Summary
Background and objectives
S100A12 is an endogenous receptor ligand for advanced glycation end products. Cardiovascular disease remains a major cause of morbidity and mortality in patients with chronic kidney disease. In this study, we report cross-sectional data on 550 hemodialysis patients and assess the relationship between plasma S100A12 level and cardiovascular disease.
Design, setting, participants, & measurements
A cross-sectional study of 550 maintenance hemodialysis patients was conducted. We investigated the past history of cardiovascular disease and quantified the plasma level of S100A12 protein in all participants.
Results
Plasma S100A12 level was higher in hemodialysis patients with cardiovascular disease (n = 197; 33.8 ± 28.1 ng/ml) than in those without it (n = 353; 20.2 ± 16.1 ng/ml; P < 0.001). In multivariate logistic regression analysis, the plasma S100A12 level (odds ratio [OR], 1.28; 95% confidence interval [CI], 1.13 to 1.44; P < 0.001) was identified as an independent factor associated with the prevalence of cardiovascular disease. The other factors associated with the prevalence of cardiovascular diseases were the presence of diabetes mellitus (OR, 2.81; 95% CI, 1.79 to 4.41; P < 0.001) and high-sensitivity CRP level (OR, 1.02; 95% CI, 1.00 to 1.05; P = 0.046). Furthermore, the plasma S100A12 level (OR, 1.30; 95% CI, 1.09 to 1.54; P = 0.004) was significantly associated with cardiovascular disease even in hemodialysis patients without diabetes mellitus (n = 348).
Conclusions
These results suggest that the plasma S100A12 protein level is strongly associated with the prevalence of cardiovascular disease in hemodialysis patients.
Introduction
Atherosclerotic cardiovascular disease (CVD) is a significant cause of morbidity and mortality in patients with chronic kidney disease (CKD), particularly hemodialysis (HD) patients. Mortality caused by CVD in patients with ESRD is 10- to 40-fold higher than that in the general population (1,2). Therefore, the assessment and prevention of CVD are major considerations for managing individuals with ESRD. Most traditional CVD risk factors, such as old age, male sex, diabetes mellitus, systolic hypertension, smoking, dyslipidemia, obesity, and positive family history, are highly prevalent in patients with CKD. However, because these traditional risk factors fail to fully explain the elevated CVD risk in patients with CKD, the metabolic milieu and the development of renal dysfunction appears to accelerate the atherosclerotic process (3). Nontraditional risk factors, such as oxidative stress and advanced glycation end products (AGE) in combination with their receptor (RAGE) have been emphasized as factors associated with atherosclerosis and may play an important role in the development of CVD in patients with CKD (1,3,4).
RAGE is a member of the Ig superfamily of cell surface molecules. Enhanced expression of RAGE in peripheral blood monocytes of patients with CKD has been observed, suggesting that RAGE may amplify the ligand-induced monocyte perturbation and contribute to monocyte-mediated vascular inflammation in CKD (5). AGEs were initially thought to be the main active ligands for RAGE, but since then several RAGE ligands including the high mobility group box proteins, S100 proteins, and amyloid fibrils have been identified (6). Although major alterations in the production of AGEs in CKD are the decreased clearance of glycation-free adducts and their markedly increased levels in plasma, the change in plasma AGE levels in patients with CKD is relatively modest (7). Therefore, ligands other than AGEs for RAGE may be more important in RAGE-mediated atherosclerosis of patients with CKD.
S100A12 is a member of the S100 family of EF-hand calcium-binding proteins (8,9). Human S100A12 is abundantly expressed and secreted from neutrophils and monocytes/macrophages (10). Engagement of RAGE by S100A12 activates nuclear factor-κB, a central transcription factor involved in inflammatory events; triggers the expression of multiple gene products contributing to the inflammatory response, such as vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 in vascular endothelial cells; and induces the migration and activation of monocytes, indicating the potential contribution of S100A12 in the development of atherosclerosis (8,11).
We have reported high plasma S100A12 levels in patients with type 2 diabetes mellitus (12). We have also examined plasma S100A12 levels in 72 patients undergoing HD and found that its mean level was 2.3-fold higher in these patients than in control subjects. Furthermore, maximum intima-media thickness of the carotid artery correlated with plasma S100A12 level in patients with HD (13). In this context, the diagnostic capacity of plasma S100A12 appears to be superior to many conventional atherosclerosis parameters because of its close correlation with local inflammatory processes that involve activation of granulocytes and monocytes (14). In this study, we report the cross-sectional data of 550 patients undergoing maintenance HD and assess the relationship between plasma S100A12 level and CVD.
Materials and Methods
Subjects
All of the procedures were performed in accordance with the guidelines of the Helsinki Declaration on Human Experimentation. This study was approved by the ethics committees on human research of our institutions, and all of the subjects provided their informed consent. All patients undergoing HD received the conventional dialysis treatment for 3 to 5 hours thrice a week with a standard bicarbonate dialysis solution. None of the subjects showed clinical evidence of malignant diseases or overt infection.
Definition of CVD
CVD was defined as follows: (1) ischemic heart disease including myocardial infarction diagnosed on the basis of elevated levels of cardiac enzymes and/or typical electrocardiography changes and angina pectoris diagnosed by typical electrocardiography changes without elevated cardiac enzymes and coronary intervention (percutaneous coronary intervention or coronary artery bypass grafting); (2) symptomatic stroke verified by computed tomography and/or magnetic resonance imaging; and (3) symptomatic peripheral vascular disease verified by lower-limb angiography and/or computed tomography angiography. The end-point committee of this study verified the above-mentioned criteria for CVD in each participant. We investigated the medical history of all participants to determine CVD on the basis of these criteria.
Laboratory Methods
Blood samples were obtained via vascular access when dialysis treatment was started on a midweek routine dialysis day. Blood samples were immediately transferred to tubes containing Na2+-EDTA (1 mg/ml) and centrifuged at 4°C. The plasma was immediately frozen and stored at −80°C until the time of assay. Urea nitrogen, serum creatinine, total cholesterol, albumin, white blood cells, hemoglobin, platelets, calcium, and phosphate levels were measured by standard clinical laboratory methods. High-sensitivity C-reactive protein (hs-CRP) was measured using an automatic analyzer (TBA-120FR; Toshiba, Tokyo, Japan).
ELISA for S100A12 Protein Quantification
A sandwich assay was performed with hCF128 monoclonal antibodies to quantify the plasma level of the S100A12 protein, as reported in our pilot study previously (12). The sample S100A12 level was determined by interpolation of absorbance values from a calibration curve. Plasma S100A12 levels in 42 healthy subjects (29% men; mean age, 50.5 years) were previously reported in our pilot study (means ± SD, 10.8 ± 6.3 ng/ml) (13). None of the control subjects had a history of hypertension, diabetes mellitus, renal disease, or CVD, and none were receiving medication at the time of the study. On the basis of the plasma S100A12 level in healthy subjects, a 10-ng/ml increase in plasma S100A12 level was adopted as an arbitrary unit of the independent variable in the subsequent multivariable regression analyses.
Statistical Analyses
The results are expressed as the means ± SD. Chi-squared test was used for categorical variables, and t test or Mann-Whitney U test was used for continuous variables. S100A12 and hs-CRP were evaluated as continuous variables. To determine the contributing factor for the prevalence of CVD, multiple logistic regression analysis was performed with a full model that included all of the candidate variables in the clinical characteristics. To determine the relationship between S100A12 level and the prevalence of CVD, 550 patients with ESRD who received maintenance HD in this study were stratified by quartiles of S100A12 level (see Table 4). A P value <0.05 was considered statistically significant. SPSS 17.0 for Windows (SPSS Inc., Chicago, IL) was used to analyze the data.
Table 4.
Multivariate logistic regression analysis of the prevalence of CVD by quartiles of plasma S100A12 level in 550 hemodialysis patients
| S100A12 | n | OR (95% CI) | P |
|---|---|---|---|
| Quartile 1 (<11.7 ng/ml) | 137 | 1.0 | |
| Quartile 2 (11.7 to 18.7 ng/ml) | 138 | 2.30 (1.22 to 4.34) | 0.01a |
| Quartile 3 (18.8 to 30.2 ng/ml) | 138 | 2.74 (1.45 to 5.18) | 0.002b |
| Quartile 4 (>30.2 ng/ml) | 137 | 5.77 (2.98 to 11.2) | <0.001b |
The values are adjusted for gender, age, duration of HD, current smoker, systolic blood pressure, hemoglobin, white blood cell and platelet count, hs-CRP, serum creatinine, albumin, sodium, potassium, calcium × phosphate and total cholesterol levels, and the presence of diabetes mellitus.
P < 0.05 versus quartile 1.
P < 0.01 versus quartile 1.
Results
Study Population
The study subjects comprised 550 patients with ESRD who received maintenance HD in our affiliated hospitals (65% men; mean age, 63.4 years; mean duration of HD, 9.7 years). The underlying renal disorders were diabetic nephropathy (DN) (36.7%) and non-DN diseases including chronic glomerulonephritis, hypertensive nephrosclerosis, polycystic kidney disease, and those of unknown etiology (63.3%). A history of CVD was noted in 197 patients (35.8%). The clinical characteristics of the patients are listed in Table 1.
Table 1.
Clinical characteristics and plasma S100A12 level in 550 hemodialysis patients
| Age (years) | 63.4 ± 12.1 |
| Men/women (n) | 355/195 |
| Current smoker/nonsmoker (n) | 65/485 |
| Duration of HD (years) | 9.7 ± 8.4 |
| Systolic BP (mmHg) | 142.9 ± 22.4 |
| Hemoglobin (g/dl) | 10.3 ± 1.08 |
| White blood cells (/mm3) | 5729 ± 1816 |
| Platelet (104/μl) | 16.8 ± 5.92 |
| hs-CRP (mg/L) | 4.10 ± 10.92 |
| Creatinine (mg/dl) | 10.5 ± 2.62 |
| Albumin (g/dl) | 3.7 ± 0.38 |
| Sodium (mEq/L) | 139.5 ± 2.95 |
| Potassium (mEq/L) | 4.6 ± 0.70 |
| Calcium (mg/dl) × phosphate (mg/dl) | 41.5 ± 10.49 |
| Total cholesterol (mg/dl) | 162.3 ± 45.8 |
| DN/non-DN (n) | 202/348 |
| CVD/non-CVD (n) | 197/353 |
| Plasma S100A12 (ng/ml) | 25.1 ± 22.4 |
The data are the means ± SD.
Increased Plasma S100A12 Level in Patients Undergoing HD
The mean plasma S100A12 level (25.1 ± 22.4 ng/ml) was higher in patients undergoing HD than in healthy subjects, as described previously in our pilot study (10.8 ± 6.3 ng/ml; P < 0.001) (13). Furthermore, the mean plasma S100A12 level in patients with DN undergoing HD was higher than that in non-DN patients undergoing HD (30.9 ± 25.4 versus 21.8 ± 19.8 ng/ml; P < 0.001). These results confirm the results from our previous pilot study (13).
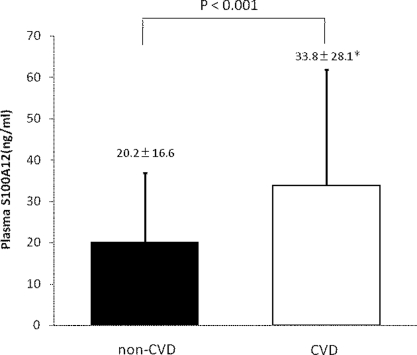
Higher Level of Plasma S100A12 in Patients Undergoing HD with a History of CVD
We found that the plasma S100A12 level in patients with a history of CVD was significantly higher than that in patients with no history of CVD (33.8 ± 28.1 versus 20.2 ± 16.6 ng/ml; P < 0.001) (Table 2 and Figure 1). The prevalence of DN, age, white blood cell count, platelet count, serum creatinine levels, serum sodium levels, and hs-CRP level were statistically different between the CVD and non-CVD groups.
Table 2.
Clinical characteristics and plasma S100A12 level of the study groups
| CVD (n = 197) | Non-CVD (n = 353) | P | |
|---|---|---|---|
| Age (years) | 66.1 ± 9.7 | 61.9 ± 13.0 | <0.001a |
| Men/women (n) | 135/62 | 220/133 | 0.145 |
| Current smoker/nonsmoker (n) | 28/169 | 37/316 | 0.194 |
| Duration of HD (years) | 9.2 ± 7.6 | 9.9 ± 8.7 | 0.321 |
| Systolic BP (mmHg) | 145.0 ± 22.9 | 141.7 ± 22.1 | 0.101 |
| Hemoglobin (g/dl) | 10.4 ± 1.2 | 10.3 ± 1.0 | 0.167 |
| White blood cells (/mm3) | 6081 ± 1807 | 5532 ± 1793 | 0.001a |
| Platelet (104/μl) | 17.6 ± 6.38 | 16.3 ± 5.60 | 0.014a |
| hs-CRP (mg/L) | 6.59 ± 10.49 | 2.70 ± 7.65 | <0.001a |
| Creatinine (mg/dl) | 10.0 ± 2.43 | 10.8 ± 2.69 | 0.001a |
| Albumin (g/dl) | 3.7 ± 0.37 | 3.7 ± 0.39 | 0.266 |
| Sodium (mEq/L) | 138.8 ± 3.02 | 139.9 ± 2.83 | <0.001a |
| Potassium (mEq/L) | 4.5 ± 0.72 | 4.6 ± 0.68 | 0.781 |
| Calcium (mg/dl) × phosphate (mg/dl) | 41.7 ± 10.40 | 41.5 ± 10.56 | 0.825 |
| Total cholesterol (mg/dl) | 163.3 ± 43.11 | 161.8 ± 47.32 | 0.715 |
| DN/non-DN (n) | 107/90 | 95/258 | <0.001a |
| Plasma S100A12 (ng/ml) | 33.8 ± 28.1 | 20.2 ± 16.6 | <0.001a |
The data are the means ± SD.
Significant independent determinants.
Figure 1.
Higher level of plasma S100A12 in patients undergoing HD. We found that plasma S100A12 level in patients with a history of CVD was significantly higher than that in patients without it (33.8 ± 28.1 versus 20.2 ± 16.6 ng/ml; P < 0.001). The data are the means ± SD.
Association of Plasma S100A12 Level with the Prevalence of CVD
As shown in Table 3, a 10-ng/ml increase in plasma S100A12 level (odds ratio [OR], 1.28; 95% confidence interval [CI], 1.13 to 1.44; P < 0.001) was identified as an independent factor associated with the prevalence of CVD. The other factors were the presence of diabetes mellitus (OR, 2.81; 95% CI, 1.79 to 4.41; P < 0.001), age (OR, 1.04; 95% CI, 1.02 to 1.06; P < 0.001), duration of HD (OR, 1.04; 95% CI, 1.01 to 1.06; P = 0.007), and hs-CRP level (OR, 1.02; 95% CI, 1.00 to 1.05, P = 0.046) (Table 3). To determine the relationship between the plasma S100A12 level and the prevalence of CVD, all of the subjects were categorized by quartiles of plasma S100A12 level. As shown in Table 4, after multivariable adjustment, a higher plasma S100A12 level was associated with an increased risk of CVD prevalence. Compared with patients in the first quartile, the level of plasma S100A12 was significantly higher in patients in the second (OR, 2.30; 95% CI, 1.22 to 4.34; P = 0.01), third (OR, 2.74: 95% CI, 1.45 to 5.18; P = 0.002), and fourth quartile (OR, 5.76: 95% CI, 2.98 to 11.5; P < 0.001).
Table 3.
Multivariate logistic regression analysis of clinical parameters and CVD
| OR | 95% CI | P | |
|---|---|---|---|
| Age (years) | 1.04 | 1.02 to 1.06 | <0.001a |
| Men | 1.51 | 0.95 to 2.42 | 0.084 |
| Current smoker | 1.25 | 0.67 to 2.34 | 0.485 |
| Duration of HD (years) | 1.04 | 1.01 to 1.06 | 0.007a |
| Systolic BP (mmHg) | 1.01 | 0.99 to 1.01 | 0.277 |
| Hemoglobin (g/dl) | 1.07 | 0.88 to 1.30 | 0.493 |
| White blood cells (/mm3) | 1.00 | 1.00 to 1.00 | 0.594 |
| Platelet (104/μl) | 1.03 | 0.99 to 1.07 | 0.169 |
| hs-CRP (mg/L) | 1.02 | 1.00 to 1.05 | 0.046a |
| Creatinine (mg/dl) | 0.91 | 0.81 to 1.01 | 0.066 |
| Albumin (g/dl) | 1.77 | 0.91 to 3.43 | 0.092 |
| Sodium (mEq/L) | 0.94 | 0.87 to 1.01 | 0.066 |
| Potassium (mEq/L) | 1.11 | 0.81 to 1.51 | 0.522 |
| Calcium (mg/dl) × phosphate (mg/dl) | 1.02 | 0.99 to 1.04 | 0.185 |
| Total cholesterol (mg/dl) | 1.02 | 0.98 to 1.07 | 0.340 |
| Diabetic nephropathy | 2.81 | 1.79 to 4.41 | <0.001a |
| Plasma S100A12 (10 ng/ml) | 1.28 | 1.13 to 1.44 | <0.001a |
Significant independent determinants.
Substudies in Diabetic and Nondiabetic Patients
We separately analyzed DN patients undergoing HD and non-DN patients undergoing HD. Notably, in patients with DN undergoing HD (n = 202), a 10-ng/ml increase in plasma S100A12 level was the strongest independent factor associated with the prevalence of CVD (OR, 1.25; 95% CI, 1.04 to 1.49; P = 0.016). Another factor was the duration of HD (OR, 1.10; 95% CI, 1.02 to 1.19, P = 0.018) (Table 5). Moreover, even in non-DN patients undergoing HD (n = 348), the plasma S100A12 level (OR, 1.30; 95% CI, 1.09 to 1.54; P = 0.004) was the most significant independent factor. Another factor for the prevalence of CVD was age (OR, 1.05; 95% CI, 1.02 to 1.08; P = 0.003) (Table 6).
Table 5.
Multivariate logistic regression analysis of clinical parameters and CVD in HD patients with DN (n = 202)
| OR | 95% CI | P | |
|---|---|---|---|
| Age (years) | 1.04 | 0.99 to 1.08 | 0.054 |
| Men | 1.40 | 0.65 to 2.99 | 0.392 |
| Current smoker | 0.99 | 0.35 to 2.84 | 0.990 |
| Duration of HD (years) | 1.10 | 1.02 to 1.19 | 0.018a |
| Systolic BP (mmHg) | 1.01 | 0.99 to 1.03 | 0.226 |
| Hemoglobin (g/dl) | 1.06 | 0.78 to 1.46 | 0.706 |
| White blood cells (/mm3) | 1.00 | 1.00 to 1.00 | 0.477 |
| Platelet (104/μl) | 1.03 | 0.96 to 1.10 | 0.472 |
| hs-CRP (mg/L) | 1.07 | 0.99 to 1.15 | 0.067 |
| Creatinine (mg/dl) | 0.87 | 0.73 to 1.04 | 0.119 |
| Albumin (g/dl) | 2.32 | 0.73 to 7.35 | 0.153 |
| Sodium (mEq/L) | 0.94 | 0.84 to 1.06 | 0.313 |
| Potassium (mEq/L) | 1.15 | 0.69 to 1.91 | 0.599 |
| Calcium (mg/dl) × phosphate (mg/dl) | 1.02 | 0.98 to 1.06 | 0.349 |
| Total cholesterol (mg/dl) | 1.08 | 0.99 to 1.17 | 0.068 |
| Plasma S100A12 (10 ng/ml) | 1.25 | 1.04 to 1.49 | 0.016a |
Significant independent determinants.
Table 6.
Multivariate logistic regression analysis of clinical parameters and CVD in HD patients with non-DN (n = 348)
| OR | 95% CI | P | |
|---|---|---|---|
| Age (years) | 1.05 | 1.02 to 1.08 | 0.003a |
| Men | 1.85 | 0.97 to 3.53 | 0.063 |
| Current smoker | 1.38 | 0.62 to 3.11 | 0.433 |
| Duration of HD (years) | 1.03 | 0.99 to 1.06 | 0.098 |
| Systolic BP (mmHg) | 1.00 | 0.99 to 1.01 | 0.977 |
| Hemoglobin (g/dl) | 1.06 | 0.82 to 1.38 | 0.648 |
| White blood cells (/mm3) | 1.00 | 1.00 to 1.00 | 0.687 |
| Platelet (104/μl) | 1.03 | 0.97 to 1.08 | 0.344 |
| hs-CRP (mg/L) | 1.01 | 0.98 to 1.04 | 0.675 |
| Creatinine (mg/dl) | 0.90 | 0.77 to 1.04 | 0.159 |
| Albumin (g/dl) | 1.56 | 0.67 to 3.67 | 0.307 |
| Sodium (mEq/L) | 0.92 | 0.84 to 1.02 | 0.108 |
| Potassium (mEq/L) | 1.03 | 0.68 to 1.57 | 0.891 |
| Calcium (mg/dl) × phosphate (mg/dl) | 1.02 | 0.99 to 1.05 | 0.269 |
| Total cholesterol (mg/dl) | 0.99 | 0.93 to 1.05 | 0.680 |
| Plasma S100A12 (10 ng/ml) | 1.30 | 1.09 to 1.54 | 0.004a |
Significant independent determinants.
Discussion
We studied 550 patients undergoing maintenance HD. The main findings of this study are as follows: (1) the plasma S100A12 level in patients undergoing HD was high; (2) among patients undergoing HD, the plasma S100A12 level in patients with CVD was significantly higher than in those without CVD; (3) the plasma S100A12 level was identified as an independent factor associated with the prevalence of CVD; (4) a higher plasma S100A12 level was associated with an increased risk of CVD; and (5) even in non-DN patients undergoing HD, the plasma S100A12 level was the most significant factor related to the prevalence of CVD.
The pathogenesis of atherosclerosis, which causes CVD, is characterized by the presence of subclinical chronic inflammation (15). Chronic inflammation is a common feature of CKD, and approximately 30% to 50% of predialysis, HD, and peritoneal dialysis patients have serologic evidence of an activated inflammatory response (16). Serum levels of CRP, the prototypical acute phase reactant, have been shown to be particularly high when renal function declines to the level of ESRD (17). A recent prospective cohort study of more than 1000 patients with CKD for a median of 2.5 years indicated that the highest CRP quartile was associated with a two-fold increase in cardiac death (18). Notably, the traditional Framingham risk factors were not associated with cardiac death. The pathogenesis of an extremely elevated CRP, which is approximately 10-fold higher in patients with ESRD than in that the normal population, has not been completely understood, but several endogenous factors such as the renin-angiotensin system, modified LDL, homocysteine, and the AGE-RAGE system have been proposed as key mediators of chronic inflammation during CKD (3,19). Thus, chronic inflammation is intimately linked to atherosclerosis in patients with CKD (19).
S100A12 has been reported to be a potential ligand for RAGE (8). Evidence has accumulated that S100A12 plays a key role in inflammation (14). An elevated serum S100A12 level has been reported in patients with several inflammatory diseases such as Kawasaki disease, rheumatoid arthritis (RA), and chronic inflammatory bowel diseases (20–22). This study of 550 patients undergoing HD showed that plasma S100A12 level was high, confirming the results of our previous pilot study of patients undergoing HD and peritoneal dialysis (13,23). Taken together with these results, the plasma S100A12 level may reflect chronic inflammation in patients with ESRD. RAGE has a circulating secretory form called soluble RAGE (sRAGE), which neutralizes the action of AGE as a functional decoy. Although the plasma sRAGE level data were not available in this study, Koyama et al. (24) reported that a low plasma sRAGE level is associated with CVD in patients with CKD. Furthermore, in patients with diabetes, low sRAGE and high S100A12 levels are associated with an increased risk for CVD. The inverse relationship between plasma S100A12 and sRAGE levels led to the hypothesis that in chronic inflammation, there is an increase in plasma S100A12 level and a decrease in plasma sRAGE level, leading to an acceleration of atherosclerosis (25). Consistent with that hypothesis, a recent report on patients with RA indicated that plasma S100A12 and sRAGE levels were associated not only with RA inflammatory factors and autoantibody formation but also with the recruitment of classical vascular risk factors to end-organ damage (26). S100A12 also had opposing effects to sRAGE in patients with RA.
The limitations of our study include its cross-sectional design, which does not permit the determination of causality. However, recent clinical and experimental studies have reported an underlying relationship between S100A12 and atherogenesis. Goyette et al. (27) clearly showed that S100A12 is localized in foam cells of coronary atherosclerotic plaques in patients with acute coronary syndrome. Furthermore, Mahajan et al. (28) reported that mRNA levels of S100A12 in peripheral blood mononuclear cells are up-regulated in nondiabetic patients with premature coronary heart disease. Bowman et al. (29) generated transgenic (TG) mice expressing S100A12 in vascular smooth muscle cells. S100A12 TG mice displayed pathologic remodeling of the aorta, and cultured aortic vascular smooth muscle cells from TG mice increased the IL-6 production and elevated measures of oxidative stress. We have shown that S100A12 production is induced by IL-6 in human macrophages (30). Thus, S100A12 expression is sufficient to activate pathogenic pathways through the modulation of oxidative stress, inflammation, and vascular remodeling. Furthermore, Thornalley et al. (7) reported that RAGE activation by S100A12 decreases expression of glyoxalase 1, which is a critical enzyme for the metabolism of glycation-free adducts such as glyoxal and methylglyoxal. Downregulation of glyoxalase 1 leads to increased local levels of glycation-free adduct and related AGE residue formation. Therefore, it is likely that RAGE activation by S100A12 is important for increasing the AGE-RAGE interaction in the vulnerable vasculature. In this study, the plasma S100A12 level was clearly associated with the prevalence of CVD, suggesting that S100A12 overproduction followed by chronic inflammation exaggerates the vicious cycle of atherosclerotic lesion formation in patients with CKD. Alternatively, this may have caused the superiority of plasma S100A12 to hs-CRP as an independent factor associated with the prevalence of CVD.
In conclusion, for the first time, in a study of 550 patients undergoing HD, we found that the plasma S100A12 level was an independent factor associated with the prevalence of CVD. We anticipate an important role for S100A12 as a novel biomarker to predict CVD in patients with HD. Prospective and interventional studies are currently ongoing to further elucidate the relationship between plasma S100A12 level and CVD.
Disclosures
None.
Acknowledgments
We thank Mr. Toshio Furuta and Ms. Ikuko Arai for providing the laboratory data and Mr. Hiroshi Omizu for supporting the statistical analyses. Part of this study was supported by Grants-in-Aid for Scientific Research from the Japanese Society for the Promotion of Science (No. 20590846) to A. K. and Y. M.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UpToDate on-line is available for additional clinical information at www.cjasn.org.
References
- 1. Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW: Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 108: 2154–2169, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Collins AJ: Cardiovascular mortality in end-stage renal disease. Am J Med Sci 325: 163–167, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Stenvinkel P, Carrero JJ, Axelsson J, Lindholm B, Heimburger O, Massy Z: Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: How do new pieces fit into the uremic puzzle? Clin J Am Soc Nephrol 3: 505–521, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kendrick J, Chonchol MB: Nontraditional risk factors for cardiovascular disease in patients with chronic kidney disease. Nat Clin Pract Nephrol 4: 672–681, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Hou FF, Ren H, Owen WF, Jr., Guo ZJ, Chen PY, Schmidt AM, Miyata T, Zhang X: Enhanced expression of receptor for advanced glycation end products in chronic kidney disease. J Am Soc Nephrol 15: 1889–1896, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Sparvero LJ, Asafu-Adjei D, Kang R, Tang D, Amin N, Im J, Rutledge R, Lin B, Amoscato AA, Zeh HJ, Lotze MT: RAGE (Receptor for Advanced Glycation Endproducts), RAGE ligands, and their role in cancer and inflammation. J Transl Med 7: 17, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thornalley PJ, Rabbani N: Highlights and hotspots of protein glycation in end-stage renal disease. Semin Dial 22: 400–404, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, Neurath MF, Slattery T, Beach D, McClary J, Nagashima M, Morser J, Stern D, Schmidt AM: RAGE mediates a novel proinflammatory axis: A central cell surface receptor for S100/calgranulin polypeptides. Cell 97: 889–901, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Donato R: S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol 33: 637–668, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Moroz OV, Dodson GG, Wilson KS, Lukanidin E, Bronstein IB: Multiple structural states of S100A12: A key to its functional diversity. Microsc Res Tech 60: 581–592, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Yang Z, Tao T, Raftery MJ, Youssef P, Di Girolamo N, Geczy CL: Proinflammatory properties of the human S100 protein S100A12. J Leukocyte Biol 69: 986–994, 2001 [PubMed] [Google Scholar]
- 12. Kosaki A, Hasegawa T, Kimura T, Iida K, Hitomi J, Matsubara H, Mori Y, Okigaki M, Toyoda N, Masaki H, Inoue-Shibata M, Nishikawa M, Iwasaka T: Increased plasma S100A12 (EN-RAGE) levels in patients with type 2 diabetes. J Clin Endocrinol Metab 89: 5423–5428, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Mori Y, Kosaki A, Kishimoto N, Kimura T, Iida K, Fukui M, Nakajima F, Nagahara M, Urakami M, Iwasaka T, Matsubara H: Increased plasma S100A12 (EN-RAGE) levels in hemodialysis patients with atherosclerosis. Am J Nephrol 29: 18–24, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Pietzsch J, Hoppmann S: Human S100A12: A novel key player in inflammation? Amino Acids 36: 381–389, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Ross R: Atherosclerosis: An inflammatory disease. N Engl J Med 340: 115–126, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Stenvinkel P: Inflammatory and atherosclerotic interactions in the depleted uremic patient. Blood Purif 19: 53–61, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Stenvinkel P, Alvestrand A: Inflammation in end-stage renal disease: Sources, consequences, and therapy. Semin Dial 15: 329–337, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Parekh RS, Plantinga LC, Kao WH, Meoni LA, Jaar BG, Fink NE, Powe NR, Coresh J, Klag MJ: The association of sudden cardiac death with inflammation and other traditional risk factors. Kidney Int 74: 1335–1342, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Hage FG, Venkataraman R, Zoghbi GJ, Perry GJ, DeMattos AM, Iskandrian AE: The scope of coronary heart disease in patients with chronic kidney disease. J Am Coll Cardiol 53: 2129–2140, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Foell D, Ichida F, Vogl T, Yu X, Chen R, Miyawaki T, Sorg C, Roth J: S100A12 (EN-RAGE) in monitoring Kawasaki disease. Lancet 361: 1270–1272, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Foell D, Kane D, Bresnihan B, Vogl T, Nacken W, Sorg C, Fitzgerald O, Roth J: Expression of the pro-inflammatory protein S100A12 (EN-RAGE) in rheumatoid and psoriatic arthritis. Rheumatology 42: 1383–1389, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Foell D, Kucharzik T, Kraft M, Vogl T, Sorg C, Domschke W, Roth J: Neutrophil derived human S100A12 (EN-RAGE) is strongly expressed during chronic active inflammatory bowel disease. Gut 52: 847–853, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Uchiyama-Tanaka Y, Mori Y, Kosaki A, Kimura T, Moriishi M, Kawanishi H, Matsubara H: Plasma S100A12 concentrations in peritoneal dialysis patients and subclinical chronic inflammatory disease. Ther Apher Dial 12: 28–32, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Koyama H, Shoji T, Fukumoto S, Shinohara K, Shoji T, Emoto M, Mori K, Tahara H, Ishimura E, Kakiya R, Tabata T, Yamamoto H, Nishizawa Y: Low circulating endogenous secretory receptor for AGEs predicts cardiovascular mortality in patients with end-stage renal disease. Arterioscler Thromb Vasc Biol 27: 147–153, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Basta G, Sironi AM, Lazzerini G, Del Turco S, Buzzigoli E, Casolaro A, Natali A, Ferrannini E, Gastaldelli A: Circulating soluble receptor for advanced glycation end products is inversely associated with glycemic control and S100A12 protein. J Clin Endocrinol Metab 91: 4628–4634, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Chen YS, Yan W, Geczy CL, Brown MA, Thomas R: Serum levels of soluble receptor for advanced glycation end products and of S100 proteins are associated with inflammatory, autoantibody, and classical risk markers of joint and vascular damage in rheumatoid arthritis. Arthritis Res Ther 11: R39, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goyette J, Yan WX, Yamen E, Chung YM, Lim SY, Hsu K, Rahimi F, Di Girolamo N, Song C, Jessup W, Kockx M, Bobryshev YV, Freedman SB, Geczy CL: Pleiotropic roles of S100A12 in coronary atherosclerotic plaque formation and rupture. J Immunol 183: 593–603, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Mahajan N, Malik N, Bahl A, Dhawan V: Receptor for advanced glycation end products (RAGE) and its inflammatory ligand EN-RAGE in non-diabetic subjects with pre-mature coronary artery disease. Atherosclerosis 207: 597–602, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Hoffmann Bowman M, Wilk J, Heydemann A, Kim G, Rehman J, Lodato JA, Raman J, McNally EM: S100A12 mediates aortic wall remodeling and aortic aneurysm. Circ Res 106: 145–154, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hasegawa T, Kosaki A, Kimura T, Matsubara H, Mori Y, Okigaki M, Masaki H, Toyoda N, Inoue-Shibata M, Kimura Y, Nishikawa M, Iwasaka T: The regulation of EN-RAGE (S100A12) gene expression in human THP-1 macrophages. Atherosclerosis 171: 211–218, 2003 [DOI] [PubMed] [Google Scholar]