Summary
Background and objectives
Uremic xerosis is a bothersome condition that is poorly responsive to moisturizing and emollient therapy.
Design, setting, participants, & measurements
A randomized, double-blind, intraindividual (left versus right comparison), multicentric clinical study was performed on 100 patients with moderate to severe uremic xerosis for 7 days, during which the patients applied twice daily an emulsion combining glycerol and paraffin (test product) on one allocated lower leg, and the emulsion alone (comparator) on the other lower leg. This was followed by an open-labeled use of the test product on all of the xerotic areas for 49 days. The main efficacy parameter was treatment response on each lower leg, as defined by a reduction from baseline of at least two grades in a five-point clinical score on day 7.
Results
Among the 99 patients analyzed, the test product was highly effective with a treatment response in 72 patients (73%), whereas 44 patients (44%) responded to the comparator (P < 0.0001, intergroup analysis). This was associated with an objective reduction in the density and thickness of the scales on day 7 (P < 0.0001 compared with the comparator) and a substantial improvement of the uremic pruritus (75%) and quality of life of the patients at study end (P < 0.001, intragroup analysis). The test product was very well tolerated, with product-related local intolerance (exacerbated pruritus, local burning, or erythema) occurring in only five patients (5%).
Conclusions
Uremic xerosis can be managed successfully when an appropriate emollient therapy is used.
Introduction
Patients undergoing maintenance renal dialysis (MRD) present several dermatologic complications, of which xerosis (rough and scaly skin) is the most common manifestation, occurring in about 75% of the MRD population (1,2). In large clinical series, the intensity of uremic xerosis varied from mild in 30 to 40% of the patients to moderate in 35 to 50% and severe in 15 to 30% of the patients (1,3). Although uremic xerosis is easy to identify, it is often neglected by physicians, and few investigators have dedicated any clinical work to this topic. Uremic xerosis is often associated with another common complication of MRD, namely uremic pruritus, and may contribute to its occurrence and severity (1,3–5).
Patients with uremic xerosis have an unmet need for an effective moisturizing and emollient therapy. Whereas mild cases may respond well to conventional emollients, patients with severe forms of uremic xerosis are reputed to be resistant to these products (6). Local intolerance to soaps and detergents is another concern (2). Here we report the results of a prospective, randomized, double-blind, and industry-sponsored clinical study in moderate-to-severe uremic xerosis patients using an emollient product combining both high hydrating and covering properties.
Materials and Methods
Patients
One hundred subjects were enrolled in four participating centers. The inclusion criteria were: male or female patients of at least 10 years of age, undergoing MRD (hemodialysis or peritoneal dialysis) because of end-stage renal disease. They all presented signs of uremic xerosis as defined by the modified El Gammal clinical score (0 = smooth skin; 1 = patches of fine, powdery scales; 2 = diffuse ashy appearance with many fine scales; 3 = moderate scaling with beginning of cracks; and 4 = intense scaling, moderate cracks) (7). Patients with a symmetrical score of at least 2 on both lower legs were included. Patients with a known allergy to one of the test ingredients and those having an intercurrent condition that might have interfered with the good conduct of the study were excluded. Patients treated with any moisturizing or emollient preparation within 7 days, having a modified dosage of antipruritics within 4 weeks or phototherapy within 8 weeks before study entry were also excluded. Participation in the study required the written informed consent of the patients. The study was performed in accordance with the Declaration of Helsinki.
Study Treatments
The test product combined glycerol 15% and paraffin 10% in an oil-in-water (o/w) emulsion. The combination of these two active ingredients was expected to be pharmacologically relevant for the target indication (uremic xerosis) on the basis of previous studies where their individual activities (hydration and epidermal barrier repair for glycerol, protective anti-irritant effects for paraffin) and their synergistic action on skin occlusion have been demonstrated (8). The comparative product was the o/w emulsion devoid of the active ingredients (comparator) that matched in color and appearance the active product. The comparator was not a completely inert material, but an emulsion with basic hydrating and emollient effects (data not shown).
Study Design
The study was performed in four investigating centers over two subsequent periods. Period I (days 0 to 7) was a comparative period evaluating the test product versus the comparative emulsion intraindividually (left lower leg versus right lower leg comparison), according to a randomized, double-blind schedule. Period II (days 7 to 56) was a noncomparative open-labeled period assessing the test product alone. In Period I, patients applied each product twice daily onto each randomly assigned lower leg, whereas the other xerotic areas remained untreated. In Period II, only the test product was applied onto all xerotic areas, with a recommended dosage of two applications per day. Study visits were carried out at baseline, day 7, day 28, and day 56.
Study Evaluation
Response to treatment was defined as a decrease of at least two grades of the El Gammal score on each lower leg at the end of Period I (day 7). To minimize interassessor variability, a photograder illustrating each grade was provided. The other evaluation parameters of xerosis included a test-side preference made by the investigators on day 7 using a three-point categorical scale (0 = right side comparable with left side; 1 = right side better than left side; 2 = left side better than right side) and the instrumental measurements of scaling on each lower leg at baseline and day 7 using the D-Squame® technique (the total surface area of all squames [SURFT] parameter measures the extent and density of the scales, and the mean optical density without threshold [MOD] parameter measures the thickness of the scales) (9). The time-course severity of uremic xerosis was assessed throughout the study on four sites (both lower legs, forearm without arteriovenous shunt, and chest) and expressed as a total score. Patient oriented evaluation consisted of the auto-assessment of global pruritus at baseline, day 28 and 56 visits using a 100-mm visual analog scale (VAS), and the assessment of the quality of life at baseline and day 56 using both the generic scale Short Form-12 (SF-12) questionnaire and the dermatology-specific Dermatology Life Quality Index (DLQI) (10). SF-12 is a multipurpose measurement of general health status comprising a physical component (physical component summary (PCS) = 50 in the overall population) and a mental component (normal mental component summary (MCS) = 50), whereas DLQI measures the effect of dermatological diseases on patient life quality (DLQI <0.5 in the population with healthy skin). At study end, global tolerance was assessed by the investigators, and product acceptability concerning efficacy, local tolerance, and cosmetics was assessed by the patients. Adverse events were recorded throughout the study.
Regulatory Statement
The study protocol was approved by local and national ethics committees before study execution. The study was conducted according to good clinical practice and monitored under standard operating procedures.
Statistical Analyses
Statistical analyses were made in the intent-to-treat population, in which missing data were replaced using standard statistical methods (e.g. last observation carried forward). Treatment response was analyzed using McNemar's test. The other qualitative efficacy parameters were evaluated using the Wilcoxon signed rank test, and the quantitative parameters were analyzed using the nonparametric Wilcoxon test. A descriptive analysis was performed for global tolerance, product acceptability, and adverse events.
Results
One hundred patients were randomized in the study. One patient withdrew his consent the day after baseline and never applied the study treatments. Accordingly, this patient was excluded from the analysis, and 99 patients were finally included in the intent-to-treat population. There was no further patient loss to follow-up.
The demographic characteristics and lesional status of the study population are shown in Table 1. The mean duration ± SEM at baseline was 7.91 ± 0.71 years for MRD and 5.40 ± 0.53 years for xerosis. A total of 74 patients (75%) had associated uremic pruritus, with a mean ± SEM duration of pruritus of 4.81 ± 0.52 years. Quality of life of the patients at baseline was significantly compromised for all scales (PCS, MCS, and DLQI; Table 1).
Table 1.
Demographic features and health status of the study population at baseline
| Variables | n = 99 |
|---|---|
| Age (years, mean ± SEM; range) | 63.16 ± 1.28; 18–89 |
| Gender (n, %) | |
| male | 53 (54%) |
| female | 46 (46%) |
| End-stage renal disease underlying disease (n, %) | |
| glomerular diseases | 25 (25%) |
| diabetic nephropathy | 15 (15%) |
| nephrosclerosis | 15 (15%) |
| polycystic kidney | 14 (14%) |
| congenital nephropathies | 1 (1%) |
| pyelonephritis | 5 (5%) |
| hypertensive nephropathy | 7 (7%) |
| others | 9 (9%) |
| unknown | 10 (10%) |
| Type of MRD (n, %) | |
| hemodialysis | 90 (91%) |
| peritoneal dialysis | 9 (9%) |
| Duration of MRD (years, mean ± SEM) | 7.91 ± 0.71 |
| Duration of xerosis (years, mean ± SEM) | 5.4 ± 0.53 |
| Clinical severity of xerosis (n, %) | |
| mild | 2 (2%) |
| moderate | 50 (51%) |
| severe to very severe | 47 (47%) |
| Total clinical score of xerosis (mean ± SEM) | 8.3 ± 0.3 |
| Association with pruritus (n, %) | |
| yes | 74 (75%) |
| no | 25 (25%) |
| Severity of pruritus (mm VAS, mean ± SEM) | 40.64 ± 3.36 |
| Quality of life scores (mean ± SEM) | |
| PCS | 30.51 ± 1.21 |
| MCS | 41.29 ± 1.24 |
| DLQI | 5.61 ± 0.57 |
Comparative data between treatment groups on day 7 are summarized in Table 2. Treatment response was observed in 72 patients (73%) on the leg treated by the test product and in 44 patients (44%) on the leg treated by the comparator. The difference between treatment groups was statistically significant (P < 0.0001). Similarly, test side preference by the investigators was in favor of the test product (60 patients, 61%), whereas the comparative emulsion was found to be better in 13 patients (13%) and comparable to the test product in 26 patients (26%; P < 0.0001). Scaling measurements by D-Squame® on day 7 confirmed that the test product was efficient by reducing from baseline the extent and density of the scales (SURFT parameter) as well as their thickness (MOD parameter) in a statistically significant manner compared with the emulsion alone (P < 0.0001).
Table 2.
Comparison of uremic xerosis severity on the lower legs between the two treatment groups after 7 days (Period I) in the study population (intent-to-treat analysis)
| Study Parameters | Test Product (n = 99) | Comparator (n = 99) | P |
|---|---|---|---|
| Treatment response (n, %) | |||
| yes | 72 (73%) | 44 (44%) | <0.0001 |
| no | 27 (27%) | 55 (56%) | |
| Instrumental severity of xerosis (arbitrary units, mean ± SEM) | |||
| SURFT | |||
| baseline | 11.37 ± 0.64 | 10.25 ± 0.55 | |
| day 7 | 3.19 ± 0.42 | 5.35 ± 0.57 | <0.0001 |
| MOD | |||
| baseline | 19.20 ± 0.98 | 18.02 ± 0.76 | |
| day 7 | 7.83 ± 0.60 | 11.56 ± 0.93 | <0.0001 |
| Test side preference | |||
| clearly better | 60 | 13 | <0.0001 |
| comparable | 26 | 26 |
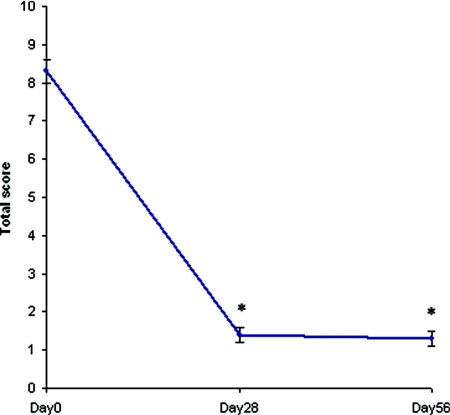
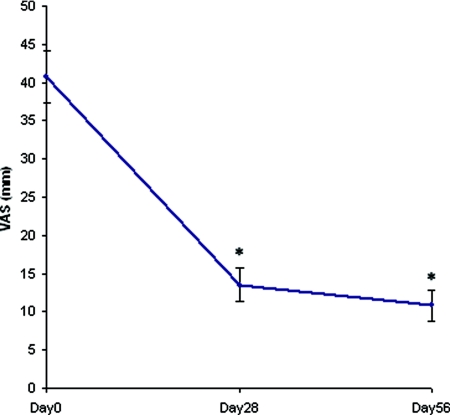
The time course of the total clinical score with the test product on the four test areas showed a dramatic decrease on day 28 (mean ± SEM, 1.4 ± 0.2), that was maintained on day 56 (mean ± SEM, 1.3 ± 0.2; P < 0.001 at the two visits, intragroup analysis; Figure 1). The overall pruritus as assessed by the patients (100-mm VAS) was also markedly ameliorated on day 28 (mean ± SEM, 13.39 ± 2.19 mm) and day 56 (mean ± SEM, 10.66 ± 2.14 mm; P < 0.0001, intragroup analysis), i.e. by about 75% at study end (Figure 2). On day 56, improvement of uremic xerosis and pruritus was associated with a marked improvement of the DLQI (mean ± SEM, 2.27 ± 0.46) and with a moderate improvement of the SF-12 (PCS, 33.89 ± 1.34; MCS, 46.72 ± 1.28). The difference between baseline scores and day 56 scores was statistically significant for all life quality scales used (P < 0.0001, intragroup analysis).
Figure 1.
Time-course severity score of uremic xerosis in the study population (n = 99). *P < 0.001 (intragroup analysis).
Figure 2.
Evolution of the severity of uremic pruritus under treatment in the study population (n = 99). *P < 0.0001 (intragroup analysis).
At study end, investigators judged global tolerance of the test product (n = 93) to be very good in 82 patients (88%), good in seven patients (8%), poor in one patient (1%), and very poor in three patients (3%). Product acceptability by the patients met with high or very high satisfaction with regard to efficacy (82 out of 98 patients, 84%), local tolerance (84 out of 90 patients, 93%), and cosmetic acceptance (82 out of 88 patients, 93%). A total of 21 adverse events were reported, five of which were considered to be related to the test product, occurring in five patients (5%). They were all local and included two exacerbations of pruritus, two local burning or hyperesthesia events, and one irritative reaction (erythema). Four adverse events were judged of mild intensity, and one was severe. Finally, all but two were resolved completely by temporarily or definitively discontinuing the product. One case was still unresolved at study end, and another case had no follow-up record.
Discussion
Uremic xerosis is a unique condition resulting from long-lasting skin dehydration and keratinization abnormalities resulting in persistent barrier dysfunction. It has features distinct from those observed in other xerotic conditions. It usually affects the whole body surface and is more severe in some areas (legs, forearms, hands, and back). Interestingly, glycerol content is decreased in the stratum corneum of uremic xerosis patients, glycerol decrease being correlated to severity of xerosis and skin barrier alteration (11).
We therefore investigated the effects of an emollient and skin protective product combining glycerol 15% and paraffin 10%. Glycerol has a rapid hydrating and smoothing effect (12,13) that can be achieved at concentrations ranging from 10 to 15% (14). Long-term use of glycerol also accelerates barrier repair (15) by improving corneodesmosome degradation and by restoring normal keratosis (16,17), as well as through its preventive action of the transition of intracellular lipids from liquid crystals to solid crystals (18–20). The mechanism of the action of glycerol has been ascribed to modulation of Aquaporin-3 channels (21). In contrast, paraffin has no effect on skin hydration and only a limited effect on transepidermal water loss (22,23) but preserves the barrier function against irritants (24). The combination of glycerol and paraffin is therefore of potential benefit in the treatment of uremic xerosis patients to rapidly compensate for the main defects that characterize this condition, i.e. glycerol deficiency with skin dehydration, barrier dysfunction, and chemically induced irritation (11,25). The relevance of their combination has been further demonstrated in studies in which the occlusive properties of paraffin were enhanced in the presence of glycerol when formulated in o/w emulsions (8,26).
Our study, which comprised a short comparative period of 7 days followed by a noncomparative, open-labeled observational period of 49 days, demonstrated that the test product combining glycerol and paraffin in an o/w formulation is a clinically effective treatment of uremic xerosis. On the basis of pharmacologic investigation (data not shown), the 7-day comparison was judged as being sufficient to show a rapid palliative effect of the product on xerotic lesions. Compared with the basic emulsion used as the comparator, a significant decrease of the xerotic lesions after a twice-daily application of the product was observed with a complete or almost complete remission of xerotic signs in 73% of the patients within 7 days of initial application (P < 0.0001, intergroup analysis). This effect was sustained 56 days after initiation of the treatment. In addition to the observed clinical effect, the benefit could be further objectively quantified using SURFT and MOD parameters (P < 0.0001, intergroup analysis).
At baseline, a majority of patients (75%) complained of uremic pruritus despite antihistamine therapy and indicated a marked loss of quality of life. At the end of the open-label observation period of the study, the test product induced marked relief of pruritus, this syndrome being amended by about 75% after 49 days of therapy (P < 0.0001, intergroup analysis). A significant improvement of the patients' quality of life was also observed (P < 0.0001). A few open studies have already documented the beneficial action of an emollient therapy in uremic pruritus (5,27,28). Overall, a marked relief of uremic pruritus was observed in 33% (various moisturizing creams) (27) to 35% and 43% (one emollient cream) (5,28) of the patients, whereas a placebo effect in uremic pruritus has been described to be decreased by around 25% (29). Our test treatment clearly outperformed these results, indicating that an effective emollient treatment of uremic xerosis can also efficiently relieve uremic pruritus. However, because pruritus and quality of life were assessed during the noncomparative observational period of the study, it was not possible to conclude whether the improvement of pruritus and quality of life could be attributed to glycerol and paraffin or to the emulsion alone.
Test product-related adverse events occurred infrequently (five cases, 5% of the patients) and were all local. They included pruritus exacerbation (2%), erythema (1%), and local pain and burning (2%). Most cases were of mild severity and resolved by discontinuing the treatment. This was further confirmed by the overall local tolerance as assessed by the investigators (96% of patients with good to very good tolerance) and a high degree of consensus among patients as to the excellent tolerability (93% of satisfaction or high satisfaction). The test product therefore demonstrated a very good local tolerance profile in uremic xerosis patients, bearing in mind that these patients are particularly prone to develop irritancy to topical products (2).
In conclusion, uremic xerosis is a poorly recognized condition that may aggravate uremic pruritus and compromise the quality of life of patients. It can be easily managed by the use of an efficient emollient and skin protective product.
Disclosures
None.
Acknowledgments
The authors are indebted to Drs. Mauro Barbareschi, Amedeo F. De Vecchi, Spyridon Liakos, Tomasz Szepietowski, and Efstratios Vakirlis, who participated in the study as co-investigators.
The authors would also like to thank Isabelle Jeu for the preparation of the manuscript and John Pimm for the English revision of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Balaskas EV, Chu M, Uldall RP, Gupta A, Oreopoulos DG: Pruritus in continuous ambulatory peritoneal dialysis and hemodialysis patients. Perit Dial Int 13: 527S–532S, 1992 [PubMed] [Google Scholar]
- 2. Szepietowski JC, Reich A, Schwartz A: Uraemic xerosis. Nephrol Dial Transplant 19: 1–4, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Szepietowski JC, Sikora M, Kusztal M, Salomon J, Magott M, Szepietowski T: Uremic pruritus: A clinical study of maintenance hemodialysis patients. J Dermatol 29: 621–627, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Gilchrest BA, Stern RS, Steinman TI, Brown RS, Arndt KA, Anderson WW: Clinical features of pruritus among patients undergoing maintenance hemodialysis. Arch Dermatol 118: 154–156, 1982 [PubMed] [Google Scholar]
- 5. Morton CA, Lafferty M, Hau C, Henderson I, Jones M, Lowe JG: Pruritus and skin hydration during dialysis. Nephrol Dial Transplant 11: 2031–2036, 1996 [DOI] [PubMed] [Google Scholar]
- 6. Ponticelli C, Bencini PL: Dermatological disorders. in Oxford Textbook of Clinical Nephrology, Oxford University Press, 1998, pp 1995–2002 [Google Scholar]
- 7. El Gammal C, Pagnoni A, Kligman AM, El Gammal S: A model to assess the efficacy of moisturisers: The quantification of soap-induced xerosis by image analysis of adhesive-coated discs (D-Squames®). Clin Exp Dermatol 21: 338–343, 1996 [PubMed] [Google Scholar]
- 8. Gloor M, Gehring W: Increase in hydration and protective function of horny layer by glycerol and a W/O emulsion: Are these effects maintained during long-term use? Contact Dermatitis 44: 123–125, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Lagarde JM, Black D, Gall Y: Image analysis of scaly skin using Dsquame® samplers: Technical and physiological validation. Int J Cosmet Science 22: 53–65, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Finlay AY, Khan GK: Dermatology life quality index (DLQI): A simple practical measure for routine clinical use. Clin Exp Dermatol 19: 210–216, 1994 [DOI] [PubMed] [Google Scholar]
- 11. Yosipovitch G, Duque MI, Patel TS, Ishiuji Y, Guzman-Sanchez DA, Dawn AG, Freedman BI, Chan YH, Crumrine D, Elias PM: Skin barrier structure and function and their relationship to pruritus in end-stage renal disease. Nephrol Dial Transplant 22: 3268–3272, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Batt MD, Fairhurst E: Hydration of the stratum corneum. Int J Cosmet Sci 8: 253–264, 1986 [DOI] [PubMed] [Google Scholar]
- 13. Batt MD, Davis WB, Fairhurst E, Gerrard WA, Ridge BD: Changes in the physical properties of the stratum corneum following treatment with glycerol. J Soc Cosmet Chem 39: 367–381, 1988 [Google Scholar]
- 14. Orth DS, Appa Y: Glycerine: A natural ingredient for moisturizing skin. In: Dry skin and moisturizers, edited by Loden M, Maibach HI. Boca Raton, CRC Press, 2000, pp 213–228 [Google Scholar]
- 15. Fluhr JW, Gloor M, Lehmann L, Lazzerini S, Distante F, Berardesca E: Glycerol accelerates recovery of barrier function in vivo. Acta Derm Venereol 79: 418–421, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Rawlings A, Harding C, Watkinson A, Banks J, Ackerman C, Sabin R: The effect of glycerol and humidity on desmosome degradation in stratum corneum. Arch Dermatol Res 287: 457–464, 1995 [DOI] [PubMed] [Google Scholar]
- 17. Chandar P, Harding CRH, Watkinson AW, Banks J, Sabin RD, Hoyberg K, Rawlings AV: A comparison of the effect of commercial moisturizers on desquamation and desmosome hydrolysis. J Invest Dermatol 106: 919, 1996 [Google Scholar]
- 18. Froebe CL, Simion FA, Ohlmeyer H, Rhein LD, Mattai J, Cagan RH, Friberg SE: Prevention of stratum corneum lipid phase transitions in vitro by glycerol: An alternative mechanism for skin moisturization. J Soc Cosmet Chem 41: 51–65, 1990 [Google Scholar]
- 19. Appa Y, Orth DS, Widjaja J, Asuncion A: Effect of glycerin on energy requirements and liquid crystallinity of model intracellular lipids [Abstract]. J Invest Dermatol 100: 587, 1993 [Google Scholar]
- 20. Mattai J, Froebe CL, Rhein LD, Simion FA, Ohlmeyer H, Su DT, Friberg SE: Prevention of model stratum corneum lipid phase transitions in vitro by cosmetic additives: Differential scanning calorimetry, optical microscopy, and water evaporation studies. J Soc Cosmet Chem 44: 89–100, 1993 [Google Scholar]
- 21. Ma T, Hara M, Sougrat R, Verbavatz JM, Verkman AS: Impaired statum corneum hydration in mice lacking epidermal water channels aquaporin-3. J Biol Chem 277: 17147–17153, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Kolbe L, Kligman AM, Stoudemayer T: Objective bioengineering methods to assess the effects of moisturizers on xerotic leg skin of elderly people. J Dermatol Treat 11: 241–245, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Tabata N, O'Goshi K, Zhen YX, Kligman AM, Tagami H: Biophysical assessment of persistent effects of moisturizers after their daily applications: Evaluation of corneotherapy. Dermatol 200: 308–313, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Wigger-Alberti W, Elsner P: Petrolatum prevents irritation in a human cumulative exposure model in vivo. Dermatol 194: 247–250, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Overgaard Olsen L, Jemec GBE: The influence of water, glycerin, paraffin oil and ethanol on skin mechanics. Acta Derm Venereol 73: 404–406, 1993 [DOI] [PubMed] [Google Scholar]
- 26. Wigger-Alberti W, Rougier A, Richard A, Elsner P: Efficacy of protective creams in a modified repeated irritation test. Acta Derm Venereol 78: 270–273, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Southi P, Commens C: Pruritus in dialysis patients. Med J Australia 146: 397–398, 1987 [DOI] [PubMed] [Google Scholar]
- 28. Kim H, Jeong S, Jeong M, Ahn J, Moon S, Lee S: The relationship of PAR2 and pruritus in end stage renal disease patients and the clinical effectiveness of soybean extracts containing moisturizer on epidermal permeability barrier in end stage renal disease patients. J Invest Dermatol 130: S56, 2010 [Google Scholar]
- 29. Murphy M, Reaich D, Pai P, Finn P, Carmichael AJ: A randomized, placebo-controlled, double-blind trial of ondansetron in renal itch. Br J Dermatol 148: 314–317, 2003 [DOI] [PubMed] [Google Scholar]