Abstract
Aims
Patients with diabetes mellitus (DM) have increased platelet reactivity and reduced platelet response to clopidogrel compared with patients without DM. Prasugrel, a more potent antiplatelet agent, is associated with greater reductions in ischaemic events compared with clopidogrel, particularly in patients with DM. The aim of this study was to perform serial pharmacodynamic assessments of prasugrel with high-dose clopidogrel in patients with DM.
Methods and results
Optimizing anti-Platelet Therapy In diabetes MellitUS (OPTIMUS)-3 was a prospective, randomized, double-blind, crossover study in patients with type 2 DM and coronary artery disease (CAD). Patients (n= 35) were randomly assigned to either prasugrel 60 mg loading dose (LD)/10 mg maintenance dose (MD) or clopidogrel 600 mg LD/150 mg MD over two 1-week treatment periods separated by a 2-week washout period. Platelet function was assessed by VerifyNow® P2Y12 assay, light transmission aggregometry, and vasodilator-stimulated phosphoprotein phosphorylation at 0, 1, 4, and 24 h and 7 days. Greater platelet inhibition by VerifyNow® P2Y12 was achieved by prasugrel compared with clopidogrel at 4 h post-LD (least squares mean, 89.3 vs. 27.7%, P< 0.0001; primary endpoint). The difference in platelet inhibition between prasugrel and clopidogrel was significant from 1 h through 7 days (P < 0.0001). Similar results were obtained using all other platelet function measures. Prasugrel resulted in fewer poor responders at all time points irrespective of definition used.
Conclusion
In patients with type 2 DM and CAD, standard-dose prasugrel is associated with greater platelet inhibition and better response profiles during both the loading and maintenance periods when compared with double-dose clopidogrel.
Clinical trial identifier: www.clinicaltrials.gov—NCT00642174
Keywords: Diabetes mellitus, Coronary disease, Platelets
Introduction
Patients with diabetes mellitus (DM) are characterized by enhanced platelet reactivity and a reduced response to oral antiplatelet medications used for the prevention of ischaemic events.1–3 Platelets from patients with DM demonstrate upregulation of several signalling pathways, which may explain the increased prevalence of low platelet inhibition following clopidogrel treatment and contribute to increased atherothrombotic risk in these patients compared with those without DM.1,4 Therefore, greater platelet P2Y12 receptor inhibition may provide more optimal platelet inhibition in this high-risk patient population. In the Optimizing anti-Platelet Therapy In diabetes MellitUS (OPTIMUS)-1 Study, a high proportion of patients had a suboptimal response to a standard dose of clopidogrel. Although a high maintenance dose (MD) of clopidogrel (150 mg per day) enhanced platelet inhibition in patients with DM presenting with elevated platelet reactivity while on standard treatment regimens, a considerable proportion of the population maintained persistently elevated platelet function despite the more aggressive P2Y12 receptor blockade.5 These results demonstrate the need for alternative platelet inhibition strategies for this high-risk patient population.
Prasugrel is a thienopyridine that produced a greater reduction in ischaemic events than clopidogrel but with increased risk of bleeding in moderate-to-high-risk patients with acute coronary syndrome undergoing percutaneous coronary interventions (PCIs) in the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis in Myocardial Infarction (TRITON-TIMI 38).6 In the subgroup of patients with DM in TRITON, a 30% relative risk reduction with prasugrel vs. clopidogrel in the primary composite endpoint of cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke was demonstrated without an increased risk of major bleeding.7 However, randomization was not stratified by diabetic status in this clinical study and there was no significant interaction between patients with and without diabetes. Additionally, two studies have examined, in non-randomized subgroup analyses, the pharmacodynamic profiles of prasugrel and clopidogrel in stable coronary artery disease (CAD) patients with DM.8,9 However, neither of these analyses was from a prospectively defined trial in a well-characterized DM population.
In patients with DM, a prospective comparison of the pharmacodynamic profiles of prasugrel with higher than approved loading dose (LD) and MD of clopidogrel is largely unexplored in a prospectively defined trial. Therefore, the aim of the present study was to compare the pharmacodynamic activity of standard-dose prasugrel with double-dose clopidogrel in patients with type 2 DM who have CAD by means of multiple platelet function assays. We hypothesized that prasugrel would achieve greater platelet inhibition compared with double-dose clopidogrel in these patients.
Methods
Patient population and study design
OPTIMUS-3 was a prospective, randomized, double-blind, double-dummy, active-controlled, two-period crossover study conducted between April 2008 and January 2009 at four academic sites in the USA. Patients with type 2 DM between 18 and 75 years of age treated with oral and/or parenteral hypoglycaemic therapy for at least 1 month were enrolled. All patients had known CAD and were taking aspirin (81–325 mg/day). Patients were ineligible to participate if any of the following criteria was met: a defined need for thienopyridine therapy (e.g. within 12 months of an acute coronary syndrome event or placement of a drug eluting stent; or within 1 month of placement of a bare metal stent); within 30 days of coronary artery bypass graft surgery or PCI without a stent placed; planned coronary revascularization; glycosylated haemoglobin (HbA1c) ≥10 mg/dL within 3 months; treatment with fibrin-specific fibrinolytic therapy <24 h or non-fibrin-specific fibrinolytic therapy <48 h prior to randomization; presence of active internal bleeding or history of bleeding diathesis or clinical findings associated with an increased risk of bleeding; history of ischaemic or haemorrhagic stroke, transient ischaemic attack, intracranial neoplasm, arteriovenous malformation, or aneurysm; body weight <60 kg; an international normalized ratio >1.5, platelet count <100 000/ mm3, or haemoglobin <10 gm/dL within 1 week of study entry.
The trial was conducted in agreement with the Declaration of Helsinki and the International Conference on Harmonisation Guidelines for Good Clinical Practice. The protocol was approved by the local Ethics Review Committee or institutional review board at each participating site, and all patients gave written informed consent before undergoing any study procedure or receiving any study treatment.
A clinical research organization (CRO) was contracted by the sponsor to hold the data and perform the data analysis after data lock and then deliver the database and the analysis results to the sponsor. An independent expert was contracted to check the quality of the blinded data prior to the data analysis by the CRO.
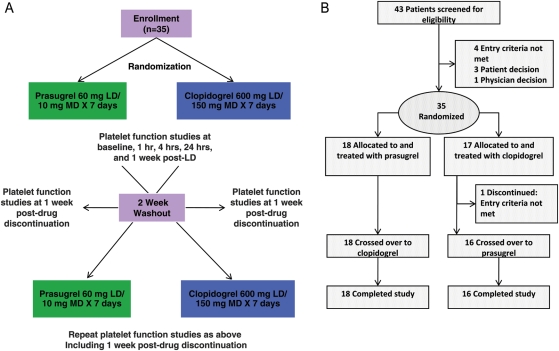
Patients who met all criteria for enrolment were randomized at the first visit to double-blind treatment of either prasugrel 60 mg LD orally followed by 10 mg/day MD for 7 days or clopidogrel 600 mg LD orally [75 mg tablets Plavix® (clopidogrel bisulfate; Bristol–Myers Squibb/sanofi-aventis, Bridgewater, NJ, USA)] followed by 150 mg/day MD for 7 days (Figure 1A). The randomization ratio was 1:1 between the two treatments within each site. Assignment to treatment was determined by a computer-generated random sequence using an interactive voice response system. Prasugrel was administered as the hydrochloride salt in 10 mg tablets (Eli Lilly and Company, Indianapolis, IN, USA). Patients were administered an equal number of identical tablets for either the LDs (six prasugrel 10 mg tablets and eight placebo tablets or eight clopidogrel 75 mg tablets and six placebo tablets) or MDs (one prasugrel 10 mg tablet and two placebo tablets or two clopidogrel 75 mg tablets and one placebo tablet). Patients continued taking their pre-study dosage of aspirin throughout the study. Platelet function studies were performed at 0 (baseline), 1, 4, and 24 h, and 7 days (24 h post last MD) after LD. After completion of the first 1-week treatment period, patients discontinued the study drug, and platelet function studies were repeated 1 and 2 weeks later, with the 2-week measure serving as the baseline (0 h) for the second treatment period. After the 2-week washout period, patients received the other study drug regimen for 1 week, during which assessment of platelet function mirrored the first period. Final platelet function studies were performed 1 week after discontinuation of therapy.
Figure 1.
(A) Study design. (B) Patient disposition.
Sample collection and platelet function assays
Blood for platelet function analyses was collected from an antecubital vein at pre-defined time points into citrated tubes (Figure 1). Platelet function assays included VerifyNow® P2Y12 (Accumetrics, Inc., San Diego, CA, USA), light transmission aggregometry (LTA), and vasodilator-stimulated phosphoprotein phosphorylation (VASP-P) expressed as the platelet reactivity index (PRI). The VerifyNow® P2Y12 assay is a rapid whole-blood point-of-care device and was utilized according to the instructions of the manufacturer as described previously.10,11 Light transmission aggregometry was performed according to standard protocols.2,3,5 Maximum platelet aggregation (MPA) was induced by 5 and 20 μM ADP and IPA% (IPA, inhibition of platelet aggregation) was calculated as (baseline aggregation response − post-dose aggregation response)/baseline aggregation response × 100. Vasodilator-stimulated phosphoprotein phosphorylation was assessed as recommended by the manufacturer5 using quantitative flow cytometry and commercially available labelled monoclonal antibodies (Biocytex, Inc., Marseille, France) at a central laboratory at the University of Massachusetts.
Study endpoints
The primary endpoint of this study was IPA measured by the VerifyNow® P2Y12 assay 4 h after the administration of the study drug LD. This time point was selected because the peak (≥95%) effect of prasugrel 60 mg occurs by 2 h post-LD and is sustained past 4 h, whereas the peak effect of a clopidogrel 600 mg LD has been measured at ≤4 h in several studies.12,13
Secondary measures included: IPA by VerifyNow® P2Y12 at all other time points; VerifyNow® P2Y12 PRU, LTA (MPA and IPA), and VASP-P PRI at all time points; and evaluation of the incidence of adverse events such as thrombosis and haemorrhage. In addition, a responder-type analysis was conducted in which a poor response to thienopyridine treatment was defined as: VerifyNow® P2Y12 PRU >23514; 20 μM ADP LTA MPA >50%5; 20 μM ADP LTA IPA ≤20%15; or VASP-P PRI >50%.16 Finally, the possibility of ‘rebound' platelet activation was evaluated in an analysis of the pharmacodynamic effect of prasugrel 6–8 days after treatment discontinuation for each study period. Rebound was considered to have occurred if, after the cessation of the study drug, a ≥20% increase in platelet reactivity above the 0 h value was obtained at the start of each treatment period. A cut-off value of 20% was chosen because it is outside the range of assay variability.
Statistical analysis
A sample size of 30 patients was required to provide at least 90% power to detect a 17.5% difference in VerifyNow® P2Y12 IPA (primary endpoint) between treatment groups with a standard deviation of 20% at a two-tailed significance level of 0.05. This difference was based on the response to MD therapy with prasugrel compared with clopidogrel in a previous study available at the time of the OPTIMUS-3 design.17 All statistical comparisons of platelet function between prasugrel and clopidogrel for the primary endpoint and secondary endpoints with continuous variables were conducted using a linear mixed-effect model with treatment group, sequence, and treatment-group-by-sequence (i.e. period) as fixed effects and patient as a random effect. Confidence intervals (CI) and all tests of statistical significance for treatment comparisons were evaluated at a two-tailed significance level of 0.05 using SAS version 9.1. Results are reported as the least-squares mean with 95% CI. Sensitivity analysis on the primary endpoint was conducted after adjusting baseline IPA values assessed by VerifyNow® P2Y12 in the mixed-effect model. Categorical variables were analysed using Fisher's exact tests.
All analyses of platelet function were conducted on the pharmacodynamic population, which was defined as all randomized subjects who received at least one dose of study drug and had blood draws for IPA assessed by VerifyNow® P2Y12 4 h after the administration of the LD. Safety analyses were conducted on the safety population, which included all patients exposed to at least one dose of the study drug. Safety was assessed by evaluating vital signs, all reports of bleeding, and all reported adverse events including thrombotic cardiovascular events, death, myocardial infarction, and stroke. Bleeding was classified as major, minor, or insignificant according to the Thrombolysis In Myocardial Infarction (TIMI) criteria.6
Results
Forty-three patients were screened and 35 were randomly assigned to either prasugrel (n= 18) or clopidogrel (n= 17) for the first treatment period (Figure 1B). Of these 35 patients, 34 completed both treatment periods and served as their own control. One patient who was initially assigned to the clopidogrel group in the first treatment period was found to have an exclusion criterion (HbA1c ≥10 mg/dL) and did not cross over to prasugrel. Baseline characteristics are described in Table 1.
Table 1.
Baseline characteristics
| Characteristic | N = 35 |
|---|---|
| Age (years), mean ± SD | 61.3 ± 8.8 |
| Male, n (%) | 24 (68.6) |
| Race, n (%) | |
| Non-Hispanic white | 15 (42.9) |
| Non-Hispanic black | 7 (20.0) |
| Hispanic | 8 (22.9) |
| Other | 5 (14.3) |
| Body mass index (kg/m2), mean ± SD | 33.1 ± 8.2 |
| Cardiovascular history, n (%) | |
| Congestive heart failure | 5 (14.3) |
| Hypertension | 33 (94.3) |
| Hypercholesterolaemia | 33 (94.3) |
| Prior myocardial infarction | 13 (37.1) |
| Prior PCI | 20 (57.1) |
| Prior coronary artery bypass graft | 8 (22.9) |
| Current smoker | 7 (20.0) |
| Obesity (body mass index ≥30) | 17 (48.6) |
| Duration of CAD (years), mean ± SD | 3.1 ± 3.3 |
| Duration of type 2 DM (years), mean ± SD | 8.5 ± 6.6 |
| Antihyperglycaemic medication, n (%) | |
| Insulin | 7 (20.0) |
| Non-insulin | 28 (80.0) |
| Aspirin dose, n (%) | |
| 81 mg/day | 22 (62.9) |
| 162 mg/day | 1 (2.9) |
| 325 mg/day | 12 (34.3) |
CAD, coronary artery disease; DM, diabetes mellitus; MI, myocardial infarction; N, number of patients; n, number in group; PCI, percutaneous intervention; SD, standard deviation.
VerifyNow® P2Y12 assay
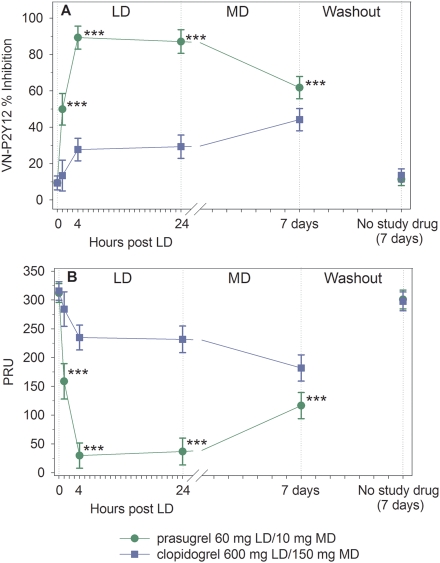
At 4 h post-LD (primary endpoint), patients treated with prasugrel had significantly greater IPA compared with clopidogrel [89.3% (95% CI = 83.0–95.6) vs. 27.7% (95% CI = 21.5–33.9), P< 0.0001] (Figure 2A). Following ∼1 week of MD therapy, the IPA was still significantly greater with daily prasugrel 10 mg compared with daily clopidogrel 150 mg [61.8% (95% CI = 55.7–67.9) vs. 44.2% (38.1–50.3), P< 0.0001]. The difference between prasugrel- and clopidogrel-treated patients was significant at all time points from 1 h post-LD through 7 days (24 h after the last MD). The difference between prasugrel- and clopidogrel-treated patients was also significant at all time points from 1 h post-LD through 7 days as measured by VerifyNow® P2Y12 PRU (Figure 2B). When measured at 1 week following discontinuation of study drug, platelet function for prasugrel- and clopidogrel-treated patients returned to near baseline (Figure 2A and B). The results were consistent irrespective of the use of insulin for glycaemic control (data not shown).
Figure 2.
Platelet function by VerifyNow® P2Y12. (A) Inhibition of platelet aggregation. (B) VerifyNow® P2Y12 PRU. Values are expressed as the least-squares mean and 95% confidence intervals. Prasugrel and clopidogrel values from each treatment period have been combined. Time 0 is the baseline value (combined for each treatment period). ***P< 0.0001.
Light transmission aggregation
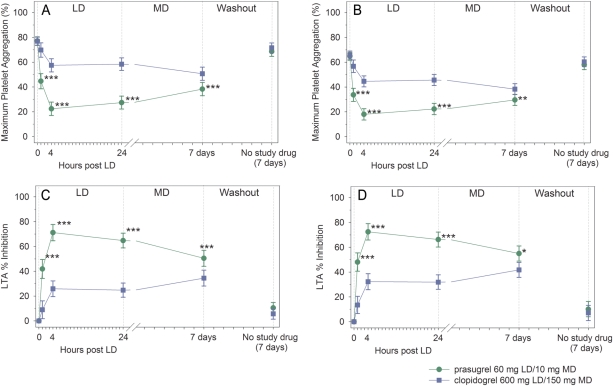
Figure 3A shows MPA to 20 μM ADP using LTA. Maximum platelet aggregation values at 0 h for patients in both treatment groups were similar. At 4 h post-LD, patients treated with prasugrel had significantly lower MPA compared with patients treated with clopidogrel [22.4% (95% CI = 17.0–27.8) vs. 57.5% (52.2–62.8), P< 0.0001]. Following ∼1 week of MD therapy, MPA was still significantly lower with prasugrel compared with clopidogrel [38.3% (95% CI = 33.0–43.6) vs. 50.7% (95% CI = 45.4–56.0), P< 0.0001]. The differences between prasugrel- and clopidogrel-treated patients were significant at all time points from 1 h post-LD through 7 days. Similar findings were observed using 5 μM ADP (Figure 3B).
Figure 3.
Platelet aggregation by light transmission aggregometry. (A) Maximum platelet aggregation to 20 μM ADP; (B) maximum platelet aggregation to 5 μM ADP; (C) inhibition of platelet aggregation in response to 20 μM ADP; (D) inhibition of platelet aggregation in response to 5 μM ADP. Values are expressed as the least-squares mean and 95% confidence intervals. Prasugrel and clopidogrel values from each treatment period have been combined. Time 0 is the baseline value (combined for each treatment period). *P= 0.0002, **P = 0.0001, ***P < 0.0001.
Figure 3C compares IPA by LTA in response to 20 μM ADP. At 4 h post-LD, IPA was significantly greater for prasugrel compared with clopidogrel [71.2% (95% CI = 64.7–77.7) vs. 25.9% (19.6–32.3), P< 0.0001]. Following ∼1 week of MD therapy, IPA was still significantly greater with prasugrel 10 mg daily compared with clopidogrel 150 mg daily [50.5% (95% CI = 44.0–56.9) vs. 34.5% (28.1–40.9), P< 0.0001]. The differences between prasugrel- and clopidogrel-treated patients were significant at all time points from 1 h post-LD through 7 days. Similar findings were observed using 5 μM ADP (Figure 3D).
Vasodilator-stimulated phosphoprotein
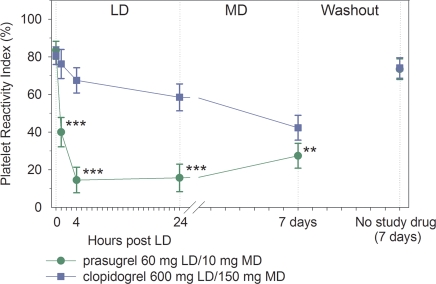
Baseline VASP PRI values for prasugrel- and clopidogrel-treated patients were similar [83.5% (95% CI = 78.8–88.3) vs. 80.6% (95% CI = 75.9–85.3), P= 0.37] (Figure 4). At 4 h post-LD, patients treated with prasugrel had significantly lower platelet reactivity by VASP PRI than patients treated with clopidogrel [14.5% (95% CI = 7.7–21.4) vs. 67.5% (95% CI = 60.8–74.2), P< 0.0001]. Following ∼1 week of MD therapy, VASP PRI was still significantly lower with prasugrel 10 mg daily compared with clopidogrel 150 mg daily [27.4% (95% CI = 20.8–34.0) vs. 42.3 (95% CI = 35.7–48.9), P= 0.0012]. The difference between prasugrel- and clopidogrel-treated patients was significant at all time points from 1 h post-LD through 7 days.
Figure 4.
Platelet function by vasodilator-stimulated phosphoprotein. Vasodilator-stimulated phosphoprotein platelet reactivity index values are expressed as the least-squares mean and 95% confidence intervals. Prasugrel and clopidogrel values from each treatment period have been combined. Time 0 is the baseline value (combined for each treatment period). **P< 0.01, ***P< 0.0001.
Response profiles
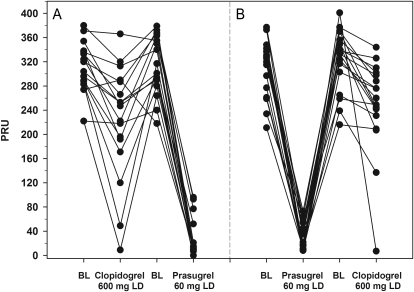
Figure 5 shows individual patient VerifyNow® P2Y12 PRU values at baseline (0 h) and 4 h post-LD during the clopidogrel-to-prasugrel crossover sequence (Figure 5A) and the prasugrel-to-clopidogrel crossover sequence (Figure 5B). After the prasugrel 60 mg LD, PRU was generally lower than that achieved after the clopidogrel 600 mg LD, regardless of whether prasugrel was administered in the first or second treatment period. Figure 5 also illustrates less variability in response to prasugrel compared with clopidogrel. Three patients treated with clopidogrel achieved response rates comparable with those treated with prasugrel.
Figure 5.
Individual response profiles. VerifyNow® P2Y12 PRU was measured at 0 h (baseline) and 4 h post-loading dose of prasugrel or clopidogrel. (A) Patients dosed with clopidogrel first and then crossed over to prasugrel. (B) Patients dosed with prasugrel first and then crossed over to clopidogrel. Baseline (0 h) values for the second treatment period were measured after the 2-week washout period. BL, baseline.
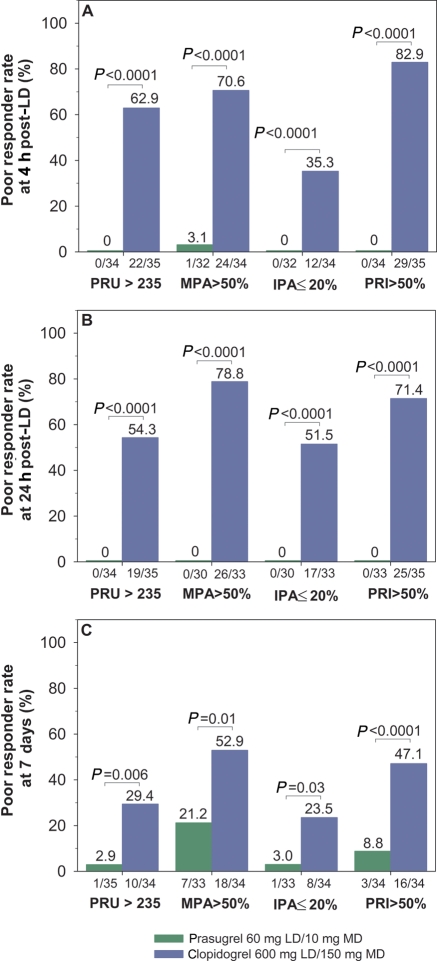
Figure 6 presents the results of the poor responder analysis at 4 and 24 h post-LD and at 7 days. Regardless of the definition used, the poor responder rate was considerably higher with clopidogrel compared with prasugrel (all P-values <0.05). At 4 h post-LD, the rate of poor responders ranged from 0 to 3.1% for prasugrel and from 35.3 to 82.9% for clopidogrel. At 24 h post-LD, the rate of prasugrel poor responders was 0% regardless of definition compared with 51.5–78.8% for clopidogrel. At 7 days, the poor responder rate ranged from 2.9 to 21.2% for prasugrel and from 23.5 to 52.9% for clopidogrel.
Figure 6.
Poor responders. Poor responder analyses were conducted at (A) 4 h post-loading dose; (B) 24 h post-loading dose; and (C) 7 days by VerifyNow® (PRU >235), light transmission aggregometry (MPA >50% and IPA ≤20%), or vasodilator-stimulated phosphoprotein phosphorylation (PRI >50%) platelet function assays. P-values for comparison between treatment groups for each assay are indicated. MPA, maximum platelet aggregation to 20 µM ADP by light transmission aggregometry; IPA, inhibition of platelet aggregation with 20 µM ADP by light transmission aggregometry.
Rebound platelet activation
One patient on prasugrel and one patient on clopidogrel met rebound criteria by MPA but not by the other assays. Similarly, one prasugrel patient met rebound criteria by VerifyNow® P2Y12 PRU; one patient on prasugrel and two patients on clopidogrel met rebound criteria according to PRI using VASP-P; and none of these patients met rebound criteria using the other assays. No patient meeting rebound criteria on clopidogrel or prasugrel met criteria with the alternative drug.
Safety
Major or minor bleeding by TIMI criteria did not occur with either treatment regimen. Two TIMI minimal bleeding events that occurred within 24 h of the last MD were observed in clopidogrel-treated patients. One prasugrel-treated patient experienced a vessel puncture site bleed. None of these episodes required intervention to control bleeding. No deaths or other serious adverse events occurred during the study. The incidence of adverse events in the prasugrel group was 14.7% (5/34), and 25.7% (9/35) in the clopidogrel group. The types of adverse events in the prasugrel and clopidogrel groups were similar (Table 2). No patients discontinued due to an adverse event.
Table 2.
Summary of adverse events in the safety population of the OPTIMUS-3 Study
| Adverse event | Prasugrel 60 mg LD/10 mg MD (n = 34), n (%) | Clopidogrel 600 mg LD/150 mg MD (n = 35), n (%) |
|---|---|---|
| Number of patients with at least one event | 5 (14.7) | 9 (25.7) |
| Number of events | 8 | 19 |
| Haematocrit decreaseda | 1 (2.9) | 0 |
| Haemoglobin decreaseda | 1 (2.9) | 0 |
| Headache | 1 (2.9) | 2 (5.7) |
| Pain in extremity | 1 (2.9) | 0 |
| Upper respiratory tract infection | 1 (2.9) | 1 (2.9) |
| Urinary tract infection | 1 (2.9) | 0 |
| Vessel puncture site haemorrhage | 1 (2.9) | 0 |
| Contusion | 0 | 1 (2.9) |
| Diarrhoea | 0 | 1 (2.9) |
| Electrolyte imbalance | 0 | 1 (2.9) |
| Epistaxis | 0 | 1 (2.9) |
| Haemorrhage | 0 | 1 (2.9) |
| Nausea | 0 | 1 (2.9) |
| Respiratory tract congestion | 0 | 1 (2.9) |
| Rhinorrhoea | 0 | 1 (2.9) |
| Sinusitis | 0 | 1 (2.9) |
| Vomiting | 0 | 1 (2.9) |
| Wart excision | 0 | 1 (2.9) |
aInvestigator defined.
Discussion
The results of the OPTIMUS-3 study confirm the hypothesis that treatment with prasugrel 60 mg LD/10 mg MD is associated with greater platelet inhibition than double-dose clopidogrel 600 mg LD/150 mg MD in a prospectively defined study in patients with type 2 DM and CAD. This study enrolled a well-characterized population of patients with DM, unlike previous studies. Platelet inhibition was corroborated by multiple platelet function assessments using different methodologies, including point-of-care testing, LTA, and flow cytometry, which encompassed a range of specificities for P2Y12-mediated platelet activation. This higher level of platelet inhibition in prasugrel-treated patients was evident at the first measurement (1 h post-LD) and continued over 24 h. Although the level of platelet inhibition after 1 week of prasugrel 10 mg MD was lower than the prasugrel LD, as the need for very high levels of platelet inhibition is probably not required in the absence of a procedure, the level of inhibition associated with the prasugrel 10 mg MD was significantly higher than with clopidogrel 150 mg MD. This study also provides additional information on clopidogrel's poor response in patients with DM, examines potential rebound after 7 days of drug withdrawal, and provides adverse event data in the study population.
Numerous studies have demonstrated the clinical benefit associated with platelet P2Y12 receptor inhibition with clopidogrel, particularly in high-risk patients.18,19 However, patients with DM continue to have a higher risk of recurrent ischaemic events, including stent thrombosis, compared with patients without DM despite the use of clopidogrel.1,18 This may be attributed, at least in part, to the greater prevalence of suboptimal P2Y12 inhibition achieved with recommended regimens of clopidogrel that has been associated with a higher risk of atherothrombotic complications.1 These observations suggest the need for more effective P2Y12 inhibition, particularly in high-risk patients such as those with DM.
The OPTIMUS-1 study demonstrated that a high-clopidogrel MD was associated with greater platelet inhibitory effects compared with standard dosing in patients with DM and CAD who presented with elevated platelet reactivity while on standard dual antiplatelet therapy. However, inadequate platelet inhibition persisted in 60% of patients.5 In the PRINCIPLE-TIMI 44 Trial, the rate of response to prasugrel 60 mg LD/10 mg MD was significantly greater than double-dose clopidogrel in the overall patient population,7 as well as in patients with DM.9 The rate of poor responders in patients with DM at 24 h post-LD was ∼40%.9 The OPTIMUS-2 study showed that adjunctive treatment with cilostazol (‘triple therapy') enhances measures of platelet P2Y12 inhibition in patients with DM and CAD to a greater extent than standard dual antiplatelet therapy with aspirin and clopidogrel.20 However, cilostazol is poorly tolerated and has an elevated prevalence of side effects that limit treatment compliance.20 Results from a subgroup of 19 patients with DM were presented by Erlinge et al.8 in a study examining the pharmacodynamic poor responder rate with high dose clopidogrel compared with prasugrel. There was a higher rate of poor responders as measured by LTA and VASP in the patients receiving clopidogrel 600 mg LD/75 mg MD compared with prasugrel 60 mg LD/10 mg MD for 28 days. These previous investigations suggested the need to explore better tolerated treatment strategies, which provide more effective P2Y12 blockade, as was assessed in the present study. The results from the OPTIMUS-3 randomized trial in patients with DM confirmed earlier pharmacodynamic observations from subgroup analyses that the rate of response to prasugrel was greater than the response to clopidogrel despite the use of double the approved clopidogrel dose.19,21 Additionally, both prasugrel and clopidogrel were well tolerated in this population with DM.
Clinical outcome data demonstrating an increase in recurrent ischaemic events following withdrawal of thienopyridine therapy suggest the potential for rebound in platelet reactivity.22 Although limited by the short duration of the current study, no consistent evidence of a rebound effect was measured at either 1 week following discontinuation of study drug or at 2 weeks when the second baseline (post crossover) was compared with the first. If rebound did exist, one would expect to find it in the early period when new, fully functional platelets would be the majority of platelets in circulation. This was an exploratory analysis and not specifically designed to examine rebound; therefore, results of our study should be interpreted with caution but are consistent with those reported by Sibbing et al.,23 who found no evidence of rebound platelet activity on clopidogrel withdrawal in a prospectively defined study. This suggests that the previously reported increase in ischaemic events observed following thienopyridine withdrawal may be the result of treatment cessation rather than rebound platelet reactivity.
Study limitations
The aim of this study was to compare the antiplatelet effects of prasugrel with double-dose clopidogrel in patients with DM; therefore, comparisons with patients without DM cannot be made. Although adequately powered for the pharmacodynamic endpoints, this study was conducted in a relatively small number of patients, thus precluding an evaluation of clinical endpoints. Additionally, the short duration of the trial did not allow for meaningful assessment of bleeding events associated with antiplatelet therapy and also limited conclusions regarding platelet function and rebound following drug withdrawal to the time frame of the study. Finally, patients included in the study were not indicated for prasugrel or clopidogrel in order to allow a washout phase without putting patients at risk. It should be underscored that in the USA and Europe, prasugrel is indicated for ACS patients undergoing PCI, a population of patients not included in this study.
Conclusions
Compared with double-dose clopidogrel (600 mg LD/150 mg MD), standard-dose prasugrel (60 mg LD/10 mg MD) resulted in greater platelet inhibition in aspirin-treated patients with type 2 DM and CAD as assessed by multiple pharmacodynamic measures in this prospective, randomized, double-blind, crossover study. Prasugrel also resulted in a better response profile with lower rates of poor responders after both the LD and MD periods, irrespective of platelet function assay or definition used. These findings may explain the clinical benefit observed with prasugrel in patients with DM.
Funding
This work was supported by Daiichi Sankyo, Inc., and Eli Lilly and Company. Funding to pay the Open Access publication charges for this article was provided by Eli Lilly and Company.
Conflict of interest: The first author (D.J.A.) prepared the initial draft of the manuscript. All authors participated in subsequent review and revisions of the manuscript. The authors had full access to the data and the ability to query the database, take full responsibility for its integrity, and have agreed to the manuscript as written. D.J.A. (corresponding author) reports: honoraria for lectures from Bristol–Myers Squibb, sanofi-aventis, Eli Lilly and Company, and Daiichi Sankyo, Inc.; consulting fees from Bristol–Myers Squibb, sanofi-aventis, Eli Lilly and Company, Daiichi Sankyo, Inc., The Medicines Company, Portola, Novartis, Medicure, Accumetrics, Arena Pharmaceuticals, Merck, Evolva, and Astra Zeneca; and research grants from GlaxoSmithKline, Otsuka, Eli Lilly and Company, Daiichi Sankyo, Inc., The Medicines Company, Portola, Accumetrics, Schering-Plough, Astra Zeneca, Eisai, and Johnson and Johnson. J.B. reports: grant support from Eli Lilly and Company, Daiichi Sankyo, Inc., and Bayer; and consulting fees from Schering-Plough, Astellas Pharma US, Inc., Eli Lilly and Company, and Daiichi Sankyo, Inc. J.F.S. reports: grant/research support from The Medicines Company, Astra Zeneca, Abbott Vascular, Bristol–Myers Squibb, Medtronic, and Eli Lilly and Company; and grant support/consulting/honoraria from Schering Plough. A.L.F. reports: grant/research support from Bristol–Myers Squibb, sanofi-aventis, Eli Lilly and Company, Daiichi Sankyo, Inc., and GLSynthesis; and consulting fees from Eli Lilly and Company. A.D.M. reports: research grants from Arena Pharmaceuticals, GLSynthesis, Eli Lilly and Company, Daiichi Sankyo, Inc., sanofi-aventis, and Bristol–Myers Squibb; member of the Data Safety Monitoring Board of Clopidogrel to Lower Arterial Thrombotic Risk in Neonates and Infants Trial (sanofi-aventis and Bristol–Myers Squibb); and consulting fees from Arena Pharmaceuticals, Eli Lilly and Company, Daiichi Sankyo, Inc., sanofi-aventis, and Bristol–Myers Squibb. J.A.J., B.Z., C.K.O, and M.B.E. are employees of, and report equity ownership or stock options in, Eli Lilly and Company. B.A.B. is an employee of Daiichi Sankyo, Inc.
Acknowledgements
Barbara Utterback (Eli Lilly and Company) provided writing and project management support for the manuscript. Diana Swanson and Caron Modeas, both of i3 Statprobe, provided technical writing and editing, respectively, of the manuscript.
Appendix
Principal investigators and study sites
D.J.A., Cardiology Research, University of Florida—Jacksonville, ACC Fifth Floor, 655 W. Eighth Street, Jacksonville, FL 32209, USA; A.D.M., University of Massachusetts Memorial Medical Center, 55 Lake Avenue North, Worcester, MA 01655, USA; J.F.S., Cardiovascular Section, Department of Medicine, University of Oklahoma Health Sciences Center, 920 Stanton L. Young Boulevard, WP 3010, Oklahoma City, OK 73104, USA; J.B., The Mount Sinai Medical Center, Room AB6-20, Sixth Floor, Mount Sinai School of Medicine Berg Building, 1428 Madison Avenue, New York, NY 10029, USA.
References
- 1.Angiolillo DJ. Antiplatelet therapy in diabetes: efficacy and limitations of current treatment strategies and future directions. Diabetes Care. 2009;32:531–540. doi: 10.2337/dc08-2064. doi:10.2337/dc08-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Ramirez C, Sabate M, Jimenez-Quevedo P, Hernandez R, Moreno R, Escaned J, Alfonso F, Banuelos C, Costa MA, Bass TA, Macaya C. Platelet function profiles in patients with type 2 diabetes and coronary artery disease on combined aspirin and clopidogrel treatment. Diabetes. 2005;54:2430–2435. doi: 10.2337/diabetes.54.8.2430. doi:10.2337/diabetes.54.8.2430. [DOI] [PubMed] [Google Scholar]
- 3.Angiolillo DJ, Bernardo E, Ramirez C, Costa MA, Sabate M, Jimenez-Quevedo P, Hernandez R, Moreno R, Escaned J, Alfonso F, Banuelos C, Bass TA, Macaya C, Fernandez-Ortiz A. Insulin therapy is associated with platelet dysfunction in patients with type 2 diabetes mellitus on dual oral antiplatelet treatment. J Am Coll Cardiol. 2006;48:298–304. doi: 10.1016/j.jacc.2006.03.038. doi:10.1016/j.jacc.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 4.Ferreira IA, Mocking AI, Feijge MA, Gorter G, van Haeften TW, Heemskerk JW, Akkerman JW. Platelet inhibition by insulin is absent in type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol. 2006;26:417–422. doi: 10.1161/01.ATV.0000199519.37089.a0. doi:10.1161/01.ATV.0000199519.37089.a0. [DOI] [PubMed] [Google Scholar]
- 5.Angiolillo DJ, Shoemaker SB, Desai B, Yuan H, Charlton RK, Bernardo E, Zenni MM, Guzman LA, Bass TA, Costa MA. Randomized comparison of a high clopidogrel maintenance dose in patients with diabetes mellitus and coronary artery disease: results of the Optimizing Antiplatelet Therapy in Diabetes Mellitus (OPTIMUS) study. Circulation. 2007;115:708–716. doi: 10.1161/CIRCULATIONAHA.106.667741. doi:10.1161/CIRCULATIONAHA.106.667741. [DOI] [PubMed] [Google Scholar]
- 6.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM TRITON TIMI. Prasugrel vs. clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. doi:10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 7.Wiviott SD, Braunwald E, Angiolillo DJ, Meisel S, Dalby AJ, Verheugt FW, Goodman SG, Corbalan R, Purdy DA, Murphy SA, McCabe CH, Antman EM. Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-Thrombolysis in Myocardial Infarction 38. Circulation. 2008;118:1626–1636. doi: 10.1161/CIRCULATIONAHA.108.791061. doi:10.1161/CIRCULATIONAHA.108.791061. [DOI] [PubMed] [Google Scholar]
- 8.Erlinge D, Varenhorst C, Braun OÖ, James S, Winters KJ, Jakubowski JA, Brandt JT, Siegbahn A, Wallentin L. Patients with poor responsiveness to thienopyridine treatment and those with diabetes have lower levels of circulating active metabolites but their platelets respond normally to the active metabolite added ex-vivo. J Am Coll Cardiol. 2008;52:1968–1977. doi: 10.1016/j.jacc.2008.07.068. doi:10.1016/j.jacc.2008.07.068. [DOI] [PubMed] [Google Scholar]
- 9.Wilson SR, Antman EM, Frelinger AL, O'Donoghue M, Neumann FJ, Michelson AD, Angiolillo DJ, Miller DL, Luo J, McCabe CH, Braunwald E, Wiviott SD. Assessment of platelet function in diabetes mellitus: observations from the PRINCIPLE-TIMI 44 trial. Circulation. 2009;120:S548–S549. [Google Scholar]
- 10.Jakubowski JA, Payne CD, Li YG, Brandt JT, Small DS, Farid NA, Salazar DE, Winters KJ. The use of the VerifyNow P2Y12 point-of-care device to monitor platelet function across a range of P2Y12 inhibition levels following prasugrel and clopidogrel administration. Thromb Haemost. 2008;99:409–415. doi: 10.1160/TH07-09-0575. [DOI] [PubMed] [Google Scholar]
- 11.Angiolillo DJ, Costa MA, Shoemaker SB, Desai B, Bernardo E, Suzuki Y, Charlton RK, Zenni MM, Guzman LA, Bass TA. Functional effects of high clopidogrel maintenance dosing in patients with inadequate platelet inhibition on standard dose treatment. Am J Cardiol. 2008;101:440–445. doi: 10.1016/j.amjcard.2007.09.087. doi:10.1016/j.amjcard.2007.09.087. [DOI] [PubMed] [Google Scholar]
- 12.Hochholzer W, Trenk D, Frundi D, Blanke P, Fischer B, Andris K, Bestehorn HP, Buttner HJ, Neumann FJ. Time dependence of platelet inhibition after a 600 mg loading dose of clopidogrel in a large, unselected cohort of candidates for percutaneous coronary intervention. Circulation. 2005;111:2560–2564. doi: 10.1161/01.CIR.0000160869.75810.98. doi:10.1161/01.CIR.0000160869.75810.98. [DOI] [PubMed] [Google Scholar]
- 13.Montalescot G, Sideris G, Meuleman C, Bal-dit-Sollier C, Lellouche N, Steg PG, Slama M, Milleron O, Collet JP, Henry P, Beygui F, Drouet L ALBION Trial Investigators. A randomized comparison of high clopidogrel loading doses in patients with non-ST-segment elevation acute coronary syndromes: the ALBION (Assessment of the Best Loading Dose of Clopidogrel to Blunt Platelet Activation, Inflammation and Ongoing Necrosis) Trial. J Am Coll Cardiol. 2006;48:931–938. doi: 10.1016/j.jacc.2006.04.090. doi:10.1016/j.jacc.2006.04.090. [DOI] [PubMed] [Google Scholar]
- 14.Price MJ, Endemann S, Gollapudi RR, Valencia R, Stinis CT, Levisay JP, Ernst A, Sawhney NS, Schatz RA, Teirstein PS. Prognostic significance of post-clopidogrel platelet reactivity assessed by a point-of-care assay on thrombotic events after drug-eluting stent implantation. Eur Heart J. 2008;29:992–1000. doi: 10.1093/eurheartj/ehn046. doi:10.1093/eurheartj/ehn046. [DOI] [PubMed] [Google Scholar]
- 15.Weerakkody GJ, Brandt JT, Payne CD, Jakubowski JA, Naganuma H, Winters KJ. Clopidogrel poor responders: an objective definition based on Bayesian classification. Platelets. 2007;18:428–435. doi: 10.1080/09537100701206790. doi:10.1080/09537100701206790. [DOI] [PubMed] [Google Scholar]
- 16.Bonello L, Camoin-Jau L, Arques S, Boyer C, Panagides D, Wittenberg O, Simeoni MC, Barragan P, Dignat-George F, Paganelli F. Adjusted clopidogrel loading doses according to vasodilator-stimulated phosphoprotein phosphorylation index decrease rate of major adverse cardiovascular events in patients with clopidogrel resistance: a multicenter randomized prospective study. J Am Coll Cardiol. 2008;51:1404–1411. doi: 10.1016/j.jacc.2007.12.044. doi:10.1016/j.jacc.2007.12.044. [DOI] [PubMed] [Google Scholar]
- 17.Varenhorst C, James S, Erlinge D, Braun OÖ, Brandt JT, Winters KJ, Jakubowski JA, Olofsson S, Wallentin L, Siegbahn A. Assessment of P2Y(12) inhibition with the point-of-care device VerifyNow P2Y12 in patients treated with prasugrel or clopidogrel coadministered with aspirin. Am Heart J. 2009;157:562.e1–562.e9. doi: 10.1016/j.ahj.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 18.Angiolillo DJ, Ueno M, Goto S. Basic principles and platelet biology and clinical implications. Circ J. 2010;74:597–607. doi: 10.1253/circj.cj-09-0982. doi:10.1253/circj.CJ-09-0982. [DOI] [PubMed] [Google Scholar]
- 19.Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Alfonso F, Macaya C, Bass TA, Costa MA. Variability in individual responsiveness to clopidogrel: clinical implications, management, and future perspectives. J Am Coll Cardiol. 2007;49:1505–1516. doi: 10.1016/j.jacc.2006.11.044. doi:10.1016/j.jacc.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 20.Angiolillo DJ, Capranzano P, Goto S, Aslam M, Desai B, Charlton RK, Suzuki Y, Box LC, Shoemaker SB, Zenni MM, Guzman LA, Bass TA. A randomized study assessing the impact of cilostazol on platelet function profiles in patients with diabetes mellitus and coronary artery disease on dual antiplatelet therapy: results of the OPTIMUS-2 study. Eur Heart J. 2008;29:2202–2211. doi: 10.1093/eurheartj/ehn287. doi:10.1093/eurheartj/ehn287. [DOI] [PubMed] [Google Scholar]
- 21.Wiviott SD, Trenk D, Frelinger AL, O'Donoghue M, Neumann FJ, Michelson AD, Angiolillo DJ, Hod H, Montalescot G, Miller DL, Jakubowski JA, Cairns R, Murphy SA, McCabe CH, Antman EM, Braunwald E. Prasugrel compared with high loading- and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation-Thrombolysis in Myocardial Infarction 44 Trial. Circulation. 2007;116:2923–2932. doi: 10.1161/CIRCULATIONAHA.107.740324. doi:10.1161/CIRCULATIONAHA.107.740324. [DOI] [PubMed] [Google Scholar]
- 22.Ho PM, Peterson ED, Wang L, Magid DJ, Fihn SD, Larsen GC, Jesse RA, Rumsfeld JS. Incidence of death and acute myocardial infarction associated with stopping clopidogrel after acute coronary syndrome. JAMA. 2008;299:532–539. doi: 10.1001/jama.299.5.532. doi:10.1001/jama.299.5.532. [DOI] [PubMed] [Google Scholar]
- 23.Sibbing D, Stegherr J, Braun S, Mehilli J, Schulz S, Seyfarth M, Kastrati A, von Beckerath N, Schomig A. A double-blind, randomized study on prevention and existence of a rebound phenomenon of platelets after cessation of clopidogrel treatment. J Am Coll Cardiol. 2010;55:558–565. doi: 10.1016/j.jacc.2009.09.038. doi:10.1016/j.jacc.2009.09.038. [DOI] [PubMed] [Google Scholar]