Abstract
Aims
The endothelium plays a role in regulating vascular tone. Acute and dynamic changes in low-flow-mediated constriction (L-FMC) and how it changes with regard to traditional flow-mediated dilatation (FMD) have not been described. We aimed to investigate the changes in brachial artery L-FMC following percutaneous coronary intervention (PCI) and during recovery from non-ST-segment elevation myocardial infarction (NSTEMI).
Methods and results
FMD was performed in accordance with a previously described technique in patients before and after PCI and in the recovery phase of NSTEMI, but in addition, L-FMC data were acquired from the last 30 s of cuff inflation. About 135 scans were performed in 96 participants (10 healthy volunteers and 86 patients). Measurement of brachial L-FMC was reproducible over hours. L-FMC was greater among patients with unstable vs. stable coronary atherosclerosis (−1.33 ±1.09% vs. −0.03 ± 1.26%, P < 0.01). Following PCI, FMD reduced (4.43 ± 2.93% vs. 1.66 ± 2.16%, P < 0.01) and L-FMC increased (−0.33 ± 0.76% vs. −1.63 ± 1.15%, P = 0.02). Furthermore, during convalescence from NSTEMI, L-FMC reduced (−1.37 ± 1.19% vs. 0.01 ± 0.82%, P = 0.02) in parallel with improvements in FMD (2.54 ± 2.19% vs. 5.15 ± 3.07%, P < 0.01).
Conclusion
Brachial L-FMC can be measured reliably. Differences were observed between patients with stable and unstable coronary disease. L-FMC was acutely increased following PCI associated with reduced FMD and, in the recovery from NSTEMI, L-FMC reduced associated with increased FMD. These novel findings characterize acute and subacute variations in brachial L-FMC. The pathophysiological and clinical implications of these observations require further study.
Keywords: Flow-mediated dilatation, Low-flow-mediated constriction, Percutaneous coronary intervention, Non-ST-segment elevation myocardial infarction
See page 784 for the editorial comment on this article (doi:10.1093/eurheartj/ehq412)
Introduction
Disturbed vascular function is important in the pathophysiology of atherosclerosis and cardiovascular disease.1 Endothelial-dependent vasodilatation can be quantified non-invasively using flow-mediated vasodilation (FMD).2 FMD is reproducible, is easily performed, correlates closely to coronary endothelial function,3 and has consequently been used extensively in order to explore the interaction between endothelial dysfunction among subjects at risk of cardiovascular disease and those with established disease.4 Although FMD allows for quantification of the endothelial response to increases in vascular shear stress and is a measure of nitric oxide bioactivity, in most studies there is no information reported about basal tone or response to low flow present prior to the measurement of FMD.5
However, with the advent of FMD techniques that include continuous imaging during both cuff occlusion (low flow) and release (hyperaemic flow) phases, a more detailed vascular profile is available. Gori et al.5 have described a novel index for assessing the response of the radial artery to low flow, which utilizes data obtained from the cuff occlusion period of an FMD scan. Synonymous to FMD, the vasoconstriction observed under conditions of reduced flow has been named low-flow-mediated vasoconstriction (L-FMC). Inclusion of L-FMC data to traditional measurement of FMD provides additional and complementary information, which, they propose, may improve the detection of patients with cardiovascular disease and profile the vascular response to exercise among healthy volunteers.6 Whether the integration of L-FMC into traditional FMD studies will provide additional information among patients with advanced symptomatic cardiovascular disease is unknown.
Furthermore, FMD can also be influenced by a number of systemic influences, including coronary angioplasty and in the early phase of acute coronary syndrome, and how L-FMC is influenced in these situations is unknown but has implications for understanding the measure. L-FMC has not been assessed within the brachial artery, which remains the site where the majority of FMD data exist.
Therefore, in this study, we aimed to primarily investigate acute changes in brachial L-FMC in patients before and after percutaneous coronary intervention (PCI) and to characterize longitudinal changes in L-FMC among a cohort of patients with non-ST-segment elevation myocardial infarction (NSTEMI) in relation to FMD. Secondarily, we aimed to investigate its relationship to changes in circulating levels of endothelin-1 (ET-1), inflammatory cytokines, and pro-inflammatory markers.
Methods
The study was approved by our local research and Ethics Committee, and all subjects provided written informed consent.
Population
Eighty-six patients attending Harefield Hospital, Royal Brompton & Harefield NHS Trust, for investigation or interventional management of symptomatic coronary artery disease were invited to participate. Study inclusion criteria included age between 25 and 80 years, stable or unstable angina pectoris, including NSTEMI, and the ability to provide written informed consent. Patients were not eligible if they presented with acute ST-segment elevation myocardial infarction, were suffering from concurrent illness, were taking immunosuppressive or immunomodulatory pharmacotherapy, or were known to have severe left ventricular systolic dysfunction (left ventricular ejection fraction <35%). Patients were removed from the study if they experienced complications during PCI. Ten healthy volunteers were recruited for the validation studies.
Measurement of flow-mediated dilatation and low-flow-mediated constriction
Participants fasted for at least 6 h prior to their initial vascular scan and continued to fast until the second scan had been performed 2 h later. Participants refrained from ingesting caffeine or smoking cigarettes for 12 h prior to the initial vascular scan and until after the second scan had been performed. Vasoactive pharmacotherapy was continued in accordance with clinical necessity. Previously published data have demonstrated that the majority of these drugs do not significantly affect peripheral endothelial function.7
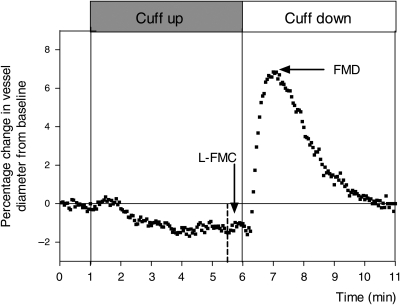
In brief, the brachial artery was imaged longitudinally using high-resolution ultrasound (Acuson 128XP/10) and followed a previously described and validated FMD technique.4 End-diastolic images were acquired continuously throughout the study (Brachial ImagerTM). Vessel diameters were calculated using commercially available automatic B-mode edge detection software (Brachial AnalyserTM). FMD was calculated as maximum percentage change in the vessel diameter from baseline following cuff release, which has recently been shown to be the most reproducible method of calculating FMD when compared with alternative techniques.8 L-FMC was calculated from the mean arterial diameter during the last 30 s before cuff release and expressed as percentage change from baseline, as reported recently5 (Figure 1). Data were expressed as both the mean of these values and also over the whole 30 s. FMD and L-FMC were summated in order to generate a composite score. Brachial artery blood flow was measured continuously during the scan.
Figure 1.
Raw data from a representative scan illustrating the measurement of low-flow-mediated constriction and flow-mediated dilatation; the brachial artery was continuously imaged for 11 min (1 min rest, 5 min cuff inflation, and 5 min cuff deflation). Low-flow-mediated constriction was calculated from data acquired during the last 30 s of cuff inflation. Flow-mediated dilatation was calculated as maximum percentage change in the vessel diameter from baseline following cuff release.
Measurement of endothelin-1, cytokines, and pro-inflammatory markers
Blood tests were performed using a conventional Vacutainer® technique via the antecubital vein and a 21-gauge needle. Fresh venepuncture was performed each time a sample was required, and blood was collected directly into an EDTA-containing tube (Becton Dickinson, Franklin Lakes, NJ, USA). Samples were then immediately centrifuged, and the resulting plasma supernatant was removed and frozen in 2 mL aliquots at −80°C for later analysis. Once collected, all samples were analysed at the same time. Levels of ET-1 were measured using a commercially available enzyme immunoassay technique (QuantiGlo™ Human Endothelin-1 Immunoassay, R&D Systems, Abingdon, UK). The detection range for ET-1 using this assay was 0.8–28 pg/mL. Levels of cytokines and pro-inflammatory markers, including high-sensitivity C-reactive protein (hs-CRP), interleukin-6 (IL-6), monocyte chemotactic protein-1 (MCP-1), CD40L, tumour necrosis factor-α (TNFα), plasminogen activator inhibitor-1 (PAI-1), matrix metalloproteinase-9 (MMP-9), myeloperoxidase (MPO), and soluble vascular cell adhesion molecule-1 (sVCAM-1), were measured using a commercially available multiplexed bead-based immunoassay (LUMINEXTM, LINCO Research, MO, USA).
Percutaneous coronary intervention protocol
PCI was performed by an experienced operator. Vascular access was via the right femoral artery. Following intravenous administration of heparin, conventional monorail stent systems were used. Wherever possible, a single stent strategy was adopted; however, multiple stents were used where necessary. The arterial sheath was removed at the end of the procedure, and a vascular closure device (AngiosealTM) was deployed.
Statistical analysis
Statistical analysis of the data was performed using SPSS version 17 and Graph Pad Prism version 4. Distribution of L-FMC data was assessed using a Shapiro–Wilk test of normality and a Q–Q plot, which suggested a normal distribution. Data are expressed as mean (±SD) or n (%). Baseline clinical characteristics between groups were assessed using an unpaired Student's t-test, χ2, or Fisher's exact test when appropriate. Two-way analysis of variance (ANOVA) was used to assess for a difference in individual data points, during the last 30 s of cuff inflation, between serial scans. A paired Student's t-test was used to assess for differences in the calculated mean L-FMC. Correlation between values of L-FMC and FMD was assessed using regression analysis. Serial changes in the levels of ET-1 and cytokines measured in the same subject were assessed using a paired Student's t-test. A P-value of less than 0.05 was considered significant.
Study design
Validation of brachial measures
Validation of FMD and L-FMC measurements in our laboratory over time was investigated in 8 patients before and after diagnostic angiography alone and after a similar time interval in 10 healthy volunteers who did not undergo any procedure.
Brachial measures in a cohort of patients with coronary atherosclerosis
Eighty-six patients admitted for diagnostic or invasive management of symptomatic coronary disease were invited to participate and underwent a measurement of FMD and L-FMC on admission to hospital.
Acute effects of percutaneous coronary intervention
The acute effects of PCI were measured in 10 patients undergoing elective procedures. Measurement of brachial FMD and L-FMC, together with circulating plasma levels of ET-1, inflammatory cytokines, and pro-inflammatory markers, was repeated 2 h post-stent deployment and compared with baseline levels.
Recovery from non-ST-segment elevation myocardial infarction
A cohort of 11 patients with NSTEMI (defined as troponin-positive) was studied in the acute phase and in the convalescent period, following either percutaneous or surgical revascularization. Measurements of brachial FMD and L-FMC were compared with circulating plasma levels of ET-1, inflammatory cytokines, and pro-inflammatory markers.
Results
Brachial flow-mediated dilatation and low-flow-mediated constriction in healthy volunteers and patients
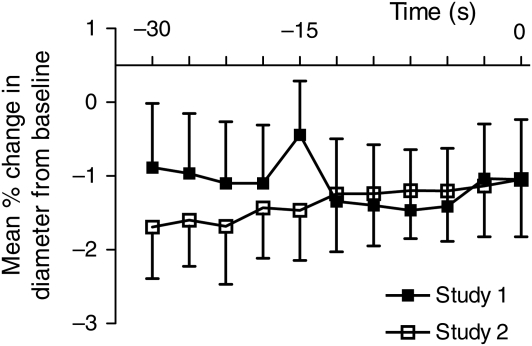
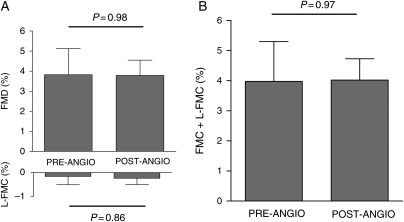
The mean age of healthy volunteers was 28 (±4) years, two were female, and no subjects were taking any regular medication. No significant changes in FMD or L-FMC were observed between the baseline and the repeated scan 2 h later (FMD, 7.53 ± 6.37% vs. 7.63 ± 4.35%, P = 0.92; L-FMC, −1.20 ± 2.52% vs. −1.34 ± 2.16%, P = 0.86, ANOVA P = 0.97) (Table 1 and Figure 2). Eight patients (four with stable atherosclerosis and four with NSTEMI) were investigated before and 2 h after angiography alone. Mean age was 58 (±5) years. All patients undergoing angiography were pre-treated with aspirin and statin medication, but did not receive clopidogrel or heparin at the time of angiography. No significant changes in FMD (3.82 ± 3.68% vs. 3.79 ± 2.15%, P = 0.98) or L-FMC (−0.18 ± 1.04% vs. −0.11 ± 0.56%, P = 0.86, ANOVA P = 0.89) were observed following angiography (Table 2 and Figure 3).
Table 1.
Reproducibility of low-flow-mediated constriction among healthy volunteers, n= 10
| Baseline | 2 h | P-value | |
|---|---|---|---|
| Baseline vessel diameter (mm) | 3.61 (±0.67) | 3.64 (±0.68) | 0.49 |
| Baseline flow (mL/min) | 16.5 (±8.4) | 23.2 (±15.4) | 0.25 |
| Hyperaemic response (%) | 1641 (±1301) | 1113 (±621) | 0.15 |
| FMD (%) | 7.53 (±6.37) | 7.63 (±4.35) | 0.92 |
| L-FMC (%) | −1.20 (±2.52) | −1.34 (±2.16) | 0.86 |
| Time to peak (min) | 6.92 (±0.39) | 6.93 (±0.25) | 0.94 |
Figure 2.
Mean percentage change in the vessel diameter compared with baseline for each individual measurement during the last 30 s before cuff deflation (at time = 0) during repeated healthy volunteer studies, P = 0.97 (n= 10).
Table 2.
Effects of angiography alone on low-flow-mediated constriction, n= 8
| Before angiography | After angiography | P-value | |
|---|---|---|---|
| Baseline vessel diameter (mm) | 4.38 (±0.94) | 4.41 (±0.76) | 0.79 |
| Baseline flow (mL/min) | 33.2 (±16.0) | 29.1 (±14.8) | 0.49 |
| Hyperaemic response (%) | 678 (±320) | 752 (±258) | 0.38 |
| FMD (%) | 3.82 (±3.68) | 3.79 (±2.15) | 0.98 |
| L-FMC (%) | −0.18 (±1.04) | −0.11 (±0.56) | 0.86 |
| GTN (%) | 8.56 (±5.34) | 7.54 (±3.47) | 0.25 |
Figure 3.
Separate (A) and composite (B) brachial flow-mediated dilatation and low-flow-mediated constriction before and after diagnostic angiography (n= 8).
Population from which study patients were drawn
A total of 115 vascular scans were performed in 86 patients with symptomatic coronary artery disease. Eighty-six scans were performed at baseline from which brachial FMD data were obtained in 65 (76%) of the studies. From these 65 baseline scans, brachial L-FMC data were available in 42 patients (65%). Reasons for study failure included difficulty in acquiring high-quality images, due to vessel tortuosity and calcification, and patient movement, which led to incomplete image acquisition and consequently exclusion of the scan. Of the 42 studies with image data that allowed for the calculation of L-FMC, 24 patients were with stable disease, whereas 18 patients were investigated 6.6 (±3.4) days following presentation to hospital with NSTEMI (peak troponin I: 2.1 ± 2.7 µg/L) (Table 3).
Table 3.
Patient characteristics
| Stable atherosclerosis (n= 24) | NSTEMI (n= 18) | P-value | |
|---|---|---|---|
| Age (years) | 61 (±10) | 57 (±10) | 0.24 |
| Male (n, %) | 21 (88) | 14 (78) | 0.68 |
| Treated with PCI (n, %) | 20 (83) | 9 (50) | 0.04 |
| Use of DES (n, %) | 16 (80) | 7 (78) | 0.90 |
| Angiography alone (n, %) | 4 (17) | 9 (50) | 0.05 |
| BMI | 29.0 (±3.8) | 29.3 (±4.8) | 0.84 |
| Number of risk factors per subject | 2.8 (±1.0) | 2.5 (±1.1) | 0.27 |
| Current smoker (n, %) | 2 (8) | 7 (39) | 0.04 |
| Hypertension (n, %) | 15 (63) | 10 (56) | 0.89 |
| Diabetes mellitus (n, %) | 6 (25) | 2 (11) | 0.50 |
| Hypercholesterolaemia (n, %) | 18 (75) | 10 (56) | 0.32 |
| Family history of IHD (n, %) | 12 (50) | 11 (61) | 0.69 |
| Other known vascular disease (n, %) | 7 (29) | 2 (11) | 0.34 |
| Prescribed statin therapy (n, %) | 21 (88) | 17 (94) | 0.82 |
| ACEI/ARB (n, %) | 18 (75) | 14 (78) | 0.88 |
| Long-acting nitrate (n, %) | 3 (13) | 1 (6) | 0.77 |
| Calcium channel blocker (n, %) | 10 (42) | 0 | 0.01 |
| Aspirin and clopidogrel (n, %) | 1 (4) | 18 (100) | <0.01 |
| Haemoglobin (g/dL) | 14.5 (±1.2) | 14.5 (±1.5) | 0.92 |
| White cell count (×109/L) | 7.6 (±1.3) | 8.6 (±3.5) | 0.23 |
| Platelet count (×109/L) | 261 (±80) | 277 (±53) | 0.50 |
| Serum creatinine (µmol/L) | 92.8 (±15.0) | 90.8 (±16.1) | 0.68 |
| Total cholesterol (mmol/L) | 4.4 (±0.8) | 4.6 (±1.0) | 0.46 |
| HDL-C (mmol/L) | 1.1 (±0.3) | 0.9 (±0.2) | 0.10 |
| TC:HDL-C ratio | 4.1 (±1.0) | 5.5 (±1.6) | 0.01 |
| TNI (µg/L) | N/A | 2.1 (±2.7) | N/A |
BMI, body mass index; RF, risk factor; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor antagonist; HDL-C, high-density lipoprotein cholesterol; TC:HDL-C, total cholesterol: high-density lipoprotein cholesterol; TNI, troponin-I.
Impact of non-ST-segment elevation myocardial infarction
Compared with subjects with stable coronary disease, patients with NSTEMI were of similar age [57 (±10) vs. 61 (±10) years, P = 0.24], had been exposed to a similar number of conventional cardiovascular risk factors [current smoker, treated hypertension, hypercholesterolaemia, diabetes, or a family history of premature coronary artery disease; 2.5 (±1.1) vs. 2.8 (±1.0), P = 0.27], were more likely to be current smokers [7 (39%) vs. 2 (8%), P = 0.04], had a higher total cholesterol: high-density lipoprotein cholesterol ratio (5.5 ± 1.6 vs. 4.1 ± 1.0, P = 0.01), and were less likely to be taking a calcium channel blocker. All patients were prescribed oral dual antiplatelet pharmacotherapy (aspirin 75 mg and clopidogrel 75 mg per day). Only one patient received pre-treatment with a long-acting nitrate, which was stopped following coronary angioplasty. All other pharmacotherapy remained unchanged following PCI. Baseline brachial artery diameter, baseline flow, hyperaemic response (HR), and peak FMD were the same between patient groups; however, L-FMC was significantly greater among subjects with unstable disease (−1.33 ± 1.09% vs. −0.03 ± 1.26%, P < 0.01) (Table 4).
Table 4.
Baseline flow-mediated dilatation and low-flow-mediated constriction in patients with stable and unstable coronary atherosclerosis
| Stable atherosclerosis (n= 24) | Unstable atherosclerosis (n= 18) | P-value | |
|---|---|---|---|
| Baseline vessel diameter (mm) | 4.43 (±1.04) | 4.35 (±1.29) | 0.83 |
| Baseline flow (mL/min) | 26.8 (±15.9) | 26.2 (±9.8) | 0.89 |
| Hyperaemic response (%) | 846 (±425) | 862 (±300) | 0.90 |
| Peak FMD (%) | 3.51 (±3.80) | 3.44 (±3.33) | 0.95 |
| L-FMC (%) | −0.03 (±1.26) | −1.33 (±1.09) | <0.01 |
Relationship of flow-mediated dilatation to low-flow-mediated constriction
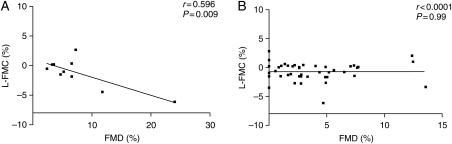
When individual baseline values of FMD were directly compared with values of L-FMC, a significant correlation of increasing FMD with increasing L-FMC was seen among healthy volunteers (r= 0.596, P = 0.009), which was not present in subjects with clinically symptomatic atherosclerosis (Figure 4).
Figure 4.
Correlation of brachial flow-mediated dilatation to low-flow-mediated constriction among (A) healthy volunteers (n= 10) and (B) patients with coronary atherosclerosis (n= 42).
Relationship of flow-mediated dilatation and low-flow-mediated constriction to burden of coronary atherosclerosis
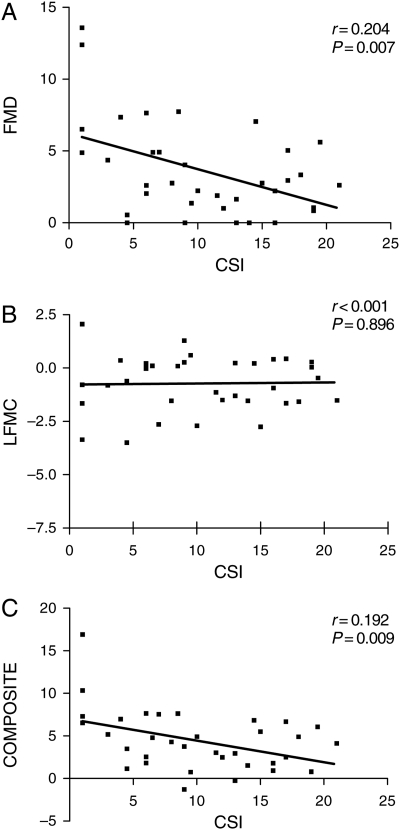
Using a previously described technique,9,10 a coronary score index (CSI) was calculated for each patient who had complete coronary angiographic data available (n= 36). FMD and the composite sum of FMD and L-FMC were observed to demonstrate a statistically significant linear relationship, with increasing CSI being related to a reduction in FMD and composite score. However, no such association was observed for L-FMC alone (Figure 5).
Figure 5.
Association of extent and severity of coronary atherosclerosis (coronary score index, CSI) with (A) flow-mediated dilatation, (B) low-flow-mediated constriction, and (C) composite score of flow-mediated dilatation plus low-flow-mediated constriction (n= 36).
Acute effects of coronary angioplasty (percutaneous coronary intervention)
In the 10 patients studied before and after elective PCI, mean age was 58 (±12) years, one patient was female, and all patients were taking statin therapy. Mean number of stents used per patient was 1.7 (±0.8), with a mean length of 19.2 mm (±4.9 mm) and a diameter of 3.1 mm (±0.5 mm). Five patients received drug-eluting stents and five received bare metal stents. All patients were placed on dual antiplatelet therapy (aspirin 75 mg and clopidogrel 75 mg per day) at least 7 days prior to admission, with one patient receiving intravenous glycoprotein IIb/IIIa inhibition immediately following PCI. No other acute changes in medication were made at the 2 h time point following PCI.
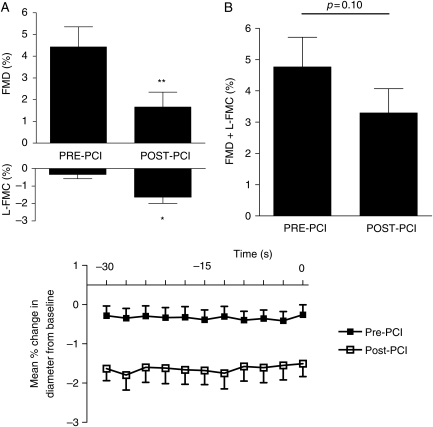
Baseline vessel diameter, baseline flow, flow during cuff inflation, and HR did not change 2 h after angioplasty (Table 5). However, there was a significant reduction in FMD (4.43 ± 2.93% vs. 1.66 ± 2.16%, P < 0.01) and a marked increase in L-FMC (−0.33 ± 0.76% vs. −1.63 ± 1.15%, P = 0.02, ANOVA <0.001) 2 h after PCI (Figure 6). Response to glyceryl trinitrate (GTN) was not altered (8.51 ± 5.21% vs. 7.38 ± 4.34%, P = 0.15).
Table 5.
Effects of percutaneous coronary intervention on flow-mediated dilatation and low-flow-mediated constriction, n= 10
| Before PCI | After PCI | P-value | |
|---|---|---|---|
| Baseline vessel diameter (mm) | 4.73 (±1.03) | 5.14 (±0.90) | 0.06 |
| Baseline flow (mL/min) | 18.7 (±7.0) | 26.1 (±9.2) | 0.09 |
| Flow during cuff up (mL/min) | 2.6 (±1.8) | 2.9 ± (1.5) | 0.30 |
| Hyperaemic response (%) | 1111 (±424) | 1012 (±293) | 0.53 |
| FMD (%) | 4.43 (±2.93) | 1.66 (±2.16) | <0.01 |
| L-FMC (%) | −0.33 (±0.76) | −1.63 (±1.15) | 0.02 |
| GTN (%) | 8.51 (±5.21) | 7.38 (±4.34) | 0.15 |
Figure 6.
Flow-mediated dilatation and low-flow-mediated constriction before and 2 h after percutaneous coronary intervention (n= 10). Upper graphs: separate (A) and composite (B) brachial flow-mediated dilatation and low-flow-mediated constriction (*P = 0.02 and **P < 0.01). Lower graph: mean percentage change in the vessel diameter compared with baseline for each individual measurement during the last 30 s before cuff deflation (at time = 0), analysis of variance, P < 0.001.
Longitudinal changes in low-flow-mediated constriction
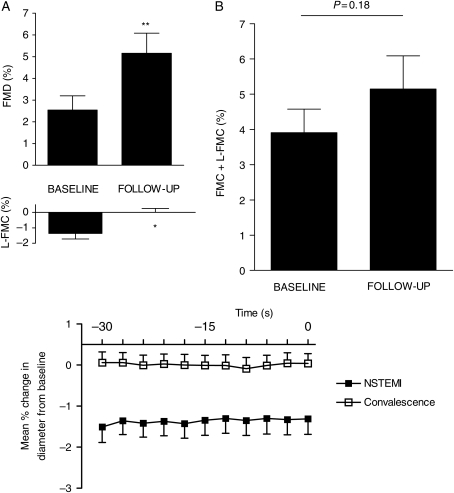
Eleven patients with NSTEMI were investigated at presentation (baseline) and after a mean of 2.5 (±1.7) months follow-up. All patients had received either percutaneous (PCI, n= 7) or surgical revascularization [coronary artery bypass graft (CABG), n= 4]. There were no changes in the baseline vessel diameter, baseline flow, flow during cuff inflation, or HR (Table 6) between initial (baseline) and repeated (follow-up) vascular studies. Clopidogrel prescription was stopped among patients who received CABG; however, all other pharmacotherapy remained unchanged between baseline and follow-up vascular studies. FMD improved significantly (from 2.54 ± 2.19% to 5.15 ± 3.07%, P < 0.01). Associated with this increase in FMD, L-FMC decreased significantly (from −1.37±1.19% to 0.01±0.82%, P = 0.02, ANOVA <0.001) (Figure 7).
Table 6.
Change in flow-mediated dilatation and low-flow-mediated constriction following convalescence from non-ST-segment elevation myocardial infarction, n= 11
| Baseline | Follow-up | P-value | |
|---|---|---|---|
| Baseline vessel diameter (mm) | 4.83 (±0.45) | 4.62 (±0.40) | 0.07 |
| Baseline flow (mL/min) | 29.2 (±8.5) | 35.9 (±20.3) | 0.17 |
| Flow during cuff up (mL/min) | 3.9 (±3.3) | 3.5 (±2.7) | 0.26 |
| Hyperaemic response (%) | 865 (±327) | 810 (±326) | 0.58 |
| FMD (%) | 2.54 (±2.19) | 5.15 (±3.07) | <0.01 |
| L-FMC (%) | −1.37 (±1.19) | 0.01 (±0.82) | 0.02 |
| GTN (%) | 7.93 (±3.09) | 8.78 (±3.47) | 0.23 |
Figure 7.
Flow-mediated dilatation and low-flow-mediated constriction at the time of non-ST-segment elevation myocardial infarction (baseline) and after a period of convalescence (follow-up), n= 11. Upper graphs: separate (A) and composite (B) brachial flow-mediated dilatation and low-flow-mediated constriction (*P = 0.02 and **P < 0.01). Lower graph: mean percentage change in the vessel diameter compared with baseline for each individual measurement during the last 30 s before cuff deflation (at time = 0), analysis of variance, P < 0.001.
Endothelin-1
Circulating plasma levels of ET-1 did not change following PCI [baseline 1.51 pg/mL (±0.53) vs. 2 h post-PCI 1.57 pg/mL (±0.38), P = 0.75] nor after a period of recovery from NSTEMI [baseline 1.47 pg/mL (±0.36) vs. follow-up 1.39 pg/mL (±0.36), P = 0.42].
Cytokines and pro-inflammatory markers
Circulating levels of inflammatory markers were measured in the same 10 patients who had vascular function assessed before and after PCI. Compared with baseline measurements, circulating levels of CD40L, sVCAM-1, PAI-1, and TNFα reduced 2 h after PCI, whereas levels of MPO increased significantly (Tables 7 and8). No statistically significant changes in the levels of circulating inflammatory markers were observed among patients investigated at the time of NSTEMI and again following a period of convalescence (Tables 7 and 8).
Table 7.
Levels of circulating inflammatory markers: before and 2 h after percutaneous coronary intervention, n = 10
| Before PCI | 2 h after PCI | P-value | |
|---|---|---|---|
| hs-CRP | 16 590 | 7915 | 0.30 |
| IL-6 | 69 (143) | 32 (64) | 0.21 |
| MCP-1 | 223 (74) | 205 (87) | 0.24 |
| CD40L | 1060 (6832) | 645 (503) | 0.01 |
| TNFα | 4.7 (0.7) | 4.2 (0.6) | 0.02 |
| PAI-1 | 23 349 (13 195) | 11 595 (3458) | 0.02 |
| MMP-9 | 76 945 (31 611) | 62 298 (23 917) | 0.18 |
| MPO | 4851 (4931) | 164 249 (110 009) | <0.01 |
| sVCAM-1 | 928 (187) | 826 (113) | 0.02 |
hs-CRP, high sensitivity C-reactive protein; IL-6, interleukin-6; MCP-1, monocyte chemotactic protein-1; PAI-1, plasminogen activator inhibitor-1; MMP-9, matrix metalloproteinase-9; MPO, myeloperoxidase; sVCAM-1, soluble vascular cell adhesion molecule-1.
All data pg/mL, expressed as mean (±standard deviation).
Table 8.
Levels of circulating inflammatory markers: at the time of non-ST-segment elevation myocardial infarction and after a period of convalescence (follow-up), n= 11
| NSTEMI | Follow-up | P-value | |
|---|---|---|---|
| IL-6 | 110 (135) | 91 (104) | 0.18 |
| MCP-1 | 181 (38) | 197 (32) | 0.07 |
| CD40L | 1209 (1735) | 1630 (2082) | 0.30 |
| TNFα | 4.3 (1.1) | 4.7 (1.3) | 0.18 |
| PAI-1 | 14 506 (11 714) | 16 845 (13 218) | 0.65 |
| MMP-9 | 116 422 (40 116) | 83 266 (40 825) | 0.06 |
| MPO | 8100 (6198) | 32 892 (56 781) | 0.16 |
| sVCAM-1 | 742 (175) | 827 (224) | 0.19 |
IL-6, interleukin-6; MCP-1, monocyte chemotactic protein-1; PAI-1, plasminogen activator inhibitor-1; MMP-9, matrix metalloproteinase-9; MPO, myeloperoxidase; sVCAM-1, soluble vascular cell adhesion molecule-1.
All data are in pg/mL, and are expressed as mean (±SD).
Discussion
The novel findings from this study are that there is an acute and dynamic increase in systemic brachial artery L-FMC following coronary intervention, which is associated with systemic endothelial dysfunction characterized by a reduction in FMD. Also, that in the natural longitudinal phase of recovery from acute coronary syndrome, L-FMC reduces from that measured at the index scan and is associated with an improvement in systemic FMD. Furthermore, we demonstrate that brachial L-FMC can be reproducibly measured over a short (2 h) time interval in healthy volunteers and patients, and that in a cohort of ‘real-world’ patients with symptomatic coronary artery disease, it is technically possible to measure this parameter in two-thirds of the individuals; these changes did not appear to relate to measurable changes in the circulating levels of ET-1 or pro-inflammatory markers.
Since its conception by Celermajer et al.,2 brachial FMD has been adopted by research groups throughout the world and used as a tool with which to measure endothelial-dependent vascular function. Subsequently, endothelial dysfunction, as determined by reduced FMD, has been shown to predict cardiovascular adversity among asymptomatic subjects exposed to cardiovascular risk factors,11 individuals presenting to hospital with chest pain,12 patients with peripheral vascular disease,13 and elderly populations.14 It also changes acutely in response to vascular insults such as systemic inflammation,15 fatty meal,16 or mental stress,17 for example. FMD describes the endothelium's ability to respond to a hyperaemic stimulus, but does not provide information regarding basal tone, which may affect the ability to respond to hyperaemia. This may be important given the observation that resting vessel diameter tends to be greater among patients than among healthy subjects,12 and as FMD is measured as a relative change from baseline, small changes in the baseline diameter may significantly affect values of FMD.
Gori et al.5 have now described the measurement of radial L-FMC in a cohort of mainly healthy volunteers or those exposed to cardiovascular risk factors. In their studies, they demonstrated that L-FMC was reproducible and may be mediated via the inhibition of vasodilator pathways, involving P450-derived endothelial-derived hyperpolarizing factor and cyclooxygenase products, such as prostaglandins. L-FMC provided data that were additional and complementary to those obtained with traditional FMD.
More recently, Gori et al.6 reported that the inclusion of L-FMC data with that obtained from traditional measurements of FMD improves the sensitivity and specificity of detecting subjects with cardiovascular disease when compared with FMD alone. Therefore, among their cohort, radial L-FMC appeared to be an easy-to-perform and reproducible technique and provided further characterizing of vascular function. However, most FMD studies use the brachial artery. Our data are therefore relevant in this context and demonstrate that it is possible to measure this novel parameter in a larger vessel. However, in our study, we excluded a third of patients due to technical difficulties. This perhaps reflected our consecutive cohort of more elderly patients with advanced symptomatic stable or unstable cardiovascular disease who often found it difficult to remain still during the scan or the differences in scanning the brachial artery. Nevertheless, in the present study, we found that brachial L-FMC was stable and could be reproducibly measured.
In our cohort, we observed a weak but significant association of FMD with L-FMC in healthy volunteers. Indeed, although Gori et al.5 could not demonstrate this relationship, their data did show a linear trend of reducing L-FMC with reducing FMD. These observations suggest that the vasoconstriction is an ‘active’ process. However, the association of FMD with L-FMC was lost among patients with atherosclerosis and, furthermore, L-FMC was significantly different between patients with stable and unstable coronary disease: with greater vasoconstriction seen in patients with NSTEMI. Added to this is the finding that in a cohort of patients studied 2 months after NSTEMI, there was an improvement in FMD and a reduction in L-FMC. Furthermore, following PCI, L-FMC was observed to increase at a time when FMD reduced. These observations suggest an enhanced L-FMC response among patients in the acute phase of NSTEMI and early after PCI and dissociation between the mechanisms that are active in health and disease. The relationship between L-FMC and FMD appears to be complex and non-linear among the setting of disease, and although impaired vasodilator function (FMD) correlates with the extent and severity of coronary atherosclerosis (CSI), this does not appear to be true for L-FMC.
The underlying mechanism of these observations is unclear. At the time of NSTEMI, phenotypic characteristics include elevated markers of inflammation and impaired endothelial function.18,19 Following the acute event, recovery of endothelial function is paralleled by a reduction in the levels of circulating inflammatory markers and predicts a favourable long-term outcome.20 It is interesting that despite significant changes in the vascular function, we were unable to demonstrate a significant early pro-inflammatory response 2h following PCI. A temporal disconnect between systemic inflammation and endothelial dysfunction has been well described using models of acute inflammation.21 It is possible that in the present study, blood sampling times may have been too early to detect the recognized systemic inflammatory response to PCI, which peaks between 24 and 48 h,22 but this suggests that the changes in the vascular function are not related directly to these cytokines. However, activation of leukocytes does occur early following PCI,23 with activated neutrophils releasing various vasoactive mediators including MPO. Indeed, in the present study, circulating levels of MPO increased significantly 2h after PCI. MPO may negatively impact on vasomotor function via oxidation of endothelium-derived NO, which may reduce NO bioavailability and impair the endothelial-dependent vasodilator response.24 Additionally, increased circulating levels of MPO following PCI were also associated with an increased vasoconstrictor (L-FMC) response. The mechanistic explanation for this effect remains unclear; however, given the data described earlier, it is possible that increased levels of MPO may help manipulate the vasomotor balance within the vascular endothelium in favour of a more pronounced vasoconstrictor response to conditions of low flow. However, no such association was demonstrated among patients recovering from NSTEMI.
ET-1 is a potent vasoconstrictor,27 and increased levels of ET-1 have been reported following PCI, possibly via a mechanism involving increased oxidative stress.25–28 Indeed, direct inhibition of the ET-A receptor by administration of the antagonists BQ-123, among healthy volunteers, reduces L-FMC of the radial artery.29 Consequently, circulating levels of ET-1 were measured in the present study. Despite an inability to demonstrate a change in ET-1 levels following PCI or convalescence from NSTEMI, we observed significant alterations in L-FMC. The endothelial function represents the expression of a final common pathway influenced by several vasoactive mediators, of which ET-1 is just one. Consequently, our data are hypothesis-generating and further studies are required in order to better understand the mechanistic process responsible for enhanced low-flow vasoconstriction.
In addition to leukocyte activation, PCI itself has also been shown to induce acute systemic endothelial dysfunction.30 However, it remains unclear whether the reduction in FMD observed at the time of NSTEMI or following PCI reflects enhanced basal tone, which may impair the vessel's ability to respond to further vasodilator stimulation such as hyperaemia during an FMD study.
Following elective PCI, although baseline vessel diameter did not change significantly, a profound reduction in FMD was observed, which occurred in conjunction with an increase in L-FMC. Gori et al.6 reported that when measuring L-FMC following isovolumetric exercise in healthy volunteers, baseline diameter was significantly greater than before exercise, which may be due to enhanced endothelial activation and may explain the observed blunting of FMD, as L-FMC was observed to increase in a compensatory fashion. In our study, although there was no statistically significant difference in the baseline diameter before and after PCI our numbers were, however, small and consequently the 9% increase in baseline diameter 2 h following PCI may have been important. This trend towards increased baseline diameter in our study may have reflected enhanced basal endothelial activation and therefore may lead to a potentially larger L-FMC. However, the measured vasodilator response to sublingual GTN was the same both before and after PCI, so changes in vessel dilatation were not fully explained by basal diameter alone. Previous observations of L-FMC have involved studies of the radial artery. Following cuff inflation, reduction in blood flow may be less in the brachial when compared with the radial artery, which may help explain the low measurements of L-FMC described in the present study. However, our findings are similar to those made among subjects with established heart disease investigated by Gori et al. It would be interesting in future studies to directly compare the brachial and radial responses among healthy volunteers and subjects with disease states.
Among patients studied 2 months following NSTEMI recovery of FMD was associated with reduced L-FMC. Importantly, in both the PCI and NSTEMI studies, there were no differences in the composite endpoint (sum of FMD and L-FMC), which questions the usefulness of the composite analysis. However, when FMD and L-FMC data are analysed separately, our observations further support the hypothesis that processes may have actively influenced the response to low flow and hyperaemic flow with reduced FMD and, importantly, enhanced L-FMC after PCI and the reverse findings after recovery from NSTEMI.
Enhanced vasoconstriction in response to reduced flow may have important pathophysiological implications in the setting of vessel occlusion. Systemic vasomotor function closely reflects coronary arterial function,3 and if an analogous response occurs in the heart, then under conditions of slow flow associated with NSTEMI, the exaggerated constriction could be detrimental, for example, contributing to the no-reflow phenomenon. It is premature to speculate that this occurs in the coronary vasculature at this stage, and further studies are required to define the coronary physiology and clinical importance of this recently described index. Furthermore, the relationship between radial and brachial L-FMC remains unclear, and it is unknown how these relate to any coronary L-FMC responses.
Limitations of our study include the small number of patients investigated. However, we did perform matched ‘before and after studies’ and have been able to demonstrate significant disturbance in the vasomotor function as measured by FMD. Furthermore, our numbers are of a magnitude similar to those of other mechanistic studies. Nevertheless, due to the small number of patients studied, we are unable to dissect out the mechanism responsible for the dynamic variation in L-FMC observed in the present study. It would be interesting among larger cohorts of patients to characterize the effect of potential influencing factors, such as the use of antiplatelet medication or drugs eluted from stents and the impact of inflammation, on this novel measure of endothelial function.
Conclusion
In conclusion, the present study demonstrates that measuring brachial artery L-FMC is easily possible in healthy volunteers and outlines the practical difficulties encountered when using this technique among patients with advanced vascular disease. Nevertheless, we demonstrate differences in L-FMC between patient groups and show that L-FMC undergoes a dynamic change following PCI and recovery from NSTEMI, which appears to be independent of circulating levels of ET-1 and pro-inflammatory markers. Further studies involving larger cohorts are required in order to clarify the clinical significance of this observation and to better define its role in risk stratification. However, understanding mechanisms thought to be important in the maintenance of basal vascular tone, and investigating these conditions where the vasoconstrictor/vasodilator balance is disturbed, may have important clinical implications.
Funding
This work was supported by The Royal Brompton & Harefield NHS Trust. No external grants were used to support this work. R.K.K. is funded by the Oxford NIHR Biomedical Research Centre.
Conflict of interest: none declared.
References
- 1.Bonetti P, Lerman L, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 2.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 3.Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrande D, Lieberman EH, Ganz P, Creager MA, Yeung AC, Selwyn AP. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26:1235–1241. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 4.Corretti M, Anderson T, Benjamin E, Celermajer D, Charbonneau F, Creager M, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilatation of the brachial artery. A report of the international brachial artery reactivity task force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 5.Gori T, Dragoni S, Lisi M, Di Stolfo G, Sonnati S, Fineschi M, Parker J. Conduit artery constriction mediated by low flow: a novel noninvasive method for the assessment of vascular function. J Am Coll Cardiol. 2008;51:1953–1958. doi: 10.1016/j.jacc.2008.01.049. [DOI] [PubMed] [Google Scholar]
- 6.Gori T, Grotti S, Dragoni S, Lisi M, Di Stolfo G, Sonnati S, Fineschi M, Parker J. Assessment of vascular function: flow-mediated constriction complements the information of flow-mediated dilatation. Heart. 2010;96:141–147. doi: 10.1136/hrt.2009.167213. [DOI] [PubMed] [Google Scholar]
- 7.Gokce N, Holbrook M, Hunter LM, Palmisano J, Vigalok E, Keaney JF, Vita JA. Acute effects of vasoactive drug treatment on brachial artery reactivity. J Am Coll Cardiol. 2002;40:761–765. doi: 10.1016/s0735-1097(02)02034-x. [DOI] [PubMed] [Google Scholar]
- 8.Donald A, Halcox J, Charakida M, Storry C, Wallace S, Cole T, Friberg P, Deanfield J. Methodological approaches to optimize reproducibility and power in clinical studies of flow-mediated dilatation. J Am Coll Cardiol. 2008;51:1959–1964. doi: 10.1016/j.jacc.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 9.Mabuchi H, Koizumi J, Shimizu M, Takeda R. Hokuriku, FH-CHD Study Group: development of coronary heart disease in familial hypercholesterolaemia. Circulation. 1989;79:225–332. doi: 10.1161/01.cir.79.2.225. [DOI] [PubMed] [Google Scholar]
- 10.Kaku B, Mizuno S, Ohsato K, Murakami T, Moriuchi I, Arai Y, Nio Y, Hirase H, Nagata M, Takahashi Y, Ohnaka M. The correlation between coronary stenosis index and flow-mediated dilation of the brachial artery. Jpn Circ J. 1998;62:425–430. doi: 10.1253/jcj.62.425. [DOI] [PubMed] [Google Scholar]
- 11.Perticone F, Ceravolo R, Pujia A, Ventura G, Iacopino S, Scozzafava A, Ferraro A, Chello M, Mastroroberto P, Verdecchia P, Schillaci G. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation. 2001;104:191–196. doi: 10.1161/01.cir.104.2.191. [DOI] [PubMed] [Google Scholar]
- 12.Neunteufl T, Heher S, Katzenschlager R, Wolfl G, Kostner K, Maurer G, Weidinger F. Late prognostic value of flow-mediated dilation in the brachial artery of patients with chest pain. Am J Cardiol. 2000;86:207–209. doi: 10.1016/s0002-9149(00)00857-2. [DOI] [PubMed] [Google Scholar]
- 13.Gokce N, Keaney J, Hunter L, Watkins M, Nedeljkovic Z, Menzoian J, Vita J. Predictive value of non-invasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol. 2003;41:1769–1775. doi: 10.1016/s0735-1097(03)00333-4. [DOI] [PubMed] [Google Scholar]
- 14.Yeboah J, Crouse J, Hsu F-C, Burke G, Herrington D. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults. Circulation. 2007;115:2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 15.Liuba P, Aburawi E, Pesonen E, Andersson S, Truedsson L, Yla-Herttuala S, Holmberg L. Residual adverse changes in arterial endothelial function and LDL oxidation after a mild systemic inflammation induced by influenza vaccination. Ann Med. 2007;39:392–399. doi: 10.1080/07853890701390111. [DOI] [PubMed] [Google Scholar]
- 16.De Koning E, Rabelink T. Endothelial function in the postprandial state. Atheroscler Suppl. 2002;3:11–16. doi: 10.1016/s1567-5688(01)00008-3. [DOI] [PubMed] [Google Scholar]
- 17.Ghiadoni L, Donald A, Cropley M, Mullen M, Oakley G, Taylor M, O'Connor G, Betteridge J, Klein N, Steptoe A, Deanfield J. Mental stress induces transient endothelial dysfunction in humans. Circulation. 2000;102:2473–2478. doi: 10.1161/01.cir.102.20.2473. [DOI] [PubMed] [Google Scholar]
- 18.Fitchtlscherer S, Breuer S, Zeiher A. Prognostic value of systemic endothelial dysfunction in patients with acute coronary syndromes¾further evidence for the existence of the ‘vulnerable' patient. Circulation. 2004;110:1926–1932. doi: 10.1161/01.CIR.0000143378.58099.8C. [DOI] [PubMed] [Google Scholar]
- 19.Karatzis E, Ikonomidis I, Vamvakou G, Papaioannou T, Protogerou A, Andreadou I, Voidonikola P, Karatzi K, Papamichael C, Lekakis J. Long-term prognostic role of flow-mediated dilatation of the brachial artery after acute coronary syndromes without ST elevation. Am J Cardiol. 2006;98:1424–1428. doi: 10.1016/j.amjcard.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 20.Fichtlscherer S, Rosenberger G, Walter D, Breuer S, Dimmeler S, Zeiher A. Elevated C-reactive protein levels and impaired endothelial vasoreactivity in patients with coronary artery disease. Circulation. 2000;102:1000–1006. doi: 10.1161/01.cir.102.9.1000. [DOI] [PubMed] [Google Scholar]
- 21.Hingorani AD, Cross J, Kharbanda RK, Mullen MJ, Bhagat K, Taylor M, Donald A, Palacios M, Griffin G, Deanfield J, MacAllister R, Vallance P. Acute systemic inflammation impairs endothelial-dependent dilatation in humans. Circulation. 2000;102:994. doi: 10.1161/01.cir.102.9.994. [DOI] [PubMed] [Google Scholar]
- 22.Azar R, McKay R, Kiernan F, Seecharran B, Feng Y, Fram D, Wu A, Waters D. Coronary angioplasty induces a systemic inflammatory response. Am J Cardiol. 1997;80:1476–1478. doi: 10.1016/s0002-9149(97)00726-1. [DOI] [PubMed] [Google Scholar]
- 23.Serrano C, Jr, Ramires J, Venturinelli M, Arie S, D'Amico E, Zweier J, Pileggi F, da Luz P. Coronary angioplasty results in leukocyte and platelet activation with adhesion molecule expression. Evidence of inflammatory responses in coronary angioplasty. J Am Coll Cardiol. 1997;29:1276–1283. doi: 10.1016/s0735-1097(97)00070-3. [DOI] [PubMed] [Google Scholar]
- 24.Eiserich J, Baldus S, Brennan M, Ma W, Zhang C, Tousson A, Castro L, Lusis A, Nauseef W, White C, Freeman B. Myeloperoxidase, a leukocyte-derived vascular NO oxidase. Science. 2002;296:2391–2394. doi: 10.1126/science.1106830. [DOI] [PubMed] [Google Scholar]
- 25.Taylor A, Bobik A, Richards M, Kaye D, Raines G, Gould P, Jennings G. Myocardial endothelin-1 release and indices of inflammation during angioplasty for acute myocardial infarction and stable coronary artery disease. Am Heart J. 2004;148:e10. doi: 10.1016/j.ahj.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 26.Hojo Y, Ikeda U, Katsuki T, Mizuno O, Fukazawa H, Kurosaki K, Fujikawa H, Shimada K. Release of endothelin 1 and angiotensin II induced by percutaneous transluminal coronary angioplasty. Catheter Cardiovasc Interv. 2000;51:42–49. doi: 10.1002/1522-726x(200009)51:1<42::aid-ccd10>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 27.Davlouros P, Simeonidou E, Tsakas S, Vlachojannis I, Alexopoulos D, Manolis A. Release of endothelin-1 from human endocardium after radiofrequency catheter ablation and coronary angioplasty: comparative results. Int J Cardiol. 2005;102:187–193. doi: 10.1016/j.ijcard.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Wainstein M, Goncalves S, Zago A, Zenker R, Burttet R, Couto Gde B, Tomazi F, Ribeiro J. Plasma endothelin-1 levels after coronary stenting in humans. Am J Cardiol. 2003;92:1211–1214. doi: 10.1016/j.amjcard.2003.07.035. [DOI] [PubMed] [Google Scholar]
- 29.Spieker L, Luscher T, Noll G. ETA receptors mediate vasoconstriction of large conduit arteries during reduced flow in humans. J Cardiovasc Pharmacol. 2003;42:315–318. doi: 10.1097/00005344-200309000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Warnholtz A, Abolfazl Ostad M, Heitzer T, Goldmann BU, Nowak G, Munzel T. Effects of tirofiban on percutaneous coronary intervention-induced endothelial dysfunction in patients with stable coronary artery disease. Am J Cardiol. 2005;95:20–23. doi: 10.1016/j.amjcard.2004.08.057. [DOI] [PubMed] [Google Scholar]