Summary
Background and objectives:
Guidelines recommend systematically screening for stenosis using various methods, but no studies so far have compared all of the options. A prospective blinded study was performed to compare the performance of several bedside tests performed during dialysis in diagnosing angiographically proven >50% fistula stenosis.
Design, setting, participants, & measurements
In an unselected population of 119 hemodialysis patients with mature fistulas, physical examination (PE) was conducted; dynamic and derived static venous pressure (VAPR), blood pump flow/arterial pressure (Qb/AP) ratio, recirculation (R), and access blood flow (Qa) were measured; and angiography was performed.
Results
Angiography identified 59 stenotic fistulas: 43 stenoses were located upstream from the venous needle (inflow stenosis), 12 were located downstream (outflow stenosis), and 4 were located at both sites. The optimal tests for identifying an inflow stenosis were Qa < 650 ml/min and the combination of a positive PE “or” Qa < 650 ml/min (accuracy 80% and 81%, respectively), the latter being preferable because it was more sensitive (85% versus 65%, respectively) for a comparable specificity (79% versus 89%, respectively). The best tests for identifying outflow stenosis were PE and VAPR, with no difference between the two (accuracy 91% and 85%, sensitivity 75% and 81%, specificity 93% and 86%, respectively), the former being preferable because it was more reproducible, easier to perform, and applicable to all fistulas.
Conclusions
This study showed that fistula stenosis can be detected and located during dialysis with a moderate-to-excellent accuracy using PE and Qa measurement as screening procedures.
Introduction
Guidelines recommend systematically screening the vascular access for hemodynamically significant stenosis to reduce thrombosis rates and improve patency (1–3). Various arteriovenous fistula (AVF) screening methods have been proposed, including physical examination (PE), measuring access blood flow (Qa), access recirculation (R), or direct or derived static dialysis pressure, and Duplex ultrasound (DU) (1), but few studies have compared their performance in detecting stenosis in this type of access (4–7) and none have comprehensively assessed all of the options. The authors of the guidelines consequently feel there is insufficient evidence to recommend one method rather than another (1) and suggest that each dialysis unit establish the screening program that best meets its needs (1,2).
Qa measurement is generally considered the most useful surveillance tool, and ultrasound dilution (UD) has become the most popular and validated method (1,3,5) because of its good accuracy and reproducibility and because it is easy to perform. On the other hand, it requires dedicated and expensive equipment, it interrupts the dialysis treatment, and it cannot be measured in AVFs without communicating branches.
Measuring dynamic venous and arterial pressures is the least expensive and easiest method of surveillance available (3,8), although very few studies have evaluated its utility in AVFs, and some found a low accuracy for stenosis detection (9), whereas others reported that measuring the ratio of dialysis blood pump flow (Qb) to negative arterial pressure (Qb/AP) can predict AVF inflow stenosis (1,8). Direct measurements of static intra-access pressures (10) have some advantages over dynamic pressures (3) but are not “user friendly” in a busy dialysis unit (1). Static venous pressure can be derived from the venous drip chamber pressure, as proposed by Frinak et al. (11), although it requires a computerized algorithm. This test (VAPR) is a valuable tool in grafts, but it has yet to be validated in AVFs. Measuring R is useful in detecting AVFs requiring revision (1), but not in predicting access dysfunction (3,8). All of these methods adopted during the dialysis session also require access cannulation with two needles, which makes them unsuitable for analyzing some AVFs with an anatomy only allowing for single-needle dialysis. DU and PE are applicable to all AVFs. DU is also noninvasive and it can measure Qa and reveal any stenosis in the body of the access in much the same way as the gold-standard angiography (3,4,12). However, it demands dedicated equipment and it is operator dependent and time-consuming, making it inconvenient as a screening procedure on a dialysis day. PE is readily available, cost-free, and not time-consuming (13,14), so it has been proposed as the backbone of any stenosis screening program (3). It has the drawback of being subjective, although it has been demonstrated that the results obtained by a nephrology fellow compare favorably with those obtained by an experienced interventional nephrologist (15). In addition, its accuracy in detecting AVF stenosis has also proved excellent (7,16,17), so a panel of experts recently said that PE only is sufficient for surveillance, with Qa and static venous pressure measurements being useful when combined with clinical judgment and clinical data suggestive of access dysfunction (18).
The aim of our study was to compare the utility of the different methods used during a dialysis session at our unit in detecting and locating stenosis in an unselected population of hemodialysis patients with a mature AVF (i.e., one that can be repeatedly cannulated and supports adequate dialysis) (19), with a view to ascertaining whether an optimal screening program can be achieved at the bedside. Secondary aims of the study were to assess the variability of the different surveillance methods and the operator dependence of PE and Qa measurements.
Materials and Methods
This blinded study was conducted in an unselected population of 119 consecutive prevalent patients with native mature AVFs attending the hemodialysis unit at Ospedale Policlinico in Verona, Italy, between December 2006 and May 2010. Forty-five percent of these AVFs had previously undergone endovascolar and/or surgical measures, but none of them had undergone revision in the 3 months before their inclusion in the study.
All subjects gave their informed consent to the study protocol, which was conducted according to the principles of the Helsinki Declaration and was approved by the local ethical committee (Progetto No. 1330).
Study Design
Stenosis prediction.
All AVFs were tested in random order over a 2-week period as follows:
PE. A PE was performed according to the model described by Beathard (13,14). The pulse augmentation test was not used systematically in our study because it is subjective (20) and may be less useful in older, relatively more rigid AVFs. The diagnostic elements of the PE used to identify stenosis are reported in Table 1. An inflow stenosis was defined as any stenosis located in the juxta-anastomotic area upstream from the arterial needle or in the body of the fistula between the arterial and venous needles. An outflow stenosis was defined as any stenosis located beyond the cannulation area downstream from the venous needle. PE was considered positive for the presence of stenosis if at least one of the signs suggestive of stenosis was detected. PE was performed within 2 to 3 minutes before access cannulation.
Dynamic venous pressure. Dynamic venous pressures were measured under two different conditions using 15-G needles: (1) in the first 5 minutes of dialysis after setting the Qb at 200 ml/min for 1 minute (VP200), and (2) every 30 minutes during the first 3 hours of dialysis at the prescribed Qb (300 to 350 ml/min) (VP300).
Static venous pressure. Derived static venous pressure was measured according to Frinak et al. (11) (VAPR) every 30 minutes during the first 3 hours of dialysis.
Dynamic arterial pressure. AP was measured every 30 minutes during the first 3 hours of dialysis at the prescribed Qb (300 to 350 ml/min) and expressed as the Qb300/AP ratio.
Access R. R was measured using the UD method with a Transonic HD03 monitor (Transonic System, Ithaca, NY) as described elsewhere (5). Each value higher than zero was considered positive.
Qa. Qa was measured using the UD method with the HD03 monitor as described elsewhere (5). Measurements were taken in triplicate, within a mean of 10 minutes (range 7 to 12 minutes).
Table 1.
Diagnostic elements of PE used to identify stenosis
| Inflow Stenosis | Outflow Stenosis |
|---|---|
| A flat access | Arm swelling |
| Excessive collapse of the venous segment upon arm elevation | No partial vein collapse upon arm elevation |
| Palpation of stenotic segments in the juxta-anastomotic or cannulation areas | Palpation of stenotic segments in the venous region beyond the cannulation area |
| Abnormal thrill (weak and/or discontinuous with only a systolic component) in the juxta-anastomotic or cannulation areas | Abnormal thrill (weak and/or discontinuous with only a systolic component) in the venous region beyond the cannulation area |
| Abnormal bruit (high pitched with a systolic component) in the juxta-anastomotic or cannulation areas | Abnormal bruit (high pitched with a systolic component) in the venous region beyond the cannulation area |
| Abnormal pulse (a weak or resistant pulse difficult to compress) in the juxta-anastomotic or cannulation areas | Abnormal pulse (a weak or resistant pulse difficult to compress) in the venous region beyond the cannulation area |
| Failure of the pulse to increase when the outflow vein was temporarily occluded |
Venous and arterial pressures and Qa measurements could not be obtained in the three patients treated using single-needle dialysis. Qa measurements were also unobtainable in another eight patients because the arterial and venous needles had to be placed in noncommunicating branches.
Access imaging.
After the previously mentioned tests, all AVFs underwent fistulography to evaluate the access from the feeding artery to the right atrium, as explained elsewhere (5), seeking for the presence of a significant stenosis, which was defined by a >50% reduction in vessel diameter compared with the adjacent segment (21,22) as ascertained by the same radiologist who was unaware of the results of the other tests.
Variability of surveillance methods.
Variability was evaluated by computing the intra-assay and the pooled intra- and interassay coefficient of variation (CV) in the initial 38 AVFs enrolled in the study. The intra-assay CV was obtained from six measurements for VP300, VAPR, and Qb300/AP and from measurements taken in triplicate during the same dialysis session for Qa. The pooled intra- and interassay CV was obtained at three consecutive sessions for dialysis pressures and at two sessions 2 weeks apart for Qa.
Operator dependence of PE and Qa.
For PE, the operator dependence was evaluated in 55 AVFs by comparing the performance of three raters: a senior nephrologist with >2 years of experience of access monitoring; a junior nephrologist with <2 years spent on access monitoring; and a nephrology fellow who had received 3 weeks of intensive, specific, theoretical, and practical training. All raters performed the PEs of the access at separate dialysis sessions within a 2-week period, and they were all unaware of the other raters' findings. All AVFs enrolled in the study during the period in which the three raters were simultaneously attending the hemodialysis unit were included in this analysis. The operator dependence of the Qa measurement was evaluated by comparing the intra-assay CV for the above three raters and an additional nephrology fellow. The junior nephrologist and the nephrology fellows had been trained on Qa measurement for only 1 week by the senior nephrologist.
Statistical Analyses
Data are given as percentages, mean ± SD, or medians (95% confidence interval [CI]). Receiver-operating characteristic (ROC) curves were analyzed to determine each test's overall screening accuracy, as measured by the area under the curve (AUC), and to identify optimal cutoffs for continuous variables. Not all of the tests considered could be applied to all of the AVFs, and ROC curve analysis was consequently likely to produce biased comparisons, so differences in diagnostic performance between tests and their optimal threshold(s) were assessed by comparing their sensitivity, specificity, and accuracy, including their 95% CIs (23), and correcting for the fact that some AVFs could not be studied using all of the tests. When a test could not be used, a random allocation was provided by tossing a coin. We also calculated the diagnostic performance of the association of two tests, according to Macaskill et al. (24). When one of the two tests could not be used, the diagnosis was based on the outcome of the other one alone. A multivariate logistic regression model was used to estimate the association between stenosis and other clinical and physiologic variables.
The Cohen kappa (κ) was also used as a measure of the level of agreement beyond chance between the results of fistulography and the various tests and between two different raters for PE (25). κ values range from 0 to 1.0, with zero indicating no agreement beyond chance and 1.0 denoting perfect agreement. κ values between 0 to 0.20 and 0.21 to 0.40 confer a poor and a fair agreement beyond chance, respectively; those between 0.41 and 0.60 a moderate agreement; and those between 0.61 and 0.80 and those exceeding 0.80 a substantial and a nearperfect agreement, respectively.
Differences between groups were tested by t test, the Wilcoxon test, or Fisher exact test, as appropriate. Analyses were carried out with SPSS version 17.0 (SPSS, Inc., Chicago, IL). Differences between ROC curves were tested with the DeLong test and those between binomial data with the McNemar test. Results were considered significant when P was ≤0.05 (two-tailed).
Results
Diagnostic Performance for Stenosis
The characteristics of the patients and their AVFs are given in Table 2. Angiography identified 59 of 119 stenotic AVFs (49.6%). The stenosis was located on the inflow side (STin) in 43 of 59 AVFs (in the juxta-anastomotic area in 41 and between the arterial and venous needles in 2), on the outflow side (STout) in 12, and at both sites in 4. The prevalence of STin and STout was 39.5% and 13.4%, respectively.
Table 2.
Characteristics of patients and their AVFs
| Number of patients | 119 |
| Gender (male/female) | 87/32 |
| Age (years) | 63.3 ± 15.5 |
| Proportion with diabetes (%) | 27.7 |
| Proportion with cardiovascular disease (%) | 48.7 |
| Single-pool Kt/V | 1.32 [1.29 to 1.37] |
| Age of AVF (months) | 24 [6 to 78] |
| Proportion of AVF with a history of endovascular and/or surgical measures (%) | 45.0% |
| Site of AVF | |
| forearm | |
| radial-cephalic | 98 |
| brachial-cephalic | 4 |
| upper arm | |
| brachial-cephalic | 7 |
| brachial-basilic | 10 |
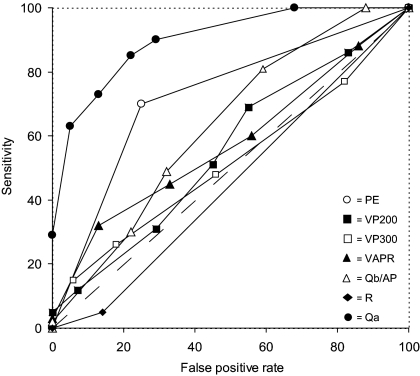
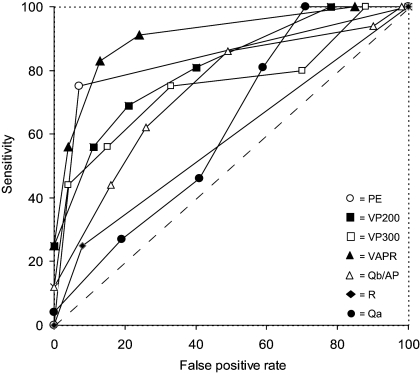
At ROC curve analysis, the only tests with a discriminatory capacity for STin were Qa, PE, and Qb300/AP (Figure 1), whereas all of the tests except R and Qa could distinguish STout (Figure 2).
Figure 1.
Diagnostic performance of the tests for inflow stenosis at ROC curve analysis. The diagnostic performance of the various tests was measured from the AUC [95% CI]. In decreasing order, the AUC for Qa (closed circles) was 0.879 [0.815 to 0.944] (P < 0.001), for PE (open circles) it was 0.726 [0.630 to 0.822] (P < 0.0001), for Qb300/AP (open triangles) it was 0.613 [0.510 to 0.717] (P = 0.040), for VP200 (closed squares) it was 0.588 [0.481 to 0.694] (P = NS), for VP300 (open squares) it was 0.543 [0.436 to 0.650] (P = NS), for VAPR (closed triangles) it was 0.510 [0.403 to 0.617] (P = NS), and for R (closed diamonds) it was 0.453 [0.341 to 0.565] (P = NS). The dashed line at a 45° angle indicates no discriminative capacity (AUC = 0.5).
Figure 2.
Diagnostic performance of the tests for outflow stenosis by ROC curve analysis. The diagnostic performance of the various tests was measured from the AUC [95% CI]. In decreasing order, the AUC for VAPR (closed triangles) was 0.853 [0.723 to 0.983] (P < 0.0001), for PE (open circles) it was 0.841 [0.712 to 0.970] (P < 0.0001), for VP200 (closed squares) it was 0.833 [0.726 to 0.940] (P < 0.0001), for VP300 (open squares) it was 0.765 [0.612 to 0.918] (P = 0.001), for Qb300/AP (open triangles) it was 0.730 [0.582 to 0.879] (P = 0.003), for R (closed diamonds) it was 0.585 [0.421 to 0.749] (P = NS), and for Qa (closed circles) it was 0.531 [0.412 to 0.649] (P = NS). The dashed line at a 45° angle indicates no discriminative capacity (AUC = 0.5).
At multivariate logistic regression analysis (in a model including the age of the patient and the AVF, history of previous AVF interventions, presence of diabetes and cardiovascular disease, single-pool Kt/V, and the tests that can significantly predict stenosis at ROC curve analysis as explanatory variables), the only variables significantly associated with STin were a positive PE (hazard ratio [HR] = 22.0 [95% CI 2.3 to 206.5], P = 0.007) and a lower Qa (HR = 0.992 [95% CI 0.988 to 0.996], P < 0.001), whereas a positive PE was the only variable significantly associated with STout (HR = 15.5 [95% CI 1.5 to 160,8], P = 0.022). The diagnostic performance for STin and STout of the tests with a discriminatory capacity at ROC curve analysis and their best thresholds are given in Tables 3 and 4. One of the three nonstenotic AVFs in which the pressures were unobtainable was considered a true negative, and two were assumed to be false positives. Two of the four stenotic AVFs in which R and Qa measurements were unobtainable were considered true positives and two were considered false negatives, whereas three of seven nonstenotic AVFs in which these tests were unobtainable were assumed to be true negative and four were false positives.
Table 3.
Diagnostic performance of the tests for inflow stenosis
| Accuracy | Cohen κ (agreement) | Sensitivity | Specificity | |
|---|---|---|---|---|
| Positive PE | 74% [65 to 86] | 0.46 [0.30 to 0.62] (moderate) | 70% [55 to 83] | 76% [65 to 86] |
| Qb300/AP < 2.6 | 58% [48 to 67] | 0.15 [0.00 to 0.33] (poor) | 57% [42 to 72] | 58% [46 to 70] |
| Qa < 900 ml/min | 73% [64 to 81] | 0.46 [0.31 to 0.62] (moderate) | 81% [67 to 91] | 68% [56 to 79] |
| Qa < 650 ml/min | 80% [73 to 86] | 0.57 [0.41 to 0.70] (moderate) | 65% [50 to 77] | 89% [79 to 95] |
| Qa < 500 ml/min | 71% [62 to 79] | 0.35 [0.19 to 0.51] (fair) | 40% [26 to 56] | 92% [83 to 97] |
| Positive PE or Qa < 900 ml/min | 76% [67 to 83] | 0.54 [0.40 to 0.67] (moderate) | 98% [89 to 100] | 61% [49 to 72] |
| Positive PE or Qa < 650 ml/min | 81% [73 to 88] | 0.62 [0.48 to 0.76] (substantial) | 85% [72 to 94] | 79% [68 to 88] |
| Positive PE and Qa < 900 ml/min | 79% [70 to 86] | 0.54 [0.39 to 0.70] (moderate) | 64% [49 to 77] | 89% [79 to 95] |
| Positive PE and Qa < 650 ml/min | 77% [69 to 84] | 0.48 [0.33 to 0.64] (moderate) | 49% [34 to 64] | 96% [88 to 99] |
| Qa < 650 ml/min or VAPR > 0.50 | 69% [61 to 78] | 0.36 [0.19 to 0.53] (fair) | 64% [48 to 77%] | 72% [60 to 82] |
Table 4.
Diagnostic performance of the tests for outflow stenosis
| Accuracy | Cohen κ (agreement) | Sensitivity | Specificity | |
|---|---|---|---|---|
| Positive PE | 91% [84 to 95] | 0.63 [0.43 to 0.83] (substantial) | 75% [48 to 93] | 93% [86 to 97] |
| Qb300/AP > 2.8 | 68% [59 to 76] | 0.21 [0.05 to 0.37] (fair) | 69% [41 to 89] | 68% [58 to 77] |
| VP200 > 80 mmHg | 76% [68 to 84] | 0.31 [0.13 to 0.50] (fair) | 69% [41 to 89] | 78% [68 to 85] |
| VP300 > 125 mmHg | 75% [66 to 82] | 0.31 [0.14 to 0.49] (fair) | 75% [48 to 93] | 75% [65 to 83] |
| VAPR > 0.50 | 85% [78 to 91] | 0.52 [0.33 to 0.72] (moderate) | 81% [54 to 96] | 86% [78 to 92] |
| Positive PE or VAPR > 0.50 | 86% [78 to 91] | 0.56 [0.38 to 0.74] (moderate) | 94% [70 to 100] | 84% [76 to 91] |
| Positive PE and VAPR > 0.50 | 92% [86 to 96] | 0.65 [0.43 to 0.86] (substantial) | 63% [35 to 85] | 97% [92 to 99] |
| Qa < 650 ml/min or VAPR > 0.50 | 69% [59 to 76] | 0.28 [0.13 to 0.42] (fair) | 87% [62 to 98] | 65% [55 to 74] |
PE and Qa at a threshold varying from <650 to <900 ml/min were equally moderate in their accuracy for STin. Qa < 900 ml/min was more sensitive than Qa < 650 ml/min (P = 0.008), whereas the latter was more specific than the former (P < 0.001). PE had a sensitivity and specificity statistically no different from Qa at any threshold. Combining PE with Qa did not improve their accuracy for STin. The “or” combinations of a positive PE with Qa < 900 ml/min and a positive PE with Qa < 650 ml/min significantly improved the sensitivity (98% and 85%, respectively) by comparison with the single tests (P ≤ 0.039), although the latter combination appears to be preferable because its specificity did not drop significantly vis-à-vis the single tests, whereas it did for the former combination (P ≤ 0.013). The “and” combination of a positive PE with a Qa at any threshold did not seem to be useful because its performance was no better than with the single tests. The best combination of Qa with VAPR (i.e., Qa < 650 ml “or” VAPR >0.5) did not seem to be useful either because its sensitivity and specificity were significantly lower than the combination of a positive PE “or” Qa < 650 ml/min (64% versus 85%, P < 0.001) and Qa < 650 ml/min (72% versus 89%, P = 0.002) respectively, although its accuracy did not differ statistically from either of the tests (69% versus 81% and 80%, respectively).
PE and VAPR >0.5 were equally very accurate for STout, with both being better than measuring dynamic venous and arterial pressures and Qa, but combining the two tests did not improve their diagnostic performance. The combination of a positive PE “or” VAPR >0.5 was slightly, but not significantly, more sensitive than the tests considered separately, whereas the “and” combination significantly improved specificity by comparison with VAPR > 0.5 (P = 0.003) but not PE. Combining Qa < 650 ml/min “or” VAPR > 0.5 led to a significant reduction in diagnostic accuracy by comparison with VAPR > 0.50 alone (69% versus 85%, P < 0.001) and a positive PE (69% versus 91%, P < 0.001) because of the drop in specificity (P < 0.001).
Variability of Surveillance Methods
All of the tests except for VAPR had intra-assay and pooled CVs <16% (Table 5).
Table 5.
Intra-assay and pooled intra- and interassay variability of the different surveillance methods (mean CV)
| VP200 | VP300 | VAPR | Qb300/AP | Qa | |
|---|---|---|---|---|---|
| Intra-assay CV | – | 9.77 | 27.02 | 12.54 | 9.78 |
| Pooled CV | 15.64 | 11.92 | 35.80 | 15.15 | 10.18 |
Operator Dependence of PE and Qa
The level of agreement of PE for stenosis was moderate between the two nephrologists (κ 0.498 ± 0.119) and only fair between the nephrology fellow and the two nephrologists (κ 0.315 ± 0.127 and 0.326 ± 0.156).
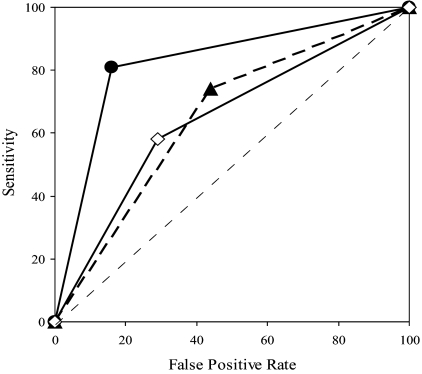
The senior nephrologist only achieved a discriminatory capacity for STin, with his AUC being higher than the other raters (Figure 3), whereas no difference between raters was found for STout (Figure 4).
Figure 3.
Diagnostic performance of PE by different raters in detecting inflow stenosis. The diagnostic performance of PE by the various raters was measured from the AUC [95% CI]. In decreasing order, the AUC for inflow stenosis was 0.826 [0.722 to 0.931] (P < 0.0001) for the senior nephrologist (closed circles), 0.649 [0.504 to 0.794] (P = 0.058) for the junior nephrologist (closed triangles), and 0.647 [0.497 to 0.796] (P = 0.064) for the nephrology fellow (open diamonds). The AUC was significantly greater for the senior nephrologist than for the other raters (P = 0.007), with no difference between the latter. The dashed line at a 45° angle indicates no discriminative capacity (AUC = 0.5).
Figure 4.
Diagnostic performance of PE by different raters in detecting outflow stenosis. The diagnostic performance of PE by the various raters was measured from the AUC [95% CI]. In decreasing order, the AUC for outflow stenosis was 0.926 [0.722 to 0.931] (P < 0.0001) for the nephrology fellow (open diamonds), 0.867 [0.728 to 1.000] (P < 0.0001) for the senior nephrologist (closed circles), and 0.831 [0.668 to 0.994] (P = 0.001) for the junior nephrologist (closed triangles). The AUC for the three raters did not differ significantly. The dashed line at a 45° angle indicates no discriminative capacity (AUC = 0.5).
There were no differences between the four raters in terms of Qa measurement CVs (Table 6).
Table 6.
Qa measurements by different raters and the corresponding CV (median [10th to 90th percentile])
| Senior Nephrologist | Junior Nephrologist | Nephrology Fellow | Nephrology Fellow | |
|---|---|---|---|---|
| Number of measurements | 52 | 32 | 44 | 40 |
| Qa (ml/min) | 953 [493 to 1901] | 1084 [512 to 1652] | 1105 [488 to 1656] | 1212 [542 to 1587] |
| CV (%) | 5.2 [2.4 to 11.0] | 5.2 [1.6 to 9.5] | 4.4 [2.3 to 11.0] | 5.8 [1.1 to 10.7] |
Discussion
Our prospective blinded study shows that a moderate-to-excellent accuracy in detecting and locating AVF stenosis can be achieved at the bedside by combining two tests—PE and Qa measurement.
Because reproducibility is important when implementing tests in clinical practice, we also assessed the variability and operator dependence of the different screening methods.
All of the tests except VAPR proved sufficiently reproducible. The least variable were the UD Qa measurements, which can also be taught quickly and were found to be operator independent. Conversely, the level of inter-rater agreement for PE was only fair to moderate in our hands. The operator dependence of PE only appeared to concern inflow stenosis (the most-experienced rater performing better than the less experienced), not outflow stenosis. This discrepancy is probably because the elements most frequently used to identify outflow stenosis (no partial vein collapse on arm elevation, arm swelling) are less subjective that those generally used to identify inflow stenosis (abnormalities in the thrill, pulse, and bruit; excessive collapse of the venous segment upon arm elevation; negative pulse augmentation test), at least in our hands. Nonetheless, our findings indicated that the necessary PE skills can be taught and improved with experience in access monitoring, supporting the notion that nephrology training and quality improvement programs should incorporate structured teaching on the PE of a native access (15).
Our PE performance for inflow and outflow stenoses was similar to that of Asif et al. (16), but inferior to that of Campos et al. (7) because of a lower sensitivity (75% versus 96%, P = 0.001). This difference can be explained by differences between the two studies in the prevalence in upper-arm AVFs; an inconsistent use of the augmentation test in our study; and, last but not least, the different skills and expertise of the different operators.
PE and Qa were the best tests for inflow stenosis, with their accuracy ranging from 73% to 80%. The diagnostic performance of PE was statistically no different from that of Qa at any threshold, whereas Qa < 900 ml/min was more sensitive than Qa < 650 ml/min (81% versus 64%, P = 0.008) and the latter was more specific than the former (89% versus 68%, P < 0.001).
Combining the two tests changed neither their accuracy nor their specificity, although the “or” combination of a positive PE with Qa < 650 ml/min or Qa < 900 ml/min significantly improved sensitivity (85% and 98%, respectively) by comparison with the tests considered alone (P < 0.04), the former combination being preferable because its specificity did not drop significantly.
These findings demonstrate that inflow stenoses can be detected at the bedside with a moderate degree of accuracy and suggest that the best strategy is to use PE as the initial screening procedure, followed by Qa measurement in PE-negative cases (if sensitivity is favored), or else to measure Qa alone (considering a threshold of Qa < 650 ml/min) if specificity is favored.
PE and VAPR > 0.5 showed an equally excellent diagnostic performance for outflow stenosis with an accuracy >85%, which did not improve further if they were combined. However, PE should be preferred to VAPR because it is easier to perform, more reproducible, and it can be applied to all AVFs.
Omitting PE and relying on a screening program on the basis of Qa and VAPR does not seem to be advantageous because combining Qa < 650 ml/min “or” VAPR > 0.50 showed a significantly lower sensitivity and specificity than the combination of PE “or” Qa < 650 ml/min (P < 0.001) and Qa < 650 ml/min (P = 0.002) for inflow stenosis, respectively, and an accuracy and specificity significantly lower than PE (P < 0.001) for outflow stenosis.
We are aware that our study has its limitations. It is a single-center study on a small group of patients mainly with forearm AVFs. Another limitation may be that it considered the intermediate outcome (stenosis) rather than the clinically more relevant AVF failure, although an early and accurate identification of stenosis may be of value because it is the foremost risk factor for AVF dysfunction and thrombosis (1,4,6). This approach may also reduce the burden of access monitoring by selecting the AVFs requiring more frequent surveillance (if any stenosis is not corrected as soon as it has been identified). It may also be useful in the event of thrombosis (1) by facilitating the choice of the optimal approach for thrombectomy.
In conclusion, our study shows that a moderate-to-excellent accuracy of ≥80% in detecting and locating AVF stenoses can be achieved during dialysis by using two tests—PE and Qa measurement (considering a threshold of <650 ml/min). PE appears to be the key element for detecting inflow and outflow stenoses, whereas measuring Qa is of value for inflow stenosis alone (for a better specificity) or after a negative PE (for a higher sensitivity).
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. National Kidney Foundation: K/DOQI Clinical practice guidelines for vascular access 2006. Am J Kidney Dis 48 [Suppl S1]: S176–S273, 2006. 16813989 [Google Scholar]
- 2. Bakran A, Mickley V, Passlick-Deetjen J: Management of the renal patient: Clinical algorithms on vascular access for hemodialysis. Available at: www.vascularaccesssociety.com Accessed 2010
- 3. Tordoir J, Canaud B, Haage P, Konner K, Basci A, Fouque D, Kooman J, Martin-Malo A, Pedrini L, Pizzarelli F, Tattersall J, Vennegoor M, Wanner C, ter Wee P, Vanholder R: EBPG on vascular access. Nephrol Dial Transplant 22 [Suppl 2]: ii88–ii117, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Schwartz C, Mitterbauer C, Boczula M, Taca T, Funovics M, Heinze G, Lorenz M, Kovarik J, Oberbauer R: Flow monitoring: Performance characteristics of ultrasound dilution versus colour Doppler ultrasound compared with fistulography. Am J Kidney Dis 42: 539–545, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Tessitore N, Bedogna V, Gammaro L, Lipari G, Poli A, Baggio E, Firpo M, Morana G, Mansueto G, Maschio G: Diagnostic accuracy of ultrasound dilution access blood flow measurement in detecting stenosis and predicting thrombosis in native forearm arteriovenous fistulae for hemodialysis. Am J Kidney Dis 42: 331–341, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Polkinghorne KR, Lau KKP, Saunders A, Atkins RC, Kerr PG: Does monthly native arteriovenous fistula blood flow surveillance detect significant stenosis—A randomized controlled trial. Nephrol Dial Transplant 21: 2498–2506, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Campos RQ, Chula DC, Perreto S, Riella, Mazza do Nascimento M: Accuracy of physical examination and intra-access pressure in the detection of stenosis in hemodialysis arteriovenous fistula. Semin Dial 21: 269–273, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Besarab A, Asif A, Roy-Chaudhury P, Spergel LM, Ravani P: The native arteriovenous fistula in 2007. Surveillance and monitoring. J Nephrol 20: 656–667, 2007 [PubMed] [Google Scholar]
- 9. Lopot F, Nejedly B, Valek M: Vascular access monitoring: Methods and procedures—Something to standardize? Blood Purif 23: 36–44, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Besarab A, Sullivan KL, Ross R, Moritz M: The utility of intra-access monitoring in detecting and correcting venous outlet stenoses prior to thrombosis. Kidney Int 47: 1364–1373, 1995 [DOI] [PubMed] [Google Scholar]
- 11. Frinak S, Zasuwa G, Dunfee T, Besarab A, Yee J: Dynamic venous access pressure ratio test for hemodialysis access monitoring. Am J Kidney Dis 40: 760–768, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Salman L, Ladino M, Alex M, Dhamija R, Merrill D, Lenz O, Contreras G, Asif A: Accuracy of ultrasound in the detection of inflow stenosis of arteriovenous fistulae: Results of a prospective study. Seminars in Dialysis 23: 117–121, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Beathard GA: Physical Examination: The Forgotten Tool. In: A Multidisciplinary Approach for Hemodialysis Access, edited by Gray R, Sands J. New York, Lippincott Williams & Wilkins, 2002, pp 111–118 [Google Scholar]
- 14. Beathard GA: An algorithm for the physical examination of early fistula failure. Semin Dial 18: 331–335, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Leon C, Asif A: Physical examination of arteriovenous fistulae by a renal fellow: Does it compare favourably to an experienced interventionalist? Semin Dial 21: 557–560, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Asif A, Leon C, Orozco-Vargas LC, Krishnamurthy G, Choi KL, Mercado C, Merrill D, Thomas I, Salman L, Artikov S, Burgoignie JJ: Accuracy of physical examination in the detection of arteriovenous fistula stenosis. Clin J Am Soc Nephrol 2: 1191–1194, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Choi JR, Kim YS, Yoon SA, Won JD, Son YS, Song WJ, Sonh HC, Kim JS, Chang YS, Bang BK, Kin YO: Accuracy of physical examination in the detection of arteriovenous fistula dysfunction. Korean J Nephrol 25: 797–802, 2006 [Google Scholar]
- 18. Paulson WD, Work J: Controversial vascular access surveillance mandate. Seminars in Dialysis 23: 92–94, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Allon M, Robin ML: Increasing arteriovenous fistulas in hemodialysis patients: Problems and solutions. Kidney Int 62: 1109–1124, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Asif A, Gadalean FN, Merrill D, Chela G, Cipleu CD, Epstein D, Roth D: Inflow stenosis in arteriovenous fistulas and grafts: A multicenter, prospective study. Kidney Int 67: 1986–1992, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Gray RJ, Sack D, Martin LG. Trerotola SO and the members of the Technology Assessment Committee: Reporting standards for percutaneous interventions in dialysis access. J Vasc Interv Radiol 3: 1405–1415, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Tonelli M, Jindal K, Hirsch D, Taylor S, Kane C, Henbrey S: Screening for subclinical stenosis in native vessel arteriovenous fistulae. J Am Soc Nephrol 12: 1729–1733, 2001 [DOI] [PubMed] [Google Scholar]
- 23. van Stralen KJ, Stel VS, Reitsma JB, Dekker FW, Zoccali C, Jager KJ: Diagnostic methods I: Sensitivity, specificity, and other measures of accuracy. Kidney Int 75: 1257–1263, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Macaskill P, Walter SD, Irwig L, Franco EL: Assessing the gain in diagnostic performance when combining two diagnostic tests. Stat Med 21: 2527–2546, 2002 [DOI] [PubMed] [Google Scholar]
- 25. McGinn T, Wyers PC, Newman T, Keitz S, Leipzig R, Guyatt G: Tips for learners of evidence-based medicine: Measures of observer variability/kappa statistics). CMAJ 171: 1369–1373, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]