Summary
Background and objectives
Depression is a risk indicator for adverse outcomes in dialysis patients, but its prognostic impact in individuals who are not yet on dialysis is unknown. This study examines whether depressive symptoms are longitudinally associated with renal function decline, new-onset chronic kidney disease (CKD), ESRD, or hospitalization with acute kidney injury (AKI).
Design, setting, participants, & measurements
Depressive symptoms were measured in a longitudinal cohort study with the 10-item Centers for Epidemiologic Studies Depression scale using a previously validated cut-off value (≥8). CKD at study entry and during follow-up was defined as an estimated GFR (eGFR) < 60 ml/min per m2. Outcomes were rapid decline in eGFR (>3 ml/min per m2 per year), new-onset CKD, ESRD (U.S. Renal Data System-based), and AKI (based on adjudicated medical record review). The median follow-up duration was 10.5 years.
Results
Depressed participants (21.2%) showed a higher prevalence of CKD at baseline compared with nondepressed participants in multivariable analysis. Depression was associated with a subsequent risk of rapid decline in eGFR, incident ESRD, and AKI, but not incident CKD in unadjusted models. In multivariable analyses, only associations of depressive symptoms with AKI remained significant.
Conclusions
Elevated depressive symptoms are associated with subsequent adverse renal disease outcomes. The depression-related elevated risk of AKI was independent of traditional renal disease risk factors and may in part be explained by the predictive value of depression for acute coronary syndromes and heart failure hospitalizations that can be complicated by AKI.
Introduction
Clinical depression and subthreshold depressive symptoms are associated with increased mortality rates in dialysis patients (1–8) and after kidney transplantation (9). However, little is known about the longitudinal association between elevated depressive symptoms with a decline in kidney function, subsequent new-onset (incident) stage 3 to 4 CKD, or clinical renal outcomes such as ESRD and acute kidney injury (AKI) in individuals who are not yet on dialysis (10,11).
The potential mechanisms involved in the longitudinal association of depression with poor renal outcomes involve biologic factors (e.g., sympathetic nervous system activation, hypertension, and diabetes mellitus) and adverse health behaviors such as medication nonadherence, smoking, and poor dietary control. In dialysis patients, the prevalence of depression is high, ranging from 20 to 35% (1–8,12–21), which in part reflects a psychologic response to substantial disease burden. Studying depressive symptoms in individuals at risk for CKD provides unique opportunities to identify early risk factors for renal disease progression that cannot be achieved by studying dialysis patients (10). In addition, the prevalence of stage 3 to 4 CKD exceeds 8 million in the United States, making it substantially more common than ESRD (22,23).
This study investigates the hypothesis that elevated levels of depressive symptoms are associated with prevalent CKD and evaluates the relationship between depressive symptoms and increased risk of clinical renal outcomes. Using a prospective design, we examined whether elevated levels of depressive symptoms are associated with an accelerated decline in estimated GFR (eGFR), new-onset CKD, and future adverse clinical renal disease outcomes (ESRD and hospitalization with AKI) in a community-based sample of individuals 65 years of age and above.
Materials and Methods
Participants and Study Design
The Cardiovascular Health Study (CHS) is a prospective, community-based, observational study of individuals 65 years of and older enrolled in four geographically distinct communities across the United States. Design and participant selection have previously been described (24). Exclusion criteria were currently institutionalized, being actively treated for malignancy, or not expected to remain in the area for 3 years. Participants were identified via Medicare enrollment lists, and 57.3% of those eligible individuals were enrolled in the CHS (25). The initial cohort of 5201 participants was recruited from 1989 to 1990, and an additional African-American cohort of 687 participants was enrolled between 1992 and 1993. Clinical information was obtained from interviews, physical examinations, and questionnaire-based assessments. Hospital discharge summaries and International Classification of Diseases, Ninth Revision (ICD-9) codes were collected for all hospitalizations during 14 years of follow-up. The study was approved by institutional review boards at each site, and informed consent was obtained from all participants. Participants were excluded from the present analyses if they had evidence of ESRD (based on clinical history or an eGFR <15 ml/min per 1.73 m2; n = 13, 0.2%) at the baseline visit, if no baseline creatinine levels were available (n = 80, 1.4%), or if depression data were missing (n = 10, 0.2%). A total of 5785/5888 (98%) participants of the total CHS cohort were included for the analyses.
Assessment of Depressive Symptoms
Depressive symptoms were assessed using the 10-item Center for Epidemiologic Studies Depression (CES-D) scale (26) during each year of follow-up. The CES-D has been validated in populations 65 years of age and older (27). Depressive symptom scores were analyzed as a continuous variable (score range, 0 to 30) and also as a categorical variable to determine persons at high risk for clinical depression, using an independently validated cut-off score of CES-D ≥ 8 (28), similar to the ≥16 cut-off for the 20-item version of the CES-D (26,27). In addition, a “sustained depressive symptoms” index was determined by calculating the area under the curve of each patient's consecutive annual depression score between baseline and the 3-year follow-up (potential score range, 0 to 90). This index was used to determine the impact of cumulative depression over time as related to changes in eGFR from baseline to follow-up.
Measures of Renal Disease Outcomes
Two categories of adverse renal disease outcomes were examined. (1) Rapid decline in eGFR and new-onset CKD based on systemic biologic markers (creatinine and cystatin C) at 3- (1992 to 1993) and 7-year (1996 to 1997) follow-up. (2) Adverse clinical renal outcomes: development of ESRD and hospitalization with AKI.
Biologic markers of CKD progression.
Creatinine.
Serum creatinine was measured using a colorimetric method (Kodak Ektachem 700 Analyzer; Eastman Kodak, Rochester, NY) at baseline and the 3- and 7-year follow-up visits. The mean coefficient of variation (CV) for monthly controls was 1.94% (range, 1.16 to 3.90%). Creatinine-based eGFR was calculated by the simplified Modification of the Diet in Renal Disease study equation, defined as eGFR = 186.3 × (serum creatinine)−1.154 × age−0.203 × 0.742 (if female) × 1.212 (if African American) (29,30). Because creatinine values vary across clinical laboratories, we calibrated the creatinine concentration in the current study to the Cleveland Clinic Laboratory using indirect calibration to National Health and Nutrition Examination Surveys (NHANES) III as described previously (31,32).
CKD status during follow-up was categorized into two groups defined by creatinine-based eGFR values (29): (1) no CKD, eGFR ≥60 ml/min per 1.73 m2 and (2) CKD, eGFR <60 ml/min per 1.73 m2. Participants with CKD stages 1 (eGFR ≥90 ml/min per 1.73 m2) and 2 (eGFR between 60 and 90 ml/min per 1.73 m2) were combined in this study because of the uncertainty about the clinical implications of values ≥60 ml/min per 1.73 m2 (33) and the lack of albuminuria and hematuria measurements in this study. Incident CKD included stage 3 (eGFR ranging from 30 to 59 ml/min per 1.73 m2), stage 4 (eGFR 15 to 29 ml/min per 1.73 m2), and stage 5 (eGFR <15 ml/min per 1.73 m2) during follow-up assessments at years 3 and 7.
The annual decline in creatinine-based eGFR (eGFR-creat) was calculated to determine the rate of deterioration of kidney function based on regression analysis (34). An annual eGFR-creat increase of >3 ml/min per 1.73 m2 per year was used as indicator of rapidly declining renal function (34).
Cystatin C.
Cystatin C, a cysteine proteinase inhibitor released in the circulation by almost all nucleated cells, was measured using a BNII nephelometer (Dade Behring, Deerfield, IL) and a particle-enhanced immunonephelometric assay (N Latex Cystatin-C; Dade Behring) (35). The assay range is 0.195 to 7.330 mg/L, and the intra-assay CVs range from 2.0 to 2.8%. We used Cystatin C because this marker may better detect small changes in kidney function than creatinine (36–38). Cystatin C–based eGFR (eGFR-cysC) was calculated as 76.7 × cystatin C−1.19 as described previously (39). Consistent with the creatinine-based annual decline in eGFR measure, we calculated the slope of annual decline in eGFR-cysC and defined a rapid decline in cystatin C as Δ eGFR-cysC >3.0 ml/min per 1.73 m2 per year (34).
Adverse clinical renal disease outcomes during follow-up.
ESRD.
ESRD was based on linkage with the U.S. Renal Data System in 2005, with available data regarding dialysis until March 31, 2003. In addition, chart reviews were performed because elderly patients may refuse dialysis or die within 90 days of starting dialysis (and those individuals are therefore not entered into the U.S. Renal Data System). Charts selected for review were those with (1) adjudicated cause of death related to renal disease; (2) adjudicated cause of death related to failure to thrive; (3) hospitalization with a dialysis ICD-9 procedure code (39.1, 54.98); (4) hospitalization with an ICD-9 modifier code for renal transplant or ESRD (V45.1, V56.x, V42.0); and (5) discharge diagnosis that included renal failure or ESRD. ESRD outcomes were defined as (1) definite (dialysis for ESRD and patient withdrew shortly after starting; dialysis but patient died shortly after starting, or progressed to ESRD and patient refused dialysis), (2) probable (evidence of progressive renal insufficiency by history and presented with either acute or chronic or ESRD followed by death between 60 and 90 days on dialysis, progressive renal insufficiency but refused dialysis and died with renal failure contributing, but some details lacking to differentiate acute or chronic CKD versus ESRD), or (3) possible (acute or moderate chronic kidney disease, without evidence of progressive kidney disease before admission and acute renal failure requiring dialysis and participant died during admission or missing details regarding the duration of dialysis). Thus, the clinical ESRD outcome consisted of individuals on dialysis and those referred for dialysis for ESRD.
AKI.
All hospitalizations during follow-up were evaluated for incident AKI. Discharge summaries, available for all hospitalizations, along with ICD-9 diagnosis codes were used to identify cases of AKI. ICD-9 codes included 584 (acute kidney injury), 584.5 to 584.9 (acute kidney injury of specified etiology), 788.9 (uremia), and 586 (renal failure, not otherwise specified). Other codes considered were 39.95 (hemodialysis), 54.98 (peritoneal dialysis), and V56.8 (peritoneal). AKI was considered as present when the discharge record had a renal ICD-9 code of interest combined with further evidence of physician-diagnosed AKI on individual medical record, such as short-term dialysis or a transient rise in creatinine from a normal to an elevated value, subsequently returning to a normal baseline level. Because the AKI outcome was chosen to reflect cases of incident AKI, care was taken to differentiate whether the diagnosis at the identified hospitalization represented AKI and not progression of CKD to ESRD (40) (30 participants with ESRD at the time of AKI were excluded from AKI analyses).
Covariates
The following measures were obtained from the clinical interview: age, gender, race, marital status, and education level. Baseline clinical examinations included presence of coronary artery disease (history of myocardial infarction, angiographic determined coronary artery disease, coronary revascularization, or angina pectoris) and standardized assessments of subclinical cardiovascular disease, BP, hypertension status, blood glucose, diabetes mellitus, and lipid levels (total cholesterol, LDL, HDL, and triglycerides). Criteria for isolated subclinical CVD (i.e., markers of CVD in the absence of any form of clinical CVD) have been previously described (41). Diabetes was defined as a fasting glucose ≥126 mg/dl or use of hypoglycaemic agents (pills and/or insulin) (24,42). The use of the following medications was also recorded: anti-hypertensives, lipid-lowering medications, and anti-depressants.
Inflammation markers included high sensitivity CRP (CV = 7.6%) (43). IL-6 (CV = 6.3%) (44–46), and fibrinogen (CV = 3.09%) (42). White blood count count was assessed using automated cell counters at local laboratories (CV = 5.50%) (47). Health behavior–related factors included smoking status, weight, height, alcohol consumption, and level of physical activity (48).
Statistical Analyses
Data are presented as mean ± SD or percentages as appropriate. If the data distributions were skewed, natural logarithmic or square root transformations were used, and data were presented as median and interquartile intervals. Comparisons between participants with versus without elevated depressive symptoms were examined using independent t tests or χ2 tests, and linear and logistic regression was used for multivariably adjusted associations of baseline depressive symptoms with eGFR and CKD status.
The longitudinal relationship between depressive symptoms at study entry and rapid decline in eGFR and new-onset (incident) stage 3 to 4 CKD (based on eGFR at 3- and 7-year follow-up) was examined using logistic regression analysis because these outcomes were obtained at set time points. Cox proportional hazards models were used to examine risk of clinical outcomes during follow-up (ESRD and AKI status adjudicated up until 2003; data were right censored at 11-year follow-up). A hierarchical approach was used for the multivariable models: (1) demographic measures (age, gender, race); (2) clinical measures (coronary heart disease, subclinical atherosclerotic disease, systolic BP, glucose levels, total cholesterol, and HDL) and inflammation-related markers (CRP, IL-6, fibrinogen); and (3) health behaviors (current smoking status, body mass index, alcohol consumption, and physical activity). Interactions between baseline depressive symptoms with CKD and other covariates at baseline were used to explore multiplicative effects of depressive symptoms with these biomedical measures. For all multivariable Cox proportional hazards models, graphical and formal methods were used to test the assumption of proportionality. Results are reported as odds ratios (ORs) and hazard ratios (HRs) with 95% confidence intervals (CIs), and a two-tailed P < 0.05 was considered statistically significant.
Results
Participant Characteristics
Elevated depressive symptoms were observed in 1225 (21.2%) participants. CES-D10 depression scores were positively skewed (mean and SD = 4.7 ± 4.6; median = 3; interquartile range = 1 to 7). Table 1 shows the associations of depression with demographic and clinical variables.
Table 1.
Participant characteristics at baseline
| Total (n = 5785) [mean ± SD or n (%)] | Low Depressive Symptoms (n = 4560) [mean ± SD or n (%)] | Elevated Depressive Symptoms (n = 1225) [mean ± SD or n (%)] | P (t test/χ2) | |
|---|---|---|---|---|
| Demographics | ||||
| age (years) | 72.8 ± 5.6 | 72.7 ± 5.5 | 73.1 ± 5.8 | 0.023 |
| gender (female) | 3322 (57.4%) | 2487 (54.5%) | 835 (68.2%) | <0.001 |
| race (African American) | 4917 (15.0%) | 8946 (13.5%) | 971 (20.7%) | <0.001 |
| married/living with spouse | 3842 (66.4%) | 3153 (69.1%) | 689 (56.2%) | <0.001 |
| education | ||||
| ≤ high school | 869 (15.1%) | 628 (13.8%) | 241 (19.7%) | <0.001 |
| high school/vocational | 2912 (50.5%) | 2266 (49.8%) | 646 (52.9%) | |
| college | 1988 (34.5%) | 1654 (36.4%) | 334 (27.4%) | |
| Clinical variables | ||||
| CAD at baseline | 1718 (29.7%) | 1402 (30.7%) | 316 (25.8%) | <0.001 |
| subclinical atherosclerosisa | 2944 (50.9%) | 2339 (51.3%) | 605 (49.4%) | 0.075 |
| systolic BP (mmHg) | 136.4 ± 21.7 | 136.2 ± 21.5 | 137.1 ± 22.5 | 0.21 |
| diastolic BP (mmHg) | 70.7 ± 11.4 | 70.8 ± 11.3 | 70.4 ± 11.7 | 0.39 |
| hypertension | 2552 (44.2%) | 1943 (42.6%) | 609 (49.8%) | <0.001 |
| anti-hypertensive medication | 2729 (47.2%) | 2070 (45.5%) | 659 (53.8%) | <0.001 |
| glucose (mg/dl) | 111.1 ± 36.9 | 110.1 ± 34.3 | 114.8 ± 45.3 | <0.001 |
| diabetes | 930 (16.1%) | 689 (15.1%) | 241 (19.7%) | <0.001 |
| total cholesterol (mg/dl) | 211.1 ± 39.2 | 210.7 ± 39.2 | 212.8 ± 39.3 | 0.10 |
| LDL (mg/dl) | 129.8 ± 35.6 | 129.8 ± 35.4 | 129.6 ± 36.5 | 0.828 |
| HDL (mg/dl) | 54.2 ± 15.8 | 53.9 ± 15.7 | 55.3 ± 15.9 | 0.008 |
| lipid-lowering medication | 316 (5.5%) | 248 (5.4%) | 68 (5.6%) | 0.881 |
| triglycerides (mg/dl)a | 120 (92 to 164) | 119 (91 to 62) | 124 (93–171) | 0.068 |
| CRP (mg/L)b | 2.54 (1.28 to 4.50) | 2.44 (1.24 to 4.34) | 2.89 (1.44 to 5.66) | <0.001 |
| IL-6 (pg/ml)b | 1.70 (1.16 to 2.59) | 1.67 (1.14 to 2.54) | 1.85 (1.24 to 2.80) | 0.009 |
| fibrinogen (mg/dl) | 323.8 ± 67.2 | 322.3 ± 66.7 | 329.5 ± 68.9 | 0.001 |
| albumin (g/dl) | 4.00 ± 0.29 | 4.00 ± 0.29 | 3.99 ± 0.30 | <0.001 |
| white blood count (103/mm3) | 6.31 ± 2.10 | 6.26 ± 2.06 | 6.49 ± 2.26 | 0.001 |
| Health behaviors and medications | ||||
| current smoking | 688 (11.9%) | 514 (11.3%) | 174 (14.2%) | 0.005 |
| alcohol consumption | ||||
| 0/wk | 2870 (49.8%) | 2172 (47.8%) | 698 (57.3%) | <0.001 |
| ≤1/wk | 1107 (19.2%) | 882 (19.4%) | 225 (18.5%) | |
| >1/wk | 1786 (31%) | 1491 (32.8%) | 295 (24.2%) | |
| body mass index (kg/m2) | 26.7 ± 4.7 | 26.6 ± 4.6 | 26.9 ± 5.2 | 0.056 |
| physical activity (kcal/wk)a | 1065 (370 to 2280 | 1132 (422–2400) | 735 (203–1843) | <0.001 |
| anti-depressive medications | 208 (3.6%) | 119 (2.6%) | 90 (7.4%) | <0.001 |
| CES-D10 depression score | 4.7 ± 4.6 | 2.7 ± 2.2 | 11.9 ± 4.1 | c |
Isolated subclinical atherosclerosis status in participants without clinical coronary artery disease.
Data are reported as median interquartile range and compared using t tests on logarithmic-transferred values.
P value not calculated because CES-D was used to determine group status.
The mean creatinine level was 0.96 ± 0.31 mg/dl, and the eGFR-creat was 78.2 ± 22.9 ml/min per 1.73 m2. A total of 1232 (21.3%) participants were classified as stage 3 CKD and 44 (0.8%) as stage 4 CKD at study entry. The mean cystatin C level was 1.05 ± 0.28 mg/L.
Association of Depressive Symptoms with CKD Severity at Baseline
The presence of elevated depressive symptoms (CES-D ≥ 8) was associated with more prevalent stage 3 to 4 CKD compared with participants without depression (305/1276 [24.9%] versus 971/4560 [21.3%]: OR = 1.23, CI = 1.06 to 1.42). When adjusting for demographics, clinical variables, and health behaviors, this association remained significant (OR = 1.19, CI = 1.01 to 1.40, P = 0.041). Continuous CES-D scores were also associated with stage 3 to 4 CKD status (OR = 1.07, CI = 1.01 to 1.13 per square-root CES-D unit, P = 0.013). The CES-D depression score for individuals with CKD was 4.9 ± 4.6 versus 4.6 ± 4.5 in individuals with an eGFR ≥60 ml/min per m2 (P = 0.030).
Depressive symptom status at baseline was associated with lower eGFR among participants with stage 3 to 4 CKD (eGFR-creat = 49.9 ± 9.5 versus 51.0 ± 8.5 ml/min per 1.73 m2, P = 0.053; eGFR-cysC = 56.1 ± 16.5 versus 58.4 ± 14.9 ml/min per 1.73 m2, P = 0.029), but not in patients without CKD (eGFR-creat: P = 0.074; eGFR-cysC: P = 0.60; P interaction eGFR-creat = 0.009; P interaction eGFR-cysC = 0.10).
Longitudinal Association Between Depressive Symptoms with New-Onset CKD
A total of 4455/5785 (77.0%) participants had a valid follow-up measure for creatinine (389 [6.7% died before follow-up and 951 [16.4%] did not have a second blood draw with a valid creatinine measure). Absence of a follow-up blood draw was associated with elevated depressive symptoms at baseline (P < 0.001).
Among the patients without CKD at baseline (eGFR ≥60 ml/min per m2; n = 3561), the annual eGFR-creat decline rate was 0.62 ± 3.92 ml/min per 1.73 m2. Table 2 shows that the presence of elevated depressive symptoms at baseline was associated with a faster decline in eGFR-creat (P = 0.015) and more frequent occurrence of a rapid eGFR-creat decline (OR = 1.31, CI = 1.07 to 1.61, P = 0.009). Similar associations were found for the sustained depressive symptoms index for the occurrence of a rapid eGFR decline (OR = 1.017, CI = 1.011 to 1.024 per unit/yr, P < 0.001), and the correlation between this index and the annual decline was small but significant (r = 0.049, P = 0.003). Depressive symptoms at baseline were not significantly associated with incident (new-onset) stage 3 to 4 CKD after 3 or 7 years. Of the 692 patients with rapid decline, 209 (30.2%) developed CKD at follow-up, and depressive symptoms were significantly related to a rapid decline to levels meeting CKD stage 3 to 4 criteria (P = 0.017). When adjusting for demographics, depression was only related to rapid decline in creatinine-based eGFR, leading to incident CKD and cystatin c-based outcomes, whereas in fully adjusted models, none of the associations were significant (Table 2).
Table 2.
Association between depression and changes in creatinine and cystatin C from baseline to follow-up in patients without CKD at baseline
| Total (n = 3561) [mean ± SD or n (%)] | Low Depressive Symptoms (n = 2897) [mean ± SD or n (%)] | Elevated Depressive Symptoms (n = 664) [mean ± SD or n (%)] | P (Unadjusted) | P (Demographic Adjusted)a | P (Fully Adjusted) | |
|---|---|---|---|---|---|---|
| Creatinine-based GFR | ||||||
| annual declineb | 0.62 ± 3.92 | 0.54 ± 3.89 | 0.95 ± 4.05 | 0.015 | 0.12 | 0.67 |
| rapid declinec | 692 (19.4%) | 539 (18.4%) | 153 (23.0%) | 0.009 | 0.13 | 0.95 |
| CKD at 3-year follow-up | 330 (9.3%) | 258 (8.9%) | 72 (10.8%) | 0.12 | 0.21 | 0.45 |
| CKD at 7-year follow-upd | 210 (9.2%) | 167 (8.8%) | 43 (11.3%) | 0.12 | 0.12 | 0.53 |
| CKD during follow-up and rapid decline | 209 (5.9%) | 157 (5.4%) | 52 (7.8%) | 0.017 | 0.040 | 0.24 |
| Cystatin C–based GFR | ||||||
| annual declineb | 1.84 ± 2.66 | 1.78 ± 2.57 | 2.10 ± 2.97 | 0.006 | 0.008 | 0.096 |
| rapid declinec | 877 (25.8%) | 686 (24.7%) | 191 (30.2%) | 0.005 | 0.020 | 0.20 |
Adjusted for demographic measures (age, gender, race), clinical measures (coronary heart disease, subclinical atherosclerotic disease, systolic BP, glucose levels, total cholesterol, and HDL), inflammation-related markers (C-reactive protein, IL-6, fibrinogen), and health behaviors (current smoking status, body mass index, alcohol consumption, and physical activity levels).
Annual eGFR decline in ml/min per 1.73 m2.
Annual eGFR decline >3 ml/min per 1.73 m2.
Based on available patients alive in 1996 to 1997 (n = 2276).
Depressive Symptoms and Adverse Clinical Renal Events during Follow-Up
ESRD developed in 110/5785 (1.9%; 77 had definite or probable ESRD and 33 had possible ESRD). As shown in Figure 1, depressive symptoms at baseline were associated with an elevated risk of ESRD: 31/1225 (2.5%) versus 79/4560 (1.7%; HR = 1.62, CI = 1.07 to 2.45). Baseline eGFR was strongly associated with ESRD during follow-up, and the association between depressive symptoms and ESRD outcomes was not significant when adjusting for baseline eGFR-creat and covariates (Table 3; Figure 1).
Figure 1.
Survival curves for the unadjusted and covariate-adjusted longitudinal association between the presence of elevated depressive symptoms at study entry and incident ESRD during follow-up. Depressive symptom status was significantly associated with the development of incident ESRD, but this association became nonsignificant when adjusting for covariates (systolic BP, glucose levels, total cholesterol, HDL, CRP, IL-6, fibrinogen, current smoking status, body mass index, alcohol consumption, and physical activity levels).
Table 3.
Multivariable models for the association between depression for adverse renal outcomes during follow-up
| Clinical Events | Unadjusted [HR (95% CI)] | Adjusted for Demographics [HR (95% CI)] | Adjusted for Demographics and eGFR [HR (95% CI)] | Adjusted for Demographics eGFR, CHD and Subclinical Atherosclerosis [HR (95% CI)] | Adjusted for All Covariatesa [HR (95% CI)] |
|---|---|---|---|---|---|
| ESRD | 1.62 (1.07 to 2.46) | 1.76 (1.15 to 2.69) | 1.46 (0.95 to 2.22) | 1.45 (0.95 to 2.22) | 1.28 (0.82 to 2.01) |
| AKI | 1.52 (1.13 to 2.04) | 1.68 (1.25 to 2.27) | 1.62 (1.20 to 2.19) | 1.52 (1.12 to 2.06) | 1.53 (1.11 to 2.10) |
Adjusted for demographics (age, gender, race), creatinine-based eGFR at study entry, CHD, and subclinical atherosclerosis, and the fully adjusted model additionally included cardiovascular disease risk factors (systolic BP, glucose levels, total cholesterol, and HDL), inflammation-related markers (C-reactive protein, IL-6, fibrinogen), and health behaviors (current smoking status, body mass index, alcohol consumption, and physical activity levels).
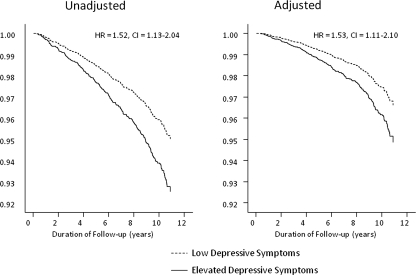
Hospitalization with AKI occurred in 225 (3.9%) participants during a median follow-up of 10.2 years (range, 5 days to 11.0 years). As shown in Figure 2, AKI occurred more often in depressed (n = 60; 5.0%) than nondepressed (n = 165; 3.7%) individuals (HR = 1.52, CI = 1.13 to 2.04). Continuous depressive symptom scores were also associated with subsequent AKI (HR = 1.26, CI = 1.12 to 1.41 per square root CES-D unit). Multivariable analyses indicated that associations with AKI remained significant (Table 3).
Figure 2.
Survival curves for the unadjusted and covariate-adjusted longitudinal association between the presence of elevated depressive symptoms at study entry and hospitalizations with AKI during follow-up. Depressive symptom status was significantly associated with AKI, which remained significant when adjusting for covariates (systolic BP, glucose levels, total cholesterol, HDL, CRP, IL-6, fibrinogen, current smoking status, body mass index, alcohol consumption, and physical activity levels).
Role of CKD Status and Comorbidities
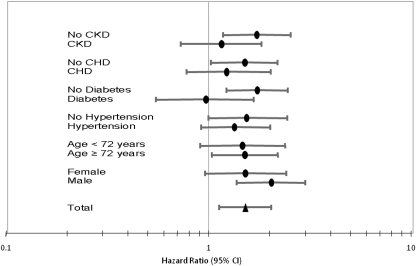
As shown in Figure 3, depressive symptoms at baseline were associated with AKI during follow-up in individuals without CKD (HR = 1.73, CI = 1.18–2.55) but not significantly for those with CKD (HR = 1.16, CI = 0.73 to 1.83; P interaction = 0.20). Exploratory analyses were conducted for other AKI risk factors. Depressive symptoms were significantly associated with AKI in individuals without coronary heart disease (HR = 1.51, CI = 1.03 to 2.19) but not in those with coronary heart disease (HR = 1.26, CI = 0.76 to 2.03). Depressive symptoms were associated with AKI in individuals without diabetes (HR = 1.73, CI = 1.23 to 2.46) but not in those with diabetes (HR = 0.97, CI = 0.55 to 1.67). AKI risks associated with depressive symptoms were stronger in men (HR = 2.04, CI = 1.38 to 3.00) than in women (HR = 1.52, CI = 0.96 to 2.42; Figure 3). The interaction terms between depressive symptom and CKD status or CV risk factors were not significant (P values between 0.08 and 0.59).
Figure 3.
Results of stratified analyses examining the relationship between depressive symptom status and incident AKI. Analyses are stratified by CKD status, presence of coronary heart disease, diabetes mellitus, hypertension, age (cut-off, 72 years), and gender.
Discussion
This longitudinal study shows that individuals with depressive symptoms are more likely to develop rapid declines in kidney function and develop clinical renal outcomes including ESRD and hospitalization with AKI during follow-up. The association of depressive symptoms with subsequent AKI was robust and remained significant when adjusting for kidney function at baseline and other potentially confounding factors. The association between depression and rapid decline in eGFR became nonsignificant in adjusted models. These findings suggest that prevalent depressive symptoms may improve the risk prediction of selected adverse renal outcomes.
These findings indicate that the presence of elevated depressive symptom levels is associated with AKI independent of coronary heart disease, cardiovascular risk factors, inflammation markers, and health behaviors. This association tended to be stronger in patients free of CKD compared with those with CKD at study entry. Exploratory subgroup analyses further indicated that these associations were also stronger in individuals without heart disease or traditional risk factors, although the interaction terms of these variables with depression were not statistically significant because of modest statistical power. Thus, the link between depression and AKI is not likely to be a direct consequence of underlying CKD or other comorbidities. We postulate that the predictive value of depression for hospitalizations with AKI may in part be explained by the predictive value of depression for acute coronary syndromes and heart failure, because related hospitalizations can be complicated by AKI. Depression may also increase the risk of other chronic diseases such as diabetes, chronic heart disease, and heart failure with their accompanying complex medical regimen. Polypharmacy may increase the risk of AKI and indicate an indirect mechanism by which depression is associated with AKI. Other pathways include poor adherence to medical regimen in depressed individuals and other adverse health behaviors such as smoking and alcohol overuse. Finally, depression may be related to delayed health care–seeking behaviors, difficulties in patient–physician communications, and possibly medical errors leading to AKI. These pathways are largely speculative, and further research is needed to identify biobehavioral mechanisms by which depression adversely affects risk of AKI and other adverse renal disease outcomes. Given the relatively rare occurrence of these events, study designs other than observational follow-up studies, such as case-crossover methodologies, may be needed to study these pathways.
Previous studies have found mixed results regarding the relationship between depression and renal disease severity indices among individuals with CKD who are not on dialysis (10,49–52). Rocco et al. (53) documented that quality of life and symptoms of psychologic distress were adversely affected by CKD in the Modification of Diet in Renal Disease study. These findings are consistent with the observations of the present investigation and show that associations between depressive symptoms and lower eGFR levels are primarily observed in individuals with CKD and not among those without CKD and that the effect size of this relationship is relatively small. Serum creatinine levels are differentially affected by kidney function and muscle mass (54), and both factors are known to decline with aging and are possibly related to depressive symptoms. Cystatin C is a potentially better predictor of GFR than creatinine (55). The optimal marker to identify increased CKD progression as related to psychologic and behavioral factors requires further investigation.
Study Limitations
Although the study was well powered to determine associations between depressive symptoms and CKD progression and AKI, the prevalence of clinical ESRD was low (approximately 5% over 10 years), which interfered with comprehensive multivariable analyses for clinical ESRD. Measures of incident CKD were based on biologic assays obtained at 3- and 7-year follow-up and did not include assessments of proteinuria. It is possible that longer, more frequent, and more comprehensive follow-ups are needed to show significant relationships. The unavailability of a follow-up blood draw was associated with depressive symptoms at baseline, which could have biased the observed relationship toward less significant associations. It is possible that residual confounding by comorbidities, disease severity, and surveillance bias may partially account for the observed associations, although the multivariable models and stratified analyses suggest that these factors are not likely to play a primary role, and associations between depression and comorbidities were primarily additive and not exponential. The AKI results may not be fully representative of all kidney damage because those who developed milder AKI may not have been hospitalized and those who were hospitalized may have had undetected AKI because chart reviews and ICD codes may not optimally detect AKI with low sensitivity (56). Polypharmacy and hospitalization-related changes in medication regimen may increase the risk of AKI, which was not controlled for in this study. To circumvent some of these limitations, we used a multiple endpoint strategy, examining both clinical endpoints (ESRD and AKI), as well as an endpoint consistently assessed across all participants (rapid decline in eGFR and incident CKD) where selection and surveillance bias is less pertinent. A final limitation is the reliance on questionnaire-based assessments for depression instead of interview-based diagnostic tools, potentially leading to false-positive classification (57) and lack of differentiation between primary and secondary (comorbidity-related) depression (58).
Conclusions
Depression may pose increased risk for the development of poor kidney function and clinical progression to ESRD and AKI. These findings underscore the partial interdependence of AKI and CKD as clinical syndromes. The association of depressive symptoms with long-term risk of AKI is independent of a broad range of covariates and therefore clinically important, whereas associations with ESRD during follow-up became nonsignificant in multivariable models. These findings show that the association between depression with adverse renal outcomes is not merely an epiphenomenon of underlying symptomatic renal disease. Future studies are needed to examine whether depression is important for patient risk stratification and whether treatment of depression will improve long-term outcomes in individuals with high vulnerability for developing renal disease.
Disclosures
None.
Acknowledgments
The first author, in collaboration with the Cardiovascular Heath Study coordinating center, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. This research was supported by Contracts N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, Grant U01 HL080295, and Grant R0-1 HL079376 from the National Heart, Lung, and Blood Institute, with additional contributions from the National Institute of Neurological Disorders and Stroke, the American Heart Association, and Grant CHS RO1 AG 027002 supporting the CHS Renal Working Group. A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Einwohner R, Bernardini J, Fried L, Piraino B: The effect of depressive symptoms on survival in peritoneal dialysis patients. Perit Dial Int 24: 256–263, 2004 [PubMed] [Google Scholar]
- 2. Kimmel PL, Peterson RA, Weihs KL, Simmens SJ, Alleyne S, Cruz I, Veis JH: Multiple measurements of depression predict mortality in a longitudinal study of chronic hemodialysis outpatients. Kidney Int 57: 2093–2098, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Ziarnik JP, Freeman CW, Sherrard DJ, Calsyn DA: Psychological correlates of survival on renal dialysis. J Nerv Ment Dis 164: 210–213, 1977 [DOI] [PubMed] [Google Scholar]
- 4. Wai L, Richmond J, Burton H, Lindsay RM: Influence of psychosocial factors on survival of home-dialysis patients. Lancet 2: 1155–1156, 1981 [DOI] [PubMed] [Google Scholar]
- 5. Burton HJ, Kline SA, Lindsay RM, Heidenheim AP: The relationship of depression to survival in chronic renal failure. Psychosom Med 48: 261–269, 1986 [DOI] [PubMed] [Google Scholar]
- 6. Shulman R, Price JD, Spinelli J: Biopsychosocial aspects of long-term survival on end-stage renal failure therapy. Psychol Med 19: 945–954, 1989 [DOI] [PubMed] [Google Scholar]
- 7. Lopes AA, Bragg J, Young E, Goodkin D, Mapes D, Combe C, Piera L, Held P, Gillespie B, Port FK: Depression as a predictor of mortality and hospitalization among hemodialysis patients in the United States and Europe. Kidney Int 62: 199–207, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Peterson RA, Kimmel PL, Sacks CR, Mesquita ML, Simmens SJ, Reiss D: Depression, perception of illness and mortality in patients with end-stage renal disease. Int J Psychiatry Med 21: 343–354, 1991 [DOI] [PubMed] [Google Scholar]
- 9. Novak M, Molnar MZ, Szeifert L, Kovacs AZ, Vamos EP, Zoller R, Keszei A, Mucsi I: Depressive symptoms and mortality in patients after kidney transplantation: A prospective prevalent cohort study. Psychosom Med 72: 527–534, 2010 [DOI] [PubMed] [Google Scholar]
- 10. Hedayati SS, Minhajuddin AT, Toto RD, Morris DW, Rush AJ: Prevalence of major depressive episode in CKD. Am J Kidney Dis 54: 424–432, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hedayati SS, Finkelstein FO: Epidemiology, diagnosis, and management of depression in patients with CKD. Am J Kidney Dis 54: 741–752, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kimmel PL: Psychosocial factors in dialysis patients. Kidney Int 59: 1599–1613, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Smith MD, Hong BA, Robson AM: Diagnosis of depression in patients with end-stage renal disease. Comparative analysis. Am J Med 79: 160–166, 1985 [DOI] [PubMed] [Google Scholar]
- 14. Watnick S, Kirwin P, Mahnensmith R, Concato J: The prevalence and treatment of depression among patients starting dialysis. Am J Kidney Dis 41: 105–110, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Walters BA, Hays RD, Spritzer KL, Fridman M, Carter WB: Health-related quality of life, depressive symptoms, anemia, and malnutrition at hemodialysis initiation. Am J Kidney Dis 40: 1185–1194, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Craven JL, Rodin GM, Littlefield C: The Beck Depression Inventory as a screening device for major depression in renal dialysis patients. Int J Psychiatry Med 18: 365–374, 1988 [DOI] [PubMed] [Google Scholar]
- 17. Israel M: Depression in dialysis patients: A review of psychological factors. Can J Psychiatry 31: 445–451, 1986 [DOI] [PubMed] [Google Scholar]
- 18. Levenson JL, Glocheski S: Psychological factors affecting end-stage renal disease. A review. Psychosomatics 32: 382–389, 1991 [DOI] [PubMed] [Google Scholar]
- 19. Finkelstein FO, Finkelstein SH: Depression in chronic dialysis patients: Assessment and treatment. Nephrol Dial Transplant 15: 1911–1913, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Kimmel PL, Weihs K, Peterson RA: Survival in hemodialysis patients: The role of depression. J Am Soc Nephrol 4: 12–27, 1993 [DOI] [PubMed] [Google Scholar]
- 21. Kimmel PL: Depression in patients with chronic renal disease: What we know and what we need to know. J Psychosom Res 53: 951–956, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS: Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 41: 1–12, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van LF, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, O'Leary DH, Psaty B, Rautaharju P, Tracy R: The Cardiovascular Health Study: Design and rationale. Ann Epidemiol 1: 263–276, 1991 [DOI] [PubMed] [Google Scholar]
- 25. Tell GS, Fried LP, Hermanson B, Manolio TA, Newman AB, Borhani NO: Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol 3: 358–366, 1993 [DOI] [PubMed] [Google Scholar]
- 26. Radloff LS: The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Measure 1: 385–401, 1977 [Google Scholar]
- 27. Andresen EM, Malmgren JA, Carter WB, Patrick DL, Radloff LS: Screening for depression in well older adults: Evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am J Prev Med 10: 77–84, 1994 [PubMed] [Google Scholar]
- 28. Schulz R, Beach SR, Ives DG, Martire LM, Ariyo AA, Kop WJ: Association between depression and mortality in older adults: The Cardiovascular Health Study. Arch Intern Med 160: 1761–1768, 2000 [DOI] [PubMed] [Google Scholar]
- 29. National Kidney Foundation: K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39:S1–S266, 2002 [PubMed] [Google Scholar]
- 30. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 31. Shlipak MG, Fried LF, Cushman M, Manolio TA, Peterson D, Stehman-Breen C, Bleyer A, Newman A, Siscovick D, Psaty B: Cardiovascular mortality risk in chronic kidney disease: Comparison of traditional and novel risk factors. JAMA 293: 1737–1745, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, Salem DN, Levey AS, Sarnak MJ: Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: A pooled analysis of community-based studies. J Am Soc Nephrol 15: 1307–1315, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Archibald G, Bartlett W, Brown A, Christie B, Elliott A, Griffith K, Pound S, Rappaport I, Robertson D, Semple Y, Slane P, Whitworth C, Williams B: UK Consensus Conference on Early Chronic Kidney Disease–6 and 7 February 2007. Nephrol Dial Transplant 22: 2455–2457, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Rifkin DE, Shlipak MG, Katz R, Fried LF, Siscovick D, Chonchol M, Newman AB, Sarnak MJ: Rapid kidney function decline and mortality risk in older adults. Arch Intern Med 168: 2212–2218, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Erlandsen EJ, Randers E, Kristensen JH: Evaluation of the Dade Behring N Latex Cystatin C assay on the Dade Behring Nephelometer II System. Scand J Clin Lab Invest 59: 1–8, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Coll E, Botey A, Alvarez L, Poch E, Quinto L, Saurina A, Vera M, Piera C, Darnell A: Serum cystatin C as a new marker for noninvasive estimation of glomerular filtration rate and as a marker for early renal impairment. Am J Kidney Dis 36: 29–34, 2000 [DOI] [PubMed] [Google Scholar]
- 37. Randers E, Erlandsen EJ, Pedersen OL, Hasling C, Danielsen H: Serum cystatin C as an endogenous parameter of the renal function in patients with normal to moderately impaired kidney function. Clin Nephrol 54: 203–209, 2000 [PubMed] [Google Scholar]
- 38. Perkins BA, Nelson RG, Ostrander BE, Blouch KL, Krolewski AS, Myers BD, Warram JH: Detection of renal function decline in patients with diabetes and normal or elevated GFR by serial measurements of serum cystatin C concentration: results of a 4-year follow-up study. J Am Soc Nephrol 16: 1404–1412, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van LF, Bruce RD, III, Zhang YL, Greene T, Levey AS: Estimating GFR using serum cystatin C alone and in combination with serum creatinine: A pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis 51: 395–406, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mittalhenkle A, Stehman-Breen CO, Shlipak MG, Fried LF, Katz R, Young BA, Seliger S, Gillen D, Newman AB, Psaty BM, Siscovick D: Cardiovascular risk factors and incident acute renal failure in older adults: The cardiovascular health study. Clin J Am Soc Nephrol 3: 450–456, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kuller L, Borhani N, Furberg C, Gardin J, Manolio T, O'Leary D, Psaty B, Robbins J: Prevalence of subclinical atherosclerosis and cardiovascular disease and association with risk factors in the Cardiovascular Health Study. Am J Epidemiol 139: 1164–1179, 1994 [DOI] [PubMed] [Google Scholar]
- 42. Tracy RP, Bovill EG, Yanez D, Psaty BM, Fried LP, Heiss G, Lee M, Polak JF, Savage PJ: Fibrinogen and factor VIII, but not factor VII, are associated with measures of subclinical cardiovascular disease in the elderly. Results from The Cardiovascular Health Study. Arterioscler Thromb Vasc Biol 15: 1269–1279, 1995 [DOI] [PubMed] [Google Scholar]
- 43. Macy EM, Hayes TE, Tracy RP: Variability in the measurement of C-reactive protein in healthy subjects: Implications for reference intervals and epidemiological applications. Clin Chem 43: 52–58, 1997 [PubMed] [Google Scholar]
- 44. Jenny NS, Tracy RP, Ogg MS, Luong lA, Kuller LH, Arnold AM, Sharrett AR, Humphries SE: In the elderly, interleukin-6 plasma levels and the −174G>C polymorphism are associated with the development of cardiovascular disease. Arterioscler Thromb Vasc Biol 22: 2066–2071, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Jr, Heimovitz H, Cohen HJ, Wallace R: Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med 106: 506–512, 1999 [DOI] [PubMed] [Google Scholar]
- 46. Zakai NA, Katz R, Jenny NS, Psaty BM, Reiner AP, Schwartz SM, Cushman M: Inflammation and hemostasis biomarkers and cardiovascular risk in the elderly: The Cardiovascular Health Study. J Thromb Haemost 5: 1128–1135, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Bovill EG, Bild DE, Heiss G, Kuller LH, Lee MH, Rock R, Wahl PW: White blood cell counts in persons aged 65 years or more from the cardiovascular health study. correlations with baseline clinical and demographic characteristics. Am J Epidemiol 143: 1107–1115, 1996 [DOI] [PubMed] [Google Scholar]
- 48. Hirsch CH, Fried LP, Harris T, Fitzpatrick A, Enright P, Schulz R: Correlates of performance-based measures of muscle function in the elderly: The cardiovascular health study. J Gerontol A Biol Sci Med Sci 52: M192–M200, 1997 [DOI] [PubMed] [Google Scholar]
- 49. Cohen SD, Patel SS, Khetpal P, Peterson RA, Kimmel PL: Pain, sleep disturbance, and quality of life in patients with chronic kidney disease. Clin J Am Soc Nephrol 2: 919–925, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Shidler NR, Peterson RA, Kimmel PL: Quality of life and psychosocial relationships in patients with chronic renal insufficiency. Am J Kidney Dis 32: 557–566, 1998 [DOI] [PubMed] [Google Scholar]
- 51. Perlman RL, Finkelstein FO, Liu L, Roys E, Kiser M, Eisele G, Burrows-Hudson S, Messana JM, Levin N, Rajagopalan S, Port FK, Wolfe RA, Saran R: Quality of life in chronic kidney disease (CKD): A cross-sectional analysis in the Renal Research Institute-CKD study. Am J Kidney Dis 45: 658–666, 2005 [DOI] [PubMed] [Google Scholar]
- 52. Odden MC, Whooley MA, Shlipak MG: Depression, stress, and quality of life in persons with chronic kidney disease: The Heart and Soul Study. Nephron Clin Pract 103: c1–c7, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rocco MV, Gassman JJ, Wang SR, Kaplan RM: Cross-sectional study of quality of life and symptoms in chronic renal disease patients: The Modification of Diet in Renal Disease Study. Am J Kidney Dis 29: 888–896, 1997 [DOI] [PubMed] [Google Scholar]
- 54. Haas M, Spargo BH, Wit EJ, Meehan SM: Etiologies and outcome of acute renal insufficiency in older adults: A renal biopsy study of 259 cases. Am J Kidney Dis 35: 433–447, 2000 [DOI] [PubMed] [Google Scholar]
- 55. Dharnidharka VR, Kwon C, Stevens G: Serum cystatin C is superior to serum creatinine as a marker of kidney function: A meta-analysis. Am J Kidney Dis 40: 221–226, 2002 [DOI] [PubMed] [Google Scholar]
- 56. Waikar SS, Wald R, Chertow GM, Curhan GC, Winkelmayer WC, Liangos O, Sosa MA, Jaber BL: Validity of International Classification of Diseases, Ninth Revision, Clinical Modification Codes for Acute Renal Failure. J Am Soc Nephrol 17: 1688–1694, 2006 [DOI] [PubMed] [Google Scholar]
- 57. Hedayati SS, Minhajuddin AT, Toto RD, Morris DW, Rush AJ: Validation of depression screening scales in patients with CKD. Am J Kidney Dis 54: 433–439, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ: Assessing depressive symptoms in five psychiatric populations: A validation study. Am J Epidemiol 106: 203–214, 1977 [DOI] [PubMed] [Google Scholar]