Summary
Background
Insulin resistance (IR) is highly prevalent in chronic hemodialysis (CHD) patients and is associated with poor cardiovascular outcomes. Hyperinsulinemic euglycemic glucose clamp (HEGC) is the gold standard for measuring IR. The comparison of commonly-used indirect indices of IR to HEGC has not been adequately performed in this population. Furthermore, the validity of newly proposed adipokine-based IR indices has not been explored.
Design, setting, participants, & measurements
This is an observational study performed in a single center, involving 12 prevalent CHD patients (50 ± 9 years old, 100% African American, 33% women, body mass index of 34.4 ± 7.6 kg/m2) who were studied three consecutive times. IR was assessed by HEGC (glucose-disposal rate [GDR]), homeostatic model assessment of IR (HOMA-IR), HOMA-IR corrected by adiponectin (HOMA-AD), leptin adiponectin ratio (LAR), QUICKI, and the McAuley's index at each time point.
Results
Eighty-three percent of the subjects displayed either glucose intolerance or overt insulin resistance by HEGC (GDR median, 5.71; interquartile range [IQR], 4.16, 6.81). LAR and HOMA-AD were the best correlates of IR measured by HEGC (r = −0.72, P < 0.001, and −0.67, P < 0.001), respectively. Fat percentage, interleukin-6, and adipokines (leptin, adiponectin, and resistin) were strongly associated with GDR. HEGC, LAR, and HOMA-AD had the best intraclass correlation coefficients.
Conclusion
IR is common in CHD patients. Adipokine-based indices are the best correlates of IR measurements by HEGC. HOMA-IR and QUICKI are reasonable alternatives. Use of these indices may allow better detection of alterations in insulin sensitivity in CHD patients.
Introduction
Insulin resistance (IR) is highly prevalent in diabetic and nondiabetic patients with chronic kidney disease (CKD) and end-stage renal disease (ESRD) (1–7) and is an established risk factor for the development of cardiovascular disease and all-cause mortality in this population (5,8–10). Recent studies indicate that many of the metabolic derangements that accompany kidney disease contribute to the development of insulin resistance through different pathways, such as chronic inflammation, metabolic acidosis, vitamin D deficiency, oxidative stress, and decreased clearance of adipocytokines. Accordingly, the assessment of insulin sensitivity in CKD patients, especially in ones on maintenance dialysis, requires consideration of these complex factors.
Although hyperinsulinemic euglycemic clamp (HEGC) is considered the gold standard for assessment of insulin sensitivity (2), the complexity of the methodology limits its use. For that reason, a number of more practical methods, such as homeostatic model assessment (HOMA) (6,11), the quantitative insulin sensitivity check index (QUICKI) (12), and McAuley's index (a triglyceride-based method) (13), are widely used in larger studies and at times in the clinical setting. Despite the recognition of “uremic insulin resistance” and its consequences, only a few studies have explored the correlation between HEGC and these commonly utilized or newly recognized indices in chronic hemodialysis (CHD) patients (1,2).
In this study, we aimed to evaluate the performance of three readily available indices of IR (HOMA, QUICKI, and the McAuley's index) and the two adipokine-based indices of IR (HOMA-AD and leptin adiponectin ratio [LAR]) relative to the gold standard “glucose-disposal rate” by HEGC in 12 CHD patients. We hypothesized that the adipokine-based indices would have a high correlation with GDR by HEGC in ESRD patients. Additionally, we explored the determinants of IR measured by HEGC in ESRD.
Materials and Methods
Subjects
Subjects were recruited from the Vanderbilt University Outpatient Dialysis units between April 2008 and January 2010. Twelve prevalent CHD patients 18 years or older were recruited for the study. Inclusion criteria included being on CHD for more than 6 months, functioning permanent dialysis access, equilibrated KT/V (the product of the urea clearance and the duration of the dialysis session normalized to the volume of distribution of urea) greater than 1.2, no active infections or chronic inflammatory conditions, and no hospitalizations within 1 month of the measurements. Patients with type 1 diabetes were excluded from the study. Patients with diabetes type 2 not taking insulin sensitizers (thiazolidinediones) were allowed in the study. Diabetes type 2 was defined as: having at least two fasting plasma glucose measurements greater or equal to 126 mg/dl or taking oral antidiabetic drugs or insulin. History of prior diabetes was also recorded. Impaired fasting glucose (IFG) was defined as fasting glucose levels between 100 and 125 mg/dl at least on two occasions. Each patient was studied three times, 8 weeks apart; however, two patients did not complete the study for personal reasons (one subject had only the baseline study and the other subject completed 2 studies). This study is part of a primary pilot and feasibility trial (NCT00656032) designed to evaluate the effect of vitamin D administration in insulin resistance. The study was approved by Vanderbilt University Medical Center Institutional Review Board, and informed consent was obtained from all patients.
Hyperinsulinemic-Euglycemic Glucose Clamp Study
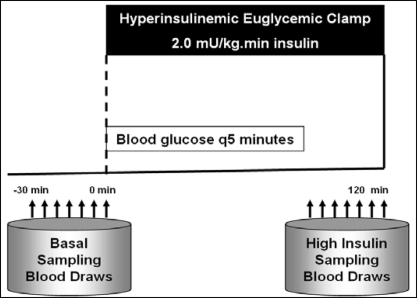
All of the studies were performed at the General Clinical Research Center at Vanderbilt University. On the morning of the clamp study (Figure 1), a fasting blood glucose measurement was obtained. Also blood was drawn to assess baseline values of inflammatory and hormonal markers. The dialysis shunt was accessed using 15-gauge fistula needles placed in opposite directions at least four finger breadths apart. The venous needle was used for the infusions of glucose, insulin, and dextrose. All of the blood samples were taken via a dialysis needle placed at the arterial side of the dialysis access. Blood samples were drawn at 5-minute intervals for 30 minutes to assess the basal levels of glucose at steady state.
Figure 1.
Hyperinsulinemic Euglycemic Clamp Procedure.
A primed continuous infusion of human regular insulin (50 units/50 ml of normal saline) was started at a rate of 2.0 mU/kg/min and maintained at that level through 120 minutes. After insulin initiation, the plasma glucose levels were allowed to drop to within 5 mg/dl of the patient's baseline glucose value and were maintained at that level throughout the study by adjusting a variable infusion of 20% dextrose. Constant monitoring of plasma glucose concentration was done every 5 minutes. Once steady state was reached and confirmed at 90 minutes, the average value of the glucose infusion rate was calculated over the last 30 minutes (M value) and than normalized to total body weight to estimate the glucose-disposal rate (GDR, mg/kg per minute) (14). GDR was calculated as an index of in vivo insulin sensitivity. We chose not to normalize the M value to fat-free mass but only to total body weight, because the latter has been studied across individuals with different weights including obese individuals (15). On the basis of literature published in the general population, GDR higher than 7.5 mg/kg per minute was considered to be an insulin-sensitive state, whereas values lower than 4.0 mg/kg per minute were considered insulin-resistant. Levels between 4.0 and 7.5 mg/kg per minute suggest “impaired glucose tolerance.”
Derived Insulin Resistance Indices
The following Insulin sensitivity indices were measured simultaneously and validated against the HEGC: homeostasis model assessment (HOMA-IR), insulin (μU/ml) × glucose (mg/dl)/405; quantitative insulin sensitivity check index (QUICKI), 1/(log glucose [mg/dl] + log insulin [μU/ml]); McAuley's index, exp (2.63 − 0.28 ln insulin [μU/ml] − 0.31 ln triglycerides [mM/ml]); homeostasis model assessment corrected by adiponectin (HOMA-AD), insulin (μU/mL) × glucose (mg/dl)/(405 × adiponectin [mg/ml]); and LAR, leptin (ng/ml)/adiponectin (mg/ml). The reference values for insulin resistance in the general population (7,16) are HOMA, ≥2.6; QUICKI, ≤0.33; and McAuley's index, ≤5.8 (16).
Blood Samples
All of the blood sampling was performed at the General Clinical Research Center and processed at Vanderbilt Cytokine and Hormonal Core facilities. Blood was drawn into Vacutainer® (Becton Dickinson, Franklin Lakes, NJ) tubes containing EDTA for plasma separation. The samples were transported on ice and immediately centrifuged at 20°C at 3000 rpm for 15 minutes. The supernatants were stored in aliquots at −80°C. Glucose concentrations were measured by using the glucose oxidase method (Glucose Analyzer 2; Beckman Coulter, Brea, CA). Insulin was measured by using a double-antibody RIA (DA RIA; Millipore, St. Charles, MO). Total adiponectin and resistin were measured using the MILLIPLEX MAP Human Serum Adipokine Panel A kit (Millipore, Billerica, MA). IL-6 concentrations were determined using cytometric bead arrays (Becton Dickinson, San Jose, CA). Two-color flow cytometric analysis was performed using a BD LSR II flow cytometer (Becton Dickinson). C-reactive protein levels were measured using the high-sensitivity particle-enhanced turbidimetric UniCel DxI Immunoassay System (Beckman Coulter). All of the other measurements (including triglycerides, HDL, LDL, and leptin) were performed using routine laboratory tests and certified methods.
Body Composition by Dual-Energy X-ray Absorptiometry
Dual-energy X-ray absorptiometry (DEXA) is considered the gold-standard method to measure body composition in hemodialysis patients (17–20). In this study we performed an assessment of body composition using a Lunar iDEXA machine, encore 2007, v.11.40.004 (General Electric, Madison, WI) and GE Lunar Body Composition Software. The Lunar system measures bone mass, fat mass, and lean body mass for the whole body and by body regions (truncal and legs). During the scan, participants lie supine, centered on the scan table within the demarcated area, with arms at their sides, palms down, and thighs separated. The legs were rotated inward with toes touching each other and strapped together to maintain this position. All metallic snaps and potential artifacts were removed. Subjects were required to remain still on the DEXA bed in the supine position for approximately 6 to 10 minutes until the whole body scan was completed. We here provide standard measurements as previously reported (17–20): whole body total fat mass, total fat mass percentage, and whole body lean body mass. The Lunar iDEXA also reports by body regions. We here use truncal fat mass percentage, which has been previously reported to be closely related to visceral fat mass in hemodialysis patients (17), and total fat mass percentage as predictors of insulin resistance.
Statistical Analyses
The data are presented as the means ± SD or median with interquartile range depending on their distribution. For the purpose of the analyses, the indices of insulin resistance and other markers were log-transformed when appropriate to yield a normal distribution before analysis. The correlation coefficient between the different indices and HEGC were assessed by Spearman correlation coefficient, and the association between the different indices and the gold standard HEGC was done using a generalized estimating equation (GEE) model to account for dependency among repeated measures. The reliability of the different indices of insulin resistance was measured using an intraclass correlation coefficient using the following formula SD2μ/SD2μ + SD2ε. SD2μ stands for the between-patient SD, and SD2ε stands for the within-patient SD. Comparison of correlation coefficients was not performed because of concerns of collinearity. Each of the proposed determinants of IR was selected a priori on the basis of previous knowledge and published literature. GEE analysis was then performed for each determinant both in an unadjusted and in an adjusted analysis for age, gender, and diabetes. The statistical analysis was performed using SPSS 18 for Windows (Chicago, IL).
Results
Baseline characteristics of study subjects are shown in Table 1. The mean age was 50 years (range, 40 to 73), 33% (n = 4) were female, and all of the patients were African American. Fifty-eight percent of patients were obese (body mass index, >30 kg/m2). The median time on dialysis was 46 months (IQR 37, 94). We classified our study participants on the basis of the American Diabetes Association criteria as diabetics, IFG, and normal fasting glucose. Two individuals had diabetes on the basis of at least two fasting glucose tests with results of >125 mg/dl (one of them was known to have diabetes and one did not), four individuals had IFG on the basis of at least two fasting glucose levels of 100 to 125 mg/dl and six had normal fasting glucose levels of <100 mg/dl. Two of the participants with IFG and one of the participants with normal fasting glucose had a previous history of diabetes that had resolved before reaching ESRD.
Table 1.
Baseline characteristics of the study subjects
| Nutritional Parameters | (n = 12) |
|---|---|
| Albumin (g/dl; median, IQR) | 3.85 (3.5, 4.2) |
| Prealbumin (mg/dl; median, IQR) | 36.85 (26.8, 46.05) |
| LDL cholesterol (mg/dl; median, IQR) | 88.5 (60.0, 106.5) |
| HDL cholesterol(mg/dl; median, IQR) | 38 (34.25, 49.50) |
| Triglycerides (mg/dl; median, IQR) | 113.5 (95, 192.25) |
| Body composition by DEXA | |
| trunk fat by DEXA (%; median, IQR) | 46.6 (39.0, 53.1) |
| total fat (%; median, IQR) | 39.9 (33.1, 48.7) |
| lean body mass (kg; median IQR) | 53.2 (45.8, 57.7) |
| body mass index (kg/m2;median, IQR) | 35 (26, 39) |
| Glucose metabolism measurements | |
| glycated hemoglobin (median, IQR) | 5.10 (5.0, 6.0) |
| fasting glucose (mg/dl; median, IQR) | 101 (88, 111) |
| fasting Insulin (μU/L; median, IQR) | 12.31 (9.32, 17.47) |
| Insulin sensitivity indices | |
| GDR (mg/kg/min; median, IQR) | 5.71 (4.16, 6.81) |
| HOMA-IR (median, IQR) | 3.29 (2.41, 3.97) |
| QUICKI (median, IQR) | 0.32 (0.31, 0.33) |
| McAuley's index (median, IQR) | 7.39 (6.55, 8.87) |
| HOMA-AD (median, IQR) | 67.46 (41.68, 132.91) |
| Leptin adiponectin ratio (median, IQR) | 1.36 (0.62, 4.67) |
| Inflammatory markers | |
| high-sensitivity C-reactive protein (mg/dl; median, IQR) | 5.85 (1.87, 8.38) |
| IL-6 (pg/ml; median, IQR) | 9.51 (4.51, 19.56) |
| IL-10 (pg/ml; median, IQR) | 6.42 (3.8, 10.04) |
| Adipokines | |
| adiponectin (μg/ml; median, IQR) | 16.51 (12.6, 32.94) |
| leptin (ng/ml; median, IQR) | 21.66 (12.07, 68.43) |
| resistin (ng/ml; median, IQR) | 34.36 (30.48, 49.1) |
Insulin Resistance
The baseline values for the different insulin resistance indices are shown in Table 1 and are as follows: GDR, median 5.7 (IQR 4.16, 6.8) mg/kg per minute; HOMA-IR, median 3.29 (IQR, 2.43, 3.97); QUICKI, median 0.32 (IQR 0.31, 0.34); McAuley's index, median 7.39 (IQR, 6.55, 8.87); HOMA-AD, median 67.46 (IQR 41.78, 132.91); and LAR, median 1.36 (IQR 0.62, 4.67). Using the averages of all measurements per each individual and the cut-off for HEGC of insulin sensitivity by GDR of <7.5 mg/kg per minute, 83% (n = 10) were either insulin resistant or glucose intolerant.
We estimated the reproducibility and reliability between the three measurements of each index using intraclass correlation coefficients (ICCs). Three of the measurements had excellent reproducibility (ICC >0.75): LAR had the highest ICC (0.97), followed by GDR (0.88) and HOMA-AD (0.81). The other three indices had good ICCs: HOMA-IR was 0.73, QUICKI was 0.69, and the McAuley's index was 0.65.
Correlations between the Different Indices of Insulin Resistance
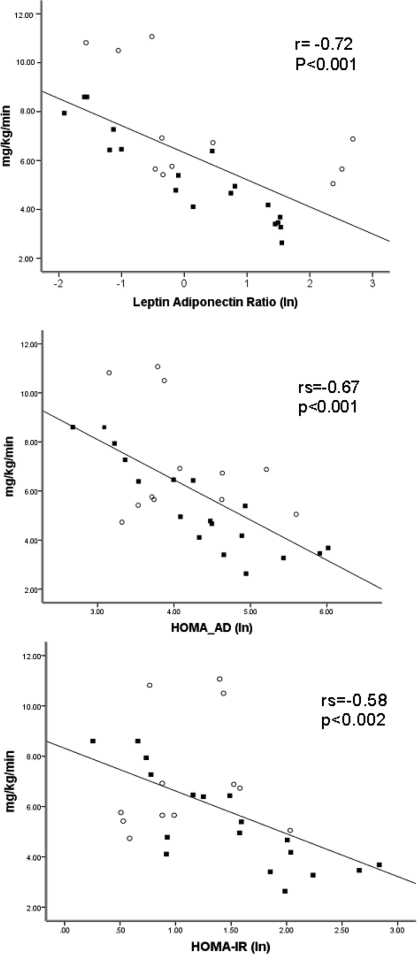
Correlation coefficients of the different indices of IR and HEGC are displayed in Table 2 and Figure 2. LAR and HOMA-AD were the best predictors of GDR by HECG: r = −0.72 (P < 0.001) and r = −0.67 (P < 0.001), respectively. However, all other indices were predictive of IR by HEGC: HOMA-IR r = −0.58 (P < 0.002), QUICKI r = −0.58 (P < 0.004), and McAuley's index r = 0.5 (P < 0.03]. Table 3 depicts the correlation coefficients between the different indirect insulin indices. HOMA-AD had excellent correlation with LAR r = 0.86 (P < 0.001), HOMA-IR r = 0.88 (P < 0.001), and QUICKI r = −0.88 (P < 0.001). HOMA-IR and QUICKI had a perfect correlation, r = 0.99 P < 0.0001. The correlation of LAR with HOMA-IR and QUICKI was lower r = 0.70 (P = 0.004) and r = −0.70 (P = 0.002), respectively. McAuley's index had the lowest correlation coefficients.
Table 2.
Correlation of the different IR indices with glucose-disposal rate by hyperinsulinemic euglycemic clamp in chronic hemodialysis patients
| IR Index | All Patients |
|
|---|---|---|
| rs | P | |
| LAR | −0.72 | <0.001 |
| HOMA-AD | −0.67 | <0.001 |
| HOMA-IR | −0.58 | <0.002 |
| QUICKI | 0.58 | <0.004 |
| McAuley's index | 0.5 | <0.03 |
The correlation coefficients are done by Spearman correlation (rs). Statistical association was tested by GEE analysis in the 10 participants with all three visits included.
Figure 2.
The data represent the best fit regression line. The P values for the association for the different indices and HEGC are derived from a GEE analysis. Only individuals with all three visits are included. The correlation coefficient is derived by Spearman correlation. The dark squares represent individuals with IFG or DM on the basis of at least two fasting glucose tests. The open circles represent individuals with normal fasting glucose levels.
Table 3.
Correlation matrix of the different indirect indices of IR
| IR Index | LAR | HOMA-AD | HOMA-IR | QUICKI | McAuley's Index |
|---|---|---|---|---|---|
| LAR | 1 | ||||
| HOMA-AD | 0.86 (P < 0.001) | 1 | |||
| HOMA-IR | 0.70 (P = 0.004) | 0.88 (P < 0.001) | 1 | ||
| QUICKI | 0.70 (P = 0.002) | −0.88 (P < 0.001) | −1 (P < 0.001) | 1 | |
| McAuley's index | −0.47 (P = 0.2) | −0.47 (P = 0.06) | −0.68 (P < 0.001) | 0.68 (P < 0.001) | 1 |
The correlation coefficients are done by Spearman correlation. Statistical association was tested by GEE analysis in the 10 participants with all three visits included.
Determinants of Insulin Resistance by HEGC
Among a number of variables known to affect insulin resistance, inflammatory markers (IL-6 [P = 0.05] and high sensitivity C-reactive protein [P = 0.03]), adipokines (adiponectin [P = 0.03], leptin [P = 0.004], and resistin [P = 0.03]), and truncal fat mass (as a surrogate marker for waist circumference or visceral fat) (P = 0.03) were significantly associated with GDR in the univariate analysis (Table 4). Age, gender, diabetic status, and lipid measurements were not associated with insulin resistance by HEGC in the univariate analysis.
Table 4.
Clinical determinants of insulin resistance measured by hyperinsulinemic euglycemic clamp
| Variable | Unadjusted Analysis |
Adjusted Analysisa |
||
|---|---|---|---|---|
| Beta Coefficient (95% CI) | P | Beta Coefficient (95% CI) | P | |
| Adipose tissue | ||||
| total fat mass percent (DEXA) (n = 12)a | −3.1 (−3.26, 3.07) | 0.31 | −0.31 (−0.427, −0.204) | <0.001 |
| truncal fat percent (DEXA) (n = 12)a | −0.12 (−0.19, −0.052) | 0.03 | −0.24 (−0.296, −0.189 | 0.01 |
| Adipokines | ||||
| adiponectin (μg/ml) | 0.14 (0.01, 0.26) | 0.03 | 0.15 (0.05, 0.25) | 0.005 |
| leptin (log; ng/ml) | −1.18 (−2.0, −0.37) | 0.004 | −2.06 (−2.54, −1.58) | <0.001 |
| resistin (log; ng/ml) | 2.76 (0.30, 5.21) | 0.03 | 3.55 (1.33, 5.76) | 0.002 |
| Inflammatory markers | ||||
| IL-6 (log; pg/ml) | −1.50 ( −2.99, −0.01) | 0.05 | −1.43 (−2.47, −0.395) | 0.007 |
| CRP (log; mg/dl) | −0.91( −1.74, −0.08) | 0.03 | −1.08 (−1.88, −0.296) | 0.007 |
| Lipids | ||||
| triglycerides (mg/dl) | −0.76 ( 2.68, 1.15) | 0.44 | −0.14 (−0.037, 0.008) | 0.21 |
| LDL (mg/dl) | −0.02 (−0.06, 0.02) | 0.35 | −0.02 (−0.07, 0.02) | 0.32 |
| HDL (mg/dl) | 0.04 ( −0.05, 0.13) | 0.37 | 0.03 (−0.05, 0.12) | 0.42 |
Adjusted for age, gender, and diabetic status.
In the multivariate analysis (adjusted for age, gender, and diabetic status), all of the adipokines including adiponectin (β 0.15, 95% confidence interval [CI] 0.05, 0.25; P = 0.005), resistin (β 3.55, 95% CI 1.33, 5.76; P = 0.002), and log-transformed leptin (β −2.06, 95% CI −2.54, −1.58; P < 0.001) were statistically significantly associated with GDR by HEGC. Similarly, inflammatory markers IL-6 (β −1.46; 95% CI −2.47, −0.395; P = 0.007) and high sensitivity C-reactive protein (β −1.08; 95% CI −1.88, −0.296; P = 0.007) were negatively correlated with GDR by HEGC in this model. Total body fat percentage by DEXA was associated with IR (β −0.31; 95% CI −0.427, −0.204; P < 0.001) as well as truncal fat percentage as a measurement of visceral fat (β −0.24; 95% CI 0.29, −0.19; P = 0.01).
Discussion
Given its strong correlation with outcomes, insulin resistance is a clinically important metabolic abnormality in CKD, and therefore its estimation requires accuracy, especially in CHD patients. Although measurement of the glucose-disposal rate by HEGC provides such precision in the sophisticated research setting, there is a need to examine more practical methods, both for use in the clinical setting and for the research applications for larger cohorts. This study was undertaken to compare several commonly-used indirect indices of IR to HEGC in CHD patients. Our results show that two adipokine-based measures of IR, specifically the HOMA-AD and leptin-adiponectin ratio, are the best correlates of IR measured by HEGC. In addition, these two indices appear to be sensitive enough to detect slight abnormalities over a broad spectrum of insulin sensitivity, including nondiabetic and diabetic CHD patients. HOMA-IR and QUICKI were also correlated with HEGC, albeit with a lesser accuracy, suggesting that these indices provide reasonable alternatives and had good correlations with HOMA-AD in CHD patients.
The results of this study confirmed the previous reports that impairments in glucose metabolism are common in CHD patients as reflected by 83% of patients having a GDR that showed either glucose intolerance or overt insulin resistance (1,2). The etiology of this abnormality is complex and multifactorial. In our study, we showed significant associations between chronic inflammation, visceral fat, adipokines, and the presence of IR using the gold standard HEGC. It is important to highlight that in our study patients, there was no association observed between lipid profile and insulin resistance, most likely because CHD patients in general have low LDL and low triglycerides levels. This observation may also explain the poor performance of McAuley's index in the CHD population.
Chronic inflammation is highly prevalent in patients with kidney disease. Pro-inflammatory cytokines, especially IL-1, IL-6, and TNF-α, play critical roles in the development of insulin resistance. TNF-α impairs insulin signaling through the phosphorylation of insulin receptor substrate-1, which can then function as an inhibitor of the tyrosine kinase activity of the insulin receptor. Administration of an IL-1 receptor antagonist in humans with type 2 diabetes mellitus improves glycemic control (21). In our study, we observed that circulating levels of IL-6 and high sensitivity C-reactive protein were independent predictors of insulin resistance, rendering inflammatory pathways as a novel target for intervention. The etiology of chronic inflammation in CHD patients is also very complex. Multiple studies have shown that one of its main determinants is truncal fat mass, a significant correlate of IR in our study cohort. It is possible that the cytokine release from this body compartment might be partially mediating the severity of insulin resistance in CHD patients.
In ESRD, obesity has been found to be paradoxically associated with better survival. However, it is likely the type and location of the specific fat may be more important. Truncal fat tissue is also the major secretory reservoir for adipokines, namely adiponectin, leptin, and resistin (22). In this study we showed that truncal fat mass percentage measured by DEXA as a surrogate marker of visceral fat was associated with insulin resistance by clamp studies. Altered secretory patterns of adipose tissue may play a crucial role in the metabolic derangements observed in advanced kidney disease and have been hypothesized to play a role in insulin resistance (23,24).
Adiponectin has insulin-sensitizing effects that are mediated through the activation of AMP-activated protein kinase, which increases fatty acid oxidation and activates the peroxisome proliferator-activated receptor α (23). Administration of adiponectin in rodents increases insulin-induced tyrosine phosphorylation of the insulin receptor in skeletal muscle, resulting in improved glucose tolerance in animals and in adiponectin-deficient mice with insulin resistance. In ESRD, the role of adiponectin in predicting poor outcomes has been conflicting and controversial (25,26), and it has been postulated that higher levels of the total or particular isoforms are a reflection of either a counterbalance response to ongoing injury or the presence of comorbidities. The role of leptin in insulin resistance is less clear because it can have both insulin-sensitizing and insulin-resistance properties (27). However, leptin potentiates secretion of TNFα and IL-6 (28), increases generation and accumulation of reactive oxygen species (29), and stimulates the synthesis of TGF-β (30). Therefore, the ability of leptin to promote cytokine signaling and growth factors may contribute to endothelial dysfunction, atherosclerosis, and insulin resistance in hyperleptinemic states such as in ESRD.
In advanced chronic kidney disease, both leptin and adiponectin are elevated because of decreased renal clearance; however, the extent of leptin accumulation is higher than that of adiponectin, creating a high leptin:adiponectin ratio when compared with the general population (31). The median leptin to adiponectin ratio in our study was 1.36 (IQR 0.62, 4.67), which is significantly higher than reported levels of healthy individuals (mean 0.6) (32). The fact that adipokines are directly involved in the pathophysiologic mechanism of insulin resistance in advanced CKD may explain its superior performance as an index for IR in this population. Moreover, given that leptin and adiponectin have pro-atherogenic and anti-atherogenic effects, respectively, their ratio has been proposed as a novel and more sensitive atherogenic index as well (31,33–35). On the other hand, it is important to note that we used total adiponectin levels rather than isoforms, which may have a differential metabolic relevance. Furthermore, because study patients are more obese than usual, this may have driven the leptin-to-adiponectin ratio toward a greater correlation than other indexes less dependent on adipose-tissue mass.
There are several strengths of this study. Most importantly, we used the gold-standard method to assess insulin resistance. This method is highly precise and reproducible as shown by the excellent intraclass correlation and provided us the opportunity to perform a thorough analysis of related metabolic pathways. We were able to test the performance of novel markers compared with the established gold standard. Second, our study included patients across a wide spectrum of insulin resistance (i.e. non diabetic and patients with history of diabetes), enabling us to more fully characterize the performance of these surrogate indices.
Limitations of this study include the relatively small number of subjects studied relative to large epidemiologic studies. This is due to the complexity and laborious nature of the HEGC procedure, and our sample size is consistent with others who have used this approach (1,2). In addition, the study was performed in African-American subjects only, and the results cannot be generalized to other populations. Similarly, 58% of our patients had a body mass index of >30 compared with 35% in the general dialysis population in the United States, which again limits generalizability. Finally, the results are not compared with a control group without CKD so that the absolute magnitude of IR in CHD cannot be assessed.
In summary, we show that insulin resistance or glucose intolerance is common in CHD patients. The major determinants of insulin resistance in our study cohort were visceral fat mass, adipokines, and proinflammatory cytokines. Consistent with these findings, we showed that insulin resistance indices that incorporate adipokines are better correlates of glucose-disposal rate by HEGC. These novel markers may potentially offer better cardiovascular risk prediction and provide better assessment of the effect of interventions that aim to improve insulin resistance in CHD patients. Interventions that down-regulate the inflammatory response and/or improve adipokine profiles may have beneficial effects in the insulin resistance observed in advanced CKD.
Disclosures.
None.
Acknowledgments
This study was supported in part by grants the Clinical Translational Science Award 1UL-1RR024975 from the National Center for Research Resources, K24 DK 62849 from the National Institute of Diabetes and Digestive and Kidney Diseases and the Center for D-Receptor Activation Research. A. Hung is supported by Veterans Administration Career Development Award 2-031-09S. The sponsors had no influence on the design, execution, and analysis of the results of the study.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Barazzoni R, Zanetti M, Stulle M, Mucci MP, Pirulli A, Dore F, Panzetta G, Vasile A, Biolo G, Guarnieri G: Higher total ghrelin levels are associated with higher insulin-mediated glucose disposal in non-diabetic maintenance hemodialysis patients. Clin Nutr 27: 142–149, 2008 [DOI] [PubMed] [Google Scholar]
- 2. DeFronzo RA: Pathogenesis of glucose intolerance in uremia. Metabolism 27: 1866–1880, 1978 [DOI] [PubMed] [Google Scholar]
- 3. Kobayashi S, Maesato K, Moriya H, Ohtake T, Ikeda T: Insulin resistance in patients with chronic kidney disease. Am J Kidney Dis 45: 275–280, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Nerpin E, Riserus U, Ingelsson E, Sundstrom J, Jobs M, Larsson A, Basu S, Arnlov J: Insulin sensitivity measured with euglycemic clamp is independently associated with glomerular filtration rate in a community-based cohort. Diabetes Care 31: 1550–1555, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shinohara K, Shoji T, Emoto M, Tahara H, Koyama H, Ishimura E, Miki T, Tabata T, Nishizawa Y: Insulin resistance as an independent predictor of cardiovascular mortality in patients with end-stage renal disease. J Am Soc Nephrol 13: 1894–1900, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Shoji T, Emoto M, Nishizawa Y: HOMA index to assess insulin resistance in renal failure patients. Nephron 89: 348–349, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Takenaka T, Kanno Y, Ohno Y, Suzuki H: Key role of insulin resistance in vascular injury among hemodialysis patients. Metabolism 56: 153–159, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Becker B, Kronenberg F, Kielstein JT, Haller H, Morath C, Ritz E, Fliser D: Renal insulin resistance syndrome, adiponectin and cardiovascular events in patients with kidney disease: The mild and moderate kidney disease study. J Am Soc Nephrol 16: 1091–1098, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Rao M, Li L, Tighiouart H, Jaber BL, Pereira BJ, Balakrishnan VS: Plasma adiponectin levels and clinical outcomes among haemodialysis patients. Nephrol Dial Transplant 23: 2619–2628, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zoccali C, Mallamaci F, Tripepi G, Benedetto FA, Cutrupi S, Parlongo S, Malatino LS, Bonanno G, Seminara G, Rapisarda F, Fatuzzo P, Buemi M, Nicocia G, Tanaka S, Ouchi N, Kihara S, Funahashi T, Matsuzawa Y: Adiponectin, metabolic risk factors, and cardiovascular events among patients with end-stage renal disease. J Am Soc Nephrol 13: 134–141, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985 [DOI] [PubMed] [Google Scholar]
- 12. Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ: Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 85: 2402–2410, 2000 [DOI] [PubMed] [Google Scholar]
- 13. McAuley KA, Williams SM, Mann JI, Walker RJ, Lewis-Barned NJ, Temple LA, Duncan AW: Diagnosing insulin resistance in the general population. Diabetes Care 24: 460–464, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Sarafidis PA, Lasaridis AN, Nilsson PM, Pikilidou MI, Stafilas PC, Kanaki A, Kazakos K, Yovos J, Bakris GL: Validity and reproducibility of HOMA-IR, 1/HOMA-IR, QUICKI and McAuley's indices in patients with hypertension and type II diabetes. J Hum Hypertens 21: 709–716, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Gniuli D, Castagneto-Gissey G, Iaconelli A, Leccesi L, Mingrone G: Fat mass largely contributes to insulin mediate glucose uptake in morbidly obese subjects. Int J Obes 2010, e-pub: 25 May 2010 [DOI] [PubMed] [Google Scholar]
- 16. Ascaso JF, Pardo S, Real JT, Lorente RI, Priego A, Carmena R: Diagnosing insulin resistance by simple quantitative methods in subjects with normal glucose metabolism. Diabetes Care 26: 3320–3325, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Axelsson J, Rashid Qureshi A, Suliman ME, Honda H, Pecoits-Filho R, Heimburger O, Lindholm B, Cederholm T, Stenvinkel P: Truncal fat mass as a contributor to inflammation in end-stage renal disease. Am J Clin Nutr 80: 1222–1229, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Bross R, Chandramohan G, Kovesdy CP, Oreopoulos A, Noori N, Golden S, Benner D, Kopple JD, Kalantar-Zadeh K: Comparing body composition assessment tests in long-term hemodialysis patients. Am J Kidney Dis 55: 885–896, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Donadio C, Halim AB, Caprio F, Grassi G, Khedr B, Mazzantini M: Single- and multi-frequency bioelectrical impedance analyses to analyse body composition in maintenance haemodialysis patients: Comparison with dual-energy x-ray absorptiometry. Physiol Meas 29: S517–S524, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Kamimura MA, Avesani CM, Cendoroglo M, Canziani ME, Draibe SA, Cuppari L: Comparison of skinfold thicknesses and bioelectrical impedance analysis with dual-energy X-ray absorptiometry for the assessment of body fat in patients on long-term haemodialysis therapy. Nephrol Dial Transplant 18: 101–105, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY: Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med 356: 1517–1526, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Scherer PE: Adipose tissue: From lipid storage compartment to endocrine organ. Diabetes 55: 1537–1545, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, Yamaguchi M, Namiki S, Nakayama R, Tabata M, Ogata H, Kubota N, Takamoto I, Hayashi YK, Yamauchi N, Waki H, Fukayama M, Nishino I, Tokuyama K, Ueki K, Oike Y, Ishii S, Hirose K, Shimizu T, Touhara K, Kadowaki T: Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature 464: 1313–1319, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Koh KK, Park SM, Quon MJ: Leptin and cardiovascular disease: Response to therapeutic interventions. Circulation 117: 3238–3249, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Drechsler C, Krane V, Winkler K, Dekker FW, Wanner C: Changes in adiponectin and the risk of sudden death, stroke, myocardial infarction, and mortality in hemodialysis patients. Kidney Int 76: 567–575, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Menon V, Li L, Wang X, Greene T, Balakrishnan V, Madero M, Pereira AA, Beck GJ, Kusek JW, Collins AJ, Levey AS, Sarnak MJ: Adiponectin and mortality in patients with chronic kidney disease. J Am Soc Nephrol 17: 2599–2606, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Cohen B, Novick D, Rubinstein M: Modulation of insulin activities by leptin. Science 274: 1185–1188, 1996 [DOI] [PubMed] [Google Scholar]
- 28. Loffreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ, Klein AS, Bulkley GB, Bao C, Noble PW, Lane MD, Diehl AM: Leptin regulates proinflammatory immune responses. FASEB J 12: 57–65, 1998 [PubMed] [Google Scholar]
- 29. Yamagishi SI, Edelstein D, Du XL, Kaneda Y, Guzman M, Brownlee M: Leptin induces mitochondrial superoxide production and monocyte chemoattractant protein-1 expression in aortic endothelial cells by increasing fatty acid oxidation via protein kinase A. J Biol Chem 276: 25096–25100, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Wolf G, Hamann A, Han DC, Helmchen U, Thaiss F, Ziyadeh FN, Stahl RA: Leptin stimulates proliferation and TGF-beta expression in renal glomerular endothelial cells: Potential role in glomerulosclerosis [seecomments]. Kidney Int 56: 860–872, 1999 [DOI] [PubMed] [Google Scholar]
- 31. Teta D, Maillard M, Halabi G, Burnier M: The leptin/adiponectin ratio: Potential implications for peritoneal dialysis. Kidney Int 73[Suppl 108]: S112–S118, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Norata GD, Raselli S, Grigore L, Garlaschelli K, Dozio E, Magni P, Catapano AL: Leptin:adiponectin ratio is an independent predictor of intima media thickness of the common carotid artery. Stroke 38: 2844–2846, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Kotani K, Shimohiro H, Sakane N: The relationship between leptin:adiponectin ratio and carotid intima-media thickness in asymptomatic women. Stroke 39: e32–e34, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Satoh N, Naruse M, Usui T, Tagami T, Suganami T, Yamada K, Kuzuya H, Shimatsu A, Ogawa Y: Leptin-to-adiponectin ratio as a potential atherogenic index in obese type 2 diabetic patients. Diabetes Care 27: 2488–2490, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Takamura N, Hayashida N, Hagane K, Kadota K, Yamasaki H, Abiru N, Ozono Y, Kamihira S, Aoyagi K, Ishibashi K, Nakazato M, Maeda T: Leptin to high-molecular-weight adiponectin ratio is independently correlated with carotid intima-media thickness in men, but not in women. Biomarkers 15:340–344, 2010 [DOI] [PubMed] [Google Scholar]