Summary
Background and objectives
Increased arterial stiffness was reported to be associated with decreased estimated GFR (eGFR). Previous studies suggested that arterial stiffness might play a role in renal function progression in patients with chronic kidney disease (CKD). The aim of this study was to investigate whether there was an independent association between brachial-ankle pulse wave velocity (baPWV), a marker of arterial stiffness, and renal function progression in CKD patients.
Design, setting, participants, & measurements
This longitudinal study enrolled 145 patients with CKD stages 3 to 5. The baPWV was measured by using an ABI-form device. The change in renal function was estimated by eGFR slope. The study endpoints were defined as commencement of dialysis or death.
Results
After a stepwise multivariate analysis, the eGFR slope was positively associated with baseline eGFR and negatively associated with hypertension and baPWV (β = −0.165, P = 0.033). Seventeen patients entering dialysis, and eight deaths were recorded. Multivariate forward Cox regression analysis identified that higher baPWV (hazard ratio, 1.001; P = 0.001), lower baseline eGFR, and higher serum phosphate level were independently associated with progression to commencement of dialysis or death.
Conclusions
Our results show an independent association between baPWV and renal function decline and progression to commencement of dialysis or death in patients with CKD. Screening CKD patients by means of baPWV may help identify a high-risk group of rapid renal function decline and progression to commencing dialysis or death.
Introduction
Chronic kidney disease (CKD) is an increasing worldwide public health problem associated with increased cardiovascular morbidity and mortality. The excess cardiovascular risk is contributed to traditional risk factors and nontraditional risk factors including arterial stiffening (1,2). Increased arterial stiffness may be responsible for cardiovascular burden in the CKD population (3,4). Previous studies have reported the association between decreased estimated GFR (eGFR) and increased arterial stiffness (5–7). Decreased eGFR may predispose to increased arterial stiffness with multiple pathogenic mechanisms involved, including deranged calcium/phosphate balance, secondary hyperparathyroidism, homocysteine, lipoprotein(a) metabolism, fluid overload, alterations in the angiotensin and endothelin systems, malnutrition, uremic toxins, oxidative stress, insulin resistance, and alterations in inflammatory and coagulation pathways (8,9). Conversely, there is evidence to suggest that arterial stiffness may play a role in renal function progression. Three studies have shown that different measures of arterial stiffness, i.e., augmentation index, radial-dorsalis pedis pulse wave velocity (PWV), and aortic PWV, were independent risk factors for the deterioration of renal function in CKD patients (10–12).
To assess arterial stiffness, many noninvasive methods have been developed, and they usually require expertise techniques (13). A clinical device, ABI-form (VP1000; Colin Co. Ltd., Komaki, Japan), has been developed to automatically and simultaneously record pulse waves of the brachial and posterior tibial arteries, using an automated oscillometric method. Using this device, we can easily and automatically calculate the brachial-ankle PWV (baPWV) (14–16). The baPWV has been reported as a good marker for arterial stiffness (15). Accordingly, the aim of this study is to investigate whether there is an independent association between baPWV and renal function decline and progression to commencement of dialysis or death in a cohort of patients with CKD stages 3 to 5.
Materials and Methods
Study Patients
The study was conducted in a regional hospital in southern Taiwan. We consecutively enrolled 179 patients with CKD stages 3 to 5 according to the National Kidney Foundation-Kidney Disease Outcomes Quality Initiative guidelines (17) from our Outpatient Department of Internal Medicine from January 2007 to May 2007. We classified our patients with evidence of kidney damage lasting for >3 months into CKD stages 3, 4, and 5, based on eGFR level (ml/min per 1.73 m2) of 30 to 59, 15 to 29, and <15, respectively. Six patients with atrial fibrillation, 4 patients with inadequate image visualization, and 13 patients with less than three eGFR measurements during the follow-up period were excluded. In addition, those patients with mortality (n = 4) or entering dialysis therapy (6 hemodialysis and 1 peritoneal dialysis) within 30 days after enrollment were also excluded to avoid incomplete observation of change in renal function. Finally, 145 patients (mean age, 68.6 ± 12.1 years; 99 men) were included in this study. The protocol was approved by our Institutional Review Board, and all enrolled patients gave written, informed consent.
Measurement of baPWV
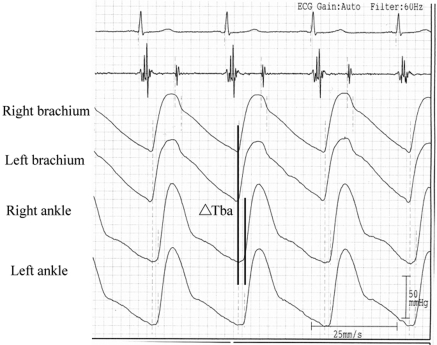
The values of baPWV were measured using an ABI-form device (VP1000; Colin Co. Ltd.), which automatically and simultaneously record pulse waves of the brachial and posterior tibial arteries, using an automated oscillometric method (14–16). The baPWV measurements were taken in a room with a temperature of around 25°C after 5 minutes of rest in the supine position. For measuring baPWV, pulse waves obtained from the brachial and tibial arteries were recorded simultaneously, and the transmission time (ΔTba), which was defined as the time interval between the initial increase in brachial and ankle waveforms, was determined (Figure 1). The transmission distance from the brachium to ankle was calculated according to body height. The path length from the suprasternal notch to the brachium (Lb) was obtained using the following equation: Lb = 0.2195 × height of the patient (in cm) − 2.0734. The path length from the suprasternal notch to the ankle (La) was obtained using the following equation: La = (0.8129 × height of the patient [in cm] + 12.328). Finally, the following equation was used to automatically obtain baPWV: baPWV = (La − Lb)/ΔTba (15). After obtaining bilateral baPWV values, the higher one was used for analysis. Systolic and diastolic BP and heart rate were measured using an appropriate cuff size by the same device. The average of systolic and diastolic BP of bilateral arms was used for analysis. The baPWV measurement was done once in each patient. The validation of this automatic device and its reproducibility have been previously published (15).
Figure 1.
For measuring baPWV, pulse waves obtained from the brachial and tibial arteries were recorded simultaneously, and the transmission time (ΔTba), which was defined as the time interval between the initial increase in brachial and ankle waveforms, was determined. The transmission distance from the brachium to ankle was calculated according to body height. The baPWV value was automatically computed as the transmission distance divided by the transmission time.
Collection of Demographic, Medical, and Laboratory Data
Demographic and medical data including age, gender, smoking history (ever versus never), and comorbid conditions were obtained from medical records and interviews with patients. Study subjects were defined as having diabetes mellitus (DM) if the fasting blood glucose level was >126 mg/dl or hypoglycemic agents were used to control blood glucose levels. Similarly, study patients were considered as having hypertension if the systolic BP was ≥140 mmHg or diastolic BP ≥90 mmHg or anti-hypertensive drugs were prescribed. Cerebrovascular disease was defined as a history of cerebrovascular accident including cerebral bleeding and infarction. Coronary artery disease was defined as a history of typical angina with positive stress test, old myocardial infarction, or having undergone coronary artery bypass surgery or angioplasty. The body mass index was calculated as the ratio of weight in kilograms divided by square of height in meters. Laboratory data were measured from fasting blood samples using an autoanalyzer (D-68298 Mannheim COBAS Integra 400; Roche Diagnostics GmbH). Serum creatinine was measured by the compensated Jaffé (kinetic alkaline picrate) method in a Roche/Integra 400 Analyzer (Roche Diagnostics, Mannheim, Germany) using a calibrator traceable to isotope-dilution mass spectrometry (18). The value of eGFR was calculated using the four-variable equation in the Modification of Diet in Renal Disease study (19). Serum intact parathyroid hormone (PTH) concentration was evaluated using a commercially available two-sided immunoradiometric assay (CIS Biointernational). Blood samples were obtained within 1 month of enrollment. Urine albumin and creatinine were measured on a spot urine sample by an autoanalyzer (COBAS Integra 400 plus; Roche Diagnostics), and albuminuria was defined as the ratio of urine albumin to creatinine of ≥30 mg/g. In addition, information regarding patient medications including aspirin, angiotensin converting enzyme inhibitors (ACEIs), angiotensin II receptor blockers (ARBs), β-blockers, calcium channel blockers, diuretics, HMG-CoA reductase inhibitors (statins), and erythropoiesis-stimulating agents (ESAs) during the study period was obtained from medical records.
Assessment of Decline in Renal Function
The decline in renal function was assessed by the eGFR slope, defined as the regression coefficient between eGFR and time in units of ml/min per 1.73 m2 per year. At least three eGFR measurements were required to estimate eGFR slope. The National Kidney Foundation-Kidney Disease Outcomes Quality Initiative guidelines suggest that eGFR is normally declined <1 ml/min per 1.73 m2 per year from a level of 125 ml/min per 1.73 m2 once adulthood is reached (18). Any reduction ≥1 ml/min per 1.73 m2 per year, i.e., slope more negative than or equal to −1 ml/min per 1.73 m2 per year (≤ −1), was considered the progression of CKD; otherwise, it was considered nonprogressive (17,20).
Definition of Study Endpoints
The study endpoints were defined as commencement of dialysis or death. In patients reaching study endpoints, renal function data were censored at the start of renal replacement therapy or death. The other patients were followed until March 2009. The mean follow-up period was 14.9 ± 3.2 months (range, 4.7 to 26.2 months).
Statistical Analyses
Statistical analysis was performed using SPSS version 12.0 (SPSS, Chicago, IL) for Windows. Data are expressed as percentages or mean ± SD. The differences between groups were checked by χ2 test for categorical variables or by independent t test for continuous variables. The relationship between two continuous variables was assessed by a bivariate correlation method (Pearson's correlation). Linear regression analysis was used to identify the factors associated with decline in kidney function. Because the formula of eGFR included age and thus simultaneous adjustments for the eGFR and age might produce confusing linearity within regression model, the parameter of age was not put in multivariate linear regression analysis. Time to combined endpoints of commencement of dialysis or death and covariates of risk factors were modeled using the Cox proportional hazards model. Age, gender, and the significant variables in univariate analysis were selected for multivariate analysis. The parameters of systolic BP, diastolic BP, and total cholesterol were not put in multivariate analysis to avoid potential colinearity. A difference was considered significant at P < 0.05.
The baPWV was partially dependent on BP (5), so it was adjusted for mean arterial pressure at the time of measurement before further analysis. Adjustment was performed by a linear regression of the two variables. The residual values were added to uncorrected baPWV to form the adjusted baPWV. Adjusted baPWV values were used for analysis.
Results
The clinical characteristics of study patients are shown in Table 1. The values of eGFR slope and baPWV of all patients were −0.57 ± 3.32 ml/min per 1.73 m2 per year and 2055.3 ± 920.5 cm/s, respectively. The average number of serum creatinine measurements during the follow-up period was 7.5 ± 3.4 (range, 3 to 19). The underlying etiology of CKD in our patients included 69 with diabetic kidney disease (47.6%), 33 with nondiabetic glomerular diseases (22.8%), 28 with tubulointerstitial diseases (19.3%), 4 cases of hypertension (2.8%), and 11 caused by other diseases (7.6%).
Table 1.
Characteristics of the study patients
| Characteristics | All patients (n = 145) |
|---|---|
| eGFR slope (ml/min per 1.73 m2 per year) | −0.57 ± 3.32 |
| Age (years) | 68.6 ± 12.1 |
| Male gender (%) | 68.3 |
| Smoking history (%) | 56.3 |
| Diabetes mellitus (%) | 49.7 |
| Hypertension (%) | 84.1 |
| Coronary artery disease (%) | 18.6 |
| Cerebrovascular disease (%) | 19.3 |
| Stage of CKD | |
| stage 3 (%) | 52.4 |
| stage 4 (%) | 28.3 |
| stage 5 (%) | 19.3 |
| Systolic BP (mmHg) | 142.4 ± 21.2 |
| Diastolic BP (mmHg) | 78.9 ± 11.5 |
| Pulse pressure (mmHg) | 64.1 ± 16.9 |
| Heart rate (beats/min) | 74.0 ± 13.7 |
| Body mass index (kg/m2) | 25.9 ± 4.8 |
| Laboratory parameters | |
| albumin (g/dl) | 3.99 ± 0.34 |
| fasting glucose (mg/dl) | 121.4 ± 45.8 |
| triglyceride (mg/dl) | 166.4 ± 98.9 |
| total cholesterol (mg/dl) | 196.9 ± 44.3 |
| HDL-cholesterol (mg/dl) | 45.7 ± 13.4 |
| LDL-cholesterol (mg/dl) | 112.1 ± 33.4 |
| hematocrit (%) | 36.6 ± 6.6 |
| basline eGFR (ml/min per 1.73 m2) | 29.9 ± 14.7 |
| calcium (mg/dl) | 9.6 ± 0.7 |
| phosphate (mg/dl) | 3.8 ± 0.8 |
| uric acid (mg/dl) | 8.3 ± 2.3 |
| PTH (pg/ml) | 65.4 ± 101.5 |
| Albuminuria (%) | 73.2 |
| baPWVa (cm/s) | 2055.3 ± 920.5 |
| Medications | |
| aspirin use (%) | 28.3 |
| ACEI and/or ARB use (%) | 78.5 |
| β-blocker use (%) | 24.5 |
| calcium channel blocker use (%) | 57.2 |
| diuretic use (%) | 40.0 |
| statin use (%) | 27.3 |
| ESA use (%) | 7.6 |
eGFR, estimated glomerular filtration rate; CKD, chronic kidney disease; HDL, high-density lipoprotein; LDL, low-density lipoprotein; PTH, parathyroid hormone; baPWV, brachial-ankle pulse wave velocity; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; ESA, erythropoiesis stimulating agent.
Data were adjusted for mean arterial pressure.
The comparison of baseline characteristics between patients with progressive (≤ −1 ml/min per 1.73 m2 per year) and nonprogressive (> −1 ml/min per 1.73 m2 per year) eGFR slope (ml/min per 1.73 m2 per year) are shown in Table 2. Compared with patients with a nonprogressive eGFR slope, patients with a progressive eGFR slope had a lower percentage of male patients, higher prevalence of a history of DM, higher systolic BP, wider pulse pressure, higher heart rate, higher fasting glucose, higher triglyceride, lower LDL-cholesterol, lower hematocrit, lower baseline eGFR, higher phosphate, higher PTH level, higher prevalence of albuminuria, and higher baPWV level. In addition, patients in the progressive group had a lower percentage of having received ACEI and/or ARB therapy.
Table 2.
Comparison of baseline characteristics between patients with progressive (≤ −1 ml/min per 1.73 m2 per year) and nonprogressive (> −1 ml/min per 1.73 m2 per year) eGFR slope (ml/min per 1.73 m2 per year)
| Characteristics | Progressive (n = 64) | Nonprogressive (n = 81) | P |
|---|---|---|---|
| eGFR slope (ml/min per 1.73 m2 per year) | −3.35 ± 2.31 | 1.64 ± 2.12 | <0.001 |
| Age (years) | 69.6 ± 12.5 | 67.7 ± 11.8 | 0.353 |
| Male gender (%) | 57.8 | 76.5 | 0.016 |
| Smoking history (%) | 63.5 | 50.6 | 0.122 |
| Diabetes mellitus (%) | 62.5 | 39.5 | 0.006 |
| Hypertension (%) | 90.6 | 79.0 | 0.057 |
| Coronary artery disease (%) | 14.1 | 22.2 | 0.210 |
| Cerebrovascular disease (%) | 23.4 | 16.0 | 0.263 |
| Stage of CKD | |||
| stage 3 (%) | 32.8 | 67.9 | <0.001 |
| stage 4 (%) | 35.9 | 22.2 | |
| stage 5 (%) | 31.3 | 9.9 | |
| Systolic BP (mmHg) | 147.4 ± 21.8 | 138.5 ± 20.1 | 0.012 |
| Diastolic BP (mmHg) | 79.5 ± 13.0 | 78.4 ± 10.3 | 0.581 |
| Pulse pressure (mmHg) | 67.9 ± 14.5 | 61.1 ± 18.2 | 0.016 |
| Heart rate (beats/min) | 77.6 ± 14.4 | 71.2 ± 12.6 | 0.005 |
| Body mass index (kg/m2) | 26.0 ± 5.6 | 25.9 ± 4.0 | 0.941 |
| Laboratory parameters | |||
| albumin (g/dl) | 3.96 ± 0.35 | 4.01 ± 0.33 | 0.362 |
| fasting glucose (mg/dl) | 131.1 ± 55.5 | 113.8 ± 34.8 | 0.032 |
| triglyceride (mg/dl) | 186.1 ± 120.2 | 150.6 ± 74.9 | 0.041 |
| total cholesterol (mg/dl) | 196.9 ± 46.6 | 195.6 ± 42.7 | 0.992 |
| HDL-cholesterol (mg/dl) | 44.9 ± 15.9 | 46.3 ± 11.3 | 0.543 |
| LDL-cholesterol (mg/dl) | 106.0 ± 32.9 | 117.1 ± 33.2 | 0.049 |
| hematocrit (%) | 33.5 ± 5.9 | 39.0 ± 6.1 | 0.001 |
| baseline eGFR (ml/min per 1.73 m2) | 23.1 ± 13.2 | 35.3 ± 13.5 | <0.001 |
| Calcium (mg/dl) | 9.5 ± 0.8 | 9.7 ± 0.6 | 0.061 |
| Phosphate (mg/dl) | 4.1 ± 1.0 | 3.6 ± 0.6 | <0.001 |
| Uric acid (mg/dl) | 8.4 ± 2.6 | 8.2 ± 2.1 | 0.669 |
| PTH (pg/ml) | 94.2 ± 144.0 | 42.7 ± 33.0 | 0.007 |
| Albuminuria (%) | 87.1 | 62.5 | 0.001 |
| baPWVa (cm/s) | 2285.4 ± 964.9 | 1871.2 ± 844.9 | 0.007 |
| Medications | |||
| aspirin use (%) | 31.7 | 26.3 | 0.471 |
| ACEI and/or ARB use (%) | 69.8 | 85.2 | 0.026 |
| β-blocker use (%) | 27.9 | 22.4 | 0.512 |
| calcium channel blocker use (%) | 62.5 | 53.1 | 0.255 |
| diuretic use (%) | 40.6 | 39.5 | 0.891 |
| statin use (%) | 23.8 | 30.0 | 0.409 |
| ESA use (%) | 10.9 | 4.9 | 0.215 |
Data were adjusted for mean arterial pressure.
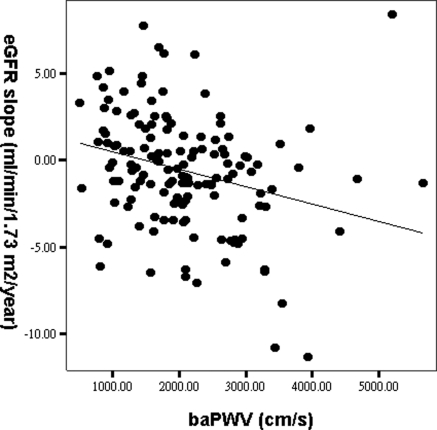
Table 3 shows the determinants of eGFR slope in all patients. In the univariate analysis, eGFR slope had a significantly positive correlation with serum hematocrit, baseline eGFR, and calcium levels and negative correlation with DM and hypertension, heart rate, log triglyceride, phosphate, log PTH level, albuminuria, and baPWV. After multiple stepwise linear regression analysis, eGFR slope was correlated independently with hypertension (β = −0.193, P = 0.011), increased baseline eGFR (β = 0.408, P < 0.001), and decreased baPWV (β = −0.165, P = 0.033). Figure 2 showed the regression plot between baPWV and eGFR slope. We further performed eGFR slope analysis using the three-variable Japanese equation (21) and found similar results, i.e., eGFR slope was significantly correlated with baseline eGFR (β = 0.392, P < 0.001), hypertension (β = −0.193, P = 0.011), and baPWV (β = −0.165, P = 0.035).
Table 3.
Determinants of eGFR slope in study patients
| Characteristics | Univariate |
Multivariate (Stepwise) |
||
|---|---|---|---|---|
| Standardized Coefficient β | P | Standardized Coefficient β | P | |
| Male versus female | 0.158 | 0.057 | — | — |
| Smoking (ever versus never) | −0.105 | 0.212 | — | — |
| Diabetes mellitus | −0.224 | 0.007 | — | — |
| Hypertension | −0.252 | 0.019 | −0.193 | 0.011 |
| Coronary artery disease | 0.072 | 0.389 | — | — |
| Cerebrovascular disease | −0.070 | 0.402 | — | — |
| Heart rate (beat/min) | −0.252 | 0.002 | — | — |
| Body mass index (kg/m2) | 0.026 | 0.759 | — | — |
| Laboratory parameters | — | — | ||
| albumin (g/dl) | 0.142 | 0.100 | — | — |
| fasting glucose (log mg/dl) | −0.117 | 0.160 | — | — |
| triglyceride (log mg/dl) | −0.208 | 0.012 | — | — |
| HDL-cholesterol (mg/dl) | 0.116 | 0.165 | — | — |
| LDL-cholesterol (mg/dl) | 0.164 | 0.051 | — | — |
| hematocrit (%) | 0.383 | <0.001 | — | — |
| baseline eGFR (ml/min per 1.73 m2) | 0.448 | <0.001 | 0.408 | <0.001 |
| calcium (mg/dl) | 0.207 | 0.013 | — | — |
| phosphate (mg/dl) | −0.291 | <0.001 | — | — |
| uric acid (mg/dl) | −0.112 | 0.180 | — | — |
| PTH (log pg/ml) | −0.347 | <0.001 | — | — |
| Albuminuria | −0.293 | <0.001 | — | — |
| baPWVa (cm/s) | −0.278 | 0.001 | −0.165 | 0.033 |
| Medications | ||||
| aspirin use (%) | −0.048 | 0.567 | — | — |
| ACEI and/or ARB use (%) | 0.100 | 0.233 | — | — |
| β-blocker use (%) | −0.077 | 0.422 | — | — |
| calcium channel blocker use (%) | −0.084 | 0.318 | — | — |
| diuretic use (%) | 0.029 | 0.729 | — | — |
| statin use (%) | −0.056 | 0.505 | — | — |
| ESA use (%) | −0.037 | 0.659 | — | — |
Adjusted R2 = 0.270. Values expressed as standardized coefficient β.
Data were adjusted for mean arterial pressure.
Figure 2.
Regression plot between baPWV and the eGFR slope (r = −0.278, P = 0.001).
The mean follow-up period was 14.9 ± 3.2 months. During the period of follow-up, 17 patients started hemodialysis and 8 patients died, including fatal cardiovascular events (n = 4), infectious disease (n = 3), and gastrointestinal bleeding (n = 1). The comparison of characteristics between patients who did and did not reach the study endpoints is shown in Table 4. Compared with patients who did not reach the study endpoints, patients who reached the study endpoints were significantly associated with higher prevalence of a history of DM, cerebrovascular disease, and albuminuria, higher systolic BP, higher pulse pressure, lower hematocrit, lower baseline eGFR, lower calcium, higher phosphate, higher baPWV levels, and higher percentages of having received diuretics and ESAs.
Table 4.
Comparison of baseline characteristics between patients who did and did not reach the combined endpoints of commencement of dialysis or death
| Characteristics | Patients with Endpoint (n = 25) | Patients without Endpoint (n = 120) | P |
|---|---|---|---|
| Age (years) | 72.2 ± 10.7 | 67.8 ± 12.3 | 0.103 |
| Male gender (%) | 68.0 | 68.3 | 0.974 |
| Smoking history (%) | 64.0 | 54.6 | 0.390 |
| Diabetes mellitus (%) | 68.0 | 45.8 | 0.044 |
| Hypertension (%) | 92.0 | 82.5 | 0.368 |
| Coronary artery disease (%) | 28.0 | 16.7 | 0.185 |
| Cerebrovascular disease (%) | 36.0 | 15.8 | 0.020 |
| Systolic BP (mmHg) | 151.9 ± 18.5 | 140.5 ± 21.3 | 0.016 |
| Diastolic BP (mmHg) | 81.4 ± 11.5 | 78.4 ± 11.5 | 0.246 |
| Pulse pressure (mmHg) | 70.5 ± 12.3 | 62.8 ± 17.5 | 0.041 |
| Heart rate (beats/min) | 77.6 ± 9.6 | 73.3 ± 14.3 | 0.161 |
| Body mass index (kg/m2) | 25.8 ± 5.0 | 26.0 ± 4.8 | 0.883 |
| Laboratory parameters | |||
| Albumin (g/dl) | 3.9 ± 0.3 | 4.0 ± 0.3 | 0.050 |
| fasting glucose (mg/dl) | 140.2 ± 61.9 | 117.5 ± 40.9 | 0.089 |
| triglyceride (mg/dl) | 205.6 ± 120.2 | 158.5 ± 92.7 | 0.080 |
| total cholesterol (mg/dl) | 193.9 ± 43.8 | 197.5 ± 44.6 | 0.719 |
| HDL-cholesterol (mg/dl) | 41.8 ± 13.2 | 46.5 ± 13.4 | 0.118 |
| LDL-cholesterol (mg/dl) | 105.8 ± 30.0 | 113.4 ± 34.0 | 0.309 |
| hematocrit (%) | 31.0 ± 4.6 | 37.7 ± 6.4 | <0.001 |
| baseline eGFR (ml/min per 1.73 m2) | 14.4 ± 10.4 | 33.2 ± 13.3 | <0.001 |
| calcium (mg/dl) | 9.3 ± 0.7 | 9.7 ± 0.7 | 0.023 |
| phosphate (mg/dl) | 4.5 ± 1.2 | 3.7 ± 0.7 | 0.002 |
| uric acid (mg/dl) | 8.5 ± 2.3 | 8.2 ± 2.3 | 0.666 |
| PTH (pg/ml) | 102.2 ± 107.1 | 57.8 ± 99.1 | 0.050 |
| Albuminuria (%) | 92.0 | 69.2 | 0.024 |
| baPWVa (cm/s) | 2405.3 ± 711.6 | 1981.7 ± 944.6 | 0.036 |
| Medications | |||
| aspirin use (%) | 37.5 | 26.9 | 0.294 |
| ACEI and/or ARB use (%) | 64.0 | 81.5 | 0.053 |
| β-blocker use (%) | 24.0 | 33.3 | 0.634 |
| calcium channel blocker use (%) | 55.8 | 64.0 | 0.453 |
| diuretic use (%) | 45.0 | 16.0 | 0.007 |
| statin use (%) | 25.0 | 27.7 | 0.784 |
| ESA use (%) | 28.0 | 3.3 | <0.001 |
Data were adjusted for mean arterial pressure.
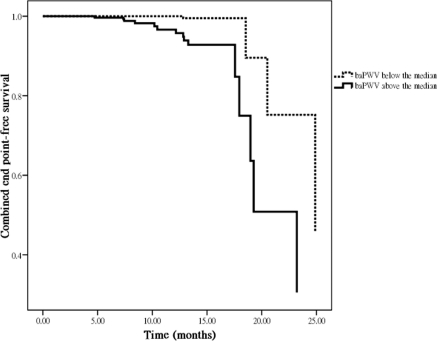
Table 5 shows a Cox proportional hazards regression analysis for progression to commencement of dialysis or death. The univariate regression analysis shows that the hazard ratio (HR) of the baPWV was 1.001 (95% confidence interval [CI], 1.000 to 1.001; P = 0.004). In addition, other variables including the presence of cerebrovascular disease, decreased serum albumin, decreased LDL-cholesterol, decreased hematocrit, decreased baseline eGFR, decreased calcium, increased phosphate, increased log PTH level, no use of ACEI and/or ARB therapy, and the use of ESAs were associated with a significant increase in progression to commencement of dialysis or death. In the multivariate forward analysis, the baPWV (HR, 1.001; 95% CI, 1.000 to 1.001; P = 0.001) and serum phosphate level (HR, 1.883; 95% CI, 1.183 to 2.997; P = 0.008) were positively associated with and baseline eGFR (HR, 0.918; 95% CI, 0.862 to 0.977; P = 0.007) was negatively associated with progression to commencement of dialysis or death. In addition, using baPWV as a categorical variable (baPWV above versus below the median of 1964 cm/s), we found the baPWV above the median was strongly associated with progression to commencement of dialysis or death (HR, 5.107; 95% CI, 1.527 to 17.086; P = 0.008; Figure 3).
Table 5.
Predictors of progression to combined endpoints of commencement of dialysis or death using Cox proportional hazards model
| Parameter | Univariate |
Multivariate (Forward) |
||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age (per 1 year) | 1.030 (0.990 to 1.072) | 0.138 | — | — |
| Male versus female | 0.851 (0.365 to 1.983) | 0.708 | — | — |
| Smoking (ever versus never) | 1.354 (0.597 to 3.070) | 0.468 | — | — |
| Diabetes mellitus | 1.438 (0.604 to 3.420) | 0.411 | — | — |
| Hypertension | 1.094 (0.245 to 4.889) | 0.906 | — | — |
| Coronary artery disease | 1.876 (0.769 to 4.579) | 0.167 | — | — |
| Cerebrovascular disease | 4.086 (1.681 to 9.933) | 0.002 | — | — |
| Heart rate (per 1 beat/min) | 1.019 (0.991 to 1.048) | 0.190 | — | — |
| Body mass index (per 1 kg/m2) | 0.918 (0.826 to 1.021) | 0.114 | — | — |
| Laboratory parameters | ||||
| albumin (per 1 g/dl) | 0.308 (0.108 to 0.882) | 0.028 | — | — |
| fasting glucose (log per 0.1 mg/dl) | 1.247 (0.976 to 1.594) | 0.077 | — | — |
| triglyceride (log per 0.1 mg/dl) | 1.039 (0.880 to 1.225) | 0.653 | — | — |
| HDL-cholesterol (per 1 mg/dl) | 0.979 (0.944 to 1.016) | 0.264 | — | — |
| LDL-cholesterol (per 1 mg/dl) | 0.987 (0.974 to 0.999) | 0.041 | — | — |
| hematocrit (per 1%) | 0.843 (0.786 to 0.905) | <0.001 | — | — |
| baseline eGFR (per 1 ml/min per 1.73 m2) | 0.873 (0.823 to 0.926) | <0.001 | 0.918 (0.862 to 0.977) | 0.007 |
| calcium (per 1 mg/dl) | 0.587 (0.399 to 0.865) | 0.007 | — | — |
| phosphate (per 1 mg/dl) | 2.108 (1.552 to 2.863) | <0.001 | 1.883 (1.183 to 2.997) | 0.008 |
| uric acid (per 1 mg/dl) | 1.066 (0.893 to 1.273) | 0.476 | — | — |
| PTH (log per 0.1 pg/ml) | 1.149 (1.043 to 1.265) | 0.005 | — | — |
| Albuminuria | 2.443 (0.551 to 10.825) | 0.240 | — | — |
| baPWVa (per 1 cm/s) | 1.001 (1.000 to 1.001) | 0.004 | 1.001 (1.000 to 1.001) | 0.001 |
| Medications | ||||
| aspirin use | 1.292 (0.561 to 2.974) | 0.547 | — | — |
| ACEI and/or ARB use | 0.381 (0.167 to 0.870) | 0.022 | — | — |
| β-blocker use | 0.719 (0.119 to 4.324) | 0.718 | — | — |
| calcium channel blocker use | 1.529 (0.672 to 3.478) | 0.311 | — | — |
| diuretic use | 0.392 (0.132 to 1.170) | 0.093 | — | — |
| statin use | 0.465 (0.167 to 1.294) | 0.142 | — | — |
| ESA use | 18.429 (6.615 to 51.348) | <0.001 | — | — |
Abbreviations are the same as in Table 1. −2 Log likelihood = 100.5; χ2 = 40.3; P <0.001.
Data were adjusted for mean arterial pressure.
Figure 3.
Adjusted combined endpoints of commencement of dialysis or death-free survival by baPWV above versus below the median (hazard ratio, 5.107; 95% confidence interval, 1.527 to 17.086; P = 0.008), corrected for age, gender, the presence of cerebrovascular disease, serum albumin, LDL-cholesterol, hematocrit, baseline eGFR, calcium, phosphate, log parathyroid hormone level, the use of angiotensin-converting enzyme inhibitors and/or angiotensin II receptor blockers, and the use of ESAs.
Discussion
In this study, we evaluated the influence of baPWV, a marker of arterial stiffness, on the decline in renal function and the risk of progression to a composite of commencing dialysis or death in a cohort of patient with moderate to advanced CKD. We found that baPWV was independently associated with renal function decline and progression to commencing dialysis or death. Other factors including hypertension, lower eGFR level, and higher phosphate level were also associated with renal function progression.
Previous studies have shown that a decreased eGFR was strongly correlated with increased arterial stiffness in CKD patients (5–7). However, those results were cross-sectional studies, and arterial stiffness was not able to predict the renal function progression. Recently, three longitudinal studies using different measurement methods of arterial stiffness showed increased arterial stiffness was an independent predictor for renal function progression in patients with CKD (10–12). Takenaka et al. (12) studied the relationship between the augmentation index and the rate of decline in annual creatinine clearance in 41 nondiabetic CKD patients with a baseline creatinine clearance level of 52.4 ml/min and found higher basal augmentation index assessed on the radial artery waveform resulted in a greater annular creatinine clearance decrement. Taal et al. (11) studied the relationship between arterial stiffness and the risk of progression to dialysis in 35 patients with CKD stages 4 and 5 with a baseline eGFR level of 13.4 ml/min per 1.73 m2 and identified two markers of arterial stiffness, augmentation index and radial-dorsalis pedis PWV, as risk factors for progression to dialysis in their relatively advanced CKD patients. Ford et al. (10) also evaluated the longitudinal relationship between change in renal function and the marker of aortic stiffness and found aortic PWV was independently associated with the rate of renal function decline in 120 CKD stage 3 and 4 patients with a baseline eGFR level of 32 ml/min per 1.73 m2. They also found aortic stiffness was associated with reaching combined end points of commencing of dialysis or ≥25% decline in eGFR. Although our measurement method was different from theirs, we also showed the significant association between baPWV with renal function progression, including a rapid eGFR decline and an increased risk for progression to commencing dialysis or death in 145 CKD stage 3 to 5 patients with a baseline eGFR level of 29.9 ml/min per 1.73 m2. Although the exact mechanisms for this association are currently unknown, it is possible that increased arterial stiffness results in greater transmission of elevated systemic BP to the glomerular capillaries, thereby exacerbating glomerular hypertension, a major determinant of progressive renal damage (22,23).
Although there were several parameters using in the assessment of arterial stiffness, the gold standard of noninvasive arterial stiffness measurement is carotid-femoral PWV (24). The carotid-femoral PWV directly reflects aortic PWV (25,26). In contrast, baPWV reflects a composite of several arterial segments, some of which are prone to arteriosclerosis alone (brachial and distal arteries) and some to both atherosclerosis and arteriosclerosis (aorta and femoral arteries). Therefore, baPWV may not be as reliable as carotid-femoral PWV in reflecting aortic PWV. However, Tanaka et al. (27) compared carotid-femoral PWV and baPWV in 2287 patients and found a strong correlation between them (r = 0.73, P < 0.001). These two parameters also exhibited similar extent of associations with cardiovascular disease risk factors and clinical events (27). In addition, Yamashina et al. (15) compared aortic PWV derived from an invasive method and baPWV in 41 patients and showed a strong correlation between them (r = 0.87, P < 0.01). Therefore, it is reasonable to use baPWV as a marker of arterial stiffness. In this study, we further confirmed that increased baPWV was a risk factor for renal function decline and progression to dialysis or death in CKD patients.
A high phosphate level was reported to be an independent risk factor for rapid renal function decline and increased mortality (28). Kasiske et al. (29) showed that a high protein diet may increase phosphate intake and accelerate renal function decline. Furthermore, a high plasma phosphate concentration may lead to the deposition of calcium phosphate crystals in either the mitochondria of tubular cells or renal interstitium, thereby causing cell damage and mitogenesis of fibroblasts, resulting in progressive loss of renal function (30). Similarly, our study also showed that increased phosphate level was associated with progression to commencement of dialysis or death in patients with CKD.
ACEIs and ARBs have been shown to reduce neointimal proliferation and vascular inflammation (31). In this study, the use of ACEIs and/or ARBs was associated with a significant decrease in progression to initiation of dialysis or death in univariate analysis. However, the use of ACEIs and/or ARBs was not significantly associated with the combined endpoint after multivariate Cox regression analysis. Thus, treatment with ACEIs and/or ARBs was not a major determinant of the combined endpoint in this study.
There were several limitations to this study. The baPWV was only measured at enrollment, and thus, we could not longitudinally assess the relationship between changes in arterial stiffness and kidney function. In addition, the number and interval of serum creatinine measurements for making the eGFR slope varied in each patient and thus the calculation of eGFR slope was not uniform in every subject. However, to decrease the chance of an unreliable estimation of eGFR slope, we excluded 13 patients with less than three eGFR measurements during the follow-up period, and 4 patients died and 7 patients started dialysis therapy within 30 days after enrollment. Finally, because eGFR tends to be less accurate in subjects with normal renal function than in those with CKD (32) and the reason of renal function decline in patients with normal renal function may be related to their primary diseases, i.e., glomerulonephritis or diabetic nephropathy, our results may be unable to apply in the patients with normal renal function.
In conclusion, our results showed that baPWV, a marker of arterial stiffness, has an independent association with renal function decline and progression to commencing dialysis or death in patients with moderate to advanced CKD. Screening CKD patients by means of baPWV may help identify a high-risk group of rapid renal function progression and progression to commencing dialysis or death.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg AX: Chronic kidney disease and mortality risk: A systematic review. J Am Soc Nephrol 17: 2034–2047, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Mitchell GF: Increased aortic stiffness: An unfavorable cardiorenal connection. Hypertension 43: 151–153, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Safar ME, London GM, Plante GE: Arterial stiffness and kidney function. Hypertension 43: 163–168, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Kawamoto R, Kohara K, Tabara Y, Miki T, Ohtsuka N, Kusunoki T, Yorimitsu N: An association between decreased estimated glomerular filtration rate and arterial stiffness. Intern Med 47: 593–598, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Ohya Y, Iseki K, Iseki C, Miyagi T, Kinjo K, Takishita S: Increased pulse wave velocity is associated with low creatinine clearance and proteinuria in a screened cohort. Am J Kidney Dis 47: 790–797, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Chen JH, Chen SC, Liu WC, Su HM, Chen CY, Mai HC, Chou MC, Chang JM: Determinants of peripheral arterial stiffness in patients with chronic kidney disease in southern Taiwan. Kaohsiung J Med Sci 25: 366–373, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Levin A, Djurdjev O, Barrett B, Burgess E, Carlisle E, Ethier J, Jindal K, Mendelssohn D, Tobe S, Singer J, Thompson C: Cardiovascular disease in patients with chronic kidney disease: Getting to the heart of the matter. Am J Kidney Dis 38: 1398–1407, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Shinohara K, Shoji T, Tsujimoto Y, Kimoto E, Tahara H, Koyama H, Emoto M, Ishimura E, Miki T, Tabata T, Nishizawa Y: Arterial stiffness in predialysis patients with uremia. Kidney Int 65: 936–943, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Ford ML, Tomlinson LA, Chapman TP, Rajkumar C, Holt SG: Aortic stiffness is independently associated with rate of renal function decline in chronic kidney disease stages 3 and 4. Hypertension 55: 1110–1115, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Taal MW, Sigrist MK, Fakis A, Fluck RJ, McIntyre CW: Markers of arterial stiffness are risk factors for progression to end-stage renal disease among patients with chronic kidney disease stages 4 and 5. Nephron Clin Pract 107: c177–c181, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Takenaka T, Mimura T, Kanno Y, Suzuki H: Qualification of arterial stiffness as a risk factor to the progression of chronic kidney diseases. Am J Nephrol 25: 417–424, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Oliver JJ, Webb DJ: Noninvasive assessment of arterial stiffness and risk of atherosclerotic events. Arterioscler Thromb Vasc Biol 23: 554–566, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Tomiyama H, Yamashina A, Arai T, Hirose K, Koji Y, Chikamori T, Hori S, Yamamoto Y, Doba N, Hinohara S: Influences of age and gender on results of noninvasive brachial-ankle pulse wave velocity measurement: A survey of 12517 subjects. Atherosclerosis 166: 303–309, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Yamashina A, Tomiyama H, Takeda K, Tsuda H, Arai T, Hirose K, Koji Y, Hori S, Yamamoto Y: Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res 25: 359–364, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Yokoyama H, Shoji T, Kimoto E, Shinohara K, Tanaka S, Koyama H, Emoto M, Nishizawa Y: Pulse wave velocity in lower-limb arteries among diabetic patients with peripheral arterial disease. J Atheroscler Thromb 10: 253–258, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Levey AS, Coresh J, Bolton K, Culleton B, Harvey KS, Ikizler TA, Johnson CA, Kausz A, Kimmel PL, Kusek J, Levin A, Minaker KL, Nelson R, Rennke H, Stettes M, Witten B: K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39: S1–S266, 2002 [PubMed] [Google Scholar]
- 18. Vickery S, Stevens PE, Dalton RN, van Lente F, Lamb EJ: Does the ID-MS traceable MDRD equation work and is it suitable for use with compensated Jaffe and enzymatic creatinine assays? Nephrol Dial Transplant 21: 2439–2445, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Jones C, Roderick P, Harris S, Rogerson M: Decline in kidney function before and after nephrology referral and the effect on survival in moderate to advanced chronic kidney disease. Nephrol Dial Transplant 21: 2133–2143, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A: Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53: 982–992, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Bidani AK, Griffin KA, Picken M, Lansky DM: Continuous telemetric blood pressure monitoring and glomerular injury in the rat remnant kidney model. Am J Physiol 265: F391–F398, 1993 [DOI] [PubMed] [Google Scholar]
- 23. Griffin KA, Picken MM, Churchill M, Churchill P, Bidani AK: Functional and structural correlates of glomerulosclerosis after renal mass reduction in the rat. J Am Soc Nephrol 11: 497–506, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Asmar R, Benetos A, Topouchian J, Laurent P, Pannier B, Brisac AM, Target R, Levy BI: Assessment of arterial distensibility by automatic pulse wave velocity measurement. Validation and clinical application studies. Hypertension 26: 485–490, 1995 [DOI] [PubMed] [Google Scholar]
- 25. Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM: Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension 38: 938–942, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM: Impact of aortic stiffness on survival in end-stage renal disease. Circulation 99: 2434–2439, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Tanaka H, Munakata M, Kawano Y, Ohishi M, Shoji T, Tomiyama H, Yamashina A, Yasuda H, Sawayama T, Ozawa T: Comparison between carotid-femoral and brachial-ankle pulse wave velocity as measures of arterial stiffness. J Hypertens 27: 2022–2027, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Voormolen N, Noordzij M, Grootendorst DC, Beetz I, Sijpkens YW, van Manen JG, Boeschoten EW, Huisman RM, Krediet RT, Dekker FW; PREPARE study group High plasma phosphate as a risk factor for decline in renal function and mortality in pre-dialysis patients. Nephrol Dial Transplant 22: 2909–2916, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Kasiske BL, Lakatua JD, Ma JZ, Louis TA: A meta-analysis of the effects of dietary protein restriction on the rate of decline in renal function. Am J Kidney Dis 31: 954–961, 1998 [DOI] [PubMed] [Google Scholar]
- 30. Khan SR: Crystal-induced inflammation of the kidneys: Results from human studies, animal models, and tissue-culture studies. Clin Exp Nephrol 8: 75–88, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Gradzki R, Dhingra RK, Port FK, Roys E, Weitzel WF, Messana JM: Use of ACE inhibitors is associated with prolonged survival of arteriovenous grafts. Am J Kidney Dis 38: 1240–1244, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Zuo L, Ma YC, Zhou YH, Wang M, Xu GB, Wang HY: Application of GFR-estimating equations in Chinese patients with chronic kidney disease. Am J Kidney Dis 45: 463–472, 2005 [DOI] [PubMed] [Google Scholar]