBX8 | Exp. 1/Exp. 2 | RING2_HUMAN | E3 ubiquitin-protein ligase RING2 | 37632 | 395/346 | 11/11 |
| CBX8 | Exp. 1/Exp. 2 | RRP5_HUMAN | Protein RRP5 homolog | 208570 | 272/61 | 11/1 |
| CBX8 | Exp. 2 | SETD2_HUMAN | Histone-lysine N-methyltransferase SETD2 | 287550 | 47 | 5 |
| CBX8 | Exp. 1 | SSF1_HUMAN | Suppressor of SWI4 1 homolog | 53161 | 66 | 2 |
| CBX8 | Exp. 1 | STAU1_HUMAN | Double-stranded RNA-binding protein Staufen homolog 1 | 63143 | 113 | 2 |
| CBX8 | Exp. 1/Exp. 2 | THOC4_HUMAN | THO complex subunit 4 | 26872 | 98/65 | 2/1 |
| CBX8 | Exp. 1/Exp. 2 | TSR1_HUMAN | Pre-rRNA-processing protein TSR1 homolog | 91752 | 100/92 | 2/2 |
| CBX8 | Exp. 2 | UBR1_HUMAN | E3 ubiquitin-protein ligase UBR1 | 200080 | 62 | 3 |
| CBX8 | Exp. 1/Exp. 2 | YBOX1_HUMAN | Nuclease-sensitive element-binding protein 1 | 35903 | 339/204 | 11/7 |
| CBX8 | Exp. 1 | YTDC2_HUMAN | YTH domain-containing protein 2 | 74853 | 98 | 2 |
| CBX8 | Exp. 2 | ZCHC3_HUMAN | Zinc finger CCHC domain-containing protein 3 | 43591 | 86 | 2 |
| CBX8 | Exp. 1/Exp. 2 | ZFR_HUMAN | Zinc finger RNA-binding protein | 116939 | 115/39 | 2/1 |
| CBX8 | Exp. 2 | ZN717_HUMAN | Zinc finger protein 717 | 105183 | 46 | 3 |
| CBX8 | Exp. 1 | ZN828_HUMAN | Zinc finger protein 828 | 89099 | 51 | 3 |
a SWISS PROTEIN data base accession.
b Mascot protein score.
c Number of different peptides.
The Chromodomain-containing CBX Proteins Associate with the Other Components of the PRC1 Protein Complex
As expected (28, 48), all known components of the PRC1 protein complex were found stably associated with CBX proteins (Table I). In particular, we identified all the human Psc orthologs (BMI1, PCGF1, PCGF2, PCGF3, PCGF5, and PCGF6), the Ph orthologs (PHC1, PHC2, and PHC3), and the two Sce orthologs (RING1 and RNF2) with a large number of peptides (Table II). However, TAP purifications using different CBX proteins do not allow the recovery of the PRC1 components with the same efficiency. Specifically, RNF2 and PHC2 were the only components co-eluted with all the CBX proteins, suggesting that these proteins might have a strong affinity for all of the five chromodomain-containing CBX proteins. The Psc ortholog BMI1 (PCGF4) is also found with all CBX proteins, but not CBX6; and RING1 is co-eluted with all CBXs except CBX2. In general, a large number of PRC1 components, including BMI1, PCGF2 (MEL18), PCGF6 (MBLR), PHC2, RING1, and RNF2, are recovered with CBX4, CBX7, and CBX8 whereas little amount of them interacts with CBX2 and CBX6. Some PRC1 components, such as PHC1 and PHC3 with CBX4, PCGF1 (NSPC1), PCGF3 (RNF3), and PCGF5 (RNF159) with CBX8, are identified in only one of 10 CBX-TAP purifications. In the case of CBX6, despite a good recovery of the bait as judged by sequence coverage, only little amount of PRC1 protein members were identified indicating that CBX6 associates preferentially with other factors than the PRC1 protein complex. However, another PcG protein, SCML2, was specifically identified with CBX6.
Table II. PcG and casein kinase 2 polypeptides associated to CBX proteins.
| CBX2 |
CBX4 |
CBX6 |
CBX7d |
CBX8 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Peptidesa | Coverageb | Peptidesa | Coverageb | Peptidesa | Coverageb | Peptidesa | Coverageb | Peptidesa | Coverageb | |
| Bait | 69 | 28% | 202 | 31% | 169 | 55% | 11 | 16% | 360 | 67% |
| PcG proteins | ||||||||||
| BMI1 | 4 | 12% | 25 | 26% | 7 | 17% | 22 | 23% | ||
| PCGF1 | 3 | 13% | ||||||||
| PCGF2 | 15 | 10% | 6 | 16% | 6 | 15% | ||||
| PCGF3 | 1 | 5% | ||||||||
| PCGF5 | 2 | 6% | ||||||||
| PCGF6 | 2 | 9% | 5 | 15% | 4 | 11% | ||||
| PHC1 | 1 | 2% | ||||||||
| PHC2 | 7 | 10% | 23 | 18% | 1 | 2% | 5 | 4% | 4 | 4% |
| PHC3 | 4 | 9% | ||||||||
| RING1 | 20 | 32% | 1 | 8% | 15 | 33% | 7 | 17% | ||
| RNF2 | 15 | 35% | 26 | 23% | 2 | 7% | 27 | 41% | 22 | 32% |
| SCML2 | 3 | 5% | ||||||||
| Casein kinase 2 | ||||||||||
| CSNK2A1 Casein kinase II subunit alpha | 4 | 7% | 3 | 13% | 3 | 12% | 7 | 17% | ||
| CSNK2A2 Casein kinase II subunit alpha' | 1 | 4% | 1 | 3% | ||||||
| CSNK2B Casein kinase II subunit beta | 3 | 16% | 3 | 9% | 2 | 9% | 4 | 20% | ||
a Number of different peptides.
b Fraction of protein recovered in peptides.
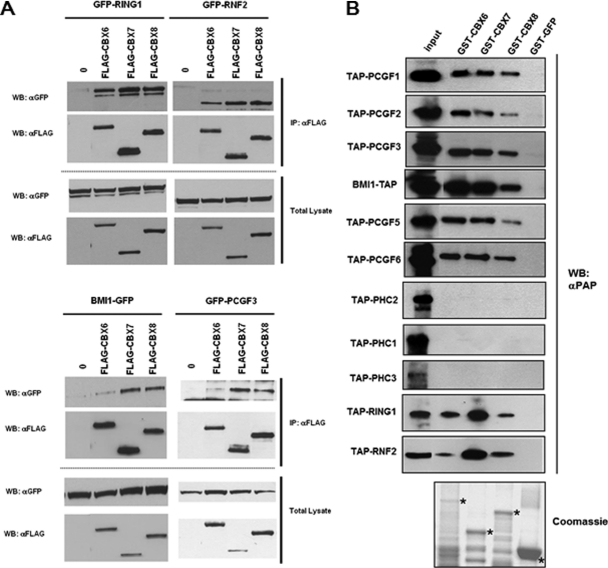
To further investigate the absence of peptides corresponding to Psc orthologs in the CBX6 TAP purification, co-immunoprecipitation experiments were performed. CBX6, CBX7, or CBX8 were co-expressed in HEK293 cells as FLAG-tagged proteins together with RING1-, RNF2-, BMI1-, or PCGF3-GFP fusion proteins. Protein extracts were immunoprecipitated using anti-FLAG antibodies covalently crosslinked to agarose beads. Following washing, bound proteins were separated on SDS-PAGE and Western blots were probed with an anti-FLAG antibody for detection of precipitated CBX proteins and an anti-GFP antibody for detection of the co-immunoprecipitated PcG proteins. Fig. 2A shows that CBX6 interacts with the Sce orthologs RING1 and RNF2 in a similar way as CBX7 and CBX8 do. In contrast, the interaction between CBX6 and the Psc orthologs BMI1 and PCGF3 appears to be significantly weaker than the interaction between CBX7 or CBX8 and BMI1 or PCGF3. Thus, this observation using co-immunoprecipitation parallels the results obtained by TAP purifications.
Fig. 2.
Comparison of CBX6, CBX7, and CBX8 interactions with the other PRC1 components. A, Co-immunoprecipitation analyses of interactions between CBX6, CBX7, CBX8, and the RING1, RNF2, BMI1, and PCGF3 components of the PRC1 protein complex show that CBX6 interacts with RING1 and RNF2 in a similar manner as CBX7 and CBX8, whereas CBX6 interaction with BMI1 and PCGF3 is significantly lower. HEK293 were transfected to express transiently FLAG-tagged CBX proteins and GFP-tagged RING1, RNF2 (upper panel), BMI1 and PCGF3 (lower panel). Proteins were immunoprecipitated (IP) with an anti-FLAG antibody linked to agarose beads, separated by SDS-PAGE, and detected (WB) using the indicated αGFP or αFLAG antibodies. Levels of transfections were analyzed in total lysates. B, GST-pull down analysis of interactions between CBX6, CBX7, CBX8, and the RING1, RNF2, BMI1, and PCGF3 components of the PRC1 protein complex showing that CBX6, as well as CBX7 and CBX8 interact with PCGF1, PCGF2, PCGF3, BMI1, PCGF5, PCGF6, RING1, and RNF2 in vitro. None of the CBX proteins shows a direct interaction with the Ph orthologs PHC1, PHC2 and PHC3 in vitro. GST-tagged CBX6, CBX7 and CBX8 proteins as well as control GST-GFP fusion were expressed in E. coli, bound to glutathione-Sepharose, and incubated with in vitro expressed TAP-tagged PRC1 components as indicated. Bound proteins were separated by SDS-PAGE and Western blots probed with peroxidase anti-peroxidase (αPAP) antibodies to reveal TAP-tagged proteins. GST-tagged proteins purified from E. coli are indicated with a star on the Coomassie stained gel following protein separation by SDS-PAGE.
Next, GST-pull down experiments were performed to determine the ability of PRC1 components to interact directly with CBX6 in vitro. Human CBX6, CBX7, and CBX8, together with a GFP control, were expressed in E. coli as recombinant GST fusion proteins, bound to glutathione-Sepharose beads and incubated with in vitro expressed TAP-tagged PRC1 components. Following extensive washings, bound proteins were eluted with glutathione, separated on SDS-PAGE and Western blots were probed with peroxidase coupled anti-peroxidase antibodies for detection of TAP-tagged PcG proteins. Fig. 2B shows that all three CBX paralogs interact directly and at similar levels with all Psc and Sce orthologs. CBX proteins show no interaction with the Ph orthologs PHC1, PHC2, and PHC3, presumably because the interaction is indirect or requires post-translational modifications not accomplished in this in vitro assay.
Taken together, these data indicate that all CBX proteins interact with the other components of the PRC1 protein complex. However, if proteins like CBX4, CBX7, and CBX8 form protein complexes with a large number of PcG factors, CBX6 and in a lesser extend CBX2, preferentially associate with a subset of these PcG proteins in vivo. In particular, despite an in vitro interaction between CBX6 and all the Psc orthologs, the chromodomain-containing CBX6 protein does not form strong protein assemblies with Psc family members in vivo.
Both Short and Long PHC2 Isoforms Associate with Chromodomain-containing CBX Proteins
In mammals, PHC2 is expressed as two isoforms, PHC2a and PHC2b of 90 kDa and 36 kDa, respectively (49; Fig. 3A). The short isoform corresponds to the last 323 C-terminal amino-acids of the longer one. In order to determine which PHC2 isoforms associate with CBX proteins, we searched in which gel slice PHC2 peptides were identified. In a CBX4-TAP purification, PHC2 peptides were identified both in a high molecular weight part of the gel (CBX4 Exp. 2, gel slice n°5; supplemental Table S1) corresponding to proteins of around 90 kDa, and in lower molecular weight parts of the gel (CBX4 Exp. 2, gel slices n°22 and 23; supplemental Table S1) at about 40kDa. Peptides identified in the high molecular weight part of the gel include a GDGNSSVPGSMEGR peptide falling in a protein region specific to the long PHC2 isoform whereas peptides identified in the lower part of the gel all match to the C-terminal moiety of the protein that includes PHC2b. This suggests that both PHC2a and PHC2b isoforms could be associated with CBX proteins. However, in all other CBX-TAP purifications, PHC2 peptides were found exclusively in the regions of the gels corresponding to proteins of around 40 kDa. The absence of TEV cleavage sites (E-X-X-Y-X-Q-S; 50) in the PHC2 protein sequence excludes the possibility that PHC2 is cleaved during the first elution step of the TAP procedure. Furthermore, all peptides match to the C-terminal moiety of the protein that includes PHC2b (Fig. 3B).
Fig. 3.
Both long and short PHC2 isoforms associate with CBX proteins. A, Schematic representation of the human PHC2 locus. Two transcripts are generated from the PHC2 locus by alternative transcriptional initiations. Exons are drawn by boxes. Gray boxes correspond to coding regions, whereas white boxes correspond to untranslated regions. B, Position of peptides identified by TAP-MS/MS on the PHC2 protein sequence. A peptide (underlined) matches to sequences specific to PHC2a long isoform and was identified specifically in a high molecular weight fraction of a TAP gel. Protein sequence of PHC2 is depicted. Part of the protein sequence corresponding to the PHC2b short isoform is in italic. The identified peptides are indicated as black boxes or underlined sequence on the PHC2 protein sequence. C, Co-immunoprecipitation analyses showing that CBX4 interacts with the PHC2a and PHC2b isoforms. HEK293 were transfected to express transiently FLAG-tagged CBX4 or CBX4[1–269] proteins together with and GFP-tagged PHC2a or PHC2b isoforms. Proteins were immunoprecipitated (IP) with an anti-FLAG antibody linked to agarose beads, separated by SDS-PAGE, and detected (WB) using the indicated αGFP or αFLAG antibodies. Levels of transfections were analyzed in total lysates.
To further demonstrate that the PHC2b short isoform could associate with CBX proteins in vivo, co-immunoprecipitation experiments were performed (Fig. 3C). Full length CBX4 as well as a truncated CBX4 version lacking the conserved Pc box (CBX4[1–269]) were co-expressed as FLAG-tagged proteins in HEK293 cells together with the long PHC2a or short PHC2b isoforms expressed as GFP fusion proteins. Protein extracts were immunoprecipitated using anti-FLAG antibodies covalently crosslinked to agarose beads, bound proteins were separated on SDS-PAGE and Western blots were probed with an anti-FLAG antibody for detection of precipitated CBX proteins and an anti-GFP antibody for detection of the co-immunoprecipitated PHC2 isoforms. Fig. 3C shows that both PHC2a and PHC2b isoforms interact with CBX4. Furthermore, the conserved Pc box appears to be required for the interaction between CBX4 and the PHC2 isoforms.
Because the apparent size of PHC2 purified by TAP in SDS-PAGE corresponds only to PHC2b in all TAP purifications but not one, we speculate that the short isoform PHC2b is preferentially part of the PRC1 protein complexes, even if PHC2a could also be present in these complexes.
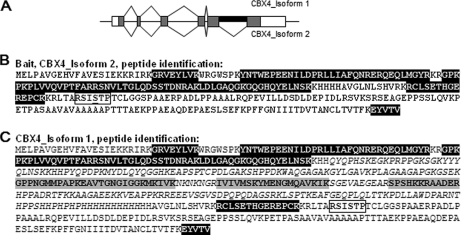
CBX4 Forms Homo-oligomers
In human cells, CBX4 is also expressed as two isoforms. The short isoform (isoform 2) results from an additional splicing event occurring within the last exon of the longer CBX4 isoform (isoform 1), leading to a 268 amino-acids deletion (Fig. 4A). For our CBX4 TAP-MS/MS experiments, we cloned the short CBX4 isoform from the EST BC014967.1 and expressed it as a TAP-tagged fusion protein in HeLa cells. TAP-MS/MS analysis identified CBX4 by peptides covering 38.6% of the total protein sequence of the bait (Fig. 4B). Interestingly, we were able to identify in addition three peptides covering the exon part specifically retained in the long isoform 1 of CBX4 (Fig. 4C). This indicates that CBX4 is able to associate as homo-oligomers in cells. Moreover, our data show that the overexpression of TAP-tagged exogenous CBX proteins does not prevent the interaction with the endogenous counterparts by displacing an equilibrium.
Fig. 4.
TAP-CBX4 purification from HeLa cells reveals CBX4 oligomerization. A, Schematic representation of the human CBX4 locus. Two transcripts are generated from the CBX4 locus by an alternative splicing event. Exons are drawn as boxes. Dark boxes correspond to coding regions, whereas white boxes correspond to untranslated regions. The part of exon 5 spliced out in CBX4_isoform 2, and specific to CBX4_isoform 1 is shown in black. B, CBX4_isoform 2 protein sequence used for protein complex purifications by TAP. The identified peptides are indicated as black boxes on the protein sequence. C, CBX4_isoform 1 protein sequence. Part of the sequence specific to CBX4_isoform 1 and spliced out in CBX4_isoform 2 is in italic. The identified peptides specific to CBX4_isoform 1 are indicated as gray boxes on the protein sequence. A putative 14-3-3 binding motif is shown in a white box. Underlined serine is mutated into an alanine in the CBX4[S164A] mutant version.
CBX Proteins are Mutually Exclusive Within the PRC1 Protein Complexes and do not Colocalize in U2-OS Cells
In our TAP-MS/MS experiments, we did not detect chromodomain-containing Pc orthologs other than the one used as the bait itself, in agreement with similar findings from other groups for CBX8 (47) or CBX7 (51). In particular, Maertens et al. used different ways including protein complex purification in presence of several Pc orthologs co-expressed, to demonstrate that CBX7 and CBX8 participate to distinct PRC1-like protein complexes. Using GST-pull down assays, we also failed to detect direct interactions between CBX4 and the other CBX proteins, except with CBX4 itself (Fig. 5A), reinforcing the idea that CBX proteins belong to different protein complexes.
Fig. 5.
CBX proteins do not interact with each other and do not colocalize. A, GST-pull down analysis of interactions between CBX4 and CBX family proteins indicates that CBX4 oligomerizes but does not interact directly with the other CBX proteins in vitro. GST-tagged CBX4 and GFP control proteins were expressed in E. coli, bound to glutathione-Sepharose, and incubated with in vitro expressed TAP-tagged CBX proteins as indicated. Bound material was washed, proteins were separated by SDS-PAGE and Western blots probed with a peroxidase anti-peroxidase (αPAP) antibody to reveal TAP-tagged proteins. B, CBX proteins show different nuclear localization patterns. U2-OS cell lines stably expressing GFP-tagged CBX proteins were fixed with paraformaldehyde and localization of fusion proteins visualized by fluorescence microscopy (right panels). Hoechst staining labels nuclei (left panels).
Next, we investigated the subnuclear localization of CBX proteins. CBX2, CBX4, CBX6, CBX7, and CBX8 were stably expressed as GFP fusion proteins in U2-OS cells. U2-OS cells were used because they were initially described as exhibiting subnuclear foci known as Polycomb bodies, where PcG proteins accumulate (52). Pools of U2-OS cells expressing GFP-CBX fusion proteins were generated by retroviral transduction. Fig. 5B shows that the subnuclear distributions of different GFP fusions are dissimilar in interphase U2-OS cell nuclei. The CBX2-GFP fusion protein is enriched in small and large foci. The CBX4 fusion protein is located in a more restricted number of foci and the fluorescence signal over the nucleus remains very weak. These foci correspond to the Polycomb bodies initially described using specific antibodies (52) The CBX6 fusion is dispersed in a granular pattern throughout the nucleus. The CBX7-GFP fusion protein is enriched in foci unevenly distributed in the nucleus. Finally the CBX8 fusion proteins accumulate in several smaller foci throughout the nucleus. These distinct localization patterns suggest that CBX proteins do not colocalize with each other. A similar observation has also been reported in ES cells by others (53).
Casein Kinase 2 is a Component of CBX Protein Assemblies
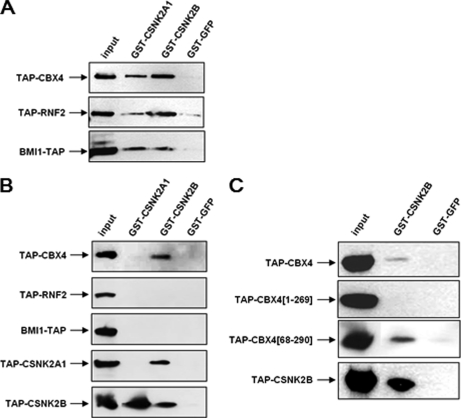
Another component identified with a high confidence in our TAP-MS/MS experiments is the casein kinase 2 (CSNK2; Tables I and II). CSNK2 is a ubiquitous serine/threonine protein kinase organized as tetrameric complexes, composed of two catalytic (CSNK2A1, CKIIα, and/or CSNK2A2, CKIIα') and two regulatory (CSNK2B, CKIIβ) subunits. Peptides corresponding to both regulatory and catalytic subunits of CSNK2 were identified associated with all CBX proteins, but not CBX6. Because CBX6 is also the family member for which less PRC1 components were found, our data suggest that CSNK2 could be directly bound to PRC1-like protein complexes.
To confirm that CSNK2 interacts with the PRC1 protein complex, the regulatory CSNK2B and a catalytic CSNK2A1 subunit of casein kinase 2, as well as a GFP control, were expressed in E. coli as GST fusion proteins and bound to glutathione-Sepharose beads. Full-length TAP-tagged CBX4, RNF2, and BMI1 PRC1 components were expressed in HEK293 cells and resulting nuclear extracts were incubated with the glutathione bound proteins. Following extensive washings, bound proteins were eluted with glutathione, separated on SDS-PAGE and Western blots were probed with peroxidase coupled anti-peroxidase antibody for detection of TAP-tagged proteins. Fig. 6A shows that all the PRC1 components tested are recovered both with CSNK2B and CSNK2A1 in this GST-pull down assay using nuclear extracts. In order to identify the direct interactions between the PRC1 components and the casein kinase 2 subunits, GST-pull down experiments were performed using individual proteins expressed in vitro. As expected (54) the regulatory CSNK2B subunit of casein kinase interacts with itself and with the catalytic subunit CSNK2A1, whereas CSNK2A1 does not form homo-oligomers (Fig. 6B). Moreover, Fig. 6B shows that CSNK2B, but not CSNK2A1 interacts specifically with the chromodomain-containing protein CBX4, but not with the Sce ortholog RNF2 nor with the Psc ortholog BMI1. To define the CBX4 domains involved in the binding to CSNK2B, TAP-tagged deletion versions of CBX4 were expressed in vitro and incubated with the GST-CSNK2B recombinant protein (Fig. 6C). Deletion of the conserved Pc box of CBX4 (construct CBX4[1–269]) abolishes the interaction with CSNK2B, whereas deletion of CBX4 chromodomain (construct CBX4[68–290]) has no effect on CBX4-CSNK2B direct interaction.
Fig. 6.
Casein kinase 2 interacts with the PRC1 protein complex. A, Casein kinase 2 subunits associate complexes with PRC1 components extracted from nuclear extracts in a GST-pull down assay. GST-tagged catalytic (CSNK2A1) and regulatory (CSNK2B) subunits of casein kinase 2 were expressed in E. coli, bound to glutathione-Sepharose, and incubated with nuclear extracts from HEK293 cells expressing TAP-tagged CBX4, RNF2, or BMI1, as indicated. Following washing, proteins were separated by SDS-PAGE and Western blots probed with peroxidase anti-peroxidase (αPAP) antibodies to reveal TAP-tagged proteins. B, CBX4 directly interacts with the regulatory subunit of casein kinase 2 in vitro. GST-tagged catalytic (CSNK2A1) and regulatory (CSNK2B) subunits of casein kinase 2 were expressed in E. coli, bound to glutathione-Sepharose, and incubated with in vitro translated TAP-tagged CBX4, RNF2, BMI1, CSNK2A1, or CSNK2B proteins, as indicated. Following extensive washings, proteins were separated by SDS-PAGE and Western blots probed with αPAP antibodies to reveal TAP-tagged proteins. Interactions between CSNK2A1 and CSNK2B, as well as CSNK2B dimerization were used as controls. C, CBX4 Pc box is required for CBX4-CSNK2B interaction in vitro. GST-tagged regulatory subunit of casein kinase 2 was expressed in E. coli, bound to glutathione-Sepharose, and incubated with in vitro translated TAP-tagged CBX4, as well as the CBX4 truncations, CBX4[1–269] having a Pc box deletion and CBX4[68–290] harboring a chromodomain deletion. Following extensive washings, proteins were separated by SDS-PAGE and Western blots probed with αPAP antibodies to reveal TAP-tagged proteins.
Taken all together, these results indicate that casein kinase 2 interacts with the PRC1 protein complexes. The interaction among these protein assemblies is mediated by direct binding of CBX to the regulatory subunit CSNK2B of casein kinase 2. Moreover, the presence of the conserved Pc box is required for this association.
CBX4 Specifically Associates with 14-3-3 Proteins
Comparison of the protein lists identified by TAP-MS/MS with the different CBX proteins revealed several CBX-specific interacting partners (Table II). Among them, 14-3-3 proteins appeared particularly abundant specifically in CBX4 TAP purifications. 14-3-3 proteins are highly conserved small acidic proteins implicated in the regulation of a wide variety of both general and specialized signaling pathways, and encoded by seven mammalian genes (α/β, ε, η, γ, τ/θ, ζ/δ, and σ) (55). All seven 14-3-3 isotypes were co-eluted specifically with CBX4 and identified with particularly high scores, but not with other CBX family members. In our duplicated experiments, we identified a total of 329 peptides counts (87 unique peptides, supplemental Table S1), including 8 peptides corresponding specifically to 14-3-3β, 101 specific peptides for 14-3-3ε, 23 for 14-3-3η, 21 for 14-3-3γ, 12 for 14-3-3τ, 28 for 14-3-3ζ, and 1 specific peptide for 14-3-3σ.
In most cases, 14-3-3 proteins regulate cellular processes by binding to specific phosphoserine (pS) and phosphothreonine (pT) motifs within target proteins (56) and two optimal 14-3-3 phosphopeptide ligands with the consensus sequences RSX(pS/T)XP and RX(Y/F)X(pS/T)XP (where X is any amino acid) have been defined (57). The search for potential binding sites for 14-3-3 proteins revealed the presence of a sequence RSISTP matching the first consensus sequence, at positions AA161–166 and AA429–434 within the short and long CBX4 isoforms, respectively (Figs. 4B, 4C). This sequence is a potential phosphorylation target site for several different kinases including AKT1, the Ataxia telangiectasia mutated (ATM) kinase, the Serine/Threonine-protein kinase CHK1, the Casein kinase 1 (CSNK1), the cGMP-dependent protein kinase (PKG), the Serine/Threonine kinase 4 (STK4), and the Ribosomal S6 kinase (RSK) (58). Sequence alignments showed that this putative 14-3-3-binding site is evolutionary conserved in CBX4 proteins, even if adjacent sequences diverge in lower vertebrates (Fig. 7A). To test whether this RSISTP sequence is indeed involved in 14-3-3 binding, we performed co-immunoprecipitation assays using CBX4 protein harboring a point mutation at the putative phosphoserine residue within the consensus binding site. In the construct CBX4[S164A], the serine at position 164 in the short isoform CBX4 is mutated into an alanine. Hemagglutinin (HA)-tagged CBX4 and CBX4[S164A] proteins were expressed in HEK293 cells together with GFP-tagged 14-3-3ζ or 14-3-3ε proteins. Protein extracts were immunoprecipitated using anti-HA antibodies covalently crosslinked to agarose beads. Following extensive washes, bound proteins were separated on SDS-PAGE and Western blots were probed with an anti-HA antibody for detection of precipitated CBX4 proteins and an anti-GFP antibody for detection of the co-immunoprecipitated 14-3-3 proteins. Co-immunoprecipitation experiments shown on Fig. 7B confirm the association of CBX4 with 14-3-3ζ and 14-3-3ε proteins. Moreover, point mutation at serine 164 abolishes this interaction, demonstrating that this conserved residue indeed belongs to a functional 14-3-3-binding site.
Fig. 7.
14-3-3 binding to CBX4 plays a role in the interaction between CBX4 and BMI1/PCGF family members but has no role on CBX4 localization at Polycomb bodies. A, Sequence alignment shows that a putative 14-3-3-binding site is conserved among the CBX4 protein sequences from various vertebrates. B, Mutation of the putative 14-3-3-binding site in CBX4 (CBX4[S164A]) abolishes 14-3-3-CBX4 interaction in a co-immunoprecipitation assay. HEK293 were transfected to express transiently HA-tagged CBX4 and CBX4[S164A] mutant proteins together with GFP-tagged 14-3-3ζ and 14-3-3ε. Proteins were immunoprecipitated (IP) with an anti-HA antibody linked to agarose beads, separated by SDS-PAGE, and detected (WB) using the indicated αGFP or αHA antibodies. Levels of protein expression were analyzed in total lysates. C, Mutation of the putative 14-3-3-binding site in CBX4 reduces CBX4 interaction with PCGF6 and BMI1, but not with RNF2 in a co-immunoprecipitation assay. HEK293 were transfected to transiently express HA-tagged CBX4 and CBX4[S164A] proteins together with GFP-tagged RNF2, PCGF6, and BMI1. Proteins were immunoprecipitated (IP) with an anti-HA antibody linked to agarose beads, separated by SDS-PAGE, and detected (WB) using the indicated αGFP or αHA antibody. Levels of protein expression were analyzed in total lysates. D, Mutation of the putative 14-3-3-binding site in CBX4 reduces CBX4 interaction with BMI1, whereas deletion of the Pc box abolishes the CBX4-RNF2 interaction in a co-immunoprecipitation assay. HEK293 were transfected to transiently express HA-tagged wild type CBX4, mutant CBX4[S164A] and deletion mutant CBX4[1–269] proteins together with GFP-tagged RNF2 and BMI1. Proteins were immunoprecipitated (IP) with an anti-HA antibody linked to agarose beads, separated by SDS-PAGE, and detected (WB) using the indicated αGFP or αHA antibodies. Levels of protein expression were analyzed in total lysates. E, Schematic representation of the CBX4 constructs used. The chromodomain is drawn as a blue triangle, the Pc box as a red circle, and the S164A mutation as a cross. F, CBX4 Pc box, but not the chromodomain, is required for CBX4 localization at Polycomb bodies. U2-OS cell lines stably expressing GFP-tagged CBX4 as well as mutated versions CBX4[S164A], CBX4[1–269] and CBX4[68–290] were fixed with paraformaldehyde and localization of fusion proteins visualized by fluorescence microscopy (upper panels). Hoechst staining labels nuclei (lower panels).
The dimeric structure of 14-3-3 proteins suggests that they could represent a simple adapter or bridging strategy, where two different target proteins bind simultaneously to each monomer of the same 14-3-3 dimer. Alternatively, 14-3-3 protein might operate by binding to specific protein target sites, thereby inducing conformational changes that will influence interactions between these target proteins and other molecule. Then, in order to check whether the loss of CBX4-14-3-3 association could modify the interaction between CBX4 and other components of the PRC1 protein complex, the interaction between HA-tagged CBX4[S164A] and GFP-tagged RNF2, PCGF6 and BMI1 proteins was measured using a co-immunoprecipitation assay. Fig. 7C shows that mutation at serine 164 which abolishes 14-3-3 association also significantly reduces the interaction of CBX4 with the Psc orthologs PCGF6 and BMI1, but has no effect on the interaction with the Sce ortholog RNF2. This effect is specific and opposite to the Pc box deletion effect. As previously described (59–61), deletion of the Pc box leads to the loss of CBX4-RNF2 interaction, whereas it has no effect on the CBX4-BMI1 association in a co-immunoprecipitation assay (Fig. 7D). We conclude that the interaction between CBX4 and 14-3-3 proteins favors CBX4-Psc proteins binding and/or its binding stability.
14-3-3 Proteins have been implicated in the regulation of the subcellular localization of many phosphorylated target proteins and in particular in the trafficking between the nucleus and the cytoplasm (55). Then, to test whether the loss of CBX4-14-3-3 association could alter CBX4 localization at Polycomb bodies in U2-OS cells, CBX4[S164A] were stably expressed as a GFP fusion protein in this line using retroviral transduction. In parallel, cell lines expressing GFP-tagged CBX4 deletion mutants CBX4[1–269] and CBX4[68–290] (see figure 7E) were generated in order to define some of the domain requirements for proper localization of CBX4 at Polycomb bodies. Fig. 7F shows that mutation at serine 164, and consequently the loss of interaction between CBX4 and 14-3-3 proteins does not affect localization at Polycomb bodies. Surprisingly, deletion of the chromodomain responsible for the binding of CBX4 to H3K27me3 residues (62) in the construct CBX4[68–290] does not lead to miss-localization of the deletion mutant. More importantly, deletion of the Pc box in the CBX4[1–269] construct leads to dispersion of the protein in a granular pattern throughout the nucleus, indicating that a major determinant for Polycomb body localization is included within a C terminus region of 21 amino acids comprising the Pc box of CBX4. Because the Pc box is involved in the interaction with several proteins, it is tempting to speculate that these protein interactions play a dominant role, over the binding of the chromodomain to H3K27me3 residues, for CBX4 localization at Polycomb bodies.
CBX Proteins Have Different Activities on Transcription
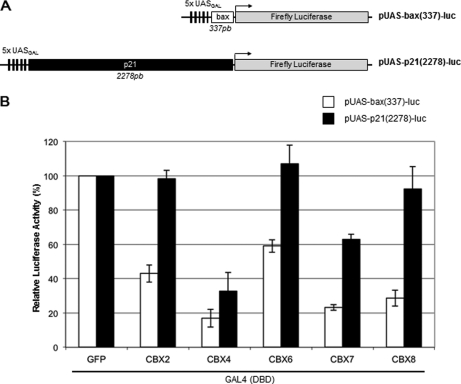
To further study the functional properties of the different CBX proteins, we assessed their ability to silence transcription when targeted to a reporter gene in vivo (Fig. 8). Several individual chromodomain-containing Pc proteins, including Drosophila Pc, CBX2, mouse and human CBX4 and CBX8 have been demonstrated to act directly as transcriptional repressors in mammalian cells (59, 61, 63–65), but we aimed here to compare their activities in parallel and in the same experimental setting. We transiently expressed GAL4 DNA binding domain (DBD)-CBX fusion constructs and assayed for firefly luciferase expression from reporter plasmids containing five UAS binding sites for the GAL4 DBD either adjacent to the 337 pb bax promoter [pUAS-bax(337)-luc] or upstream of the 2278 pb p21 promoter [pUAS-p21(2278)-luc] (Fig. 8A). These promoters were chosen for their strong basal expression in order to assay for CBX silencing activities. Transfection efficiencies were normalized using a co-transfected reference reporter vector containing the Renilla luciferase gene under the control of the Ubiquitin C promoter and repressive activities were normalized to the effect of a GAL4 DBD-GFP fusion control construct. Fig. 8B shows that cotransfection of GAL4 DBD-CBX constructs and pUAS-bax(337)-luc resulted in a marked repression of luciferase activity for all CBX proteins, even if CBX6 shows less silencing effects. In contrast, CBX4 and CBX7 exert a significant repressive effect on pUAS-p21(2278)-luc expression, whereas CBX2, CBX6, and CBX8 have no or only marginal effects. We could not discriminate whether differences in the response of the two constructs pUAS-bax(337)-luc and pUAS-p21(2278)-luc are because of distance effects or differences in the binding factors interacting with the two promoter constructs. However, our results shed light on differences of silencing activities harbored by the CBX proteins in the same assay.
Fig. 8.
CBX proteins repress transcription of a luciferase reporter gene in transfected mammalian cells. A, Schematic representation of the luciferase reporter plasmids used in the silencing assay. pUAS-bax(337)-luc contains five UAS binding sites for the GAL4 DBD upstream of a 337 pb bax promoter driving the expression of the firefly reporter gene. pUAS-p21(2278)-luc possesses five UAS (GAL4) sites upstream of a 2278 pb p21 promoter driving the luciferase gene. B, CBX effects on transcriptional repression. HEK293 cells were transfected either with pUAS-bax(337)-luc (white bars) or with pUAS-p21(2278)-luc (black bars), together with pUbi-Rluc reference plasmid and GAL4(DBD)-CBX2, GAL4(DBD)-CBX4, GAL4(DBD)-CBX6, GAL4(DBD)-CBX7, GAL4(DBD)-CBX8, or control GAL4(DBD)-GFP expression vectors. Firefly luciferase expression was determined 48 h following transfection and normalized to Renilla luciferase levels from the pUbi-Rluc reference plasmid. Results are expressed as normalized luciferase levels (arbitrary units) relative to those obtained in the presence of the GAL4(DBD)-GFP expression vector. Values represent the average of five independent experiments, with the S.D. values indicated.
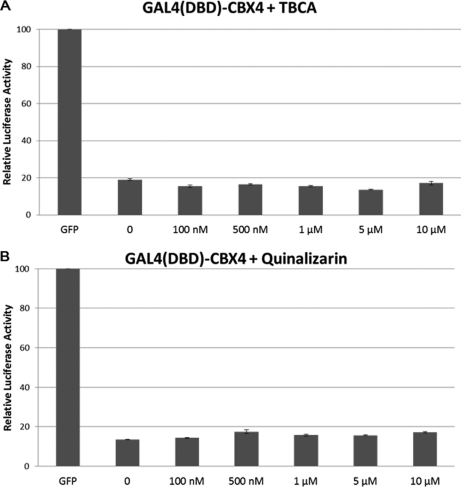
Casein Kinase 2 Inhibitors Show no Effect on CBX4 Transcriptional Silencing Activity
In order to determine whether Casein kinase 2 (CSNK2) plays a role in the CBX proteins-mediated silencing activity we used the GAL4-dependent transcriptional assay in presence of the CSNK2 inhibitors, (E)-3-(2,3,4,5-Tetrabromophenyl)acrylic acid (TBCA) and 1,2,5,8-Tetrahydrohy-9,10-anthraquinone (Quinalizarin) that are two cell-permeable compounds acting as potent, highly selective, and ATP-competitive inhibitors of CSNK2 (66, 67). We transiently expressed pUAS-bax(337)-luc in presence of an expression vector driving the expression of a GAL4 DNA binding domain (DBD)-CBX4 effector or GAL4 DBD-GFP control constructs and apply various concentrations of TBCA or Quinalizarin for 24 h before assaying for the luciferase activity. Fig. 9 shows that CBX4 exerts a strong repressive effect on pUAS-bax(337)-luc expression, and addition of TBCA or Quinalizarin has no effect on the CBX4-mediated silencing of pUAS-bax(337)-luc. Thus, using this assay, we could not assign any role on transcriptional repression to the CBX partner, casein kinase 2.
Fig. 9.
Casein kinase 2 inhibitors show no effect on CBX4 transcriptional silencing activity. HEK293 cells were transfected with pUAS-bax(337)-luc, together with the pUbi-Rluc reference plasmid and GAL4(DBD)-CBX4, or control GAL4(DBD)-GFP expression vectors. Twenty four hours following transfection, various concentrations, as indicated, of the casein kinase 2 inhibitors TBCA (A) or Quinalizarin (B) were added to cell culture media. Firefly luciferase expression was determined 24 h later and normalized to Renilla luciferase levels from the pUbi-Rluc reference plasmid. Results are expressed as normalized luciferase levels (arbitrary units) relative to those obtained in the presence of the GAL4(DBD)-GFP expression vector (GFP) in presence of the corresponding amounts of TBCA or Quinalizarin. Values represent the average of three independent experiments, with the S.D. values indicated.
DISCUSSION
Chromatin-associated Polycomb group (PcG) proteins implement repression of hundreds of genes that encode crucial developmental regulators in higher eukaryotes. Biochemical studies in Drosophila as well as in mammalian cells have revealed that PcG proteins form at least two classes of protein complexes named Polycomb repressive complexes 1 and 2 (PRC1 and PRC2). PRC2 trimethylates histone H3 on Lysine 27 (H3K27me3), which is a central epigenetic mark at PcG-silenced chromatin. PRC1 contains four core subunits homologous to Drosophila Polycomb (Pc), Sex combs extra (Sce), Polyhomeotic (Ph), and Posterior sex combs (Psc) proteins. Each of these proteins has multiple orthologs in vertebrates classified respectively as the CBX, RING1/RNF2, PHC, and BMI1/PCGF families. Mammalian genomes encode five CBX family proteins (CBX2, CBX4, CBX6, CBX7, and CBX8) that contain a highly conserved chromodomain at the amino terminus (59, 63, 68, 69). This chromodomain binds H3K7me3 residues (62) and therefore targets PRC1 protein complexes at these sites. CBX family proteins also contain a conserved Pc box near the carboxyl terminus that can mediate interactions with the RNF2 subunit of the PRC1 complex (59–61). Different CBX proteins are believed to have distinct biological functions (47, 68, 70, 71). However, the properties of these CBX proteins have not been systematically compared in cells. Here, we applied the efficient TAP-MS/MS approach to identify interacting partners of CBX family proteins under the same experimental conditions. HeLa-derived cell lines expressing similar amounts of TAP-tagged CBX proteins were generated using retroviral transduction. CBX-associated protein complex purifications and protein identifications by MS/MS were performed under identical standardized conditions. Then, the relative abundance of a given protein in the different CBX purifications performed under identical conditions is central to this comparative interaction proteomics analysis. The combination of tandem affinity purification and tandem mass spectrometry results in the identification of a large number of polypeptides binding to each CBX family proteins. Most contaminants that unspecifically bind during the purification procedure could be removed by filtering proteins identified in a dataset of more than 30 TAP-MS/MS experiments performed in HeLa cells.
Our analysis identified with high confidence about 20 proteins co-eluted with CBX2 and CBX7 tagged proteins, about 40 with CBX4, and around 60 with CBX6 and CBX8. The number of identified proteins correlates with the Coomassie-stained intensity of the respective protein gels following TAP purification. As expected, all the other PRC1 components were found in our analyses. However, the different CBX proteins are mutually exclusive within the PRC1 protein complexes. We and others (51) failed to find an interaction between two distinct CBX proteins using different biochemical approaches. In agreement with the existence of distinct CBX-containing PRC1 protein complexes, we observed that CBX proteins do not show the same distribution within the U2-OS cell nuclei, as reported also in ES cells (53). In addition, Vincenz and Kerppola (72) reported the use of bimolecular fluorescence complementation (BiFC) analysis of CBX proteins interactions with H3 to visualize the subnuclear localization of the CBX family members in ES cells. BiFC complexes formed by the different CBX proteins with H3 in ES cells have unique distributions both in interphase nuclei and on metaphase chromosomes indicating that BiFC complexes formed by different CBX proteins bind to different chromosomal regions and have distinct target gene specificities. Thus, CBX proteins do not form protein assemblies in vivo and do not colocalize in cells.
Moreover, the number of distinct PRC1 components copurified depends on the CBX family member used as a bait. In particular, a large number of these components including BMI1, PCGF2, PCGF6, PHC2, RING1, and RNF2, were co-eluted only with CBX4, CBX7, and CBX8, whereas a limited number of them were found to interact with CBX2 and CBX6 in vivo. These differences suggest that the distinct CBX proteins might have different affinities for the other PRC1 components. PHC2 and RNF2 were the only PRC1 components found in interaction with all CBX family members. Interestingly, most of the identified peptides corresponding to PHC2 matched within the part of the protein sequence comprising PHC2 short isoform (PHC2b, NP_004418.2) sequence, and only one of them was specific to the long isoform (PHC2a, NP_93157.1). This, together with the identification of most of the PHC2 polypeptides at around 40 kDa in the TAP gels suggests that the short isoform PHC2b preferentially associates within the PRC1 protein complexes.
CBX6 has been found to interact with a limited number of PRC1 components including PHC2, RNF2, and RING1, but none of the BMI1/PCGF family members could be detected in the CBX6 TAP-MS/MS study. This result can not be explained by a weak expression of the TAP-tagged protein or a low recovery of protein complexes during the purification because the number of identified peptides corresponding to CBX6 remains in the same range as the number of identified peptides corresponding to other CBX proteins in parallel purifications. Moreover, Coomassie-stained intensity of CBX6 TAP gels is as strong as those of CBX4 and CBX8 TAP gels, and significantly stronger than Coomassie-stained intensity of the CBX7 TAP gels, for which most of the PRC1 components were identified. Our observation is in agreement with other studies showing that CBX6 is co-eluted with RNF2 (48), but not with BMI1 and PCGF2 (51). However, this is in total contrast with our in vitro binding assays based on GST-pull down experiments. We have shown that CBX6 strongly interacts with all BMI1/PCGF family proteins in vitro, whereas the binding appears weaker in co-immunoprecipitation experiments in vivo, where both CBX6 and BMI1/PCGF proteins were overexpressed. Finally, size-exclusion chromatography indicates that TAP-tagged CBX6 is still involved in high-molecular-weight protein complexes suggesting that in vivo, CBX6 is preferentially part of protein complexes different than the canonical PRC1 complexes containing BMI1/PCGF family members.
In our CBX4 TAP-MS/MS experiments, we used a short isoform of CBX4 (CBX4_isoform 2) in which part of exon 5 is spliced out. However, several peptides specific for the long isoform of CBX4 (CBX4_isoform 1) were identified by mass spectrometry, indicating on one hand that overexpression of TAP-CBX4 do not prevent the recovery of endogenous CBX proteins by displacing an equilibrium, and on the other hand that CBX4 forms oligomers. Furthermore, our in vitro binding assays based on GST-pull down experiments indicate that CBX4 oligomerization is direct and may occur in vitro. It has been previously shown that the Drosophila PRC1 member Ph is able to form homotypic interaction (73, 74) as we show it is also the case for CBX4. However, we could not determine whether several CBX subunits are part of the PRC1 protein complexes or whether CBX oligomers form distinct protein complexes. Nevertheless, because CBX proteins are part of high molecular-weight complexes and form oligomers, CBX-containing protein complexes might contact several adjacent or distant H3K27me3 marked nucleosomes.
Casein kinase 2 (CSNK2) was identified with high confidence with all CBX proteins, but not CBX6 by TAP-MS/MS. Because CBX6 is the family member showing the weakest associations with PRC1 components, we hypothesize that CSNK2 could directly bind to PRC1 protein complexes. Moreover, all subunits of CSNK2 were also co-eluted with RNF2 in another study (48).
Casein kinase 2 is a highly conserved serine/threonine protein kinase organized as a tetramer consisting in two catalytic subunits and two regulatory subunits. In yeast Saccharomyces cerevisiae, the holoenzyme also contains four subunits (Cka1, Cka2, Ckb1, and Ckb2) and has many known substrates including transcription factors and all RNA polymerases. In global protein-protein interaction studies in S. cerevisiae using chromosomally TAP-tagged open reading frames expressed from the endogenous promoter, one or more yeast casein kinase 2 subunits were found in 15 out of the 589 purifications and in 11 of 232 protein complexes deduced from these primary data (75, 76). Four of these complexes (70, 113, 137, and 156) contained all four subunits of casein kinase 2. Three of these complexes are categorized as having a role in transcription, DNA maintenance and/or chromatin organization whereas the fourth is involved in RNA metabolism. Complex 70 contains several chromatin remodeling enzymes (the chromodomain-containing protein Chd1, Isw1, Isw2), Complex 113 includes a large number of RNA polymerase II elongation factors, such as the core subunits of the FACT transcriptional elongation complex (Spt16 and Pob3), and Complex 156 contains numerous components of the U3 snoRNP. The presence of casein kinase 2 in such protein complexes suggests a global role for casein kinase 2 in chromatin organization and/or gene expression. In this context, the binding of CSNK2 to PRC1 protein complexes is not fully surprising.
Using GST-tagged CSNK2 subunits immobilized on glutathione-Sepharose beads, we could demonstrate PRC1 components binding. In addition, in vitro binding assays based on GST-pull down experiments showed that the regulatory subunit CSNK2B of casein kinase 2 directly interacts with CBX4, and that the C-terminal Pc box of CBX4 is required for this interaction. Thus, our results point out that CSNK2 is able to bind to PRC1 protein complexes, therefore conferring to these complexes a kinase activity in addition to the E3 ubiquitin ligase activity conveyed by RNF2. However, we could not assign a role in CBX-mediated transcriptional silencing to casein kinase 2 and the potential function of CSNK2 in Polycomb repression remains unknown.
The study of co-eluted proteins identified a limited number of proteins specifically interacting with one CBX family member but not with the others. These proteins include PSME3, PARP1, and JHD2A (KDM3A) with CBX2, 14-3-3 with CBX4, UBE20 with CBX6, or RPP5 and ZFR with CBX8. Among them, 14-3-3 proteins are of particular interest because they appear to be major CBX4 interacting partners because the number of 14-3-3 identified peptides, corresponding to all seven isotypes is particularly high. 14-3-3 proteins are involved in the control of numerous cellular processes through binding to target proteins that will control protein assembling or protein trafficking. CBX4 contains the sequence RSISTP which corresponds to a 14-3-3-binding site. Mutation in this motif abolishes CBX4-14-3-3 interaction and significantly reduces BMI1/PCGF family proteins binding to CBX4 in a co-immunoprecipitation assay. However, CBX4-RNF2 interaction is not affected by the mutation of this 14-3-3 binding site. Thus, our results indicate that 14-3-3 binding to CBX4 might regulate the recruitment or the stability of factors such as the BMI1/PCGF proteins, but not RNF2 within the CBX4-PRC1 protein complex, presumably via a CBX4 conformational change upon 14-3-3 binding. In contrast loss of CBX4-14-3-3 interaction does not impact CBX4 subnuclear localization at Polycomb bodies indicating that 14-3-3 might not play a role in CBX4 trafficking as it does for other nuclear proteins. The 14-3-3-binding site present in CBX4 is a potential phosphorylation target site for several kinases including AKT1, ATM, CHK1, CSNK1, PKG, STK4, or RSK (58), suggesting that CBX4-PRC1 protein complex formation or stability could be controlled by a phosphorylation event within a signaling pathway. In an attempt to mimic the phosphorylation of the 14-3-3-binding site, a CBX4[S164E] mutant has been engineered. However, co-immunoprecipitation experiments showed that the mutation S164E does not increase, but abolishes 14-3-3 binding, suggesting that glutamate is not phosphomimic in this context (data not shown).
Our studies provide evidences that the CBX family proteins define distinct protein complexes that interact with different efficiencies with the other PRC1 components and bind specifically additional proteins. Consistent with the involvement in distinct protein complexes, CBX proteins silence transcription at different levels when targeted to a reporter gene in vivo. All CBX proteins repress a reporter gene expression when targeted upstream to a 337 bp bax promoter using the GAL4 system. However, CBX6 and to a lesser extend CBX2 show weaker silencing effects. Thus, the silencing activity of CBX proteins targeted at the 337 bp bax promoter correlates with their abilities to interact with numerous PCR1 components. However, when CBX proteins are targeted upstream to a 2278 pb p21 promoter, CBX4 and CBX7 are still able to repress transcriptional activity of the reporter construct whereas CBX2, CBX6, and CBX8 have no or little silencing effects. Because CBX8 interacts with a high number of PCR1 components, our results indicate that PRC1 components-binding properties are not the only determinants playing a role in silencing activities harbored by CBX family proteins in this assay.
Polycomb bodies were initially identified as discrete nuclear structures using antibodies that specifically recognize three human PcG proteins, CBX4, RING1 and BMI1 (52). Our study of CBX subcellular localization as GFP fusion proteins in U2-OS cells, as well as studies from others in ES cells (53) indicate that different CBX family members do not accumulate in the same nuclear foci. Thus, Polycomb bodies probably designate distinct subnuclear entities. However, along our study to check whether 14-3-3 proteins could be involved in CBX4 subnuclear localization at Polycomb bodies in U2-OS cells, we reported that deletion of the CBX4 Pc box leads to dispersion of the protein in a granular pattern throughout the nucleus, whereas deletion of the chromodomain has no effect on protein localization at Polycomb bodies. In current models, the CBX chromodomain would bind to H3K27me3 residues therefore targeting PRC1 protein complexes at these epigenetic marks (19, 77, 78). However, our results show that the chromodomain is not necessary to target CBX4 at Polycomb bodies whereas the Pc box is required for Polycomb bodies' localization of CBX4. Because the Pc box is involved in the interaction with numerous proteins, we speculate that these protein interactions play a crucial role in CBX4 targeting, at least at Polycomb bodies. Differences in CBX protein interactions and the existence of distinct CBX-PRC1 complexes would then explain the distinct subnuclear localization of the CBX family proteins.
Acknowledgments
We are particularly grateful to Christian Rolando, Adeline Page, and the Centre Commun de Mesures de Spectrométrie de Masse de l'Université de Lille 1 Sciences et Technologies for technical expertise in mass spectrometry. We thank Laurent Héliot, Nicolas Fourré and the Biophotonic Core Facility of Lille (BiCFaL, Interdisciplinary Research Institute) for their advices in cellular imaging. We are grateful to David Bernard and Jesus Gil for the gift of the CBX7 cDNA and to Didier Monté for the GAL4(UAS) luciferase reporter plasmids.
Footnotes
* This work was supported by the CNRS, l'Université de Lille 1 Sciences et Technologies, and by the Ministère de la Recherche et de l'Enseignement Supérieur, the Région Nord-Pas de Calais and the European Regional Developmental Funds through the ”Contrat de Projets Etat-Région (CPER) 2007-2013.” JV was supported by a fellowship from the French Ministère de la Recherche et de l'Enseignement Supérieur.
 This article contains supplemental Tables S1, S2, S3.
This article contains supplemental Tables S1, S2, S3.
1 The abbreviations used are:
- PcG
- Polycomb group
- TAP
- Tandem affinity purification
- LC
- liquid chromatography
- MS/MS
- Tandem mass spectrometry
- HA
- Hemagglutinin
- GST
- Glutathione S-transferase
- PRC
- Polycomb repressive complex
- Pc box
- Polycomb box
- Pc
- Polycomb
- Sce
- Sex combs extra
- Ph
- Polyhomeotic
- Psc
- Posterior sex combs
- GFP
- Green fluorescent protein
- DBD
- DNA binding domain
- UAS
- Upstream activating sequence
- SDS-PAGE
- Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- ORF
- Open reading frame
- PBS
- Phosphate buffer saline
- TEV
- Tobacco etch virus
- BiFC
- Bimolecular fluorescence complementation
- WB
- Western blot
- IP
- immunoprecipitation
- CBX
- Chromobox homolog
- PCGF
- Polycomb group ring finger
- BMI
- B lymphoma Mo-MLV insertion
- RNF
- Ring Finger protein
- PHC
- Polyhomeotic homolog.
REFERENCES
- 1. Kennison J. A. (1995) The Polycomb and trithorax group proteins of Drosophila: trans-regulators of homeotic gene function. Annu. Rev. Genet. 29, 289–303 [DOI] [PubMed] [Google Scholar]
- 2. Jürgens G. (1985) A group of genes controlling the spatial expression of the bithorax complex in Drosophila. Nature 316, 153–155 [Google Scholar]
- 3. Yu B. D., Hess J. L., Horning S. E., Brown G. A., Korsmeyer S. J. (1995) Altered Hox expression and segmental identity in Mll-mutant mice. Nature 378, 505–508 [DOI] [PubMed] [Google Scholar]
- 4. Akasaka T., Kanno M., Balling R., Mieza M. A., Taniguchi M., Koseki H. (1996) A role for mel-18, a Polycomb group-related vertebrate gene, during the anteroposterior specification of the axial skeleton. Development 122, 1513–1522 [DOI] [PubMed] [Google Scholar]
- 5. van der Lugt N. M., Alkema M., Berns A., Deschamps J. (1996) The Polycomb-group homolog Bmi-1 is a regulator of murine Hox gene expression. Mech. Dev. 58, 153–164 [DOI] [PubMed] [Google Scholar]
- 6. Coré N., Bel S., Gaunt S. J., Aurrand-Lions M., Pearce J., Fisher A., Djabali M. (1997) Altered cellular proliferation and mesoderm patterning in Polycomb-M33-deficient mice. Development 124, 721–729 [DOI] [PubMed] [Google Scholar]
- 7. Satijn D. P., Hamer K. M., den Blaauwen J., Otte A. P. (2001) The Polycomb Group protein EED interacts with YY1, and both proteins induce neural tissue in Xenopus embryos. Mol. Cell. Biol. 21, 1360–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schartz Y. B, Kahn T. G., Nix D. A., Li X. Y., Bourgon R., Biggin M., Pirrotta V. (2006) Genome-wide analysis of Polycomb targets in Drosophila melanogaster. Nat. Genet. 38, 700–705 [DOI] [PubMed] [Google Scholar]
- 9. Nègre N., Hennetin J., Sun L. V., Lavrov S., Bellis M., White K. P., Cavalli G. (2006) Chromosomal distribution of PcG proteins during Drosophila development. PLoS Biol. 4, e170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tolhuis B., de Wit E., Muijrers I., Teunissen H., Talhout W., van Steensel B., van Lohuizen M. (2006) Genome-wide profiling of PRC1 and PRC2 Polycomb chromatin binding in Drosophila melanogaster. Nat. Genet. 38, 694–699 [DOI] [PubMed] [Google Scholar]
- 11. Bracken A. P., Dietrich N., Pasini D., Hansen K. H., Helin K. (2006) Genome-wide mapping of Polycomb target genes unravels their roles in cell fate transitions. Genes Dev. 20, 1123–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boyer L. A., Plath K., Zeitlinger J., Brambrink T., Medeiros L. A., Lee T. I., Levine S. S., Wernig M., Tajonar A., Ray M. K., Bell G. W., Otte A. P., Vidal M., Gifford D. K., Young R. A., Jaenisch R. (2006) Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature 441, 349–353 [DOI] [PubMed] [Google Scholar]
- 13. Lee T. I., Jenner R. G., Boyer L. A., Guenther M. G., Levine S. S., Kumar R. M., Chevalier B., Johnstone S. E., Cole M. F., Isono K., Koseki H., Fuchikami T., Abe K., Murray H. L., Zucker J. P., Yuan B., Bell G. W., Herbolsheimer E., Hannett N. M., Sun K., Odom D. T., Otte A. P., Volkert T. L., Bartel D. P., Melton D. A., Gifford D. K., Jaenisch R., Young R. A. (2006) Control of developmental regulators by Polycomb in human embryonic stem cells. Cell 125, 301–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Squazzo S. L., O'Geen H., Komashko V. M., Krig S. R., Jin V. X., Jang S. W., Margueron R., Reinberg D., Green R., Farnham P. J. (2006) Suz12 binds to silenced regions of the genome in a cell-type-specific manner. Genome Res. 16, 890–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shao Z., Raible F., Mollaaghabab,a R., Guyon J. R., Wu C. T., Bender W., Kingston R. E. (1999) Stabilization of chromatin structure by PRC1, a Polycomb complex. Cell 98, 37–46 [DOI] [PubMed] [Google Scholar]
- 16. Kuzmichev A., Nishioka K., Erdjument-Bromage H., Tempst P., Reinberg D. (2002) Histone methyltransferase activity associated with a human multiprotein complex containing the enhancer of Zeste protein. Genes Dev. 16, 2893–2905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cao R., Wang L., Wang H., Xia L., Erdjument-Bromage H., Tempst P., Jones R. S., Zhang Y. (2002) Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 298, 1039–1043 [DOI] [PubMed] [Google Scholar]
- 18. Ringrose L., Paro R. (2004) Epigenetic regulation of cellular memory by the Polycomb and trithorax group proteins. Annu. Rev. Genet. 38, 413–443 [DOI] [PubMed] [Google Scholar]
- 19. Völkel P., Angrand P. O. (2007) The control of histone lysine methylation in epigenetic regulation. Biochimie 89, 1–20 [DOI] [PubMed] [Google Scholar]
- 20. Müller J., Hart C. M., Francis N. J., Vargas M. L., Sengupta A., Wild B., Miller E. L., O'Connor M. B., Kingston R. E., Simon J. A. (2002) Histone methyltransferase activity of the Drosophila Polycomb group repressor complex. Cell 111, 197–208 [DOI] [PubMed] [Google Scholar]
- 21. Nekrasov M., Klymenko T., Fraterman S., Papp B., Oktaba K., Köcher T., Cohen A., Stunnenberg H. G., Wilm M., Müller J. (2007) Pcl-PRC2 is needed to generate high levels of H3-K27 trimethylation at Polycomb target genes. EMBO J. 26, 4078–4088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Francis N. J., Saurin A. J., Shao Z., Kingston R. E. (2001) Reconstitution of a functional core Polycomb repressive complex. Mol. Cell 8, 545–556 [DOI] [PubMed] [Google Scholar]
- 23. Fischle W., Wang Y., Jacobs S. A., Kim Y., Allis C. D., Khorasanizadeh S. (2003) Molecular basis for the discrimination of repressive methyl-lysine marks in histone H3 by polycomb and HP1 chromodomains. Genes Dev. 17, 1870–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Min J., Zhang Y., Xu R. M. (2003) Structural basis for specific binding of polycomb chromodomain to histone H3 methylated at lys 27. Genes Dev. 17, 1823–1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang H., Wang L., Erdjument-Bromage H., Vidal M., Tempst P., Jones R. S., Zhang Y. (2004) Role of histone H2A ubiquitination in polycomb silencing. Nature 431, 873–978 [DOI] [PubMed] [Google Scholar]
- 26. Cao R., Tsukada Y., Zhang Y. (2005) Role of Bmi-1 and Ring1A in H2A ubiquitylation and Hox gene silencing. Mol. Cell 20, 845–854 [DOI] [PubMed] [Google Scholar]
- 27. Francis N. J., Kingston R. E., Woodcock C. L. (2004) Chromatin compaction by a Polycomb group protein complex. Science 306, 1574–1577 [DOI] [PubMed] [Google Scholar]
- 28. Levine S. S., Weiss A., Erdjument-Bromage H., Shao Z., Tempst P., Kingston R. E. (2002) The core of the Polycomb repressive complex is compositionally and functionally conserved in flies and human. Mol. Cell. Biol. 22, 6070–6078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dellino G. I., Schwartz Y. B., Farkas G., McCabe D., Elgin S. C., Pirrotta V. (2004) Polycomb silencing blocks transcription initiation. Mol. Cell 13, 887–893 [DOI] [PubMed] [Google Scholar]
- 30. Stock J. K., Giadrossi S., Casanova M., Brookes E., Vidal M., Koseki H., Brockdorff N., Fisher A. G., Pombo A. (2007) Ring1-mediated ubiquitination of H2A restrains poised RNA polymerase II at bivalent gene in mouse ES cells. Nat. Cell Biol. 9, 1428–1435 [DOI] [PubMed] [Google Scholar]
- 31. Zhou W., Zhu P., Wang J., Pascual G., Ohgi K. A., Lozach J., Glass C. K., Rosenfeld M. G. (2008) Histone H2A monoubiquitination represses transcription by inhibiting RNA polymerase II transcriptional elongation. Mol. Cell 29, 69–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jacobs J. J., van Lohuizen M. (2002) Polycomb repression: from cellular memory to cellular proliferation and cancer. Biochim. Biophys. Acta, 1602, 151–161 [DOI] [PubMed] [Google Scholar]
- 33. van der Lugt N. M., Domen J., Linders K., van Roon M., Robanus-Maandag E., te Riele H., van der Valk M., Deschamps J., Sofroniew M., van Lohuizen M. (1994) Posterior transformation, neurological abnormalities, and severe hematopoietic defects in mice with a targeted deletion of the bmi-1 proto-oncogene. Genes Dev. 8, 757–769 [DOI] [PubMed] [Google Scholar]
- 34. Takihara Y., Tomotsune D., Shirai M., Katoh-Fukui Y., Nishii K., Motaleb M. A., Nomura M., Tsuchiya R., Fujita Y., Shibata Y., Higashinakagawa T., Shimada K. (1997) Targeted disruption of the mouse homologue of the Drosophila polyhomeotic gene leads to altered anteroposterior patterning and neural crest defects. Development 124, 3673–3682 [DOI] [PubMed] [Google Scholar]
- 35. del Mar, Lorente M., Marcos-Gutiérrez C., Pérez C., Schoorlemmer J., Ramírez A., Magin T., Vidal M. (2000) Loss- and gain-of-function mutations show a Polycomb group function for Ring1A in mice. Development 127, 5093–5100 [DOI] [PubMed] [Google Scholar]
- 36. Suzuki M., Mizutani-Koseki Y., Fujimura Y., Miyagishima H., Kaneko T., Takada Y., Akasaka T., Tanzawa H., Takihara Y., Nakano M., Masumoto H., Vidal M., Isono K., Koseki H. (2002) Involvement of the Polycomb-group gene Ring1B in the specification of the anterior-posterior axis in mice. Development 129, 4171–4183 [DOI] [PubMed] [Google Scholar]
- 37. Voncken J. W., Roelen B. A., Roefs M., de Vries S., Verhoeven E., Marino S., Deschamps J., van Lohuizen M. (2003) Rnf2 (Ring1b) deficiency causes gastrulation arrest and cell cycle inhibition. Proc. Natl. Acad. Sci. U.S.A. 100, 2468–2473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Isono K., Fujimura Y., Shinga J., Yamaki M., O-Wang J., Takihara Y., Murahashi Y., Takada Y., Mizutani-Koseki Y., Koseki H. (2005) Mammalian polyhomeotic homologues Phc2 and Phc1 act in synergy to mediate Polycomb repression of Hox genes. Mol. Cell. Biol. 25, 6694–6706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schoeftner S., Sengupta A. K., Kubicek S., Mechtler K., Spahn L., Koseki H., Jenuwein T., Wutz A. (2006) Recruitment of PRC1 function at the initiation of X inactivation independent of PRC2 and silencing. EMBO J. 25, 3110–3122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pasini D., Bracken A. P., Hansen J. B., Capillo M., Helin K. (2007) The Polycomb group protein Suz12 is required for embryonic stem cell differentiation. Mol. Cell. Biol. 27, 3769–3779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rigaut G., Shevchenko A., Rutz B., Wilm M., Mann M., Séraphin B. (1999) A generic protein purification method for protein complex characterization and proteome exploration. Nat. Biotechnol. 17, 1030–1032 [DOI] [PubMed] [Google Scholar]
- 42. Bouwmeester T., Bauch A., Ruffner H., Angrand P. O., Bergamini G., Croughton K., Cruciat C., Eberhart D., Gagneur J., Ghidelli S., Hopf C., Huhse B., Mangano R., Michon A. M., Schirle M., Schlegl J., Schwab M., Stein M., Bauer A., Casari G., Drewes G., Gavin A. C., Jackson D. B., Joberty G., Neubauer G., Rick J. M., Kuster B., Superti-Furga G. (2004) A physical and functional map of the human TNF-alpha/NF-kappa B signal transduction pathway. Nat. Cell Biol. 6, 97–105 [DOI] [PubMed] [Google Scholar]
- 43. Angrand P. O., Segura I., Völkel P., Ghidelli S., Terry T. M., Brajenovic M. K., Vintersten K., Klein R., Superti-Furga G., Drewes G., Kuster B., Bouwmeester T., Acker-Palmer A. (2006) Transgenic mouse proteomics identifies new 14-3-3 associated proteins involved in cytoskeletal rearrangements and cell signalling. Mol. Cell. Proteomics 5, 2211–2227 [DOI] [PubMed] [Google Scholar]
- 44. Souza P. P., Völkel P., Trinel D., Vandamme J., Rosnoblet C., Héliot L., Angrand P. O. (2009) The histone methyltransferase SUV420H2 and heterochromatin proteins HP1 interact but show different dynamic behaviours. BMC Cell Biol. 10, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gingras A. C., Aebersold R., Raught B. (2005) Advances in protein complex analysis using mass spectrometry. J. Physiol. 563, 11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Völkel P., Le Faou P., Angrand P. O. (2010) Interaction proteomics: characterization of protein complexes using tandem affinity purification-mass spectrometry. Biochem. Soc. Trans. 38, 883–887 [DOI] [PubMed] [Google Scholar]
- 47. Dietrich N., Bracken A. P., Trinh E., Schjerling C. K., Koseki H., Rappsilber J., Helin K., Hansen K. H. (2007) Bypass of senescence by the polycomb group protein CBX8 through direct binding to the INK4A-ARF locus. EMBO J. 26, 1637–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sánchez C., Sánchez I., Demmers J. A., Rodriguez P., Strouboulis J., Vidal M. (2007) Proteomics analysis of Ring1B/Rnf2 interactors identifies a novel complex with the Fbxl10/Jhdm1B histone demethylase and the Bcl6 interacting corepressor. Mol. Cell. Proteomics 6, 820–834 [DOI] [PubMed] [Google Scholar]
- 49. Yamaki M., Isono K., Takada Y., Abe K., Akasaka T., Tanzawa H., Koseki H. (2002) The mouse edr2 (mph2) gene has two forms of mRNA encoding 90- and 36-kda polypeptides. Gene 288, 103–110 [DOI] [PubMed] [Google Scholar]
- 50. Dougherty W. G., Parks T. D., Cary S. M., Bazan J. F., Fletterick R. J. (1989) Characterization of the catalytic residues of the tobacco etch virus 49-kDa proteinase. Virology 172, 302–310 [DOI] [PubMed] [Google Scholar]
- 51. Maertens G. N., El Messaoudi-Aubert S., Racek T., Stock J. K., Nicholls J., Rodriguez-Niedenführ M., Gil J., Peters G. (2009) Several distinct Polycomb complexes regulate and co-localize on the INK4a tumor suppressor locus. PLoS ONE 4, e6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Saurin A. J., Shiels C., Williamson J., Satijn D. P. E., Otte A. P., Sheer D., Freemont P. S. (1998) The human Polycomb group complex associates with pericentromeric heterochromatin to form a novel nuclear domain. J. Cell Biol. 142, 887–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ren X., Vincenz C., Kerppola T. K. (2008) Changes in the distributions and dynamics of Polycomb repressive complexes during embryonic stem cell differentiation. Mol. Cell. Biol. 28, 2884–2895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kim M. S., Lee Y. T., Kim J. M., Cha J. Y., Bae Y. S. (1998) Characterization of protein interaction among subunits of protein kinase CKII in vivo and in vitro. Mol. Cells 8, 43–48 [PubMed] [Google Scholar]
- 55. Mackintosh C. (2004) Dynamic interactions between 14-3-3 proteins and phosphoproteins regulate diverse cellular processes. Biochem. J. 381, 329–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Muslin A. J., Tanner J. W., Allen P. M., Shaw A. S. (1996) Interaction of 14-3-3 with signalling proteins is mediated by recognition of phosphoserine. Cell 84, 889–897 [DOI] [PubMed] [Google Scholar]
- 57. Yaffe M. B., Rittinger K., Volinia S., Caron P. R., Aitken A., Leffers H., Gamblin S. J., Smerdon S. J., Cantley L. C. (1997) The structural basis of 14-3-3:phosphopetide binding specificity. Cell 91, 961–971 [DOI] [PubMed] [Google Scholar]
- 58. Wong Y. H., Lee T. Y., Liang H. K., Huang C. M., Wang T. Y., Yang Y. H., Chu C. H., Huang H. D., Ko M. T., Hwang J. K. (2007) KinasePhos 2.0: a web server for identifying protein kinase-specific phosphorylation sites based on sequences and coupling patterns. Nucleic Acids Res. 35, W588–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bárdos J. I., Saurin A. J., Tissot C., Duprez E., Freemont P. S. (2000) HPC3 is a new human ortholog that interacts and associates with RING1 and Bmi1 and has transcriptional repression properties. J. Biol. Chem. 275, 28785–28792 [DOI] [PubMed] [Google Scholar]
- 60. Satijn D. P., Otte A. P. (1999) RING1 interacts with multiple Polycomb-group proteins and displays tumorigenic activity. Mol. Cell. Biol. 19, 57–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schoorlemmer J., Marcos-Gutiérrez C., Were F., Martínez R., García E., Satijn D. P., Otte A. P., Vidal M. (1997) Ring1A is a transcriptional repressor that interacts with the Polycomb-M33 protein and is repressed at rhombomere boundaries in the mouse hindbrain. EMBO J. 16, 5930–5942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bernstein E., Duncan E. M., Masui O., Gil J., Heard E., Allis C. D. (2006) Mouse Polycomb proteins bind differently to methylated histone H3 and RNA and are enriched infacultative heterochromatin. Mol. Cell. Biol. 26, 2560–2569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Alkema M. J., Jacobs J., Voncken J. W., Jenkins N. A., Copeland N. G., Satijn D. P., Otte A. P., Berns A., van Lohuizen M. (1997) MPc2, a new murine homolog of the Drosophila polycomb protein is a member of the mouse polycomb transcriptional repressor complex. J. Mol. Biol. 273, 993–1003 [DOI] [PubMed] [Google Scholar]
- 64. Satijn D. P., Olson D. J., van der Vlag J., Hamer K. M., Lambrechts C., Masselink H., Gunster M. J., Sewalt R. G., van Driel R., Otte A. P. (1997) Interference with the expression of a novel human polycomb protein, hPc2, results in cellular transformation and apoptosis. Mol. Cell. Biol. 17, 6076–6086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bunker C. A., Kingston R. E. (1994) Transcriptional repression by Drosophila and mammalian Polycomb group proteins in transfected mammalian cells. Mol. Cell. Biol. 14, 1721–1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pagano M. A., Poletto G., Di Maira G., Cozza G., Ruzzene M., Sarno S., Bain J., Elliott M., Moro S., Zagotto G., Meggio F., Pinna L. A. (2007) Tetrabromocinnamic acid (TBCA) and related compounds represent a new class of specific protein kinase CK2 inhibitors. Chembiochem. 8, 129–139 [DOI] [PubMed] [Google Scholar]
- 67. Cozza G., Mazzorana M., Papinutto E., Bain J., Elliott M., di Maira G., Gianoncelli A., Pagano M. A., Sarno S., Ruzzene M., Battistutta R., Meggio F., Moro S., Zagotto G., Pinna L. A. (2009) Quinalizarin as a potent, selective and cell-permeable inhibitor of protein kinase CK2. Biochem. J. 421, 387–395 [DOI] [PubMed] [Google Scholar]
- 68. Gil J., Bernard D., Martínez D., Beach D. (2004) Polycomb CBX7 has a unifying role in cellular lifespan. Nat. Cell Biol. 6, 67–72 [DOI] [PubMed] [Google Scholar]
- 69. Pearce J. J., Singh P. B., Gaunt S. J. (1992) The mouse has a Polycomb-like chromobox gene. Development 114, 921–929 [DOI] [PubMed] [Google Scholar]
- 70. Kagey M. H., Melhuish T. A., Wotton D. (2003) The Polycomb protein Pc2 is a SUMO E3. Cell 113, 127–137 [DOI] [PubMed] [Google Scholar]
- 71. Scott C. L., Gil J., Hernando E., Teruya-Feldstein J., Narita M., Martínez D., Visakorpi T., Mu D., Cordon-Cardo C., Peters G., Beach D., Lowe S. W. (2007) Role of the chromobox protein CBX7 in lymphomagenesis. Proc. Natl. Acad. Sci. U.S.A. 104, 5389–5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Vincenz C., Kerppola T. K. (2008) Different Polycomb group CBX family proteins associate with distinct regions of chromatin using nonhomologous protein sequences. Proc. Natl. Acad. Sci. U.S.A. 105, 16572–16577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Peterson A. J., Kyba M., Bornemann D., Morgan K., Brock H. W., Simon J. (1997) A domain shared by the Polycomb group proteins Scm and ph mediates heterotypic and homotypic interactions. Mol. Cell. Biol. 17, 6683–6692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kim C. A., Gingery M., Pilpa R. M., Bowie J. U. (2002) The SAM domain of polyhomeotic forms a helical polymer. Nat. Struct. Biol. 9, 453–457 [DOI] [PubMed] [Google Scholar]
- 75. Gavin A. C., Bösche M., Krause R., Grandi P., Marzioch M., Bauer A., Schultz J., Rick J. M., Michon A. M., Cruciat C. M., Remor M., Höfert C., Schelder M., Brajenovic M., Ruffner H., Merino A., Klein K., Hudak M., Dickson D., Rudi T., Gnau V., Bauch A., Bastuck S., Huhse B., Leutwein C., Heurtier M.A., Copley R. R., Edelmann A., Querfurth E., Rybin V., Drewes G., Raida M., Bouwmeester T., Bork P., Seraphin B., Kuster B., Neubauer G., Superti-Furga G. (2002) Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature 415, 141–147 [DOI] [PubMed] [Google Scholar]
- 76. Gavin A. C., Aloy P., Grandi P., Krause R., Boesche M., Marzioch M., Rau C., Jensen L. J., Bastuck S., Dümpelfeld B., Edelmann A., Heurtier M. A., Hoffman V., Hoefert C., Klein K., Hudak M., Michon A. M., Schelder M., Schirle M., Remor M., Rudi T., Hooper S., Bauer A., Bouwmeester T., Casari G., Drewes G., Neubauer G., Rick J. M., Kuster B., Bork P., Russell R. B., Superti-Furga G. (2006) Proteome survey reveals modularity of the yeast cell machinery. Nature 440, 631–636 [DOI] [PubMed] [Google Scholar]
- 77. Simon J. A., Kingston R. E. (2009) Mechanisms of Polycomb gene silencing: knows and unknows. Nat. Rev. Mol. Cell Biol. 10, 697–708 [DOI] [PubMed] [Google Scholar]
- 78. Kerppola T. K. (2009) Polycomb group complexes – many combinations, many functions. Trends Cell Biol. 19, 692–704 [DOI] [PMC free article] [PubMed] [Google Scholar]