Summary
Background and objectives
The serum proteins sclerostin and Dickkopf-1 (Dkk-1) are soluble inhibitors of canonical wnt signaling and were recently identified as components of parathyroid hormone (PTH) signal transduction. This study investigated the associations between sclerostin and Dkk-1 with histomorphometric parameters of bone turnover, mineralization, and volume in stage 5 chronic kidney disease patients on dialysis (CKD-5D).
Design, setting, participants, & measurements
In a cross-sectional study, 60 CKD-5D patients underwent bone biopsies followed by histomorphometry. Levels of sclerostin, Dkk-1, and intact PTH (iPTH) were determined in blood.
Results
Serum levels of sclerostin and iPTH correlated negatively. In unadjusted analyses, sclerostin correlated negatively with histomorphometric parameters of turnover, osteoblastic number, and function. In adjusted analyses, sclerostin remained a strong predictor of parameters of bone turnover and osteoblast number. An observed correlation between sclerostin and cancellous bone volume was lost in regression analyses. Sclerostin was superior to iPTH for the positive prediction of high bone turnover and number of osteoblasts. In contrast, iPTH was superior to sclerostin for the negative prediction for high bone turnover and had similar predictive values than sclerostin for the number of osteoblasts. Serum levels of Dkk-1 did not correlate with iPTH or with any histomorphometric parameter.
Conclusions
Our data describe a promising role for serum measurements of sclerostin in addition to iPTH in the diagnosis of high bone turnover in CKD-5D patients, whereas measurements of Dkk-1 do not seem to be useful for this purpose.
Introduction
Chronic kidney disease (CKD) is associated with abnormalities in bone and mineral metabolism (1). At the tissue level, renal osteodystrophy (ROD) represents histopathologic changes observed in bone and is typically characterized by changes in bone turnover, volume, and mineralization. On the molecular level, one of the critical pathways for regulating the balance between bone formation and resorption in patients with ROD is parathyroid hormone (PTH) signaling (2).
Canonical wnt signaling is a molecular pathway known to be crucial for the regulation of bone physiology. Wnt ligands bind to the frizzled-LRP5/6 membrane receptor complex, leading to increased bone formation (3). The effect of canonical wnt signaling on bone is mediated by stimulation of stem cell and preosteoblast proliferation, induction of osteoblastogenesis, and inhibition of osteoblast and osteocyte apoptosis (4).
Sclerostin and Dickkopf-1 (Dkk-1) are two soluble inhibitors of wnt signaling that bind to the LRP5/6 co-receptors and impede formation of an active wnt receptor complex (3). Sclerostin is a member of the cystine-knot family of proteins and is produced by osteocytes (5,6). Sclerostin has been shown to be expressed at the sites of bone formation in bone and cartilage (7). Knockout or loss of sclerostin leads to increased bone formation in mice and to bone overgrowth found in sclerosteosis/Van Buchem's disease in humans (8–10). In preclinical studies using rats and primates, sclerostin neutralizing monoclonal antibody treatment showed osteoanabolic effects with marked increases in bone formation and bone strength (11,12). Moreover, a recent clinical study evaluating the efficacy of a sclerostin antibody in postmenopausal women reported an increase in N-terminal propeptide of type I collagen and a 6% increase in lumbar spine bone mineral density (13).
Dkk-1 is expressed in many tissues during embryogenesis, including bone (14). Aberrant expression of Dkk-1 has been found in diseases that impair bone health, such as multiple myeloma (15). Attenuation of Dkk-1 levels by a heterozygous gene knockout or neutralization of Dkk-1 by anti-Dkk-1 antibody treatment leads to increased bone formation and bone volume (16,17).
Preclinical studies suggest that there is a close cross-talk between PTH and wnt signaling. Activation of the PTH receptor leads to downregulation of sclerostin and Dkk-1 and activation of intracellular wnt signal transduction (18–21). Thus, the action of PTH on bone is at least partly mediated through regulation of sclerostin and Dkk-1.
In light of the specific regulatory properties of sclerostin and Dkk-1 for osteoblastic proliferation and differentiation, we evaluated the associations between serum levels of sclerostin and Dkk-1 and histomorphometric parameters of bone turnover, volume, and mineralization in patients with stage 5 CKD on dialysis.
Materials and Methods
Patients and Study Design
For this cross-sectional study, bone biopsy was done and blood was drawn in 60 CKD stage 5 white patients on chronic maintenance hemodialysis in the United States. Bone biopsies and blood drawings were performed for research purposes. The study was conducted according to the Declaration of Helsinki, and the protocol was reviewed and approved by the Institutional Review Boards of the University of Kentucky, Lexington, KY. All patients gave informed consent. Causes for development of stage 5 CKD requiring dialysis therapy were diabetes mellitus (22%), hypertension (30%), glomerular disease (8%), cystic kidney disease (7%), and unknown (33%).
Inclusion criteria were age ≥18 years, maintenance hemodialysis three times a week, adequate hemodialysis (Kt/V ≥ 1.2), naïve or on steady dose of vitamin D analogs for ≥6 months, willingness, and mental competence to participate in the study.
Exclusion criteria were renal transplantation, pregnancy or lactation, history of parathyroidectomy, use of calcimimetics, and life-threatening comorbid conditions such as malignancy, active infection, and hepatic disease.
Mineralized Bone Histology and Bone Histomorphometry
For double labeling of bone, patients received oral tetracycline hydrochloride 500 mg twice daily for 2 days, followed by a 10-day tetracycline-free interval and another course of tetracycline hydrochloride at the same dosage for 4 days. Anterior iliac crest bone biopsies were performed after an additional 4 days (bone samples: 0.3 cm diameter × 2 cm length). Iliac crest bone samples were fixed with ethanol at room temperature, dehydrated, and embedded in methylmethacrylate as described previously (22). Serial sections of 3 and 7 μm thickness were cut with a microtome (Model HM360; Microm, Walldorf, Germany) equipped with a carbide-edged knife. Sections were stained with modified Masson-Goldner trichrome stain (23), aurin tricarboxylic acid stain (24), and solochrome azurine stain (25). Unstained sections were prepared for phase-contrast and fluorescence light microscopy. Bone histomorphometry for static and dynamic parameters of bone structure, formation, and resorption was done at a magnification of ×200 using the Osteoplan II system (C. Zeiss, New York, NY). Activation frequency (Ac.f.) and bone formation rate/bone surface (BFR/BS) were calculated for assessment of bone turnover, whereas bone volume/tissue volume (BV/TV) was calculated for assessment of mineralized bone volume. For cortical bone, cortical width was measured; cortical porosity was determined by tracing the total cortex and all Haversian canals and computing the ratio between canal area over total cortical tissue area. All measured histomorphometric parameters were in compliance with the recommendations of the nomenclature committee of the American Society of Bone and Mineral Research (26). All bone samples were processed and analyzed at the Bone Diagnostic and Research Laboratory, University of Kentucky (Lexington, KY).
Laboratory Measurements
Blood was drawn at time of bone biopsy. After centrifugation, serum was aliquoted in cryovials, stored at −80°C, and analyzed without freeze/thaw cycles. Sclerostin was measured by an ELISA. Briefly, microtiter plates (MaxiSorp; Nunc-Thermo Fisher Scientific, Waltham, MA) were coated with 100 μl of monoclonal anti-sclerostin antibody (MAB1406; R&D Systems, Minneapolis, MN) at a concentration of 2 μg/ml in carbonate buffer (pH 9.6) and were incubated at 4°C overnight. Plates were washed with PBS containing 0.05% Tween 20 (Sigma-Aldrich, Vienna, Austria) and blocked with PBS containing 0.05% Tween and 1% human serum albumin (Sigma-Aldrich). Fifty microliters of serum was loaded per well, incubated overnight at 4°C, washed, and incubated for 1 hour at 37°C, followed by incubation at 4°C for 1 hour with a biotinylated polyclonal anti-sclerostin antibody (BAF 1406; R&D Systems) diluted to a concentration of 0.5 μg/ml in dilution buffer. Wells were washed with PBS/Tween, followed by the addition of 100 μl of a 1:20,000 dilution of streptavidin–horseradish peroxidase (Endogen-Thermo Fisher Scientific, Waltham, MA). Color development was achieved with the tetramethylbenzidine substrate system (Chemicon-Millipore, Billerica, MA). Serial dilutions of recombinant human sclerostin (1406-ST; R&D Systems) were used to establish a standard curve. Normal values in 44 healthy volunteers age 19 to 76 years are between 131 and 1156 pg/ml; intra- and interassay coefficients of variation are 7.5 and 6.3%, respectively.
Dkk-1 was measured using a commercially available ELISA (Biomedica, Vienna, Austria) according to manufacturer's recommendations. Normal values in 44 healthy volunteers age 19 to 76 years are between 28 and 256 pmol/L; intra- and interassay coefficients of variation are 7 and 9%, respectively.
Intact PTH (iPTH) level was measured by a radioimmunometric assay (Scantibodies, Santee, CA): normal range is 14 to 66 pg/ml; intra- and interassay coefficients of variation are <5 and <7%, respectively. Blood chemistry measurements for calcium and phosphorus were done using automated techniques.
Statistical Analyses
Continuous variables are expressed as mean ± SD and frequency counts as percentages. P ≤ 0.05 was considered statistically significant. Spearman correlation coefficients were used to assess the bivariate relationships between demographic, serum biochemical, and bone parameters. Multiple linear regression analysis was used to determine independent associations between demographic, biochemical parameters, and bone histomorphometric parameters of bone turnover, mineralization, and volume. Independent variables for inclusion were selected using a backward stepwise algorithm with P ≤ 0.20 to enter the model and P ≤ 0.05 to remain in the model. The classification of “low,” “normal,” and “high” bone turnover was based on our normative database (27–29). The outcome group “low” bone turnover was defined as Ac.f. <0.49/yr and BFR/BS <1.8 mm3/cm2 per year. The outcome group “normal” bone turnover was defined as Ac.f. = 0.49 to 0.72/yr and BFR/BS = 1.8 to 3.8 mm3/cm2 per year. The outcome group “high” bone turnover was defined as Ac.f. >0.72/yr and BFR/BS >3.8 mm3/cm2 per year. Sclerostin and iPTH thresholds that achieve empiric specificities of at least 80% were calculated, and the corresponding sensitivities and positive and negative predictive values were estimated. All parameters of bone histology were log-transformed for analyses. All statistical calculations were performed with R version 2.7.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Demographic and laboratory parameters of the study population are presented in Table 1. Our data showed a statistically significant inverse correlation between sclerostin and iPTH (ρ = −0.34, P = 0.01), whereas sclerostin and serum calcium correlated positively (ρ = 0.26, P = 0.04). No correlation was found between sclerostin and Dkk-1 (ρ = −0.04, P = 0.74) or other biochemical and demographic parameters.
Table 1.
Demographic and biochemical characteristics of the study population
| n = 60 | |
|---|---|
| Age (years) | 61 ± 12 |
| Gender (female, male, %) | 50, 50 |
| HD duration (months) | 75 ± 34 |
| Diabetes (%) | 22 |
| Vitamin D analog treatment (%) | 40 |
| Sclerostin i.s. (pg/ml) | 2055 ± 1239 |
| Dkk-1 i.s. (pmol/l) | 49.9 ± 20.4 |
| iPTH i.s. (pg/ml) | 287 ± 342 |
| Calcium i.s. (mg/dl) | 9.02 ± 0.71 |
| Phosphorus i.s. (mg/dl) | 4.73 ± 1.02 |
HD, hemodialysis; i.s., in serum.
Sclerostin and Bone Turnover Parameters
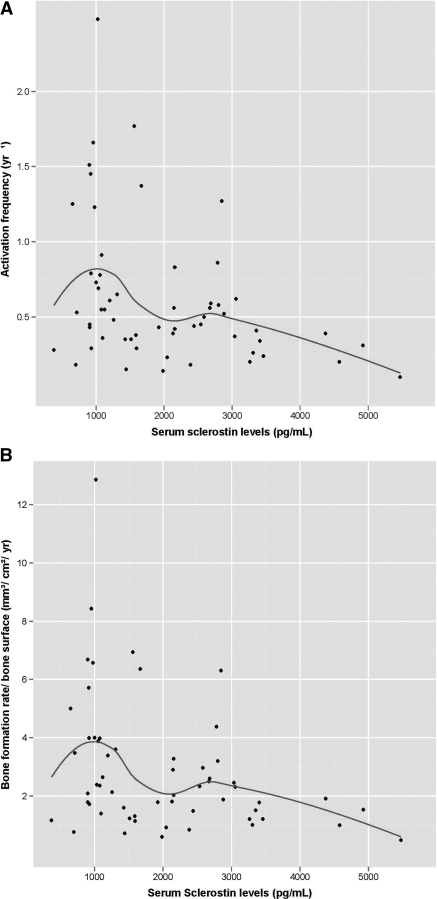
Sclerostin showed significant negative associations with parameters of bone turnover Ac.f. and BFR/BS in unadjusted analyses (Table 2; Figure 1, A and B). The cut-off points, sensitivities, and positive and negative predictive values at 80% specificity of sclerostin for Ac.f. and BFR/BS are shown in Table 3. After adjusting for age, gender, hemodialysis duration, presence of diabetes mellitus, and vitamin D treatment, sclerostin remained a strong predictor of bone turnover parameters (Ac.f.: β-estimate = 0.001, P < 0.003; BFR/BS: β-estimate = 0.001, P = 0.007).
Table 2.
Correlation coefficients (ρ) between demographic, biochemical, and bone histomorphometric parameters
| Sclerostin | Dkk-1 | iPTH | Age | HD Duration | Calcium | Phosphorus | |
|---|---|---|---|---|---|---|---|
| Ac.f. | −0.36a | 0.16 | 0.22b | −0.09 | 0.02 | 0.01 | 0.02 |
| BFR/BS | −0.35a | 0.15 | 0.23b | −0.11 | 0.01 | −0.01 | 0.04 |
| BFR/Ob | 0.31a | −0.05 | −0.26a | 0.22b | 0.01 | 0.11 | 0.04 |
| NOb/BPm | −0.45a | 0.09 | 0.34a | −0.25a | −0.03 | −0.13 | 0.03 |
| NOc/BPm | −0.24b | −0.06 | 0.25a | −0.20b | −0.05 | −0.07 | 0.01 |
| E.De | −0.32a | −0.02 | −0.02 | −0.23b | −0.03 | −0.13 | −0.30a |
| BV/TV | −0.29a | −0.06 | 0.35a | −0.24b | 0.01 | −0.14 | −0.12 |
| Ct.Po | −0.02 | −0.20 | 0.31b | −0.19 | −0.22 | −0.16 | 0.05 |
| Ct.Wi | −0.15 | 0.01 | 0.20 | −0.24 | 0.04 | −0.26b | 0.01 |
| O.Th | −0.42a | 0.01 | 0.27a | −0.16 | 0.05 | −0.51a | −0.22b |
| Mlt | −0.20b | −0.03 | 0.07 | −0.01 | 0.10 | −0.47a | −0.27a |
HD duration, hemodialysis duration; E.De, erosion depth; Ct.Po, cortical porosity; Ct.Wi, cortical width; O.Th, osteoid thickness; Mlt, mineralization lag time.
P < 0.05.
P < 0.2 for entry into regression analysis.
Figure 1.
(A and B) Scatter plot of serum concentrations of sclerostin (pg/ml) at different levels of Ac.f. (A) and different levels of BFR/BS (B).
Table 3.
Cut-off thresholds that achieve at least 80% specificity for predicting bone histomorphometric abnormalities with corresponding Sens., PPV, and NPV
| Assay | Bone Parameter | Low |
High |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Cut-off | Sens. | PPV | NPV | Cut-off | Sens. | PPV | NPV | ||
| Sclerostin (pg/ml) | Ac.f. | 2784 | 0.33 | 0.67 | 0.53 | 2387 | 0.44 | 0.90 | 0.35 |
| BFR/BS | 2800 | 0.35 | 0.57 | 0.66 | 1925 | 0.59 | 0.93 | 0.40 | |
| NOb/BPm | 3036 | 0.33 | 0.08 | 0.96 | 1588 | 0.62 | 0.97 | 0.32 | |
| BV/TV | 2849 | 0.29 | 0.57 | 0.57 | 3058 | 0.19 | 0.64 | 0.39 | |
| iPTH (pg/ml) | Ac.f. | 356 | 0.39 | 0.60 | 0.64 | 382 | 0.42 | 0.41 | 0.81 |
| BFR/BS | 335 | 0.40 | 0.72 | 0.53 | 391 | 0.43 | 0.38 | 0.84 | |
| NOb/BPm | 163 | 0.62 | 0.97 | 0.23 | 335 | 0.74 | 0.44 | 0.94 | |
| BV/TV | 335 | 0.35 | 0.75 | 0.44 | 434 | 0.26 | 0.33 | 0.76 | |
Sens., sensitivity; PPV, positive predictive value; NPV, negative predictive value.
Sclerostin and Cellular Parameters of Bone Formation and Resorption, Bone Volume, and Mineralization
Although unadjusted analyses showed statistically significant associations between sclerostin and osteoblast numbers (NOb/BPm), as well as osteoblast function measured by bone formation rate/osteoblast (BFR/Ob; Table 2), only the association with osteoblast number remained significant in linear regression analyses (NOb/BPm: β-estimate = 0.001, P < 0.01). No statistically significant associations between sclerostin and parameters of NOc/BPm or function could be found. A relationship between sclerostin and BV/TV could not be shown in linear regression despite a weak correlation in unadjusted analysis (Table 2). Sclerostin was not associated with parameters of bone mineralization. The cut-off points, sensitivities, and positive and negative predictive values at 80% specificity of sclerostin for NOb/BPm are shown in Table 3.
iPTH and Bone Parameters
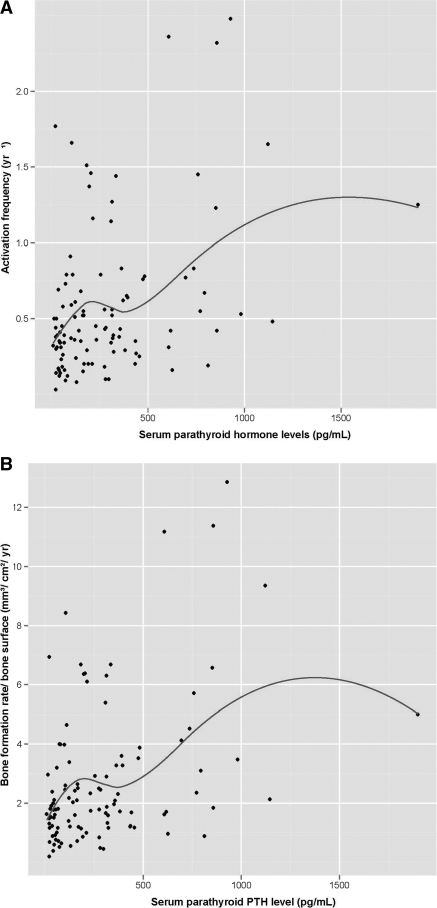
The association between bone turnover parameters (Ac.f. and BFR/BS) and iPTH are shown in Figure 2, A and B. In unadjusted analyses (Table 2), iPTH showed statistically significant positive correlations with NOb/BPm and NOc/BPm and a significant negative correlation with BFR/Ob. The associations with osteoblast number and function were maintained in linear regression analyses after adjustment for age, gender, hemodialysis duration, presence of diabetes mellitus, and vitamin D therapy (NOb/BPm: β-estimate = 0.001, P = 0.006; BFR/Ob: β-estimate = 0.001, P = 0.006), whereas the association with osteoclast number was lost. The weak but statistically significant correlations between iPTH and BV/TV and iPTH and cortical porosity observed in unadjusted analyses (Table 2) were lost in linear regression analyses. The cut-off points, sensitivities, and positive and negative predictive values at 80% specificity for iPTH are shown in Table 3.
Figure 2.
(A and B) Scatter plot of serum concentrations of iPTH (pg/ml) at different levels of Ac.f. (A) and different levels of BFR/BS (B).
Comparison between Sclerostin and iPTH for Predicting Bone Parameters
Sclerostin was superior to iPTH for the positive prediction of high Ac.f., BFR/BS, and NOb/BPm (Table 3). In contrast, iPTH was superior to sclerostin for the negative prediction of the same parameters. Negative and positive predictive values for sclerostin and iPTH remained <0.72 for low bone turnover (measured by Ac.f. and BFR/BS). Only NOb/BPm in low bone turnover was negatively predicted by sclerostin and positively by iPTH.
Dkk-1 and Bone Parameters
Dkk-1 did not show any statistically significant association with parameters of bone turnover, resorption, formation, volume, or mineralization in unadjusted and adjusted analyses (Table 2).
Discussion
To our knowledge, this is the first study reporting associations between serum sclerostin levels and bone histomorphometric parameters in stage 5 CKD patients on dialysis. Sclerostin levels in our study were higher (2055 ± 1239 pg/ml) than values reported in patients without CKD (premenopausal women, 480 ± 150 pg/ml; postmenopausal women, 1160 ± 380 pg/ml) (30). This observation may be explained by increased retention of sclerostin caused by lack of renal function and/or increased sclerostin production associated with renal osteodystrophy.
We found a statistically significant negative correlation between sclerostin and iPTH, expanding on recently reported findings in postmenopausal women without CKD (30). This negative correlation is in agreement with the negative regulatory function of sclerostin in the intracellular transduction of the PTH signal described in vitro and in vivo (18–20). Sclerostin, however, showed stronger correlations with parameters of bone turnover (measured by Ac.f and BFR/BS) than iPTH in unadjusted analyses. More importantly, only sclerostin was associated with bone turnover in adjusted analyses, pointing to a superior value of sclerostin as a noninvasive indicator of bone turnover than iPTH. However, this superiority was only observed for prediction of high bone turnover. Sclerostin had positive predictive values >0.9 for all three histomorphometric parameters (Ac.f, BFR/BS, and NOb/BPm). In contrast, iPTH showed high values for negative prediction of high bone turnover. Based on these results, one could envision using iPTH for routine screening applications followed by use of sclerostin for diagnosis of high bone turnover.
For low bone turnover, negative and positive predictive values of sclerostin and iPTH did not reach levels >0.72, which indicates that both noninvasive parameters are not helpful for screening or diagnosis of this entity.
Despite reports that PTH signal transduction downregulates Dkk-1 expression in vitro, treatment with recombinant PTH has been reported to increase serum levels of Dkk-1 in osteoporotic patients (20,31,32). Moreover, Dkk-1 expression has been reported to be positively correlated with bone mineral density in bone biopsy specimens of postmenopausal women and also with bone loss in arthritic patients (33,34). Thus, the contribution of Dkk-1 to bone biology in humans remains to be elucidated. Our data show that serum measurements of Dkk-1 are not associated with structural and cellular bone parameters in stage 5 CKD patients on dialysis. This might be related to a poor correlation between concentrations of circulating and cell membrane bound Dkk-1 or to other factors that influence the production and/or breakdown of Dkk-1 in this patient population.
In summary, our data describe a promising role for serum measurements of sclerostin in addition to iPTH in the diagnosis of high bone turnover in stage 5 CKD patients, whereas measurements of Dkk-1 do not seem to be useful for this purpose. It is of note that Dkk-1 values in this study's CKD population did not differ from those in the general population, whereas sclerostin values were clearly higher.
Disclosures
None.
Acknowledgments
This study was supported by the Dean's Clinical Research Scholar Program, University of Kentucky, Grant 1012112710 (to J.H.), NIH RO1 DK080770-01 (to H.H.M.), and the Kentucky Nephrology Research Trust (to M.-C.M.-F.). We thank Guodong Wang, MD, Richard Wheaton, and Julia Van Willigen for technical support and M. M. Naveen for review of the literature.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Sclerostin: Just One More Player in Renal Bone Disease?” on pages 700–703.
Access to UpToDate on-line is available for additional clinical information at www.cjasn.org.
References
- 1. Malluche H, Faugere MC: Renal bone disease 1990: An unmet challenge for the nephrologist. Kidney Int 38: 193–211, 1990 [DOI] [PubMed] [Google Scholar]
- 2. Malluche HH, Koszewski N, Monier-Faugere MC, Williams JP, Mawad H: Influence of the parathyroid glands on bone metabolism. Eur J Clin Invest 36[Suppl 2]: 23–33, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Baron R, Rawadi G: Targeting the Wnt/beta-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology 148: 2635–2643, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Krishnan V, Bryant HU, Macdougald OA: Regulation of bone mass by Wnt signaling. J Clin Invest 116: 1202–1209, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Veverka V, Henry AJ, Slocombe PM, Ventom A, Mulloy B, Muskett FW, Muzylak M, Greenslade K, Moore A, Zhang L, Gong J, Qian X, Paszty C, Taylor RJ, Robinson MK, Carr MD: Characterization of the structural features and interactions of sclerostin: Molecular insight into a key regulator of Wnt-mediated bone formation. J Biol Chem 284: 10890–10900, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, Shpektor D, Jonas M, Kovacevich BR, Staehling-Hampton K, Appleby M, Brunkow ME, Latham JA: Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J 22: 6267–6276, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kusu N, Laurikkala J, Imanishi M, Usui H, Konishi M, Miyake A, Thesleff I, Itoh N: Sclerostin is a novel secreted osteoclast-derived bone morphogenetic protein antagonist with unique ligand specificity. J Biol Chem 278: 24113–24117, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, D'Agostin D, Kurahara C, Gao Y, Cao J, Gong J, Asuncion F, Barrero M, Warmington K, Dwyer D, Stolina M, Morony S, Sarosi I, Kostenuik PJ, Lacey DL, Simonet WS, Ke HZ, Paszty C: Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res 23: 860–869, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Balemans W, Ebeling M, Patel N, Van Hul E, Olson P, Dioszegi M, Lacza C, Wuyts W, Van Den Ende J, Willems P, Paes-Alves AF, Hill S, Bueno M, Ramos FJ, Tacconi P, Dikkers FG, Stratakis C, Lindpaintner K, Vickery B, Foernzler D, Van Hul W: Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum Mol Genet 10: 537–543, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Balemans W, Patel N, Ebeling M, Van Hul E, Wuyts W, Lacza C, Dioszegi M, Dikkers FG, Hildering P, Willems PJ, Verheij JB, Lindpaintner K, Vickery B, Foernzler D, Van Hul W: Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet 39: 91–97, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li X, Ominsky MS, Warmington KS, Morony S, Gong J, Cao J, Gao Y, Shalhoub V, Tipton B, Haldankar R, Chen Q, Winters A, Boone T, Geng Z, Niu QT, Ke HZ, Kostenuik PJ, Simonet WS, Lacey DL, Paszty C: Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res 24: 578–588, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Ominsky MS, Vlasseros F, Jolette J, Smith SY, Stouch B, Doellgast G, Gong J, Gao Y, Cao J, Graham K, Tipton B, Cai J, Deshpande R, Zhou L, Hale MD, Lightwood DJ, Henry AJ, Popplewell AG, Moore AR, Robinson MK, Lacey DL, Simonet WS, Paszty C: Two doses of sclerostin antibody in cynomolgus monkeys increases bone formation, bone mineral density, and bone strength. J Bone Miner Res 25: 948–959, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Padhi D, Stouch B, Jang G, Fang L, Darling M, Glise H, Robinson M, Harris S, Posvar E: Anti-sclerostin antibody increases markers of bone formation in healthy postmenopausal women [abstract]. J Bone Miner Metab 22[Suppl 1]: 37, 2007 [Google Scholar]
- 14. Pinzone JJ, Hall BM, Thudi NK, Vonau M, Qiang YW, Rosol TJ, Shaughnessy JD, Jr.: The role of Dickkopf-1 in bone development, homeostasis, and disease. Blood 113: 517–525, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, Shaughnessy JD, Jr.: The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med 349: 2483–2494, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Morvan F, Boulukos K, Clement-Lacroix P, Roman Roman S, Suc-Royer I, Vayssiere B, Ammann P, Martin P, Pinho S, Pognonec P, Mollat P, Niehrs C, Baron R, Rawadi G: Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J Bone Miner Res 21: 934–945, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Fulciniti M, Tassone P, Hideshima T, Vallet S, Nanjappa P, Ettenberg SA, Shen Z, Patel N, Tai YT, Chauhan D, Mitsiades C, Prabhala R, Raje N, Anderson KC, Stover DR, Munshi NC: Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood 114: 371–379, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bellido T, Ali AA, Gubrij I, Plotkin LI, Fu Q, O'Brien CA, Manolagas SC, Jilka RL: Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: A novel mechanism for hormonal control of osteoblastogenesis. Endocrinology 146: 4577–4583, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Keller H, Kneissel M: SOST is a target gene for PTH in bone. Bone 37: 148–158, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Kulkarni NH, Halladay DL, Miles RR, Gilbert LM, Frolik CA, Galvin RJ, Martin TJ, Gillespie MT, Onyia JE: Effects of parathyroid hormone on Wnt signaling pathway in bone. J Cell Biochem 95: 1178–1190, 2005 [DOI] [PubMed] [Google Scholar]
- 21. O'Brien CA, Plotkin LI, Galli C, Goellner JJ, Gortazar AR, Allen MR, Robling AG, Bouxsein M, Schipani E, Turner CH, Jilka RL, Weinstein RS, Manolagas SC, Bellido T: Control of bone mass and remodeling by PTH receptor signaling in osteocytes. PLoS One 3: e2942, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Malluche HH, Faugere MC: Atlas of Mineralized Bone Histology, New York, Karger, 1986 [Google Scholar]
- 23. Goldner J: A modification of the Masson trichrome technique for routine laboratory purposes. Am J Pathol 14: 237–243, 1938 [PMC free article] [PubMed] [Google Scholar]
- 24. Lillie PD, Fullmer HM: Histopathologic Technique and Practical Histochemistry, New York, McGraw Hill, 1976 [Google Scholar]
- 25. Denton J, Freemont AJ, Ball J: Detection of distribution of aluminum in bone. J Clin Pathol 37: 136–142, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche HH, Meunier PJ, Ott SM, Recker RR: Bone histomorphometry: standardization of nomenclature, symbols and units. J Bone Miner Res 6: 595–610, 1987 [DOI] [PubMed] [Google Scholar]
- 27. Sawaya BP, Butros R, Naqvi S, Geng Z, Mawad H, Friedler R, Fanti P, Monier-Faugere MC, Malluche HH: Differences in bone turnover and intact PTH levels between African American and Caucasian patients with end-stage renal disease. Kidney Int 64: 737–742, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Malluche HH, Monier-Faugere MC, Wang G, Fraza OJ, Charytan C, Coburn JW, Coyne DW, Kaplan MR, Baker N, McCary LC, Turner SA, Goodman WG: An assessment of cinacalcet HCl effects on bone histology in dialysis patients with secondary hyperparathyroidism. Clin Nephrol 69: 269–278, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Ferreira A, Frazao JM, Monier-Faugere MC, Gil C, Galvao J, Oliveira C, Baldaia J, Rodrigues I, Santos C, Ribeiro S, Hoenger RM, Duggal A, Malluche HH: Effects of sevelamer hydrochloride and calcium carbonate on renal osteodystrophy in hemodialysis patients. J Am Soc Nephrol 19: 405–412, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mirza FS, Padhi ID, Raisz LG, Lorenzo JA: Serum sclerostin levels negatively correlate with parathyroid hormone levels and free estrogen index in postmenopausal women. J Clin Endocrinol Metab 95: 1991–1997, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guo J, Liu M, Yang D, Bouxsein ML, Saito H, Galvin RJ, Kuhstoss SA, Thomas CC, Schipani E, Baron R, Bringhurst FR, Kronenberg HM: Suppression of Wnt signaling by Dkk1 attenuates PTH-mediated stromal cell response and new bone formation. Cell Metab 11: 161–171, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Anastasilakis AD, Polyzos SA, Avramidis A, Toulis KA, Papatheodorou A, Terpos E: The effect of teriparatide on serum Dickkopf-1 levels in postmenopausal women with established osteoporosis. Clin Endocrinol (Oxf) 72: 752–757, 2010 [DOI] [PubMed] [Google Scholar]
- 33. Reppe S, Refvem H, Gautvik VT, Olstad OK, Hovring PI, Reinholt FP, Holden M, Frigessi A, Jemtland R, Gautvik KM: Eight genes are highly associated with BMD variation in postmenopausal Caucasian women. Bone 46: 604–612, 2010 [DOI] [PubMed] [Google Scholar]
- 34. Garnero P, Tabassi NC, Voorzanger-Rousselot N: Circulating dickkopf-1 and radiological progression in patients with early rheumatoid arthritis treated with etanercept. J Rheumatol 35: 2313–2315, 2008 [DOI] [PubMed] [Google Scholar]