Summary
Background and objectives
Recent studies suggest an overall association between chronic kidney disease (CKD) and periodontal disease, but it is unknown whether this association is similar across various subpopulations.
Design, setting, participants, & measurements
This study was a cross-sectional analysis of 2001 to 2004 National Health and Nutrition Examination Survey data. CKD was defined as a urinary albumin-to-creatinine ratio ≥30 mg/g or estimated GFR of 15 to 59 ml/min per 1.73 m2. Adjusted odds ratios were calculated using multivariable logistic regression with U.S. population-based weighting.
Results
These analyses included 6199 dentate adult participants (aged 21 to 75 years) with periodontal exams. The estimated prevalences of moderate/severe periodontal disease and CKD were 5.3% and 10.6%, respectively. Periodontal disease was associated with >2-fold higher risk of CKD that was moderately attenuated after adjustment for age, gender, race/ethnicity, tobacco use, hypertension, diabetes, educational attainment, poverty index ratio, and dental care use. There were no statistically significant interactions between periodontal disease and race/ethnicity, educational attainment, or poverty status. Less-than-recommended dental care use was associated with periodontal disease and CKD and was increasingly prevalent among nonwhites, lower educational attainment, and lower poverty status.
Conclusions
The association between periodontal disease and CKD is not significantly different among subgroups. However, because nonwhites, those with a lower educational level, and the poor less frequently report use of recommended dental care, the association between periodontal disease and kidney function over time may become stronger among these groups and warrants further investigation.
Introduction
Chronic kidney disease (CKD) is an important public health issue, affecting an estimated 13% to 16% of the U.S. population and accounting for nearly 28% of Medicare spending in 2007 (1). Although CKD risk factors such as poorly controlled diabetes and hypertension are well established, efforts to reduce these risk factors alone have not yet resulted in a decrease in the prevalence of CKD (2). Therefore, identifying and treating nontraditional modifiable risk factors for CKD could have a tremendous effect on the societal burden of CKD.
Periodontal disease, a chronic bacterial infection of the oral cavity, may be such a risk factor. Periodontal pathogens have been shown to have the ability to adhere to, invade, and proliferate in coronary endothelial cells (3,4), leading to atheroma formation (5,6) and impaired vasculature relaxation (7). Because cardiovascular disease and CKD share many risk factors, it is biologically plausible that periodontal disease exerts similar effects within the vasculature of the kidney.
Recent studies have shown an association between periodontal disease and CKD, with periodontal disease being associated with a 1.6- to 2.0-fold greater risk of CKD (8–10). Importantly, periodontal disease disproportionately affects nonwhites, those with low income, and those with a lower educational level (11–13). For example, in a nationally representative sample, Borrell et al. (13) found that the prevalence of periodontal disease among non-Hispanic blacks was more than twice that of non-Hispanic whites (7.2% versus 3.0%), and the risk of periodontal disease was 2-fold greater among non-Hispanic blacks, those with low income, and those with less than a high school education. Furthermore, higher age-adjusted incidence rates of CKD (14–17) and faster progression to end-stage renal disease (18–24) among nonwhites and the poor have also been observed. Because periodontal disease and CKD are associated with racial/ethnic and social disparities, we hypothesized that any CKD risk associated with periodontal disease might vary among these subgroups. Therefore, we sought to characterize the association of periodontal disease and CKD among race/ethnicity, educational attainment, and poverty status subgroups within a nationally representative sample of adults.
Materials and Methods
Study Population
The population for this study was drawn from the National Health and Nutrition Examination Survey (NHANES). NHANES is a well established representative survey of noninstitutionalized civilian residents in the United States conducted by the National Center for Health Statistics of the Centers for Disease Control and Prevention. The survey consists of a standardized in-home interview followed by a physical examination and blood and urine collection at a mobile examination center. All participants give written informed consent. The protocol was approved by the National Center for Health Statistics Research Ethics Review Board.
Our study was limited to dentate non-Hispanic white, non-Hispanic black, or Mexican-American participants aged 21 to 75 years from 2001 to 2004 with detailed periodontal exams. NHANES did not include periodontal examinations after 2004. Participants missing dental or kidney function measures were excluded. Participants who were edentulous or pregnant were excluded.
Measurements
Study-trained dentists conducted the periodontal examinations (25,26). Clinical attachment loss and pocket probe depths were recorded midbuccal, mesiobuccal, and distobuccal for each tooth. In the oral health questionnaire, participants were asked about their last dental visit (“About how long has it been since you last visited the dentist? Include all types of dentists, such as orthodontics, oral surgeons, and all other dental specialists, as well as dental hygienists.”) and reason for last dental visit (“What was the main reason you last visited the dentist?”).
Serum creatinine concentration was measured by the modified kinetic method of Jaffe using different analyzers in different survey years. Creatinine levels were corrected as specified in NHANES documentation (27,28) and were used in formulas for estimates of kidney function. Random spot urine samples were obtained, and urine albumin and creatinine were measured using frozen specimens. Urine albumin was measured using solid-phase fluorescence immunoassay. Urine creatinine was measured using the modified Jaffe kinetic method in the same laboratory.
Definitions
We defined periodontal disease as a dichotomous variable: none/mild or moderate/severe disease. In accordance with the Centers for Disease Control and Prevention/American Academy of Periodontology (CDC/AAP) definition, participants were considered to have moderate/severe disease if there were two or more interproximal tooth sites not on the same tooth with a level of attachment of ≥4 mm or pocket probe depths ≥5 mm. This definition was developed in an effort to standardize the clinical case definition of periodontal disease for population-based studies (29). We created a composite variable for dental care use from self-reported last dental visit and reason for last dental visit. Dental care use was defined as “as recommended” if the last dental visit was reported <1 year prior and for a routine reason (went in on own or was called in by dentist for check-up, examination, or cleaning). Dental care use was considered “less than recommended” if the last dental visit was >1 year ago, for a problem (something was wrong, bothering, or hurting or went for treatment of a condition that a dentist discovered at an earlier check-up or examination), or for another reason.
We defined CKD as the presence of reduced kidney function (estimated GFR [eGFR] 15 to 59 ml/min per 1.73 m2) and/or albuminuria (urinary albumin-to-creatinine ratio ≥ 30 mg/g). eGFR was calculated according to the Modification of Diet in Renal Disease study equation for calibrated creatinine: eGFR = 175 × [(calibrated serum creatinine in mg/dl)−1.154] × age−0.203 × (0.742 if female) × (1.21 if African American) (30).
We categorized self-reported age into four groups (21 to 34, 35 to 49, 50 to 64, and 65 to 75 years) and self-reported race/ethnicity as non-Hispanic white, non-Hispanic black, or Mexican American. Self-reported significant tobacco use (none, past, ongoing) included cigarettes, snuff, and chewing tobacco. Lifetime use of >100 cigarettes or 20 units of snuff or chewing tobacco was considered significant use. Body mass index (in kg/m2) was calculated using height and weight measured during the exam. BP (at least three auscultatory measurements) was measured during the exam. Hypertension was defined as the participant reporting having ever been told by a healthcare provider that they had hypertension or by the finding of an average of second and third readings of BP ≥140 mmHg systolic or ≥90 mmHg diastolic. We defined diabetes as the participant reporting being told by a provider that they had diabetes or by the conservative finding of a glycosylated hemoglobin >8% to minimize misclassification due to participant unawareness of diabetes diagnosis. Educational attainment was categorized as more than high school, high school or high school equivalent, and less than high school. We defined poverty status using the U.S. Census Bureau's poverty index ratio, the ratio of family income to federal poverty level: ≤1.00 is considered below the poverty level. We defined poverty status as a three-level variable: poverty index ratio ≤1.00, 1.01 to 3.49, or ≥3.50.
Statistical Analyses
We compared participant characteristics for periodontal disease categories using χ2 analyses. We used multivariable logistic regression to assess the presence, direction, strength, and independence of the association between periodontal disease and CKD. Covariates were added to the unadjusted regression model in a stepwise fashion if they were associated (P < 0.05) with periodontal disease and CKD in our sample population (i.e., true confounders). Next, we tested for interactions between periodontal disease and race/ethnicity, poverty status, or educational attainment within the fully adjusted model. Finally we described self-reported dental use among race/ethnicity, educational attainment, and poverty status subgroups and tested for the association of dental use with periodontal disease.
We repeated analyses with CKD defined by GFR estimated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (31). Finally, we conducted sensitivity analyses with periodontal disease as defined by other authors examining the association of periodontal disease with CKD (8,10). All analyses were performed using the svy commands in Stata v. 11.0 (College Station, TX) to account for study design weights, strata, and primary sampling units.
Results
We included 6199 dentate, adult (age 21 to 75 years) participants from NHANES 2001 to 2004 who met our study criteria. From a total denominator of 21,161 participants who were non-Hispanic white, non-Hispanic black, or Mexican American, we excluded 12,236 participants under the age of 21 years, 618 pregnant participants, 1613 missing kidney function measures, and 495 missing dental measures. The overall estimated prevalences of moderate/severe periodontal disease and CKD were 5.3% and 10.6%, respectively. Being older, male, nonwhite, less educated, and poor were all associated with moderate/severe versus no/mild periodontal disease (Table 1). Ongoing or past tobacco use, diabetes, and hypertension were more common among participants with moderate/severe versus no/mild periodontal disease. Recommended dental care was less frequently reported among participants with moderate/severe versus no/mild periodontal disease.
Table 1.
U.S. population characteristics (point estimate prevalence) among dentate adults (aged 21 to 75 years) by severity of periodontal disease
| Characteristic | Percent in Category |
P | ||
|---|---|---|---|---|
| Entire Population | None/Mild Periodontal Disease | Moderate/Severe Periodontal Disease | ||
| CKD | 10.6 | 10.0 | 21.6 | <0.001 |
| Age, years | <0.001 | |||
| 21 to 34 | 29.4 | 30.7 | 5.9 | |
| 35 to 49 | 37.4 | 37.5 | 35.6 | |
| 50 to 64 | 23.8 | 23.0 | 37.9 | |
| 65 to 75 | 9.5 | 8.9 | 20.6 | |
| Men | 50.1 | 49.2 | 66.2 | <0.001 |
| Race/ethnicity | <0.001 | |||
| non-Hispanic White | 79.7 | 80.2 | 70.5 | |
| non-Hispanic Black | 11.7 | 11.2 | 20.0 | |
| Mexican American | 8.7 | 8.6 | 9.5 | |
| Tobacco use | <0.001 | |||
| none | 47.8 | 49.0 | 26.1 | |
| past | 24.3 | 24.0 | 29.2 | |
| ongoing | 27.9 | 27.0 | 44.7 | |
| Diabetes | 5.9 | 5.5 | 12.5 | <0.001 |
| control: among participants with diabetes, % with glycosylated hemoglobin > 8% | 28.1 | 27.9 | 30.1 | 0.709 |
| Hypertension | 48.3 | 47.8 | 57.7 | 0.008 |
| C-reactive protein, mg/dl | 0.121 | |||
| ≤1 | 91.4 | 91.5 | 88.4 | |
| 1 to 3 | 7.4 | 7.3 | 9.6 | |
| >3 | 1.3 | 1.2 | 2.1 | |
| Body mass index, kg/m2 | 0.126 | |||
| ≤24.9 | 33.1 | 33.4 | 27.5 | |
| 25.0 to 29.9 | 34.2 | 34.0 | 38.1 | |
| ≥30.0 | 32.7 | 32.6 | 34.4 | |
| Educational attainment | <0.001 | |||
| more than high school | 59.1 | 60.0 | 42.9 | |
| high school | 25.9 | 25.8 | 27.1 | |
| less than high school | 15.0 | 14.2 | 30.0 | |
| Poverty index ratio | <0.001 | |||
| ≥3.50 | 47.8 | 48.6 | 32.2 | |
| 1.01 to 3.49 | 41.1 | 40.5 | 50.8 | |
| ≤1.00 (at or below poverty line) | 11.2 | 10.8 | 17.1 | |
| Dental care use | <0.001 | |||
| as recommended | 43.0 | 44.1 | 23.1 | |
| less than recommended | 57.0 | 55.9 | 76.9 | |
Total percent category by characteristic may exceed 100% because of rounding. CKD, chronic kidney disease (eGFR15 to 59 ml/min per 1.73m2 or urine albumin-to-creatnine ratio ≤ 30 mg/g).
In unadjusted logistic regression models, the odds of participants with moderate/severe periodontal disease having CKD were more than twice that of those with no/mild periodontal disease (Table 2). The strength of the association was modestly attenuated but remained statistically significant after adjustment for age, gender, tobacco use, diabetes, and hypertension. There was further attenuation after adjusting for race/ethnicity, educational attainment, poverty status, and dental care use, but the association remained statistically significant. Although stratified analyses by race/ethnicity, poverty status, or educational attainment revealed some significant associations, tests for interaction were NS (Table 3).
Table 2.
Association of periodontal disease with CKD (eGFR 15 to 59 ml/min per 1.73 m2) or urine albumin-to-creatinine ratio ≥ 30 mg/g)
| OR | 95% Confidence Interval | P | |
|---|---|---|---|
| Unadjusted | 2.50 | 1.96, 3.19 | <0.001 |
| Adjusted for age and gender | 1.74 | 1.32, 2.30 | <0.001 |
| Adjusted for above plus tobacco use, hypertension, and diabetes | 1.59 | 1.20, 2.10 | 0.002 |
| Adjusted for above plus race/ethnicity, poverty status, and educational attainment | 1.55 | 1.16, 2.07 | 0.004 |
| Adjusted for above plus dental care use | 1.51 | 1.13, 2.02 | 0.006 |
Table 3.
Association of periodontal disease within subgroups and CKD, fully adjusted model
| Subgroup | OR | 95% Confidence Interval | P |
|---|---|---|---|
| Race/ethnicity | |||
| non-Hispanic white | 1.72 | 1.15, 2.56 | 0.010 |
| non-Hispanic black | 0.96 | 0.54, 1.69 | 0.874 |
| Mexican Americans | 1.35 | 0.69, 2.61 | 0.364 |
| Test for interaction | 0.243 | ||
| Poverty status | |||
| PIR ≥ 3.5 | 1.75 | 0.90, 3.35 | 0.094 |
| PIR 1.01 to 3.49 | 1.17 | 0.73, 1.90 | 0.490 |
| PIR ≤ 1 | 2.57 | 1.25, 5.28 | 0.012 |
| Test for interaction | 0.259 | ||
| Educational attainment | |||
| more than high school | 1.60 | 0.97, 2.64 | 0.066 |
| high school | 2.00 | 1.24, 3.23 | 0.006 |
| less than high school | 1.13 | 0.71, 1.78 | 0.593 |
| Test for interaction | 0.107 |
Full model adjusted for age, gender, tobacco use, hypertension, diabetes, race/ethnicity, poverty status, educational attainment, and dental care use. PIR, poverty index ratio.
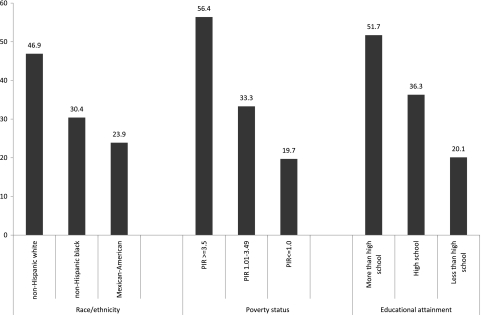
Fewer than half of the participants reported receiving recommended dental care. Non-Hispanic blacks and Mexican Americans less frequently reported recommended dental care use than non-Hispanic whites (Figure 1). Those with lower educational attainment and lower poverty status less frequently reported receipt of recommended dental care. Those reporting less-than-recommended dental care use were twice as likely to have moderate/severe periodontal disease than those reporting recommended dental care use after adjustment for other covariates (odds ratio [OR] 1.99, 1.37 to 2.89, P = 0.001).
Figure 1.
Percentage of participants reporting recommended (routine visit within last year) dental care use by subgroup. PIR, poverty index ratio.
In separate analyses using the CKD-EPI equation to define CKD, 9.2% of the population had CKD. Of participants with no/mild periodontal disease, 8.6% had CKD whereas 20.5% of those with moderate/severe periodontal disease had CKD. The overall association between periodontal disease and CKD was stronger than that using the Modification of Diet in Renal Disease equation (unadjusted OR 2.73, 2.23 to 3.35, P < 0.001) and was modestly attenuated after adjusting for age and gender (OR 1.91, 1.49 to 2.45, P < 0.001); after additionally adjusting for tobacco use, diabetes, and hypertension (OR 1.75, 1.35 to 2.26, P < 0.001); after additionally adjusting for race/ethnicity, poverty status, and educational attainment (OR 1.63, 1.22 to 2.16, P = 0.001); and lastly, after additionally adjusting for dental care use (OR 1.59, 1.19 to 2.11, P = 0.002). There were no significant interactions between periodontal disease and race/ethnicity, poverty status, or educational attainment for the association with CKD (data not shown).
Our sensitivity analysis using Kshirsagar and colleagues' (8) case definition for periodontal disease (two or more interproximal sites on different teeth with ≥4 mm or clinical attachment level) yielded nearly identical results (OR 1.52, 1.13 to 2.04, P = 0.008 in the fully adjusted model). In our sensitivity analysis using Fisher and colleagues' (10) case definition, which requires the presence of bleeding on probing with the periodontal examination (an indicator of active inflammation) on the same one or more sites with loss of attachment of ≥4 mm, periodontal disease was associated with CKD in the unadjusted model (OR 2.00, 1.45 to 2.75, P < 0.001). This association was attenuated with adjustment for comorbidities (OR 1.42, 0.99 to 2.04, P = 0.056).
Discussion
In a recent nationally representative sample of U.S. adults, we observed a significant association between periodontal disease and CKD. This association was attenuated but remained statistically significant after adjustment for confounders. Consistent with prior nationally representative (11,13) and cohort (12) studies, we found that periodontal disease disproportionately affects nonwhites, those with low income, and those with a lower educational level. Therefore, we postulated that the association of CKD and periodontal disease would be stronger among these subgroups.
In stratified analyses, the association appeared strongest among non-Hispanic whites and the very poor, but tests for interaction of periodontal disease by subgroup and CKD were NS, arguing against a differential biologic mechanism. We do not know if the relationship was actually stronger in these groups, and our study was not sufficiently powered to detect the difference or if the observed relationship was the result of sampling variation. Alternatively, the finding among the very poor may be part of a very complex, unmeasured set of factors more common to the group (e.g., environmental exposure to specific periodontal pathogens and oral hygiene) that, taken together, may increase risk of CKD.
However, of note, the self-reported lack of recommended dental care use—as more frequently reported by nonwhites, those with a lower educational level, and the poor—was associated with periodontal disease and CKD. Although adjustment for dental care use did not fully attenuate the association between periodontal disease and CKD, this finding suggests that periodontal disease prevention or treatment may play a role in attenuating the development or progression of CKD and warrants further investigation.
Our findings are similar to those among all adults in NHANES III (1988 to 1994), in whom periodontal disease and edentulism were associated with a 1.6 and 1.9 greater risk of CKD, respectively (10). Among middle-aged dentate adults in the Atherosclerosis Risk in Communities study, severe periodontal disease was associated with a 2-fold greater risk of CKD (8). Although our results show a similar association between periodontal disease and CKD, our study further informs the field by additionally accounting for dental care use and exploring subgroup differences. Furthermore, we used the CDC/AAP definition for periodontal disease, which was developed in an effort to standardize the clinical case definition of periodontal disease for population-based studies. Our sensitivity analysis using the Kshirsagar et al. (8) definition yielded similar results, likely because of its similarity to the CDC/AAP definition. On the other hand, the results from the sensitivity analysis using the Fisher et al. (10) definition likely reflect its requirement of active inflammation, which may reflect earlier periodontal disease that has yet to impair kidney function independent of comorbid conditions.
The biologic plausibility of periodontal disease leading to impaired kidney function through endothelial injury is supported by an extensive cardiovascular literature. Interestingly, treatment of periodontal disease has recently been shown to positively affect a surrogate marker for clinical cardiovascular disease through sustained increases in brachial artery dilation and blood flow (32). Given that epidemiologic studies have demonstrated an overall association between periodontal disease and cardiovascular disease (33) similar in direction and magnitude to that found between periodontal disease and CKD, it is important to explore to what extent treatment of periodontal disease may affect kidney function.
A limitation of our cross-sectional study is that we did not have data on persistent albuminuria. Because we included albuminuria in our definition of CKD, we may have overestimated the prevalence of CKD. On the other hand, because NHANES periodontal examinations included only three tooth sites (rather than a full six-site examination), we likely also underestimated the prevalence of periodontal disease. Another limitation is that our measure of dental care use is based on participant recall and is therefore subject to recall and social desirability bias. Furthermore, even if reported accurately, the last year may not be typical of an individual's usual pattern of accessing dental care. This may have skewed our analyses against estimating the true effect of recommended dental care use. Additionally, all observational studies are subject to possible confounding due to unknown or unmeasured factors. Finally, cross-sectional studies such as this do not allow for causal inference, and reverse causation (CKD causing periodontal disease), or the coexistence of the two due to common underlying risk factors, cannot be excluded.
In summary, the association between periodontal disease and CKD is not significantly different among subgroups. However, because less-than-recommended dental care use is strongly associated with periodontal disease and is most common among nonwhites, those with a lower educational level, and the poor, the effect of periodontal disease over time may be more profound among these groups. Longitudinal studies are needed to determine to what extent periodontal disease is a true risk factor for CKD and to what extent periodontal disease treatment affects the trajectory of kidney function over time.
Disclosures
None.
Acknowledgments
We thank the participants and staff of the NHANES survey. We also thank Gary Armitage for guidance regarding periodontal disease case definitions. The CDC CKD Surveillance Team consists of members from the University of California–San Francisco (Neil Powe, Laura Plantinga, Kirsten Bibbins-Domingo, Josef Coresh, Alan Go, Chi-Yuan Hsu, Lesley Stevens, Deidra Crews, and Vanessa Grubbs), the University of Michigan (Rajiv Saran, Elizabeth Hedgeman, Brenda Gillespie, William Herman, Friedrich Port, Bruce Robinson, Vahakn Shahinian, Michael Heung, Jerry Yee, and Eric Young), and CDC (Desmond Williams, Nilka Ríos Burrows, Mark Eberhardt, Paul Eggers, Nicole Flowers, Linda Geiss, Susan Hailpern, Regina Jordan, Juanita Mondeshire, Bernice Moore, Gary Myers, Meda Pavkov, Deborah Rolka, Sharon Saydah, Anton Schoolwerth, Rodolfo Valdez, and Larry Waller). This project was supported under a cooperative agreement from CDC through the Association of American Medical Colleges (AAMC), grant number 5U36CD319276, AAMC ID number MM-1143-10/10. Publication and report contents are solely the responsibility of the authors and do not necessarily represent the official views of the AAMC or CDC. Dr. Grubbs is supported by a Diversity Supplement to grant R01 DK70939 from the National Institute of Diabetes and Digestive and Kidney Disease, Bethesda, Maryland. Dr. Powe is partially supported by grant K24DK02643 from the National Institute of Diabetes and Digestive and Kidney Disease, Bethesda, Maryland. Dr. Crews is supported by the Harold Amos Medical Faculty Development Program of the Robert Wood Johnson Foundation, Princeton, New Jersey. Drs. Bibbins-Domingo and Powe and Ms. Plantinga are members of the University of California–San Francisco Center for Vulnerable Populations at San Francisco General Hospital and Trauma Center.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. U.S. Renal Data System: USRDS 2009 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institute of Health, National Institute of Diabetes and Digestive and Kidney Disease, 2009 [Google Scholar]
- 2. Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Deshpande RG, Khan MB, Genco CA: Invasion of aortic and heart endothelial cells by Porphyromonas gingivalis. Infect Immun 66: 5337–5343, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dorn BR, Dunn WA, Jr, Progulske-Fox A: Invasion of human coronary artery cells by periodontal pathogens. Infect Immun 67: 5792–5798, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Qi M, Miyakawa H, Kuramitsu HK: Porphyromonas gingivalis induces murine macrophage foam cell formation. Microb Pathog 35: 259–267, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Giacona MB, Papapanou PN, Lamster IB, Rong LL, D'Agati VD, Schmidt AM, Lalla E: Porphyromonas gingivalis induces its uptake by human macrophages and promotes foam cell formation in vitro. FEMS Microbiol Lett 241: 95–101, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Amar S, Gokce N, Morgan S, Loukideli M, Van Dyke TE, Vita JA: Periodontal disease is associated with brachial artery endothelial dysfunction and systemic inflammation. Arterioscler Thromb Vasc Biol 23: 1245–1249, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Kshirsagar AV, Moss KL, Elter JR, Beck JD, Offenbacher S, Falk RJ: Periodontal disease is associated with renal insufficiency in the Atherosclerosis Risk in Communities (ARIC) study. Am J Kidney Dis 45: 650–657, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Fisher MA, Taylor GW: A prediction model for chronic kidney disease includes periodontal disease. J Periodontol 80: 16–23, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fisher MA, Taylor GW, Shelton BJ, Jamerson KA, Rahman M, Ojo AO, Sehgal AR: Periodontal disease and other nontraditional risk factors for CKD. Am J Kidney Dis 51: 45–52, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Albandar JM, Brunelle JA, Kingman A: Destructive periodontal disease in adults 30 years of age and older in the United States, 1988–1994. J Periodontol 70: 13–29, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Borrell LN, Beck JD, Heiss G: Socioeconomic disadvantage and periodontal disease: The Dental Atherosclerosis Risk in Communities study. Am J Public Health 96: 332–339, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Borrell LN, Crawford ND: Social disparities in periodontitis among United States adults 1999–2004. Community Dent Oral Epidemiol 36: 383–391, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Hall YN, Choi AI, Chertow GM, Bindman AB: Chronic kidney disease in the urban poor. Clin J Am Soc Nephrol 5: 828–835, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krop JS, Coresh J, Chambless LE, Shahar E, Watson RL, Szklo M, Brancati FL: A community-based study of explanatory factors for the excess risk for early renal function decline in blacks vs whites with diabetes: The Atherosclerosis Risk in Communities study. Arch Intern Med 159: 1777–1783, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Stehman-Breen CO, Gillen D, Steffes M, Jacobs DR, Jr, Lewis CE, Kiefe CI, Siscovick D: Racial differences in early-onset renal disease among young adults: The coronary artery risk development in young adults (CARDIA) study. J Am Soc Nephrol 14: 2352–2357, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Tarver-Carr ME, Powe NR, Eberhardt MS, LaVeist TA, Kington RS, Coresh J, Brancati FL: Excess risk of chronic kidney disease among African-American versus white subjects in the United States: A population-based study of potential explanatory factors. J Am Soc Nephrol 13: 2363–2370, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Brancati FL, Whittle JC, Whelton PK, Seidler AJ, Klag MJ: The excess incidence of diabetic end-stage renal disease among blacks. A population-based study of potential explanatory factors. JAMA 268: 3079–3084, 1992 [PubMed] [Google Scholar]
- 19. Cowie CC, Port FK, Wolfe RA, Savage PJ, Moll PP, Hawthrone VM: Disparities in incidence of diabetic end-stage renal disease according to race and type of diabetes. N Engl J Med 321: 1074–1079, 1989 [DOI] [PubMed] [Google Scholar]
- 20. Easterling RE: Racial factors in the incidence and causation of end-stage renal disease (ESRD). Trans Am Soc Artif Intern Organs 23: 28–33, 1977 [DOI] [PubMed] [Google Scholar]
- 21. Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Stamler J: End-stage renal disease in African-American and white men. 16-year MRFIT findings. JAMA 277: 1293–1298, 1997 [PubMed] [Google Scholar]
- 22. McClellan W, Tuttle E, Issa A: Racial differences in the incidence of hypertensive end-stage renal disease (ESRD) are not entirely explained by differences in the prevalence of hypertension. Am J Kidney Dis 12: 285–290, 1988 [DOI] [PubMed] [Google Scholar]
- 23. Rostand SG, Kirk KA, Rutsky EA, Pate BA: Racial differences in the incidence of treatment for end-stage renal disease. N Engl J Med 306: 1276–1279, 1982 [DOI] [PubMed] [Google Scholar]
- 24. Whittle JC, Whelton PK, Seidler AJ, Klag MJ: Does racial variation in risk factors explain black-white differences in the incidence of hypertensive end-stage renal disease? Arch Intern Med 151: 1359–1364, 1991 [PubMed] [Google Scholar]
- 25. National Health and Nutrition Examination Survey: Dental Examiners Procedures Manual, Hyattsville MD, Centers for Disease Control and Prevention, National Center for Health Statistics, 2001 [Google Scholar]
- 26. National Health and Nutrition Examination Survey: Interviewer Procedures Manual I-IV, Hyattsville, MD, Centers for Disease Control and Prevention, National Center for Health Statistics, 2001 [Google Scholar]
- 27. Selvin E, Manzi J, Stevens LA, Van Lente F, Lacher DA, Levey AS, Coresh J: Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988–1994, 1999–2004. Am J Kidney Dis 50: 918–926, 2007 [DOI] [PubMed] [Google Scholar]
- 28. National Health and Nutrition Examination Survey: Analytic and Reporting Guidelines, Hyattsville, MD, Centers for Disease Control and Prevention, National Center for Health Statistics, 2005 [Google Scholar]
- 29. Page RC, Eke PI: Case definitions for use in population-based surveillance of periodontitis. J Periodontol 78: 1387–1399, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente F: Expressing the Modification of Diet in Renal Disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 53: 766–772, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J: A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tonetti MS, D'Aiuto F, Nibali L, Donald A, Storry C, Parkar M, Suvan J, Hingorani AD, Vallance P, Deanfield J: Treatment of periodontitis and endothelial function. N Engl J Med 356: 911–920, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Humphrey LL, Fu R, Buckley DI, Freeman M, Helfand M: Periodontal disease and coronary heart disease incidence: A systematic review and meta-analysis. J Gen Intern Med 23: 2079–2086, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]