Abstract
Aims
To evaluate efficacy and safety of RLY5016 (a non-absorbed, orally administered, potassium [K+]-binding polymer) on serum K+ levels in patients with chronic heart failure (HF) receiving standard therapy and spironolactone.
Methods and results
One hundred and five patients with HF and a history of hyperkalaemia resulting in discontinuation of a renin–angiotensin–aldosterone system inhibitor/blocker and/or beta-adrenergic blocking agent or chronic kidney disease (CKD) with an estimated glomerular filtration rate of <60 mL/min were randomized to double-blind treatment with 30 g/day RLY5016 or placebo for 4 weeks. Spironolactone, initiated at 25 mg/day, was increased to 50 mg/day on Day 15 if K+ was ≤5.1 mEq/L. Endpoints included the change from baseline in serum K+ at the end of treatment (primary); the proportion of patients with hyperkalaemia (K+ >5.5 mEq/L); and the proportion titrated to spironolactone 50 mg/day. Safety assessments included adverse events (AEs) and clinical laboratory tests. RLY5016 (n= 55) and placebo (n= 49) patients had similar baseline characteristics. At the end of treatment, compared with placebo, RLY5016 had significantly lowered serum K+ levels with a difference between groups of −0.45 mEq/L (P < 0.001); a lower incidence of hyperkalaemia (7.3% RLY5016 vs. 24.5% placebo, P= 0.015); and a higher proportion of patients on spironolactone 50 mg/day (91% RLY5016 vs. 74% placebo, P= 0.019). In patients with CKD (n= 66), the difference in K+ between groups was −0.52 mEq/L (P= 0.031), and the incidence of hyperkalaemia was 6.7% RLY5016 vs. 38.5% placebo (P= 0.041). Adverse events were mainly gastrointestinal, and mild or moderate in severity. Adverse events resulting in study withdrawal were similar (7% RLY5016, 6% placebo). There were no drug-related serious AEs. Hypokalaemia (K+ <3.5 mEq/L) occurred in 6% of RLY5016 patients vs. 0% of placebo patients (P= 0.094).
Conclusion
RLY5016 prevented hyperkalaemia and was relatively well tolerated in patients with HF receiving standard therapy and spironolactone (25–50 mg/day) (ClinicalTrials.gov registry identifier: NCT00868439).
Keywords: Heart failure, Chronic kidney disease, Hyperkalaemia, Aldosterone antagonist, RLY5016, Potassium-binding polymer
See page 791 for the editorial comment on this article (doi:10.1093/eurheartj/ehr058)
Introduction
The use of aldosterone antagonists (AAs), when combined with angiotensin converting enzyme inhibitors (ACE-Is) or angiotensin receptor blockers (ARBs), has been associated with an improvement in survival and a reduction in hospitalizations for heart failure (HF) patients with chronic severe HF and a reduced left ventricular ejection fraction (HFREF) as well as in patients with HFREF post-myocardial infarction (MI).1,2 In addition, multiple renin–angiotensin–aldosterone system (RAAS) blockers including direct renin inhibitors may be beneficial in patients with chronic kidney disease (CKD) and albuminuria, as well as in patients with resistant hypertension and other cardiovascular disorders.3–6
However, these agents are associated with an increased incidence of hyperkalaemia, especially in patients with concomitant CKD.7–9 Patients with CKD have been shown to have an attenuated ability to excrete K+.10 Dietary K+ intake, use of pharmaceutical agents that impair the ability to excrete K+, such as non-steroidal anti-inflammatory drugs or RAAS blocking agents, can increase serum K+ to critical levels. Although the incidence of hyperkalaemia in large-scale randomized trials in HFREF patients receiving an ACE-I or ARB plus an AA is relatively low,11 the patients in such trials were generally pre-selected by criteria that excluded CKD. In clinical practice where patient selection and serial monitoring of serum K+ adheres to the criteria used in large-scale clinical trials, the incidence of hyperkalaemia is low.12 However, in clinical practice where patient selection and serial monitoring of serum K+ may be less rigorous, the incidence of hyperkalaemia is increased, at times resulting in kidney failure and death.13,14 The potential risks of inducing hyperkalaemia by multiple RAAS inhibitors/blockers has resulted in the use of less than target or maximally tolerated doses of these life saving agents, especially in patients with concomitant CKD.
RLY5016 is a non-absorbed polymer designed to bind K+ in the gastrointestinal (GI) tract and thereby reduce serum K+. The aim of this study is to determine the efficacy, safety, and tolerability of RLY5016 in a prospective randomized double-blind pilot study in patients at risk of developing hyperkalaemia, receiving standard therapy for HF and who were initiating AA therapy with spironolactone.
Methods
Patients
Eligible patients were ≥18 years of age, had a history of chronic HF, an indication to initiate spironolactone therapy, per the investigator's clinical judgment, a serum K+ concentration of 4.3–5.1 mEq/L at screening. In addition, they must have had either (i) CKD [with estimated glomerular filtration rate (eGFR) determined by a local laboratory of <60 mL/min] and were receiving one or more HF therapies (ACE-Is, ARBs, beta-blockers); or (ii) a documented history of hyperkalaemia that led to discontinuation of therapy with an AA, ACE-I, ARB, or beta-blocker within 6 months prior to the baseline visit. Patients were excluded if they had severe GI disorders, major GI surgery, bowel obstruction, swallowing disorders, significant primary valvular disease, known obstructive or restrictive cadiomyopathy, uncontrolled or unstable arrhythmia, episode of unstable angina within 3 months prior to baseline, acute coronary syndrome, transient ischaemic attack, a QTc value of >500 ms (using Bazett's correction formula), recent or anticipated cardiac surgery or intervention, kidney transplantation or need for transplantation, receiving dialysis or anticipated need for dialysis during the study, sustained systolic blood pressure >170 or < 90 mmHg, elevated liver enzymes (more than three times the upper limit of normal), or any condition that had the potential to interfere with study compliance or jeopardize the safety of the patient.
Study protocol
This 4-week, double-blind, randomized, placebo-controlled, parallel-group study was conducted at 38 centres in the USA, Germany, the Czech Republic, Poland, the Ukraine, Russia, and Georgia. All participating sites received approval from their locally appointed ethics committee (or equivalent); all patients provided informed written consent, and the study was performed in accordance with current local and national regulations, the International Conference on Harmonisation Good Clinical Practice guidelines, and other applicable requirements governing the conduct of human clinical trials. The study was registered with ClinicalTrials.gov, with an identifier of NCT00868439.
Patients who completed screening and satisfied the eligibility criteria proceeded to baseline assessments, which included review of medical and medication histories, a physical examination, including weight, resting vital signs, and 12-lead electrocardiogram (ECG), determination of serum K+ (performed by both local and central laboratories), and clinical laboratory tests (including serum chemistry, haematology, and urinalysis); in addition, women of child-bearing potential had a serum pregnancy test.
Following baseline assessments, patients who continued to meet eligibility criteria were randomized 1:1 to RLY5016 or placebo treatment in a blinded fashion. Patients were instructed to take 15 g of study drug orally in the morning and evening (for a total daily dose of 30 g) and to mix study drug (supplied as a powder) with water or a low-potassium food prior to administration. Patients were also instructed to start spironolactone at a dose of 25 mg/day. After 2 weeks (i.e. on Day 15), spironolactone was increased to 50 mg/day if the patient's serum K+ was >3.5 to ≤5.1 mEq/L; the dose remained at 25 mg/day if the serum K+ level was >5.1 to ≤5.5 mEq/L; and patients were discontinued from the study if their serum K+ was ≤3.5 or >5.5 mEq/L.
Prohibited medications during the study included polymer-based drugs, other phosphate or K+ binders, K+ sparing medications, antacids, calcium or K+ supplements, and intravenous cardioactive medications.
Throughout the 4-week treatment period, assessments of efficacy and safety were performed routinely. Serum K+ was monitored at each clinic visit on Days 3, 7, 14, 17, 21, and 28. Serum chemistry, body weight, and vital signs were assessed on Days 7, 14, 21, and 28; haematology on Days 14 and 28; and 12-lead ECGs and assessments of concomitant medications and adverse events (AEs) were performed at each clinic visit.
All AEs encountered during the study and through 7 days following completion of study treatment were recorded.
Clinical endpoints
The primary efficacy endpoint was the mean change of serum K+ from baseline to the end of the study (Day 28). Secondary endpoints included the proportion of patients with serum K+ >5.5 mEq/L at any time during the trial and the proportion of patients whose spironolactone dose could be increased to 50 mg/day. Serum K+ data measured at a central laboratory were used for the efficacy analysis. The safety of RLY5016 was assessed by the incidence of AEs or clinically significant changes from baseline in clinical laboratory values, vital signs, and ECG parameters.
Statistical analysis
A sample size of ∼100 patients (50 patients per treatment group) was determined to ensure at least 90 patients (45 patients per treatment group) would be available with primary efficacy data for analysis. This sample size had 90% power to detect a difference of 0.7 mEq/L in the mean change of serum K+ from baseline to the endpoint [last observation carried forward (LOCF)] between two treatment groups. This calculation was based on a two-sided two-sample t-test with a 1:1 sample size allocation ratio, a standard deviation (SD) of 1 mEq/L, and a significance level of α = 0.05.
Efficacy and safety analyses were performed on the modified intent-to-treat (mITT) population, defined as all randomized patients who received study medication and had available efficacy data. All mITT patients were included in the primary and secondary efficacy analyses. Baseline measurement was the last available measurement obtained prior to the start of treatment. The endpoint was obtained at Day 28 post-treatment or was derived based on the LOCF method for missing data.
A parallel lines analysis of covariance (ANCOVA) model was used for the analysis of the primary efficacy measurement. This ANCOVA model included treatment and region factors, and baseline serum K+ as a covariate. The least squares (LS) estimate of the mean change in serum K+ of each treatment and its 95% confidence interval (CI) was calculated. In addition, the LS estimate of the difference between RLY5016 treatment and placebo treatment (RLY5016 treatment minus placebo) and its 95% CI was calculated. Patients from all study centres were pooled for the analysis of the categorical outcome data. For the analysis of the dichotomous outcome data, a two-sample Z-test on two proportions between RLY5016 treatment and placebo treatment was performed. The difference between two proportions (RLY5016 treatment minus placebo) and its 95% CI were calculated. Adverse event analyses were performed for the safety population, which included all patients who received at least one dose of study drug. The two-sided Fisher exact test was used to compare the incidence of AEs between two treatment groups. Statistical analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
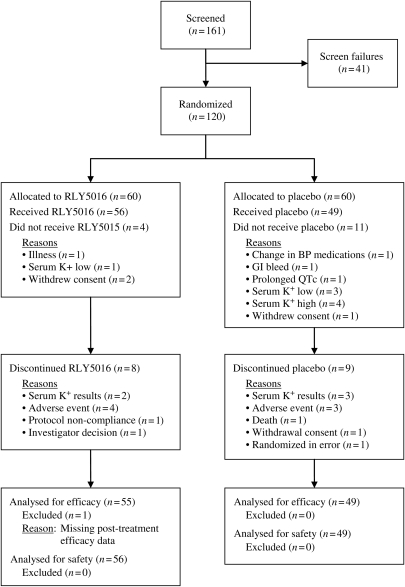
Study data were collected between June 2009 and November 2009. A total of 120 patients were randomized (60 to each treatment group) and 105 patients (63 men and 42 women with a mean age of 68 years) received at least one dose of study drug (56 received RLY5016 and 49 placebo) (Figure 1). One patient randomized to RLY5016 received one dose of study drug but did not return to the clinic within the protocol-specified time period and was therefore not evaluable for efficacy. This patient was excluded from the mITT population, but included in the safety population.
Figure 1.
Patient flow diagram.
The two treatment groups were balanced with respect to baseline characteristics (Table 1). Patients had a history of HF with a mean duration of just over 4 years, and an ejection fraction of ∼40%. Most patients were classified as NYHA class II or III. About one-third of patients had diabetes and the baseline eGFR for all patients was 81 ± 33 mL/min (calculated based on assessments by a central laboratory). At baseline, four patients (4%) had severe renal impairment (eGFR <30 mL/min) and 28 patients (27%) had an eGFR <60 mL/min. Eleven patients (15%) had HF with preserved ejection fraction (HFPEF; LVEF ≥50%), 5 in placebo and 6 in the RLY5016 group. Two RLY5016-treated patients with HFPEF were included due to a history of hyperkalaemia that resulted in discontinuation of a RAAS blocker or beta-adrenergic blocking agent.
Table 1.
Patient baseline characteristics
| Parameter | RLY5016 (n= 55) | Placebo (n= 49) |
|---|---|---|
| Demographics | ||
| Age (years) | 68 ± 9 | 68 ± 11 |
| Male, n (%) | 29 (53) | 34 (69) |
| Caucasian, n (%) | 53 (95) | 48 (98) |
| BMI (kg/m2) | 28 ± 6 | 27 ± 4 |
| Cardiac history and parameters | ||
| HF duration (years) | 5 ± 5 | 4 ± 3 |
| NT-proBNP (pg/mL) | 1395 ± 1955 | 2339 ± 5432 |
| Median NT-proBNP (pg/mL) | 824 | 756 |
| Left ventricular ejection fraction (%) | 40 ± 12 | 41 ± 12 |
| NYHA Class, n (%) | ||
| I | 2 (4) | 1 (2) |
| II | 29 (53) | 28 (57) |
| III | 24 (44) | 20 (41) |
| IV | 0 (0) | 0 (0) |
| Heart rate (b.p.m.) | 70 ± 11 | 70 ± 11 |
| Systolic blood pressure (mmHg) | 128 ± 13 | 128 ± 12 |
| Diastolic blood pressure (mmHg) | 78 ± 8 | 78 ± 8 |
| Other factors | ||
| History of diabetes, n (%) | 15 (27) | 18 (37) |
| eGFR (mL/min) | 84 ± 35 | 78 ± 32 |
| Medication at randomization | ||
| Diuretic | 41 (75) | 36 (74) |
| Digitalis glycoside | 10 (18) | 4 (8) |
| Anti-platelet | 37 (66) | 32 (65) |
| ACE-I | 45 (82) | 28 (57) |
| Maximum dose of ACE-I, n (%) | 6 (13) | 2 (7) |
| ARB | 9 (16) | 12 (24) |
| Maximum dose of ARB, n (%) | 4 (44) | 1 (8) |
| β–Blocker | 45 (82) | 46 (94) |
| Maximum dose of β-blocker, n (%) | 8 (18) | 5 (11) |
| ACE-I, ARB, or β-blocker only | 13 (24) | 9 (18) |
| ACE-I or ARB + β-blocker | 40 (73) | 37 (76) |
| ACE-I + ARB + β–blocker | 2 (4) | 1 (2) |
| No RAAS inhibitors or β-blocker | 0 (0) | 2 (4) |
| Entry criteria, n (%) | ||
| (1) CKD with eGFR <60 mL/min | 27 (50%) | 30 (63%) |
| (2) History of hyperkalaemia | 22 (41%) | 15 (31%) |
| Both (1) and (2) | 5 (9%) | 3 (6%) |
Data are presented as mean ± SD unless stated otherwise.
ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HF, heart failure; NYHA, New York Heart Association; RAAS, renin–angiotensin–aldosterone system.
At baseline, all but two patients (both in the placebo group) were receiving a RAAS inhibitor or beta-blocker. Slightly more than half of the patients were on dual therapy of an ACE-I and a beta-blocker (60% RLY5016 and 53% placebo), 18% of patients were on dual therapy with an ARB and a beta-blocker (13% RLY5016 and 22% placebo), and no patients were on both ACE-I and ARB. Few patients were on monotherapy alone (11%) and 3% of patients were on triple therapy (ACE-I, ARB, and beta-blocker). An equal proportion of patients (74%) received diuretics (15% thiazide, 57% loop) in both the RLY5016 and placebo groups. The two placebo patients who were not taking a RAAS or beta-blocker had a history of hyperkalaemia that required discontinuation of these medications.
Overall, 88 (84%) patients completed the study and 17 (8 in the RLY5016 group and 9 in the placebo group) prematurely terminated from the study. Reasons for discontinuation were attributed to AEs (four patients from the RLY5016 group and two from the placebo group); death (one patient in the placebo group); protocol-specified discontinuation criteria (two RLY5016 patients and three placebo patients); protocol non-compliance (one RLY5016 patient); investigator decision (one RLY5016 patient); randomization error (one placebo patient); and elective withdrawal (one placebo patient). Compliance with study drug and spironolactone was measured by the examination of bottles returned to the clinic at each visit (every 4–7 days); the compliance was > 97% in both placebo and active groups.
Efficacy
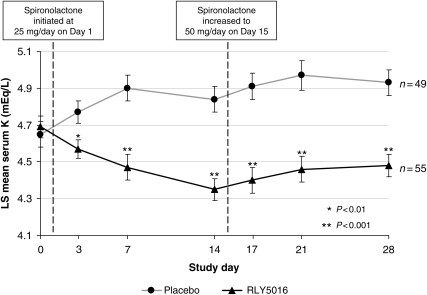
At baseline, mean serum K+ values were not different between treatment groups: 4.69 mEq/L for the RLY5016 group and 4.65 mEq/L for the placebo group (between-group P= 0.664). At the end of the treatment period, RLY5016 had significantly lowered serum K+ levels relative to placebo, with a difference between groups of −0.45 mEq/L (P < 0.001). Following the start of dosing with spironolactone and study drug on Day 1, serum K+ values decreased in patients treated with RLY5016 and increased in patients on placebo (Figure 2). The difference in response between treatment groups was statistically significant at every measured time point, starting at Day 3 (2 days after initiation of study medication) and continuing through Day 28, despite an increase in spironolactone (from 25 to 50 mg/day) at Day 15. At Day 28 (the end of treatment), patients in the RLY5016 group had a mean (LS) change in serum K+ from baseline of −0.22 mEq/L, whereas patients in the placebo group had a mean change of +0.23 mEq/L.
Figure 2.
Least squares mean (SEM) of last observation carried forward (LOCF) serum potassium for the intent-to-treat population by study visit on Day 15, the spironolactone dose was increased in patients who had serum K+ levels ≤5.1 mEq/L. * indicates P < 0.01, and ** indicates P < 0.001. Note: Data were imputed based on LOCF for seven RLY5016-treated patients and nine placebo-treated patients due to early termination from the study.
At all times during the treatment period, fewer patients in the RLY5016 treatment group developed hyperkalaemia (with a serum K+ value >5.5 mEq/L) compared with placebo (7 vs. 25%, P= 0.015; Table 2). However, more RLY5016-treated patients had hypokalaemia (K+ <3.5 mEq/L) than placebo patients (6 and 0%, respectively), and significantly more RLY5016 patients had K+ values <4.0 mEq/L than placebo patients (47 vs. 10%, respectively; P< 0.001; Table 2). In addition, significantly more patients in the RLY5016 group were able to have their spironolactone dose increased compared with patients in the placebo group (91 vs. 74%, P= 0.019; Table 2).
Table 2.
Summary of incidence of hyperkalaemia, hypokalaemia, hypomagnesaemia, and increase of spironolactone dose
| No. (%) of patients |
P-value | ||
|---|---|---|---|
| RLY5016 30 g/day (n= 55) | Placebo (n= 49) | ||
| Serum potassium >5.5 mEq/La | 4 (7) | 12 (25) | 0.015 |
| Serum potassium <4.0 mEq/L | 26 (47) | 5 (10) | <0.001 |
| Serum potassium <3.5 mEq/L | 3 (6) | 0 (0) | 0.094 |
| Serum magnesium <1.8 mg/dL | 13 (24) | 1 (2) | 0.001 |
| Spironolactone dose increased | 50 (91) | 36 (74) | 0.019 |
aAt any study visit.
In the subset of patients with baseline eGFR <60 mL/min (15 treated with RLY5016 and 13 placebo), the mean change in serum K+ to the end of study was different between treatment groups (P= 0.031): −0.14 vs. +0.38 mEq/L in the RLY5016 and placebo groups, respectively (Table 3). Consistent with the analysis of serum K+ for all patients, RLY5016-treated patients with a baseline eGFR <60 mL/min showed greater reduction in mean serum K+ compared with placebo as early as Day 3. The differences in response between treatment groups reached statistical significance on Day 7, which continued throughout the remainder of the study despite the fact that more RLY5016 patients received a higher mean dose of spironolactone compared with placebo patients. For patients with a baseline eGFR ≥60 mL/min, the mean change in serum K+ from baseline to the end of treatment was also significantly different between treatment groups (−0.35, P= 0.001; Table 3).
Table 3.
Summary of least squares mean change ±SEM from baseline in serum potassium by baseline estimated glomerular filtration rate
| Baseline eGFR (mL/min) | N/N (RLY5016/Placebo) | RLY5016 30 g/day | Placebo | Difference (mEq/L) | P-value |
|---|---|---|---|---|---|
| <60 | 15/13 | −0.14 ± 0.15 | 0.38 ± 0.16 | −0.52 ± 0.23 | 0.031 |
| ≥60 | 40/36 | −0.32 ± 0.07 | 0.02 ± 0.07 | −0.35 ± 0.10 | 0.001 |
SEM, standard error of least squares mean change.
The incidence of hyperkalaemia in the subgroup of HF patients with CKD was significantly reduced with RLY5016 treatment compared with placebo (P= 0.041 for patients with eGFR <60 mL/min). The incidence of hyperkalaemia in patients with an eGFR ≥60 mL/min was lower with RLY5016 treatment compared with placebo, although the difference was not significant (P= 0.13) (Table 4). Although not statistically significant, 87% of HF patients with CKD treated with RLY5016 could increase their spironolactone dose compared with 69% of the placebo-treated cohorts with eGFR <60 mL/min.
Table 4.
Incidence of hyperkalaemia by baseline estimated glomerular filtration rate
| Baseline eGFR (mL/min) | No. (%) of patients with hyperkalaemia (serum potassium > 5.5 mEq/L) at any study visit |
||
|---|---|---|---|
| RLY5016 30 g/day | Placebo | P-value | |
| <60 | 1/15 (6.7) | 5/13 (38.5) | 0.041 |
| ≥60 | 3/40 (7.5) | 7/36 (19.4) | 0.125 |
| All patients (eGFR = 81±33) | 4/55 (7.3) | 12/49 (24.5) | 0.015 |
The efficacy results for the subgroups based on baseline eGFR were estimated from an ANCOVA model that included treatment, region, baseline eGFR group, GFR group by treatment interaction factors, and baseline value as covariates. Based on this analysis, for patients with a baseline eGFR <60 and ≥60 mL/min, there was no significant GFR-by-treatment interaction (P= 0.61).
Among the subset of patients who entered the study under the criteria of a previous history of hyperkalaemia [22 treated with RLY5016 and 15 placebo (Table 1)], the difference in the mean change from baseline to last observation in serum K+ between treatment groups was −0.34 and +0.09 mEq/L for the RLY5016 and placebo groups, respectively (P= 0.058) (Table 5). The incidence of hyperkalaemia in this subset was 9.1% in the RLY5016 group vs. 20.0% in the placebo group (P= 0.34).
Table 5.
Summary of least squares mean change ±SEM from baseline in serum potassium and incidence of hyperkalaemia by entry criteria of history of hyperkalaemia
| Analysis | Patients with a history of hyperkalaemia at study entry |
|||
|---|---|---|---|---|
| RLY5016 30 g/day (N= 22) | Placebo (N= 15) | Difference (mEq/L) | P-value | |
| Change from baseline in serum potassium (mEq/L) | −0.34 ± 0.08 | 0.09 ± 0.10 | −0.25 ± 0.13 | 0.058 |
| Incidence of hyperkalaemia (serum potassium > 5.5 mEq/L), n (%) | 2 (9.1) | 3 (20.0) | Not applicable | 0.341 |
SEM, standard error of least squares mean change.
Safety
Thirty (54%) patients in the RLY5016 treatment group and 15 (31%) in the placebo group experienced at least one AE (Table 6). The most common AEs were GI disorders (e.g. flatulence, diarrhea, constipation, and vomiting), which were reported with higher frequency in the RLY5016 group compared with the placebo group (21 vs. 6%, respectively). The majority of AEs were graded by the investigator as mild or moderate in intensity. A similar proportion of patients in each treatment group had an AE that led to discontinuation of study drug (7% RLY5016, 6% placebo).
Table 6.
Safety summary
| Safety parameter | No. (%) of patients |
|
|---|---|---|
| RLY5016 (N= 56) | Placebo (N= 49) | |
| Any adverse event | 30 (54) | 15 (31) |
| Gastrointestinal disorders | 12 (21) | 3 (6) |
| Flatulence | 4 (7) | 0 |
| Diarrhea | 3 (5) | 1 (2) |
| Constipation | 3 (5) | 0 |
| Vomiting | 2 (4) | 0 |
| Any serious adverse event | 2 (4) | 2 (4) |
| Related serious adverse event | 0 | 0 |
| Any adverse event leading to discontinuation of study drug | 4 (7) | 3 (6) |
Four patients (two in each treatment group) had an SAE. In the RLY5016 group, one patient had three SAEs, which included worsening coronary artery disease (CAD), atrial fibrillation, and a non-ST segment elevation MI; a second patient had worsening CAD. In the placebo group, one patient had an SAE of knee gout, and a second patient had sudden cardiac arrest that resulted in death. None of the SAEs was considered by the investigator to be study drug related.
There were no clinically meaningful treatment-related changes in most laboratory tests (e.g. serum chemistry, haematology, and urinalysis). In addition to potassium (Table 2), serum chemistry testing included evaluation of calcium, iron, magnesium, phosphorous, and sodium. Significant changes were noted for serum K+ (as anticipated) and serum magnesium. Although mean serum magnesium values were within normal limits, a small but statistically significant decrease from baseline was observed (−0.22 vs. 0.01 mg/dL for the RLY5016 and placebo groups respectively, P < 0.001). Serum magnesium <1.8 mg/dL during the treatment period was seen in 13 (24%) RLY5016-treated patients and 1 (2.1%) placebo-treated patient. Additionally, hypomagnesaemia (serum Mg2+ <1.8 mg/dL) occurred in two of the three hypokalaemic patients (serum K+ < 3.5 mEq/L). There was no increase in the incidence of ventricular arrhythmias associated with either the development of hypokalaemia or hypomagnesaemia in these patients.
Both RLY5016- and placebo-treated patients had significant changes in mean serum creatinine from baseline to the end of study drug treatment at Day 28: the RLY5016 group had a mean increase of 0.10 mg/dL (P= 0.012), and the placebo group had a mean increase of 0.16 mg/dL (P= 0.001). The difference between the two treatment groups was not significant (−0.07 mg/dL, P= 0.218). For the subgroup of patients with concomitant CKD, neither treatment group had a significant change from baseline serum creatinine, and there was no significant difference between treatment groups. For the subgroup of patients with a baseline eGFR ≥60 mL/min, the mean change in serum creatinine within the RLY5106 group from baseline to the end of treatment was an increase of 0.10 mg/dL (P= 0.008); the change in the placebo group was an increase of 0.016 mg/dL (P= 0.001); and the difference between groups was not significant (P= 0.262). For patients who developed hyperkalaemia in the placebo group, there was a mean increase in serum creatinine of 0.07 mg/dL, whereas in patients who developed hyperkalaemia in the RLY5016 group, the mean serum creatinine decreased by 0.05 mg/dL (P= NS compared with placebo).
There were no significant changes in mean eGFR from baseline to the end of treatment at Day 28, either within treatment groups or between treatment groups (the mean difference between groups was 1.52 mL/min, P= 0.63). Consistent results were observed for subgroups based on baseline eGFR (< 60 and ≥60 mL/min). For patients with a baseline eGFR <60 mL/min, the difference between treatment groups was 3.31 mL/min (P= 0.61), and for patients with a baseline eGFR ≥60 mL/min, the difference between groups was 1.06 mL/min (P= 0.78).
There were no significant changes on blood pressure or heart rate from baseline to the end of treatment at Day 28 between treatment groups. Furthermore, there were no clinically meaningful treatment-related changes observed for ECGs or physical examinations.
Discussion
This first prospective, placebo-controlled, double-blind trial of RLY5016 to prevent hyperkalaemia in chronic HF patients receiving standard therapy, including an ACE-I or ARB and a beta-adrenergic blocking agent in addition to spironolactone 25–50 mg/day, demonstrated that RLY5016 significantly decreased serum K+, reduced the incidence of hyperkalaemia, and increased the proportion of patients in whom the dose of spironolactone could be increased to 50 mg/day. These results were obtained in patients with HF and concomitant CKD, as well as in patients with HF and a history of hyperkalaemia requiring prior discontinuation of a RAAS blocking agent.
A 30 g/day dose of RLY5016 significantly reduced serum K+ relative to placebo, with a difference between treatment groups of 0.45 mEq/L. Furthermore, RLY5016 prevented the development of hyperkalaemia (serum K+ >5.5 mEq/L), and allowed a significantly greater percentage of patients to be up-titrated from 25 mg/day to 50 mg/day of spironolactone compared with placebo (91 vs. 74%, Table 2). The significant reduction in serum K+ in patients randomized to 30 g/day of RLY5016 was observed within 2 days of initiating treatment and persisted throughout the 4-week course of the study (Figure 2).
In the subgroup of patients with HF and a history of hyperkalaemia requiring prior discontinuation of a RAAS blocker, but without concomitant CKD, the mean increase in serum K+ and the incidence of hyperkalaemia following administration of spironolactone 25–50 mg/day was relatively low in patients assigned to placebo (Table 5). The explanation for the relatively small increase in serum K+ and low incidence of hyperkalaemia despite administration of 25–50 mg/day of spironolactone in these patients is uncertain. In contrast, in the subgroup of patients with HF and concomitant CKD (baseline eGFR <60 mL/min), the incidence of hyperkalaemia after receiving spironolactone was 39% among patients taking placebo compared with 7% among patients taking RLY5016 (Table 4).
RLY5016 at 30 g/day was relatively well tolerated over the 4-week course of the study. Gastrointestinal side effects, generally mild or moderate in nature, were the most common side effects reported and occurred at a higher incidence on RLY5016 compared with placebo (Table 6). Treatment with RLY5016 caused a 6% incidence of hypokalaemia (serum K+ <3.5 mEq/L) and a 47% incidence of a serum K+ <4.0 mEq/L (Table 2), a difference of 37% compared with the incidence in the placebo group. A serum K+ <3.5 mEq/L has long been recognized as an important risk factor for death in patients with HFREF.15 Recent data have suggested that a serum K+ <4.0 mEq/L, and even a serum K+ <4.0 but >3.5 mEq/L, may also be associated with an increase in mortality in patients with HFREF and concomitant CKD.16 Additionally, hypomagnesaemia (serum Mg2+ <1.8 mg/dL) occurred in 24% of the patients and in two of the three hypokalaemic patients (serum K+ <3.5 mEq/L). Hypomagnesaemia is associated with ventricular arrhythmias and may increase mortality risk in patients with hypokalaemia.
RLY5016 has a novel chemical composition that promotes ionization of the polymeric potassium-binding moiety under pH conditions present along the extent of the GI tract, particularly in the colon. As a result of this chemical structure, RLY5016 exchanges monovalent (Na+) and divalent cations (Ca2+, Mg2+) through the length of the GI tract and preferentially binds K+ in the colon where the concentration of this cation is substantially higher than that of Na+, Ca2+, or Mg2+.17,18 The net effect of RLY5016-potassium binding in the colon is a reduction in serum K+ under hyperkalaemic conditions, where increased K+ secretion through BK channels represents an adaptive response to elevated serum K+.19
The RLY5016 polymer is synthesized as a 100 µm bead, with optimized flow and viscosity properties. RLY5016 is not administered with a cathartic, has not been associated with the occurrence of bowel necrosis, and has not been found to interfere with the absorption of drugs that are commonly administrated to patients with HF and CKD, with the exception of a 30% reduction in the bioavailability of valsartan and rosiglitazone in preclinical co-administration studies in rats (data on file).
RLY5016 binds soluble potassium in the GI tract, thereby effecting movement of K+ from the serum into the intestinal lumen and ultimately reducing total body potassium by increasing fecal potassium excretion. Previous studies have demonstrated that the relationship between total body potassium and serum K+ is non-linear.20 When serum K+ exceeds a ‘buffering zone’ in the normal range (serum K+ of 4.0–5.0 mEq/L), then relatively small changes in total body potassium cause exaggerated changes in serum K+. Analysis of data from previous RLY5016 preclinical and clinical studies have shown that a fixed dose of RLY5016 has a greater serum K+ reducing effect at higher serum K+ values than at lower serum K+ values (data on file). Based on the relationship between serum K+ and changes in total body K+ (mEq),20 it is predicted that at a serum K+ of 6.5 mEq/L, a 30 g/day dose of RLY5016 that provides ∼25 mEq/day extra excretion of fecal K+ will reduce serum K+ to 5.5 mEq/L, whereas at a serum K+ level of 4.0 mEq/L the same dose of RLY5016 will reduce serum K+ to only 3.8 mEq/L. This suggests that RLY5016 may not cause hypokalaemia in patients with levels of serum K+ greater than selected for the present study, such as those with hyperkalaemia.
Given the characteristics of the study, several limitations are apparent. LVEF was not a criterion for entry and on average, the patients in this study had less severe HF (and a higher LVEF than those included in RALES), therefore some of the patients did not meet current ESC/AHA/ACC-HF guideline recommendations for initiation of AA treatment. Additionally, the sample size was relatively small, the duration of the study was limited to 4 weeks of treatment, and a single dose of RLY5016 was evaluated.
The risk of a serum K+ >5.5 mEq/L was emphasized by a recent study of over 245 000 patients in the Veterans Administration system who had a measurement of serum K+ and on follow-up were found to have a significantly increased risk of death (with an odds ratio of 10.32) within 1 day of having a serum K+ >5.5 mEq/L.21 While CKD has been shown to be an important predictor of hyperkalaemia in patients receiving multiple RAAS blockers, recent studies have found that patients with HF and normal kidney function, especially those with the NR3C2 215G genotype, may also develop hyperkalaemia22 despite the finding that serum K+ tends to decrease with the severity of HF in patients with normal kidney function.23 While the acute treatment of hyperkalaemia associated with ECG changes characteristic of hyperkalaemia and or ventricular arrhythmias will likely continue to rely on the use of dialysis and other accepted strategies, the current results with RLY5016 in patients with HF and CKD suggest that it may be possible to avoid the need for dialysis in some patients. Longer term studies with a greater numbers of participants will be necessary to determine the long-term efficacy and tolerability of RLY5016 both for the prevention of hyperkalaemia as well as its treatment once it occurs. In addition, dose ranging studies will be required to avoid trading the risks of hyperkalaemia for those of hypokalaemia and hypomagnesaemia.
Funding
This work was supported by Relypsa, Inc. Funding to pay the Open Access publication charge for this article was provided by Relypsa Inc.
Conflict of interest: B.P.: Relypsa*; AstraZeneca; AuraSense*; Bayer; BG Medicine*; Boehringer Ingelheim; Forest Laboratories; GE Healthcare; Medtronic; Merck; Nile Therapeutics*; Novartis; Pfizer; Takeda. S.A.: Relypsa. D.A.B.: Relypsa*, Amgen, Cytochroma, Genzyme. D.K.: Relypsa*, Alteon; BMS/Lantheus Medical Imaging, Boston Scientific, Encysive, Gilead, Medtronic, Novartis. F.Z.: Relypsa; AstraZeneca; Boehringer Ingelheim; Boston Scientific; Novartis; Pfizer; Resmed; Servier; Takeda. I-Z.H.: Relypsa*, Anthera*. Grants and honoraria were received from the companies listed. An asterisk (*) indicates stock options.
Appendix
Steering Committee: Stefan D. Anker (Berlin Germany), David A. Bushinsky (Rochester, NY, USA), Dalane W. Kitzman (Winston-Salem, NC, USA), Bertram Pitt (Ann Arbor, MI, USA), and Faiez Zannad (Nancy, France).
Investigators: Alexander Adler (Peoria, IL, USA), Evgenia Akatova (Moscow, Russia), Inder Anand (Minneapolis, MN, USA), Ragavendra Baliga (Columbus, OH, USA), Subhash Banerjee (Dallas, TX, USA), Olga Barbarash (Kemerovo, Russia), Vakhtang Chumburidze (Tblisi, Georgia), Boris M. Goloshchekin (Saint Petersburg, Russia), Ivan Gordeev (Moscow, Russia), Joanne Holland (Northport, NY, USA), David Hotchkiss (Port Charlotte, FL, USA), Marie Iacona (Buffalo, NY, USA), Irakli Khintibidze (Tblisi, Georgia), Ivan Málek (Prague, Czech Republic), Mohammed Natour (Heidelberg, Germany), Kakhi Paposhvili (Tblisi, Georgia), Alexander Parkhomenko (Kyiv, Ukraine), Jonathan Roberts (Miami, FL, USA), Tamaz Shaburishvili (Tblisi, Georgia), Jindřich Špinar (Brno, Czech Republic), Yevgenia Svyshchenko (Kyiv Ukraine), Hanna Szwed (Warsaw, Poland), Alexander Vishnevsky (Saint Petersburg, Russia), Rolf Wachter (Goettingen, Germany), James Zebrack (Salt Lake City, UT, USA).
Contract Research Organizations: Medpace (Cincinnati, OH, USA), Worldwide Clinical Trials (King of Prussia, PA, USA).
Statistical Analysis: Yu-Kun Chiang, PhD (San Jose, CA).
Other contributors: Detlef Albrecht (Santa Clara, CA, USA), Mike Burdick (Santa Clara, CA, USA), Jerry Buysse (Santa Clara, CA, USA), Michael (Jamie) Cope (Santa Clara, CA, USA), Sherin Halfon (Santa Clara, CA, USA), Ming Jone (Santa Clara, CA, USA), Yuri Stasiv (Santa Clara, CA, USA), Suzette Warren (Santa Clara, CA, USA), and PEARL-HF Investigators.
References
- 1.Pitt B. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. doi:10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 2.Zannad F, McMurray JJV, Drexler H, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pitt B. Rationale and design of the Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure (EMPHASIS-HF) Eur J Heart Fail. 2010;12:617–622. doi: 10.1093/eurjhf/hfq049. [DOI] [PubMed] [Google Scholar]
- 3.Bianchi S, Bigazzi R, Campese VM. Long-term effects of spironolactone on proteinuria and kidney function in patients with chronic kidney disease. Kidney Int. 2006;70:2116–2123. doi: 10.1038/sj.ki.5001854. [DOI] [PubMed] [Google Scholar]
- 4.Epstein M, Williams GH, Weinberger M, Lewin A, Krause S, Mukherjee R, Patni R, Beckerman B. Selective aldosterone blockade with eplerenone reduces albuminuria in patients with type 2 diabetes. Clin J Am Soc Nephrol. 2006;1:940–951. doi: 10.2215/CJN.00240106. doi:10.2215/CJN.00240106. [DOI] [PubMed] [Google Scholar]
- 5.Palmer BF. Hypertension management in patients with chronic kidney disease. Curr Hypertens Rep. 2008;10:367–373. doi: 10.1007/s11906-008-0069-z. doi:10.1007/s11906-008-0069-z. [DOI] [PubMed] [Google Scholar]
- 6.Schrier RW, Masoumi A, Elhassan E. Aldosterone: role in edematous disorders, hypertension, chronic renal failure, and metabolic syndrome. Clin J Am Soc Nephrol. 2010;5:1132–1140. doi: 10.2215/CJN.01410210. [DOI] [PubMed] [Google Scholar]
- 7.Sica DA. Hyperkalemia risk in chronic kidney disease. deterrent to the use of aldosterone receptor antagonism or not. Hypertension. 2009;53:754–760. doi: 10.1161/HYPERTENSIONAHA.108.128017. [DOI] [PubMed] [Google Scholar]
- 8.Poggio R, Grancelli HO, Miriuka SG. Understanding the risk of hyperkalaemia in heart failure: role of aldosterone antagonism. Postgrad Med J. 2010;86:136–142. doi: 10.1136/pgmj.2008.072058. doi:10.1136/pgmj.2008.072058. [DOI] [PubMed] [Google Scholar]
- 9.Slagman MC, Navis G, Laverman GD. Dual blockade of the renin-angiotensin-aldosterone system in cardiac and renal disease. Curr Opin Nephrol Hypertens. 2010;19:140–152. doi: 10.1097/MNH.0b013e3283361887. [DOI] [PubMed] [Google Scholar]
- 10.Sorensen MV, Matos JE, Praetorius HA, Leipziger J. Colonic potassium handling. Pflugers Arch. 2010;459:645–656. doi: 10.1007/s00424-009-0781-9. doi:10.1007/s00424-009-0781-9. [DOI] [PubMed] [Google Scholar]
- 11.Weir MR, Rolfe M. Potassium homeostasis and renin-angiotensin-aldosterone system inhibitors. Clin J Am Soc Nephrol. 2010;5:531–538. doi: 10.2215/CJN.07821109. doi:10.2215/CJN.07821109. [DOI] [PubMed] [Google Scholar]
- 12.Wei L, Struthers AD, Fahey T, Watson AD, Macdonald TM. Spironolactone use and renal toxicity: population based longitudinal analysis. BMJ. 2010;340:c1768. doi: 10.1136/bmj.c1768. doi:10.1136/bmj.c1768. [DOI] [PubMed] [Google Scholar]
- 13.Lopes RJ, Lourenco AP, Mascarenhas J, Azevedo A, Bettencourt P. Safety of spironolactone use in ambulatory heart failure patients. Clin Cardiol. 2008;31:509–513. doi: 10.1002/clc.20284. doi:10.1002/clc.20284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, Laupacis A, Redelmeier DA. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. 2004;351:543–551. doi: 10.1056/NEJMoa040135. doi:10.1056/NEJMoa040135. [DOI] [PubMed] [Google Scholar]
- 15.Mandal AK. Hypokalemia and hyperkalemia. Med Clin North Am. 1997;81:611–639. doi: 10.1016/s0025-7125(05)70536-8. doi:10.1016/S0025-7125(05)70536-8. [DOI] [PubMed] [Google Scholar]
- 16.Bowling CB, Pitt B, Ahmed MI, Aban IB, Sanders PW, Mujib M, Campbell RC, Love TE, Aronow WS, Allman RM, Bakris GL, Ahmed A. Hypokalemia and outcomes in patients with chronic heart failure and chronic kidney disease: findings from propensity-matched studies. Circ Heart Fail. 2010;3:253–260. doi: 10.1161/CIRCHEARTFAILURE.109.899526. doi:10.1161/CIRCHEARTFAILURE.109.899526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wrong O, Metcalfegibson A. The electrolyte content faeces. Proc R Soc Med. 1965;58:1007–1009. doi: 10.1177/003591576505801203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fordtran JS, Locklear TW. Ionic constituents and osmolality of gastric and small-intestinal fluids after eating. Am J Dig Dis. 1966;11:503–521. doi: 10.1007/BF02233563. doi:10.1007/BF02233563. [DOI] [PubMed] [Google Scholar]
- 19.Sørensen MV, Matos JE, Praetorius HA, Leipziger J. Colonic potassium handling. Pflugers Arch. 2010;459:645–656. doi: 10.1007/s00424-009-0781-9. doi:10.1007/s00424-009-0781-9. [DOI] [PubMed] [Google Scholar]
- 20.Brown RS. Potassium homeostasis and clinical implications. Am J Med. 1984;77:3–10. doi: 10.1016/s0002-9343(84)80002-9. doi:10.1016/S0002-9343(84)80002-9. [DOI] [PubMed] [Google Scholar]
- 21.Einhorn LM, Zhan M, Hsu VD, Walker LD, Moen MF, Seliger SL, Weir MR, Fink JC. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169:1156–1162. doi: 10.1001/archinternmed.2009.132. doi:10.1001/archinternmed.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cavallari LH, Groo VL, Viana MA, Dai Y, Patel SR, Stamos TD. Association of aldosterone concentration and mineralocorticoid receptor genotype with potassium response to spironolactone in patients with heart failure. Pharmacotherapy. 2010;30:1–9. doi: 10.1592/phco.30.1.1. doi:10.1592/phco.30.1.1. [DOI] [PubMed] [Google Scholar]
- 23.Ahmed A, Zannad F, Love TE, Tallaj J, Gheorghiade M, Ekundayo OJ, Pitt B. A propensity-matched study of the association of low serum potassium levels and mortality in chronic heart failure. Eur Heart J. 2007;28:1334–1343. doi: 10.1093/eurheartj/ehm091. doi:10.1093/eurheartj/ehm091. [DOI] [PMC free article] [PubMed] [Google Scholar]