Summary
Background and objectives
A multidisciplinary team (MDT) approach to chronic kidney disease (CKD) may help optimize care of CKD and comorbidities. We implemented an MDT quality improvement project for persons with stage 3 CKD and comorbid diabetes and/or hypertension. Our objective was to decrease the rate of decline of GFR.
Design, setting, participants, & measurements
We used a 4-year historical cohort to compare 1769 persons referred for usual nephrology care versus 233 referred for MDT care within an integrated, not-for-profit Health Maintenance Organization (HMO). Usual care consisted of referral to an outside nephrologist. The MDT consisted of an HMO-based nephrologist, pharmacy specialist, diabetes educator, dietitian, social worker, and nephrology nurse. Both groups received usual primary care. The primary outcome was rate of decline of GFR. Secondary outcomes were LDL, hemoglobin A1c, and BP.
Results
In multivariate repeated-measures analyses, MDT care was associated with a mean annual decline in GFR of 1.2 versus 2.5 ml/min per 1.73 m2 for usual care. In stratified analyses, the significant difference in GFR decline persisted only in those who completed their referrals. There were no differences in the secondary outcomes between groups.
Conclusions
In this integrated care setting, MDT care resulted in a slower decline in GFR than usual care. This occurred despite a lack of significant differences for secondary disease-specific measures, suggesting that other differences in the MDT population or care process accounted for the slower decline in GFR in the MDT group.
Introduction
It is estimated that >16 million adults in the United States have stage 3 or higher chronic kidney disease (CKD) as defined by a decrease in GFR of <60 ml/min per 1.73m2 (1). In 2007, approximately 500,000 people were treated by means of renal replacement therapy (dialysis or transplantation) for ESRD in the United States (2), and the size of the prevalent ESRD population is projected to increase to 700,000 by 2015 and potentially to >2 million by 2030 (3). Therefore, it is important to identify strategies to delay the progression of CKD to ESRD.
Persons with CKD have a range of comorbid conditions. Some of these, such as hypertension and diabetes (DM), are risk factors for renal disease. Others, such as anemia, malnutrition, and metabolic bone disease are a result of CKD. Still others, such as coronary heart disease, are often co-prevalent because of shared risk factors. Comorbidities are a major cause of mortality among CKD patients (4). All of these comorbidities have been associated with adverse outcomes among patients with CKD (5–8), and optimal management of such comorbidities improves health outcomes of those with CKD (5,9–13).
There is evidence that treatment of common comorbid conditions improves health outcomes such as decreasing cardiovascular events and mortality in patients with CKD (14,15). However, care of complex CKD patients is often fragmented among specialists, primary care clinicians, and members of patient-education teams. As a result, a more cohesive multidisciplinary team (MDT) approach to CKD has been advocated as a means to optimize care of comorbidities and CKD, as well as to facilitate the transition to management of ESRD (16,17). Such team-based care is a foundation of the Chronic Care model, which calls for productive interactions between informed patients and proactive practice teams to improve health outcomes for persons with chronic medical conditions (18–20). However, it is unclear whether team-based approaches to CKD care offer definitive benefit. Although some comprehensive approaches to CKD have shown improved survival and stabilization or slowing of CKD progression, others have not changed the progression of CKD (21–24). Furthermore, the composition of MDT CKD care teams, the outcomes studied, and length of follow-up time all have been variable, limiting meaningful comparisons. This suggests that, although promising, MDT approaches to managing CKD deserve further investigation (15,25).
We report on the process and results of a quality improvement (QI) project designed to care for persons with stage 3 CKD. Our project differs from previous studies in that it assesses rate of change of renal function over time and secondary process outcomes for comorbid conditions managed by the MDT. In addition, we specifically targeted a population at risk because of comorbid diabetes and/or hypertension. The goal of the QI project was to decrease the rate of decline in GFR through comprehensive, integrated, multidisciplinary care. In this project, we compared usual care consisting of primary care plus nephrology referral, with enhanced care using primary care plus the MDT.
Materials and Methods
Project Setting and Population
The target population consisted of an historical cohort of community-dwelling members of a large, group model, integrated, not-for-profit Health Maintenance Organization (HMO) who were referred for nephrology care by their primary care physician during the period March 1, 2005 through June 1, 2009. Adult patients with stage 3 CKD and at least one of two specific comorbid conditions were eligible for inclusion. The required comorbid conditions included DM and/or hypertension; most participants had additional chronic conditions. Stage 3 CKD was defined as having at least two GFR values between 30 and 59 ml/min per 1.73 m2 measured 90 or more days apart (26). We excluded those with dementia, age greater than 90, those enrolled in an intensive heart failure management program, and those in palliative or hospice care programs on the premise that they would be unlikely to actively participate in an MDT care program. The MDT program was only available in English. The Institutional Review Board of the participating institution reviewed and approved the project.
Description of Usual Care and MDT QI Interventions
Usual care for CKD consisted of shared care between a primary care physician (PCP) and a consulting nephrologist outside of the integrated care plan. PCPs routinely managed the chronic conditions of diabetes, cardiovascular disease, and hypertension (among many others) using resources available through the integrated system including the option to access chronic care nurses such as diabetes educators and/or relevant specialty care providers. PCPs referred to one of three contracted nephrology specialty groups across the metropolitan area outside of the integrated system. The consulting nephrologist then mailed a visit summary to the PCP, which was scanned into the electronic medical record (EMR). These patients did not need additional referrals for subsequent nephrology care.
As part of the QI initiative, a multidisciplinary nephrology team was implemented within the integrated care plan. The MDT consisted of a nephrologist, renal clinical pharmacy specialist, diabetes nurse educator, renal dietitian, social worker, and nephrology nurse. Clinicians were hired to be part of the MDT based on experience in managing their area of expertise within CKD, as well as based on previous experience in team-based care, which is standard for chronic disease care within the integrated system. The MDT team then met weekly to review their ongoing processes of care. The components of the team care included an educational class with review of patient-education materials on CKD, medication therapy management and medication reconciliation, nephrology consultation including medical recommendations for hypertension, DM, and CVD comorbidities, as well as metabolic abnormalities consistent with CKD such as anemia and bone and mineral metabolism, depression screen using the Patient Health Questionnaire (PHQ-9) instrument (with appropriate treatment and/or referral), and dietary assessment with recommendations. A comprehensive care plan based on input from all team members was developed for team use and also shared with patients. After an initial assessment, the MDT visits were individualized to meet patient needs because not all patients required all services offered within the team at each visit.
Patient self-management was specifically encouraged by the MDT team and included obtaining necessary laboratory tests, keeping home BP and/or home blood sugar logs that were called, faxed, or E-mailed (secure) to the renal nurse, and keeping appointments (follow-up appointment intervals routinely ranged from 1 to 6 months). Patients were contacted by phone or E-mail to adjust medications and review behavioral or lifestyle recommendations. Through the EMR, PCPs received electronic, same-time copies of MDT clinic notes and notification of any outstanding clinical issues.
Patients were assigned to either internal nephrology care with the MDT or usual care based on available internal clinic capacity and the zip code of their residence. At any given time, if there was capacity at the MDT clinic, persons referred for nephrology care who resided in zip codes closest to the MDT clinic were preferentially offered care at that site for patient convenience. Patients were also assigned to the most convenient external nephrology care sites by zip code for usual care. This patient allocation procedure set up a quasi-experimental design permitting comparison of outcomes between patients who attended the MDT clinic and those assigned to usual care.
Analysis Plan
The primary outcome was change in GFR over time as a function of referral to the MDT program versus usual care. Secondary outcomes were adjusted final values of the disease-specific quality measures of LDL and hemoglobin A1c, as well as percent time at goal BP, as a function of referral to the MDT program versus usual care. There was some crossover in which approximately 7% of patients referred for external nephrology care also attended one or more MDT visits. The bias created by this crossover should decrease the observed effect of the MDT and thus our results are relatively conservative.
Measures
GFR was estimated using the four-variable Modification in Diet and Renal Disease equation (27). Initial GFR was defined as the second of two GFR values of 30 to 59 ml/min per 1.73 m2 measured 90 or more days apart. Final GFR was defined as the last GFR before either the end of the project period or being censored from the cohort. We censored participation in the cohort if a participant died, disenrolled from the health care plan, or started renal replacement therapy. Diagnoses for initial inclusion criteria were based on manual chart review by the nephrology team. Morbidity scores were calculated using the Quan adaptation of the Elixhauser morbidity measure (28). Initiation of renal replacement therapy, if it occurred, was identified by a reimbursement claim for dialysis. Secondary outcomes were defined as follows: percentage of time at a goal BP of <130/80; final A1c adjusted for baseline A1c in the subsample of persons with diabetes; and final LDL adjusted for initial LDL measure. All values were measured closest to baseline GFR and closest to, but not exceeding, the final date of enrollment.
We described participants in terms of baseline GFR, demographic information, MDT care versus external nephrology care, morbidity score, initial body mass index, overall morbidity level, duration of nephrology care, and rate of hospitalization during the follow-up period. We then assessed bivariate associations between these characteristics and change in GFR over time. In an approximation of an “intention to treat” analysis, we included all referrals for nephrology care—whether eligible for usual care or the QI intervention—in the analysis, regardless of whether the patient actually attended any visits at that site.
We used mixed effect modeling to assess both change in GFR and secondary outcomes as a function of participation in the QI program. Multivariate analyses were adjusted for baseline GFR, number of GFR measurements, number of primary care visits, visit to any nephrologist, amount of follow-up time, age, race, gender, morbidity score, and body mass index. All analyses were conducted using SAS version 9.1.3.
Results
We identified 2002 HMO members who received a nephrology referral between March 1, 2005 and June 1, 2009, who met the enrollment criteria above. Mean age of participants was 68 years, on average they had 4.5 chronic conditions, and they were followed for an average of 2.0 years. Characteristics of the program population are listed in Table 1. The MDT and usual care patients were equivalent in age, gender, race, ethnicity, length of follow-up, and biologic values (other than body mass index) at baseline. However, some significant differences were observed. Compared with usual care patients, MDT patients had on average fewer chronic conditions (although their average body mass index was significantly higher) and a lower rate of primary care visits, but were more likely to disenroll from the integrated care plan. In addition, fewer MDT patients initiated dialysis than did usual care patients, and they had lower rates of hospitalization during follow-up.
Table 1.
Characteristics of study population
| Total Sample | MDT Care | Usual Care | Pa | |
|---|---|---|---|---|
| N | 2,002 | 233 | 1769 | |
| Age, mean (SD) | 68.22 (12.18) | 67.62 (11.28) | 68.29 (12.29) | 0.2224 |
| Female | 888 (44.36) | 111 (47.64) | 777 (43.92) | 0.2831 |
| White | 1,365 (68.18) | 152 (65.24) | 1,213 (68.57) | 0.3259 |
| African American | 144 (7.19) | 22 (9.44) | 122 (6.90) | |
| Other/unknown race | 493 (24.63) | 59 (25.32) | 434 (24.53) | |
| Hispanic | 176 (8.79) | 20 (8.58) | 156 (8.82) | 0.7296 |
| Non-Hispanic | 1,460 (72.93) | 166 (71.24) | 1,294 (73.15) | |
| Unknown | 366 (18.28) | 47 (20.17) | 319 (18.03) | |
| Follow-up time in years, mean (SD) | 1.95 (1.05) | 1.95 (1.09) | 1.95 (1.04) | 0.9501 |
| Loss to follow-up | ||||
| death | 103 (5.14) | 9 (3.86) | 94 (5.31) | 0.3459 |
| dialysis | 197 (9.84) | 13 (5.58) | 184 (10.40) | 0.0202 |
| health plan disenrollment | 313 (15.63) | 53 (22.75) | 260 (14.70) | 0.0015 |
| MDT visitb | ||||
| any visit | 345 (17.23) | 219 (93.99) | 126 (7.12) | <0.0001 |
| annualized visit rate, mean (SD) | 1.91 (1.78) | 1.78 (1.89) | 2.17 (1.54) | 0.0004 |
| Outside nephrology visit | ||||
| any visit | 1,540 (76.92) | 1,540 (87.05) | ||
| annualized visit rate, mean (SD) | 2.20 (2.11) | 2.20 (2.11) | ||
| Primary care visits | ||||
| any visit | 1,939 (96.85) | 229 (98.28) | 1,710 (96.66) | 0.1834 |
| annualized visit rate, mean (SD) | 5.69 (6.69) | 4.29 (36.97) | 5.88 (6.95) | 0.0032 |
| Hospitalizations | ||||
| any inpatient hospitalization | 762 (39.56) | 76 (32.62) | 716 (40.47) | 0.0211 |
| annualized visit rate, mean (SD) | 0.55 (1.18) | 0.32 (0.85) | 0.57 (1.22) | <0.0001 |
| Number of chronic conditions,c mean (SD) | 4.45 (2.73) | 3.78 (2.19) | 4.53 (2.78) | 0.0002 |
| Diagnosis of diabetes | 878 | 114 (48.93) | 764 (43.19) | 0.0970 |
| Baseline A1c value (%), mean (SD) | 7.7 (1.7) | 7.9 (1.9) | 7.7 (1.7) | 0.4252 |
| Baseline GFR (ml/min per 1.73 m2) | 40.51 (6.56) | 40.89 (6.54) | 40.46 (6.56) | 0.3232 |
| Baseline at BP goal (%) | 47.6 (26.3) | 43.1 (23.5) | 48.2 (26.6) | 0.6396 |
| Baseline LDL (mg/dl) | 95.6 (35.9) | 91.5 (33.9) | 96.0 (36.1) | 0.1604 |
| BMI, mean (SD) | 29.83 (6.46) | 31.39 (6.34) | 29.63 (6.44) | <0.0001 |
P values were obtained from χ2 (categorical), or t test or Mann Whitney U-test (continuous) statistics.
Approximately 7% of the usual care group attended one or more MDT group visits.
Based on the Quan adaptation of the Elixhauser morbidity index.(28)
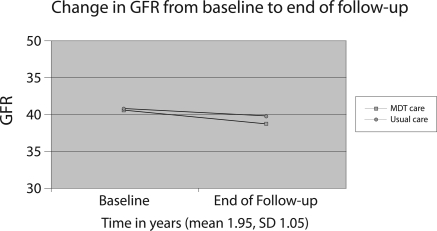
In both unadjusted and adjusted analyses, the rate of decline in GFR was lower in the MDT group (Tables 2 and 3). In multivariate repeated-measures analyses, referral to the internal MDT was associated with a mean decline in GFR of 1.2 ml/min per 1.73 m2 compared with a mean GFR decline of 2.5 ml/min per 1.73 m2 in members referred to the other three outside referral sites (P = 0.0001; Table 3; Figure 1). In stratified analyses of patients who completed their nephrology referrals and attended at least one visit with a nephrologist versus those who did not complete their referrals, the significant difference in decline in GFR between the MDT patients and usual care patients persisted in the completed referral group but not in the group who did not complete their referrals (Tables 2 and 3).
Table 2.
Unadjusted annual change in GFR: MDT versus usual care
| Referral Status | Estimated Change in GFR from |
|||||
|---|---|---|---|---|---|---|
| Total Sample |
Completed Referral |
Did Not Complete Referral |
||||
| Estimate (SE) | P | Estimate (SE) | P | Estimate (SE) | P | |
| Main effects, slopes | ||||||
| MDT care | −1.12 | 0.0003 | −1.09 (0.32) | 0.0007 | −1.64 (1.35) | 0.2241 |
| usual care | −2.47 | <0.0001 | −2.44 (0.12) | <0.0001 | −2.78 (0.029) | <0.0001 |
| Difference in slopes (MDT versus usual care) | 1.35 | <0.0001 | 1.34 (0.34) | <0.0001 | 1.13 (1.38) | 0.4131 |
Table 3.
Adjusted annual change in GFR: MDT versus usual carea
| Referral Status | Estimated Change in GFR from Baseline |
|||||
|---|---|---|---|---|---|---|
| Total Sample |
Completed Referral |
Did Not Complete Referral |
||||
| Estimate (SE) | P | Estimate (SE) | P | Estimate (SE) | P | |
| Main effects, slopes | ||||||
| MDT care | −1.17 | 0.0003 | −1.15 (0.33) | 0.0005 | −1.79 (1.38) | 0.1962 |
| usual care | −2.52 | <0.0001 | −2.48 (0.12) | <0.0001 | −2.83 (0.31) | <0.0001 |
| Difference in slopes (MDT versus usual care) | 1.35 | <0.0001 | 1.33 (0.35) | 0.0001 | 1.04 (1.41) | 0.4625 |
Adjusted for nephrology site, follow-up time, race, age, baseline GFR, gender, number of chronic conditions, body mass index, number of GFR measurements, and number of primary care visits.
Figure 1.
Change in GFR over time: MDT versus usual care (1). *Adjusted for nephrology site, follow-up time, race, age, baseline GFR, gender, number of chronic conditions, body mass index, number of GFR measurements, and number of primary care visits.
In adjusted analyses, there were no differences in the secondary outcomes of BP, A1c, or LDL between the MDT patients and the usual care patients (Table 4).
Table 4.
Adjusted model of disease-specific quality outcomes
| Percent Time at Normal BP |
Final LDL |
Final A1Ca |
||||
|---|---|---|---|---|---|---|
| Estimateb | Pc | Estimateb | Pc | Estimateb | Pc | |
| Referral status | ||||||
| MDT care | 41.35 | 0.1600 | 88.87 | 0.4143 | 7.30 | 0.7848 |
| Usual care | 45.38 | 91.59 | 7.37 | |||
Diabetic subjects only.
Adjusted for multidisciplinary team visit counts, outside nephrology visit counts, race, age, follow-up time, gender, morbidity score, body mass index, primary care visit counts, and baseline values.
P value for final adjusted difference between MDT and usual care.
Discussion
This quality improvement initiative showed that, in an integrated care setting, comprehensive, team-based, multidisciplinary care for CKD and associated comorbidities was associated with a slower decline in GFR over time and a lower percentage of patients initiating dialysis than usual care consisting of PCP management using outside nephrology consultation. These results were observed despite a lack of significant differences for secondary process of care measures for diabetes, hypertension, and coronary artery disease.
These findings suggest that there may have been other inherent differences in either the MDT population or MDT care process compared with the usual care subcohort that accounted for the slower decline in GFR in the MDT group. Both prescription of, and adherence to, a range of medications (such as angiotensin converting enzyme inhibitors, angiotensin receptor blockers, nonsteroidal anti-inflammatory agents, among others) may have differed across groups. Although control of diabetes and hypertension are primary predictors of change in GFR over time (29), the MDT care in this program also included assessment and management of multiple additional components of CKD care. For example, although there was no difference in prevalence of depression at baseline between the two groups, the team included a social worker who may have diagnosed and recommended treatment for additional incident cases of depression. Persons with CKD have a high prevalence of depression, which is independently associated with poor outcomes in CKD.(30,31) In addition the team provided coaching on details of self-management such as nutritional counseling—a missing piece for many patients with CKD (32).
There may also be intangible benefits to the informational continuity of care provided through an integrated system compared with receiving nephrology care at an outside site. Although outside nephrologists did have “read-only” access to patients' electronic medical records, the MDT team had the ability to electronically dialogue with other clinicians (e.g., cardiology, endocrinology, or primary care) and thereby address issues important to patients but not directly related to CKD care (e.g., difficulty with exercising because of pain from osteoarthritis).
In addition to differences in care delivery content and process, there may have been patient-level differences between the MDT and usual care groups. Those patients interested in participating in a comprehensive care management strategy may be more likely to have a higher level of engagement with recommended lifestyle changes (e.g., diet modification and medication adherence). The MDT group was also noted to have fewer comorbid chronic medical conditions at baseline—although this adjustment was incorporated into the analyses (see Table 1)—and lower rates of hospitalization during the program. Finally, there are multiple biopsychosical factors that may potentially influence processes of self-care for complex patients including perceived disease burden, social support, level of physical functioning, and others (33–35). Such factors may also contribute to a relatively slower decline in GFR over time.
There have been calls for increasing integrated care for CKD patients to specifically include management of cardiovascular risk factors; shared care between specialists, primary care clinicians, and allied health workers such as dieticians and pharmacists; and an emphasis on patient self-management support (14,16,17). However, there is debate in the literature about the benefit of multidisciplinary team care for CKD patients. Different MDT interventions in different populations and settings have produced conflicting results (21–23). Our findings lend support to the concept of MDT care, although they do not clarify the specific components of that care that effect improvement in health outcomes.
There are several limitations to our quality improvement assessment. As mentioned above, it was conducted in an integrated care environment, and the results may be less generalizable to other settings. However, the MDT concept may become increasingly applicable across settings with the broader implementation of electronic medical records and of integrated care consistent with the Patient Centered Medical Home model. This project was designed as a quality improvement initiative and not a randomized clinical trial. Therefore, allocation of the patients to nephrology care was based on zip code of residence rather than true randomization, and to the extent that geographic location may be associated with socioeconomic status and health status, this practice may have biased the results. We attempted to account for this limitation by adjusting for patient level variables (e.g., demographics and comorbidities) and by approximating an intention to treat analysis and including all patients referred for nephrology consultation regardless of whether or not they were actually seen by the consultant. Approximately 7% of the usual care group received one or more MDT visits; however, this crossover should bias our results toward the null and result in a more conservative estimate of the MDT program. In addition, there may have been provider practice variation within each of the external nephrology practices, whereas there was only one nephrologist in the MDT practice. Finally, other outcomes warrant consideration in a complete assessment of the effects of MDT care. Specifically, because of low rates of baseline proteinuria assessment in primary care before nephrology referral, we were unable to accurately assess changes in proteinuria over time as a function of enrollment in the MDT program. This important outcome should be assessed in future studies of MDT care, as should longer-term outcomes of renal replacement therapy, mortality, cardiovascular events, and formal cost-effectiveness analyses.
The population of persons with CKD is growing rapidly. Progressive CKD has negative effects on quality of life and on healthcare resources. Therefore, it is important to explore different mechanisms of delivering care to this complex patient population. Our findings suggest that specific groups of CKD patients may benefit from comprehensive MDT CKD care as manifested by delayed progressive decline in renal function and that these improved health outcomes may be facilitated within an integrated health care system. Furthermore, the benefit of slower decline in CKD did not seem to be completely mediated by improved control of hypertension or diabetes. Remaining challenges are to identify subsets of persons with CKD who are likely to benefit from such MDT teams and to determine the ideal components and duration of MDT care.
Disclosures
None.
Acknowledgments
We would like to acknowledge the valuable clinical contributions of Christine Schaefer, RN, MSN, CDE, BCADM, Heather Samuels, LPN, Jennifer Heffern, LCSW, Ericka Sanstedt, LSCW, Cassie Greene, RD, CDE, and Susan Spies, RN, and the statistical expertise of Chan Zeng, PhD. Portions of this material were presented in poster format at the annual meeting of the American Society of Nephrology, October 27 to November 1, 2009, San Diego, CA. An abstract on portions of this material has been submitted to the annual HMO Research Network Conference, March 2011.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van LF, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 2. United States Renal Data System: USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2010, Tables CII and DII [Google Scholar]
- 3. Whaley-Connell AT, Sowers JR, Stevens LA, McFarlane SI, Shlipak MG, Norris KC, Chen SC, Qiu Y, Wang C, Li S, Vassalotti JA, Collins AJ: CKD in the United States: Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES) 1999–2004. Am J Kidney Dis 51: S13–S20, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Gullion CM, Keith DS, Nichols GA, Smith DH: Impact of comorbidities on mortality in managed care patients with CKD. Am J Kidney Dis 48: 212–220, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Nissenson AR, Collins AJ, Hurley J, Petersen H, Pereira BJ, Steinberg EP: Opportunities for improving the care of patients with chronic renal insufficiency: Current practice patterns. J Am Soc Nephrol 12: 1713–1720, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Kausz AT, Khan SS, Abichandani R, Kazmi WH, Obrador GT, Ruthazer R, Pereira BJ: Management of patients with chronic renal insufficiency in the Northeastern United States. J Am Soc Nephrol 12: 1501–1507, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Craig JC, Barratt A, Cumming R, Irwig L, Salkeld G: Feasibility study of the early detection and treatment of renal disease by mass screening. Intern Med J 32: 6–14, 2002 [PubMed] [Google Scholar]
- 8. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 9. National Kidney Foundation: K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39: S1–S266, 2002 [PubMed] [Google Scholar]
- 10. Muntner P, Hamm LL, Kusek JW, Chen J, Whelton PK, He J: The prevalence of nontraditional risk factors for coronary heart disease in patients with chronic kidney disease. Ann Intern Med 140: 9–17, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Muntner P, He J, Hamm L, Loria C, Whelton PK: Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. J Am Soc Nephrol 13: 745–753, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Levey AS, Beto JA, Coronado BE, Eknoyan G, Foley RN, Kasiske BL, Klag MJ, Mailloux LU, Manske CL, Meyer KB, Parfrey PS, Pfeffer MA, Wenger NK, Wilson PW, Wright JT, Jr.: Controlling the epidemic of cardiovascular disease in chronic renal disease: what do we know? What do we need to learn? Where do we go from here? National Kidney Foundation Task Force on Cardiovascular Disease. Am J Kidney Dis 32: 853–906, 1998 [DOI] [PubMed] [Google Scholar]
- 13. Eknoyan G: On the epidemic of cardiovascular disease in patients with chronic renal disease and progressive renal failure: A first step to improve the outcomes. Am J Kidney Dis 32: S1–S4, 1998 [DOI] [PubMed] [Google Scholar]
- 14. Curtis BM, Levin A, Parfrey PS: Multiple risk factor intervention in chronic kidney disease: Management of cardiac disease in chronic kidney disease patients. Med Clin North Am 89: 511–523, 2005 [DOI] [PubMed] [Google Scholar]
- 15. James MT, Hemmelgarn BR, Tonelli M: Early recognition and prevention of chronic kidney disease. Lancet 375: 1296–1309, 2010 [DOI] [PubMed] [Google Scholar]
- 16. St Peter WL, Schoolwerth AC, McGowan T, McClellan WM: Chronic kidney disease: issues and establishing programs and clinics for improved patient outcomes. Am J Kidney Dis 41: 903–924, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Stevens LA, Levin A: Translating research findings of chronic kidney disease management to clinical practice: Challenges and opportunities. Adv Ren Replace Ther 11: 66–75, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Wagner EH: The role of patient care teams in chronic disease management. BMJ 320: 569–572, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bodenheimer T, Wagner EH, Grumbach K: Improving primary care for patients with chronic illness. JAMA 288: 1775–1779, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Bodenheimer T, Wagner EH, Grumbach K: Improving primary care for patients with chronic illness: The chronic care model, Part 2. JAMA 288: 1909–1914, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Ghossein C, Serrano A, Rammohan M, Batlle D: The role of comprehensive renal clinic in chronic kidney disease stabilization and management: The Northwestern experience. Semin Nephrol 22: 526–532, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Harris LE, Luft FC, Rudy DW, Kesterson JG, Tierney WM: Effects of multidisciplinary case management in patients with chronic renal insufficiency. Am J Med 105: 464–471, 1998 [DOI] [PubMed] [Google Scholar]
- 23. Hemmelgarn BR, Manns BJ, Zhang J, Tonelli M, Klarenbach S, Walsh M, Culleton BF: Association between multidisciplinary care and survival for elderly patients with chronic kidney disease. J Am Soc Nephrol 18: 993–999, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Curtis BM, Ravani P, Malberti F, Kennett F, Taylor PA, Djurdjev O, Levin A: The short- and long-term impact of multi-disciplinary clinics in addition to standard nephrology care on patient outcomes. Nephrol Dial Transplant 20: 147–154, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Wrone EM, Hornberger J: Evaluating the consequences of multidisciplinary case management for patients with chronic renal failure. Am J Med 105: 546–548, 1998 [DOI] [PubMed] [Google Scholar]
- 26. Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH: Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 164: 659–663, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi J-C, Saunders LD, Beck CA, Feasby TE, Ghali WA: Coding algorithms for defining comorbidities in ICD-9-Cm and ICD-10 administrative data. Med Care 43: 1130–1139, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Hanratty R, Chonchol M, Miriam DL, Beaty BL, Estacio RO, Mackenzie TD, Hurley LP, Linas SL, Steiner JF, Havranek EP: Incident chronic kidney disease and the rate of kidney function decline in individuals with hypertension. Nephrol Dial Transplant 25: 801–807, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hedayati SS, Minhajuddin AT, Afshar M, Toto RD, Trivedi MH, Rush AJ: Association between major depressive episodes in patients with chronic kidney disease and initiation of dialysis, hospitalization, or death. JAMA 303: 1946–1953, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hedayati SS, Minhajuddin AT, Toto RD, Morris DW, Rush AJ: Prevalence of major depressive episode in CKD. Am J Kidney Dis 54: 424–432, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thomas N, Bryar R, Makanjuola D: Development of a self-management package for people with diabetes at risk of chronic kidney disease (CKD). J Ren Care 34: 151–158, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Bayliss EA, Steiner JF, Fernald DH, Crane LA, Main DS: Descriptions of barriers to self-care by persons with comorbid chronic diseases. Ann Family Med 1: 15–21, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Noel PH, Frueh C, Larme A, Pugh J: Collaborative care needs and preferences of primary care patients with multimorbidity. Health Expectations 8: 54–63, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kerr EA, Heisler M, Krein SL, Kabeto M, Langa KM, Weir D, Piette JD: Beyond comorbidity counts: How do comorbidity type and severity influence diabetes patients' treatment priorities and self-management? J Gen Intern Med 22: 1635–1640, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]