Summary
Background and objectives
Heart failure occurs frequently in end-stage renal disease patients. However, there are no prospective, longitudinal follow-up data on its prevalence, severity, and risk factors in long-term peritoneal dialysis (PD) patients.
Design, setting, participants, & measurements
A prospective observational study was conducted in 220 long-term PD patients followed up for 4 years or until death. Echocardiography was obtained at baseline. Primary study end points were heart failure and mortality.
Results
Eighty-six patients had a previous history of heart failure at study entry. The cumulative 4-year survival probability was 37.4% and 64.7% for patients with and without previous heart failure, respectively (P < 0.0001). During follow-up, 87 patients (40.9%) developed heart failure, of which 53 were recurrence and 34 were new-onset heart failure. Diabetes, background atherosclerotic vascular disease, systolic hypertension, left ventricular (LV) mass index, systolic dysfunction, and hypoalbuminemia were significant risk factors predicting heart failure in the entire cohort. Diabetes and LV mass and volume index were significant predictors of new-onset heart failure. Systolic hypertension, LV volume index, and hypoalbuminemia were significant predictors of recurrent heart failure.
Conclusions
Heart failure is a highly prevalent complication in long-term PD patients and predicts adverse clinical outcomes. More attention should be focused on improving BP and volume control and identifying treatment strategies that effectively lower atherosclerotic burden and reverse LV hypertrophy, remodeling, and systolic dysfunction in PD patients.
Introduction
Cardiovascular disease is the leading cause of mortality in end-stage renal disease (ESRD) and manifests variously as acute myocardial infarction, cerebrovascular events, heart failure, sustained arrhythmia, or sudden cardiac death, among which heart failure is one of the most frequent. According to a previous study by Harnett and co-workers performed in predominantly hemodialysis patients, close to one third of the patients had heart failure at initiation of dialysis and more than half had recurrence during dialysis. Even among patients with no previous heart failure, 25% of them developed subsequent heart failure during their course on dialysis (1). Furthermore, heart failure present on initiation of dialysis is a strong and independent predictor of short-term (90 days) (2) and long-term mortality (1). The presence of previous heart failure also increased the risk of cardiovascular death in prevalent peritoneal dialysis (PD) patients by more than threefold (3).
Old age, pre-existing heart disease (systolic dysfunction and ischemic heart disease), and chronic uremia have been suggested to be important risk factors for heart failure in hemodialysis patients (1). However, there are so far no prospective, longitudinal, follow-up data on heart failure in PD patients. Furthermore, there is no study that examined the importance of diastolic dysfunction in relation to heart failure in PD patients.
Given this background, the study presented here aimed first to determine the prevalence, severity, risk factors, and outcomes of heart failure in long-term PD patients over 4 years of prospective follow-up. Second, we evaluated factors predicting new-onset and recurrent heart failure in these patients.
Materials and Methods
Study Design and Study Subjects
We performed a prospective, longitudinal, 4-year follow-up study in a cohort of 220 prevalent PD patients recruited from a single regional dialysis center in Hong Kong between 1999 and 2004. The study protocol complies with the Declaration of Helsinki and has obtained full approval from the local clinical research ethics committee. All patients provided informed consent before study entry.
Patients were considered eligible for study inclusion if they have been on continuous PD treatment for ≥3 months. Patients with chronic rheumatic heart disease, congenital heart disease, underlying active malignancy, or active systemic lupus erythematosus requiring steroid or other immunosuppressive treatment; patients who refused to give consent; and patients with incomplete data (total n = 50) were excluded from the study. Those excluded represented 18.5% of the total PD population in the center.
Echocardiography and Tissue Doppler Imaging
Echocardiography was performed at study entry using a GE-VingMed System 5 echocardiographic machine (GE-VingMed Sound AB, Horten, Norway) with a 3.3-mHz multiphase array probe in subjects lying in the left decubitus position by a single experienced cardiologist blinded to all clinical details of patients as described previously (4). Myocardial velocities were recorded using tissue Doppler imaging (TDI) as described previously (5,6).
Clinical Variables
Patients' demographics and clinical data including background kidney disease, diabetes, atherosclerotic vascular disease (AVD), and previous history of heart failure were obtained at study entry by direct patient enquiry and further confirmation by review of hospitalization records from the computerized medical record system of the Hong Kong Hospital Authority. Background AVD was defined as the presence of coronary artery disease (indicated by history of angina, previous myocardial infarction with or without history of coronary artery bypass surgery, or percutaneous coronary intervention), cerebrovascular disease (indicated by history of cerebrovascular event), or peripheral vascular disease (indicated by the presence of intermittent claudication or resting leg pain together with clinical signs of peripheral vascular disease with or without history of amputation or revascularization). Previous history of heart failure included only episodes that were clearly documented to require hospitalization and was defined as the presence of symptoms and signs of heart failure including dyspnoea, raised jugular venous pressure, and basal crepitations together with radiographic evidence of pulmonary venous congestion or interstitial edema and resolution of symptoms, signs, and radiographic changes with hypertonic PD exchanges. With a mercury sphygmomanometer, systolic and diastolic BP were measured once on every follow-up visit after patient rested for 15 minutes on either arm at 8-week intervals for 12 months preceding study entry and were then averaged to give the final systolic and diastolic BP.
Biochemical Measurements
Fasting EDTA, heparinized, and clotted blood samples were collected at study entry for measurement of plasma lipid profile, albumin, urea, creatinine, calcium, phosphorus, serum intact parathyroid hormone, high-sensitivity C-reactive protein, and blood hemoglobin using methods as described previously (4). Serum N-terminal pro-brain natriuretic peptide was quantified by electrochemiluminescence immunoassay on an Elecsys 2010 analyzer (Roche Diagnostics Corporation, Indianapolis, IN) with a measuring range from 5 to 35,000 pg/ml. For samples with N-terminal pro-brain natriuretic peptide concentrations above the measuring range, the final concentrations were taken as 35,000 pg/ml.
Assessment of Residual Renal Function and Dialysis Indices
Twenty-four hour urine and dialysate was collected for measurement of residual GFR and total weekly urea and creatinine clearance using standard methods (7,8). The transport status of the peritoneal membrane was characterized by a standard peritoneal equilibration test using a 4-hour dwell of a 2.27% glucose-containing PD solution.
Outcome Measures
All patients were followed prospectively for 4 years from the day of baseline assessments or until patients underwent kidney transplant, transferred to hemodialysis, or death. If a patient died within 3 months of transfer to hemodialysis, then he or she was not censored because the early mortality was considered to reflect health status during the period of failing PD treatment. The outcome measures evaluated were death, heart failure, composite end point of death and heart failure, and new-onset and recurrent heart failure. Heart failure was defined as such to include only episodes that were clearly documented to require hospitalization. Essentially, heart failure was diagnosed by the attending physician on the basis of the presence of all three clinical criteria: (1) symptoms and signs of heart failure including dyspnoea, raised jugular venous pressure, and basal crepitations; (2) radiographic evidence of pulmonary venous congestion or interstitial edema (1); and (3) resolution of symptoms, signs, and radiographic changes with hypertonic PD exchanges. In patients who presented to the outpatient clinic with milder symptoms including ankle edema or facial puffiness and not requiring hospitalization, the episode would not be counted as heart failure. This information was captured prospectively from the computerized medical record system of the Hong Kong Hospital Authority and the Renal Registry Database, which keep detailed records of all hospitalization episodes.
Statistical Analyses
Continuous data were expressed as mean ± SD or median (interquartile range) and categorical data were expressed as percentages. Comparisons between groups were done by the t test or Mann–Whitney U test, depending on data distribution. Stepwise logistic regression analysis was performed to evaluate the associations of different factors with presence of background heart failure at study entry. Factors predicting first episode of heart failure, composite end point of heart failure and mortality, and new-onset and recurrent heart failure were evaluated using univariate and multivariable stepwise Cox regression analysis. Essentially, all survival analysis was based on the first heart failure episode observed in each patient during the 4 years of follow-up. Factors with P < 0.1 on univariate analysis were considered in the multivariate analysis. Age, gender, and duration of dialysis were considered as important confounding covariates and were included in all multivariate analysis irrespective of their P values on univariate analysis. Cumulative overall survival probability in relation to the presence or absence of background history of heart failure was generated by the Kaplan–Meier method, and between-group survival was compared by the log-rank test. P < 0.05 was considered to be statistically significant. Statistical analyses were performed using SPSS version 16.0 (SPSS, Inc., Chicago, IL).
Results
Characteristics of Study Population and Factors Associated with Background History of Heart Failure
Of the 220 PD patients, 86 patients (39.1%) had a previous history of heart failure. The underlying causes of kidney disease were chronic glomerulonephritis (n = 70, 31.8%), diabetic nephropathy (n = 55, 25%), hypertensive nephrosclerosis (n = 27, 12.3%), polycystic kidney disease (n = 12, 5.5%), obstructive uropathy (n = 12, 5.5%), tubulointerstitial disease (n = 6, 2.7%), and not known in 38 patients (17.3%). The daily PD regimen was 6-L exchanges in 158 patients (71.8%), 8 L in 51 patients (23.8%), 10 L in 6 patients (2.7%), 4.5 L in 4 patients (1.8%), and 5 L in 1 patient (0.5%). Left ventricular (LV) hypertrophy was present in 208 patients (94.5%). One hundred and thirty-seven patients (62.3%) had an elevated early transmitral flow velocity (E) to early diastolic mitral annular velocity (Em) ratio ≥15 and 37 patients (16.8%) had mitral annular calcification. The other baseline characteristics are shown in Table 1 with comparisons between patients with and without background history of heart failure. Of the 86 patients with a background history of heart failure, 56 patients were on daily 6-L exchanges, 25 were on 8-L exchanges, 3 were on 10-L exchanges, and 1 patient each was on daily 4.5- and 5-L exchanges. Of the 134 patients with no previous heart failure, 102 patients were on daily 6-L exchanges, 26 were on 8-L exchanges, 3 were on 10-L exchanges, and 3 were on 4.5-L exchanges. All patients were dialyzed using conventional lactate-buffered glucose-based PD solutions. Use of erythropoietin (P = 0.09), vitamin D analogs (P = 0.5), β-blockers (P = 0.9), calcium-channel blockers (P = 0.2), and statins (P = 0.2) did not differ between patients with and without background history of heart failure. Angiotensin converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) were used more often in patients with background heart failure (36%) than those with no heart failure (17.9%, P = 0.002). Table 2 details variables that are independently related to a background diagnosis of heart failure in the multiple logistic regression analysis.
Table 1.
Baseline characteristics
| Characteristic | With Background History of Heart Failure at Study Entry (n = 86) | No Background History of Heart Failure at Study Entry (n = 134) | P |
|---|---|---|---|
| Male gender, n (%) | 47 (54.7) | 62 (46.3) | 0.2 |
| Age, years | 56 ± 12 | 55 ± 12 | 0.5 |
| Positive smoking history, n (%) | 34 (39.5) | 47 (35.1) | 0.5 |
| Diabetes, n (%) | 40 (46.5) | 26 (19.4) | <0.001 |
| Background AVD, n (%) | 37 (43) | 15 (11.2) | <0.001 |
| Body mass index, kg/m2 | 23.6 ± 3.7 | 22.7 ± 3.1 | 0.06 |
| Systolic BP, mmHg | 149 ± 19 | 145 ± 16 | 0.1 |
| Diastolic BP, mmHg | 81 ± 11 | 83 ± 9 | 0.1 |
| Duration of dialysis, monthsa | 26 (13, 49.3) | 28.5 (17, 54.5) | 0.2 |
| Daily PD exchanges, L | 6.7 ± 1.1 | 6.4 ± 1.0 | 0.09 |
| Total weekly Kt/V | 1.76 ± 0.47 | 1.85 ± 0.41 | 0.1 |
| Total weekly creatinine clearance, L/wk per 1.73 m2 | 52.9 ± 1.9 | 58.5 ± 2.3 | 0.06 |
| Residual GFR, ml/min per 1.73 m2a | 0.22 (0, 1.34) | 0.75 (0, 2.02) | 0.06 |
| Daily urine volume, La | 0.1 (0, 0.5) | 0.25 (0, 0.6) | 0.04 |
| High peritoneal transport, n (%) | 12 (14.0) | 22 (16.7) | 0.6 |
| Total daily net ultrafiltration, L | 1.29 ± 1.18 | 1.33 ± 0.82 | 0.8 |
| Hemoglobin, g/dl | 8.67 ± 1.63 | 9.49 ± 1.70 | <0.001 |
| Albumin, g/L | 27 ± 5 | 30 ± 5 | <0.001 |
| Calcium × phosphorus, mmol2/L2 | 4.34 ± 1.30 | 4.24 ± 1.23 | 0.5 |
| LDL-cholesterol, mmol/L | 3.25 ± 1.02 | 3.32 ± 0.97 | 0.6 |
| Triglyceride, mmol/L | 1.97 ± 1.31 | 2.17 ± 1.58 | 0.4 |
| Intact parathyroid hormone, pmol/La | 39.1 (18.9, 66.3) | 42.7 (17.0, 79.4) | 0.8 |
| NT-pro-BNP, pg/ml | 13,231 (5120, 350,00) | 3206 (1408, 9433) | <0.001 |
| C-reactive protein, mg/La | 3.18 (1.05, 10.43) | 2.48 (0.80, 8.20) | 0.4 |
| LV mass index, g/m2 | 262 ± 88 | 199 ± 73 | <0.001 |
| LV volume index, ml/m2 | 75.1 ± 22.3 | 59.6 ± 16.5 | <0.001 |
| LV EF, % | 49.7 ± 9.2 | 54.5 ± 7.5 | <0.001 |
| n (%) with systolic dysfunction (defined as EF ≤ 50%) | 39 (45.3) | 32 (24.2) | 0.001 |
| E, cm/s | 88 ± 32 | 73 ± 21 | <0.001 |
| A, cm/s | 91 ± 28 | 92 ± 23 | 0.8 |
| E/A ratio | 1.08 ± 0.63 | 0.83 ± 0.37 | <0.001 |
| DT, ms | 230 ± 130 | 250 ± 80 | 0.2 |
| IVRT, ms | 112 ± 86 | 107 ± 27 | 0.6 |
| E/Em ratio | 24.1 ± 11.3 | 17.5 ± 7.5 | <0.001 |
Continuous data expressed as mean ± SD unless specified otherwise. NT-pro-BNP, N-terminal pro-brain natriuretic peptide; A, late diastolic transmitral flow velocity; DT, deceleration time; IVRT, isovolumetric relaxation time.
Median (interquartile range).
Table 2.
Stepwise multiple logistic regression analysis of factors independently associated with a background history of heart failure
| Factors | Odds Ratio (95% CI) | P |
|---|---|---|
| Diabetes | 3.14 (1.47 to 6.69) | 0.003 |
| Background AVD | 3.88 (1.73 to 8.71) | 0.001 |
| LV mass index (g/m2) | 1.006 (1.001 to 1.010) | 0.01 |
| Age (years) | 0.96 (0.93 to 0.99) | 0.02 |
| Serum albumin (g/L) | 0.92 (0.86 to 0.99) | 0.02 |
| E/Em ratio | 1.05 (1.01 to 1.09) | 0.02 |
| Male gender | 1.96 (0.96 to 4.01) | 0.07 |
| Hemoglobin (g/dl) | 0.83 (0.67 to 1.03) | 0.09 |
Out of the model with dialysis vintage, daily urine volume, body mass index, residual GFR, and LV EF. Age, gender, and dialysis vintage were regarded as important confounding covariates and were considered in the multiple regression analysis irrespective of their P values in univariate analysis.
Clinical Outcomes in Relation to Background History of Heart Failure
During follow-up, 84 patients (38.2%) died, of which 48 died from cardiovascular causes and 36 deaths were noncardiovascular. The detailed causes of cardiovascular death were 14 cerebrovascular events, 7 with acute myocardial infarction, 22 with sudden cardiac death, 4 with heart failure, and 1 with sustained arrhythmia. Five patients died from foot infection secondary to severe peripheral vascular disease. The average mortality rate and heart failure event rate of this cohort were 95 per 1000 patients per year and 102 per 1000 patients per year, respectively. Kaplan–Meier survival analysis shows that patients with a background history of heart failure had significantly lower cumulative overall survival probability (P < 0.0001) than those with no previous heart failure (Figure 1). The median (interquartile range) survival was 25.2 (13.0, 48.7) and 44.9 (22.8, 48.7) months, respectively, for patients with and without background history of heart failure.
Figure 1.
Kaplan–Meier estimates of cumulative overall survival probability in relation to the presence or absence of a background history of heart failure in PD patients.
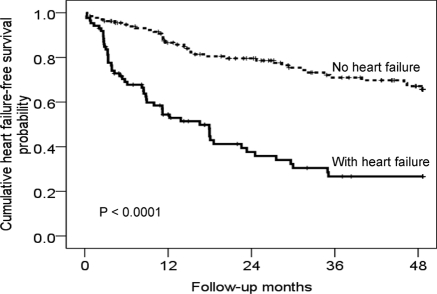
Prevalence, Outcomes, and Risk Factors Predicting Heart Failure Over 4-yr of Prospective Follow-Up
During follow-up, 90 PD patients (40%) were complicated with one or more episodes of heart failure. Figure 2 shows the Kaplan–Meier estimates of heart failure event-free survival probability for patients with and without background history of heart failure. Table 3 details the associations of different risk factors in predicting heart failure in the entire cohort in univariate Cox regression analysis. In the multivariable Cox regression analysis, systolic BP, LV mass index, low serum albumin, diabetes, a low LV ejection fraction (EF), and background AVD were independently predictive of heart failure (Table 4). Adding the covariate “previous history of heart failure” displaced LV EF from the multivariable Cox regression model and reduced the significance of LV mass index, serum albumin, and background AVD in predicting future risk of heart failure (Table 4). One hundred and thirty-three patients reached the composite end point of mortality and heart failure at 4 years. Serum albumin (hazard ratio [HR] 0.92, 95% confidence interval [CI] 0.89 to 0.96, P < 0.001), diabetes (HR 1.59, 95% CI 1.09 to 2.33, P = 0.016), systolic BP (HR 1.02, 95% CI 1.01 to 1.03, P < 0.001), LV mass index (HR 1.005, 95% CI 1.003 to 1.007, P < 0.001), background AVD (HR 1.77, 95% CI 1.18 to 2.68, P = 0.006), residual GFR (HR 0.88, 95% CI 0.78 to 0.99, P = 0.029), duration of dialysis (HR 0.99, 95% CI 0.99 to 1.00, P = 0.046), and LV EF (HR 0.98, 95% CI 0.95 to 1.00, P = 0.028) were independent predictors for the composite end point of mortality and heart failure. Adding LV volume index (HR 1.018, 95% CI 1.008 to 1.027, P < 0.001) displaced LV EF from the multivariable Cox regression model for the composite end point of mortality and heart failure whereas LV mass index retained independent significance (HR 1.004, 95% CI 1.001 to 1.006, P = 0.002).
Figure 2.
Kaplan–Meier estimates of cumulative heart failure event-free survival probability in relation to the presence or absence of a background history of heart failure in PD patients.
Table 3.
Univariate Cox regression analysis of risk factors predicting heart failure during 4 years of follow-up in the entire cohort of PD patients
| Factors | HR (95% CI) | P |
|---|---|---|
| Age (years) | 1.01 (0.99 to 1.03) | 0.4 |
| Male gender | 1.19 (0.79 to 1.80) | 0.4 |
| Vintage of dialysis (months) | 1.00 (0.99 to 1.00) | 0.3 |
| Diabetes mellitus | 2.46 (1.61 to 3.74) | <0.001 |
| Background AVD | 2.42 (1.56 to 3.77) | <0.001 |
| Previous history of heart failure | 3.68 (2.41 to 5.63) | <0.001 |
| Body mass index (kg/m2) | 1.05 (0.99 to 1.11) | 0.1 |
| Hemoglobin (g/dl) | 0.72 (0.65 to 0.85) | <0.001 |
| Serum albumin (g/L) | 0.94 (0.90 to 0.98) | 0.002 |
| C-reactive protein (mg/L) | 1.00 (0.98 to 1.02) | 1.0 |
| LDL-cholesterol (mmol/L) | 0.87 (0.69 to 1.09) | 0.2 |
| Triglyceride (mmol/L) | 0.96 (0.82 to 1.12) | 0.6 |
| Calcium × phosphorus (mmol2/L2) | 1.11 (0.94 to 1.31) | 0.2 |
| Intact parathyroid hormone (pmol/L) | 1.00 (1.00 to 1.01) | 1.0 |
| PD Kt/V | 0.75 (0.43 to 1.33) | 0.3 |
| Residual GFR (ml/min per 1.73 m2) | 0.89 (0.78 to 1.02) | 0.09 |
| Daily urine volume (L) | 0.82 (0.53 to 1.27) | 0.4 |
| Daily total net ultrafiltration (L) | 0.83 (0.68 to 1.02) | 0.07 |
| High peritoneal transport | 1.39 (0.82 to 2.35) | 0.2 |
| Systolic BP (mmHg) | 1.02 (1.01 to 1.04) | <0.001 |
| Diastolic BP (mmHg) | 1.00 (0.98 to 1.02) | 1.0 |
| LV mass index (g/m2) | 1.007 (1.004 to 1.009) | <0.001 |
| LV EF (%) | 0.95 (0.93 to 0.97) | <0.001 |
| E/Em ratio | 1.04 (1.02 to 1.05) | <0.001 |
Table 4.
Multivariable Cox regression analysis of factors predicting heart failure during 4 years of follow-up in the entire cohort of PD patients considering and not considering “previous history of heart failure” as a covariate
| Factors | Unit Increase | Model Not Including Previous History of Heart Failure |
Model Including Previous History of Heart Failure |
||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Systolic BP | 1 mmHg | 1.03 | 1.01 to 1.04 | <0.001 | 1.03 | 1.01 to 1.04 | <0.001 |
| LV mass index | 1 g/m2 | 1.005 | 1.002 to 1.008 | <0.001 | 1.004 | 1.001 to 1.007 | 0.003 |
| Serum albumin | 1 g/L | 0.94 | 0.90 to 0.99 | 0.01 | 0.96 | 0.91 to 1.003 | 0.068 |
| Diabetes mellitus | – | 1.71 | 1.09 to 2.68 | 0.02 | 1.75 | 1.13 to 2.72 | 0.013 |
| LV EF | 1% | 0.97 | 0.95 to 1.00 | 0.02 | – | – | |
| Background AVD | – | 1.70 | 1.06 to 2.74 | 0.03 | 1.51 | 0.94 to 2.42 | 0.092 |
| Previous history of heart failure | – | – | – | – | 2.41 | 1.48 to 3.91 | <0.001 |
Out of the model with age, male gender, dialysis vintage, hemoglobin, residual GFR, total net daily ultrafiltration, and E/Em ratio. Age, gender, and dialysis vintage were regarded as important confounding covariates and were considered in the multivariate analysis irrespective of their P values in univariate analysis.
New-Onset Heart Failure and Associated Risk Factors
Thirty-seven (27.6%) of 134 patients with no previous heart failure developed new-onset heart failure during follow-up. Compared with patients with no new-onset heart failure, those with new-onset heart failure (n = 37) showed higher prevalence of diabetes (35.1% versus 13.4%; P < 0.005), a trend toward more background AVD (18.9% versus 8.2%; P < 0.1), higher LV mass index (221 ± 88 versus 190 ± 66 g/m2; P < 0.05), higher LV end-diastolic volume index (65.3 ± 18.4 versus 57.3 ± 15.1 ml/m2; P < 0.05), and lower LV EF (52.3 ± 9.6 versus 55.0 ± 5.8%; P < 0.05). In the multivariable Cox regression analysis, diabetes mellitus and LV mass and volume index were significant, independent predictors of new-onset heart failure (Table 5).
Table 5.
Final multivariable Cox regression models of factors predicting new-onset heart failure and recurrent heart failure during 4 years of follow-up
| Factors | HR (95% CI) | P |
|---|---|---|
| New-onset heart failure | ||
| diabetes mellitus | 2.93 (1.46 to 5.87) | 0.002 |
| LV mass index (g/m2) | 1.005 (1.000 to 1.010) | 0.070 |
| LV volume index (ml/m2) | 1.027 (1.004 to 1.050) | 0.019 |
| Recurrent heart failure | ||
| serum albumin (g/L) | 0.91 (0.86 to 0.97) | 0.002 |
| systolic BP (mmHg) | 1.04 (1.02 to 1.06) | <0.001 |
| LV volume index (ml/m2) | 1.012 (1.001 to 1.024) | 0.03 |
Recurrent Heart Failure and Associated Risk Factors
Of the 86 patients with previous heart failure, 53 patients (61.6%) developed recurrent heart failure during follow-up. Compared with patients with no recurrent heart failure (n = 33), those with recurrent heart failure (n = 53) showed higher systolic BP (154 ± 17 versus 140 ± 17 mmHg; P < 0.001), a trend toward higher LV end-diastolic volume index (78.8 ± 22.9 versus 69.3 ± 20.4 ml/m2; P < 0.1); lower hemoglobin (8.22 ± 1.46 versus 9.38 ± 1.63 g/dl; P < 0.001), and lower serum albumin (26 ± 4 versus 29 ± 5 g/L; P < 0.01). In the multivariable Cox regression analysis, hypoalbuminemia, high systolic BP, and LV volume index were significant, independent predictors of recurrent heart failure (Table 5).
Discussion
This study is so far the largest and longest prospective follow-up study that examines the clinical problem of heart failure in long-term PD patients. The results form an important basis for planning further intervention studies in PD patients. Our study shows that heart failure is an equally highly prevalent complication (39.1%) in long-term PD patients. Twenty-eight percent of patients developed new-onset heart failure, whereas 62% of those with previous heart failure had an episode of worsening heart failure requiring hospitalization during the 4 years of follow-up. These prevalence data are comparable to those reported in incident hemodialysis patients (1). Furthermore, PD patients with a previous history of heart failure have a very poor prognosis.
Diabetes, background AVD, LV hypertrophy, an elevated E/Em ratio estimated by TDI, and hypoalbuminemia were all significant independent factors related to a background history of heart failure. Patients with a background history of heart failure also had a higher E/A ratio. Although no direct measure of volume status was performed in this study, E/Em ratio measured using TDI has been suggested to provide better estimates of LV filling pressure than other methods (9) and predicts LV filling in different groups of patients (10–12). A reduced Em reflects poor annular recoil, which is due in part to reduced stored energy from the previous systole and is associated with reduced early diastolic suction and thus reduced early diastolic filling. A reduced Em (and therefore an elevated E/Em ratio) likely reflects true myocardial dysfunction and not volume overload alone. More recently, an E/Em ratio ≥15 was adopted by the European Society of Cardiology to define diastolic heart failure (13). However, there is so far no comparative study on the diagnostic value of the E/Em ratio in dialysis patients compared with heart failure patients without renal failure. In an ESRD population, an E/Em ratio ≥15 was associated with a sensitivity of 82% and a specificity of 88% in predicting LV end-diastolic pressure >15 mmHg (14). Our recent study shows that increasing age, systolic dysfunction, LV volume index, and loss of residual renal function are all significant, independent predictors of an E/Em ratio ≥15 in ESRD. An elevated E/Em ratio also predicts long-term mortality and cardiovascular death in PD patients (4). In this study, the mean E/Em ratio of our patients is particularly high, indicating the severity of cardiac involvement. Although E/Em loses significance to LV mass index and EF on multivariate analysis for heart failure, this does not refute the importance of an elevated LV filling pressure. Rather, these data suggest a close relation between an elevated LV filling pressure, cardiac hypertrophy, and systolic dysfunction in PD patients. On the other hand, hypoalbuminemia has been suggested to reflect fluid excess other than being a nutrition marker (15,16). Thus, an elevated E/Em ratio together with hypoalbuminemia may suggest elements of volume overload in these patients and explain their associations with heart failure. A previous study demonstrated more frequent LV hypertrophy in continuous ambulatory PD than hemodialysis patients and that LV hypertrophy is associated with volume overload, hypertension, and hypoalbuminemia (17).
In this study, risk factors that predict risk of developing heart failure in PD patients were background AVD, systolic dysfunction, diabetes, hypoalbuminemia, LV hypertrophy and systolic hypertension. The covariate “previous history of heart failure” displaced LV EF from the model and reduced the significance of LV mass index, serum albumin, and background AVD in predicting future risk of heart failure. These data clearly indicate close relations between LV hypertrophy, systolic dysfunction, background AVD, and hypoalbuminemia with a previous history of heart failure in PD patients. These observations are similar to findings in incident hemodialysis patients (1). It is likely that systolic hypertension and hypoalbuminemia (significant predictors of heart failure) are partly reflecting fluid overload. Notably, our subgroup analysis shows that irrespective of whether it is new-onset or recurrent heart failure, hypertension and chronic volume overload (which predispose to LV remodeling with LV dilation and hypertrophy) are key risk factors predicting heart failure in long-term PD patients. Lower sodium and fluid removal are associated with poorer PD patient survival and that hypertensive PD patients are more prone to hospitalization for cardiovascular events (18). Taken together, our data suggest that improving BP and volume control may serve as an important treatment strategy in lowering the incidence of heart failure in chronic PD patients and warrant further investigation. We observed a trend toward lower PD exchange volume, Kt/V, creatinine clearance, residual GFR, and urine volume for those with heart failure as compared with those without, suggesting that lower exchange volume and dialysis as well as lower fluid removal may be associated with heart failure in PD patients.
Our data also raised the importance of the need for regular echocardiography to identify high-risk PD patients for earlier, active intervention. In addition, the relationship between hypoalbuminemia and increased risk of recurrent heart failure is noteworthy. Malnutrition is an important predictor of mortality in ESRD patients, including PD patients (19,20). Studies in the general and dialysis population demonstrated important associations between malnutrition and heart failure (21–24). However, it remains currently unknown whether improving nutrition status may prevent heart failure.
In this cohort, ACEIs or ARBs were prescribed more often among PD patients with a background history of heart failure, which may be explained by their well established cardioprotective benefits in the general population (25,26). On the other hand, we observed no significant association between β-blocker use and a background history of heart failure. This suggests that β-blockers were underused in our PD patients despite clinical trials showing their cardioprotective effect in dialysis patients (27,28). More awareness should be made to increase β-blocker use in PD patients with heart failure. Despite relatively low hemoglobin in our PD population, anemia still shows a significant relation with heart failure. This is in keeping with previous studies suggesting that anemia is an independent predictor of heart failure and contributes to morbidity and mortality in heart failure (29,30). In Hong Kong, erythropoietin is largely a self-financed drug item; thus, the use of erythropoietin remains relatively low although hemoglobin is on the low side. The average Kt/V of our PD patients is over 1.7 and is in keeping with the recommended minimum target Kt/V for PD patients (31). In this study, 30% of patients had diabetes. This is similar to the prevalence of diabetes in dialysis patients in Hong Kong at the time of the study. Thus, we believe our nonselective study population is likely a representative cohort. However, given the relatively small number of diabetic patients, our results will require confirmation in larger studies.
Our study has several potential limitations. First, this study included prevalent but not incident PD patients that may introduce survival bias. Second, volume status was not directly assessed in these patients because we currently lack a gold standard measure of volume status in PD patients. Instead, we estimated the E/Em ratio by Doppler echocardiography, which has a good correlation with LV filling pressure (6), and we measured BP, which indirectly reflects volume status. Third, the diagnosis of heart failure not only included episodes due to ventricular dysfunction but also those due to extracellular volume overload because it is extremely difficult to separate the effects of volume overload secondary to renal failure from those of heart failure, especially in patients with a normal LV EF in practical terms. All parameters related to increased ventricular and atrial pressures and LV wall stress (including natriuretic peptides) will be raised in both conditions. In PD patients, the prevalence of LV hypertrophy is very high, and most of these patients have diastolic dysfunction and elevated LV filling pressure, thus resulting in impaired LV filling. This will give rise to increased pulmonary venous flow reversal as in volume overload. Thus, the pulmonary venous flow will not be able to differentiate between heart failure due to intrinsic myocardial dysfunction and that of volume overload in ESRD patients (13). Nevertheless, in the presence of LV hypertrophy, an enlarged left atrium, diastolic dysfunction, and impaired LV long axis function (reduced mitral annular motion), it is not unreasonable to suppose that some proportion of the problem is due to true heart failure rather than volume overload alone due to renal failure. In addition, long-standing volume overload may lead to diastolic dysfunction and heart failure. Fourth, only episodes that were severe enough to require hospitalization were included in this analysis. Subclinical fluid overload that did not develop into overt clinical heart failure was not included. This may have underestimated the true prevalence of heart failure in this population.
In conclusion, our study demonstrated a very high prevalence of heart failure and recurrence in long-term PD patients. In addition, heart failure was associated with a very poor prognosis. More attention should be focused on reducing the atherosclerotic disease burden, systolic dysfunction, LV volume overload and hypertrophy, hypertension, and hypoalbuminemia because they are all important factors that predict heart failure in PD patients.
Disclosures
None.
Acknowledgments
This study was supported by the Hong Kong Health Service Research Grant (grant number 6901023), of which Angela Yee-Moon Wang is the principal investigator.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Harnett JD, Foley RN, Kent GM, Barre PE, Murray D, Parfrey PS: Congestive heart failure in dialysis patients: Prevalence, incidence, prognosis and risk factors. Kidney Int 47: 884–890, 1995 [DOI] [PubMed] [Google Scholar]
- 2. Soucie JM, McClellan WM: Early death in dialysis patients: Risk factors and impact on incidence and mortality rates. J Am Soc Nephrol 7: 2169–2175, 1996 [DOI] [PubMed] [Google Scholar]
- 3. Wang AY, Woo J, Lam CW, Wang M, Sea MM, Lui SF, Li PK, Sanderson J: Is a single time point C-reactive protein predictive of outcome in peritoneal dialysis patients? J Am Soc Nephrol 14: 1871–1879, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Wang AY, Wang M, Lam CW, Chan IH, Zhang Y, Sanderson JE: Left ventricular filling pressure by Doppler echocardiography in patients with end-stage renal disease. Hypertension 52: 107–114, 2008. 18474835 [Google Scholar]
- 5. Sohn DW, Chai IH, Lee DJ, Kim HC, Kim HS, Oh BH, Lee MM, Park YB, Choi YS, Seo JD, Lee YW: Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol 30: 474–480, 1997 [DOI] [PubMed] [Google Scholar]
- 6. Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quinones MA: Doppler tissue imaging: A noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol 30: 1527–1533, 1997 [DOI] [PubMed] [Google Scholar]
- 7. van Olden RW, Guchelaar HJ, Struijk DG, Krediet RT, Arisz L: Acute effects of high-dose furosemide on residual renal function in CAPD patients. Perit Dial Int 23: 339–347, 2003 [PubMed] [Google Scholar]
- 8. Nolph KD, Moore HL, Twardowski ZJ, Khanna R, Prowant B, Meyer M, Ponferrada L: Cross-sectional assessment of weekly urea and creatinine clearances in patients on continuous ambulatory peritoneal dialysis. ASAIO J 38: M139–M142, 1992 [DOI] [PubMed] [Google Scholar]
- 9. Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ: Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation 102: 1788–1794, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Nagueh SF, Kopelen HA, Quinones MA: Assessment of left ventricular filling pressures by Doppler in the presence of atrial fibrillation. Circulation 94: 2138–2145, 1996 [DOI] [PubMed] [Google Scholar]
- 11. Nagueh SF, Mikati I, Kopelen HA, Middleton KJ, Quinones MA, Zoghbi WA: Doppler estimation of left ventricular filling pressure in sinus tachycardia. A new application of tissue Doppler imaging. Circulation 98: 1644–1650, 1998 [DOI] [PubMed] [Google Scholar]
- 12. Nagueh SF, Lakkis NM, Middleton KJ, Spencer WH, III, Zoghbi WA, Quinones MA: Doppler estimation of left ventricular filling pressures in patients with hypertrophic cardiomyopathy. Circulation 99: 254–261, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De KG, Leite-Moreira AF, Borbely A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL: How to diagnose diastolic heart failure: A consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 28: 2539–2550, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Sharma R, Pellerin D, Gaze DC, Mehta RL, Gregson H, Streather CP, Collinson PO, Brecker SJ: Mitral peak Doppler E-wave to peak mitral annulus velocity ratio is an accurate estimate of left ventricular filling pressure and predicts mortality in end-stage renal disease. J Am Soc Echocardiogr 19: 266–273, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Cigarran S, Barril G, Cirugeda A, Bernis C, Aguilera A, Sanz P, Herraez I, Alegre L, Selgas R: Hypoalbuminemia is also a marker of fluid excess determined by bioelectrical impedance parameters in dialysis patients. Ther Apher Dial 11: 114–120, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Dumler F: Hypoalbuminemia is a marker of overhydration in chronic maintenance patients on dialysis. ASAIO J 49: 282–286, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Enia G, Mallamaci F, Benedetto FA, Panuccio V, Parlongo S, Cutrupi S, Giacone G, Cottini E, Tripepi G, Malatino LS, Zoccali C: Long-term CAPD patients are volume expanded and display more severe left ventricular hypertrophy than haemodialysis patients. Nephrol Dial Transplant 16: 1459–1464, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Ates K, Nergizoglu G, Keven K, Sen A, Kutlay S, Erturk S, Duman N, Karatan O, Ertuğ AE: Effect of fluid and sodium removal on mortality in peritoneal dialysis patients. Kidney Int 60: 767–776, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Avram MM, Goldwasser P, Erroa M, Fein PA: Predictors of survival in continuous ambulatory peritoneal dialysis patients: The importance of prealbumin and other nutritional and metabolic markers. Am J Kidney Dis 23: 91–98, 1994 [DOI] [PubMed] [Google Scholar]
- 20. Adequacy of dialysis and nutrition in continuous peritoneal dialysis: Association with clinical outcomes. Canada-USA (CANUSA) Peritoneal Dialysis Study Group. J Am Soc Nephrol 7: 198–207, 1996 [DOI] [PubMed] [Google Scholar]
- 21. Freeman LM, Roubenoff R: The nutrition implications of cardiac cachexia. Nutr Rev 52: 340–347, 1994 [DOI] [PubMed] [Google Scholar]
- 22. Mijan-de-la-Torre A: Recent insights on chronic heart failure, cachexia and nutrition. Curr Opin Clin Nutr Metab Care 12: 251–257, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Segall L, Mardare NG, Ungureanu S, Busuioc M, Nistor I, Enache R, Marian S, Covic A: Nutritional status evaluation and survival in haemodialysis patients in one centre from Romania. Nephrol Dial Transplant 24: 2536–2540, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Spiegel DM, Raggi P, Smits G, Block GA: Factors associated with mortality in patients new to haemodialysis. Nephrol Dial Transplant 22: 3568–3572, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G: Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 342: 145–153, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. The SOLVD Investigattors. N Engl J Med 327: 685–691, 1992 [DOI] [PubMed] [Google Scholar]
- 27. Cice G, Ferrara L, D'Andrea A, D'Isa S, Di BA, Cittadini A, Russo PE, Golino P, Calabro R: Carvedilol increases two-year survivalin dialysis patients with dilated cardiomyopathy: A prospective, placebo-controlled trial. J Am Coll Cardiol 41: 1438–1444, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Cice G, Ferrara L, Di BA, Russo PE, Marinelli G, Pavese F, Iacono A: Dilated cardiomyopathy in dialysis patients—Beneficial effects of carvedilol: A double-blind, placebo-controlled trial. J Am Coll Cardiol 37: 407–411, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Felker GM, Adams KF, Jr, Gattis WA, O'Connor CM: Anemia as a risk factor and therapeutic target in heart failure. J Am Coll Cardiol 44: 959–966, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Wexler D, Silverberg D, Blum M, Sheps D, Keren G, Wollman Y, Schwartz D, Iaina A: Anaemia as a contributor to morbidity and mortality in congestive heart failure. Nephrol Dial Transplant 20[Suppl 7]: vii11–vii15, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Lo WK, Bargman JM, Burkart J, Krediet RT, Pollock C, Kawanishi H, Blake PG: Guideline on targets for solute and fluid removal in adult patients on chronic peritoneal dialysis. Perit Dial Int 26: 520–522, 2006 [PubMed] [Google Scholar]