Abstract
GTP cyclohydrolase I (GTPCH) is the rate-limiting enzyme for biosynthesis of tetrahydrobiopterin (BH4), an obligate cofactor for NO synthases and aromatic amino acid hydroxylases. BH4 can limit its own synthesis by triggering decameric GTPCH to assemble in an inhibitory complex with two GTPCH feedback regulatory protein (GFRP) pentamers. Subsequent phenylalanine binding to the GTPCH·GFRP inhibitory complex converts it to a stimulatory complex. An N-terminal inhibitory peptide in GTPCH may also contribute to autoregulation of GTPCH activity, but mechanisms are undefined. To characterize potential regulatory actions of the N-terminal peptide in rat GTPCH, we expressed, purified, and characterized a truncation mutant, devoid of 45 N-terminal amino acids (Δ45-GTPCH) and contrasted its catalytic and GFRP binding properties to wild type GTPCH (wt-GTPCH). Contrary to prior reports, we show that GFRP binds wt-GTPCH in the absence of any small molecule effector, resulting in allosteric stimulation of GTPCH activity: a 20% increase in Vmax, 50% decrease in KmGTP, and increase in Hill coefficient to 1.6, from 1.0. These features of GFRP-stimulated wt-GTPCH activity were phenocopied by Δ45-GTPCH in the absence of bound GFRP. Addition of GFRP to Δ45-GTPCH failed to elicit complex formation or a substantial further increase in GTPCH catalytic activity. Expression of Δ45-GTPCH in HEK-293 cells elicited 3-fold greater BH4 accumulation than an equivalent of wt-GTPCH. Together, results indicate that the N-terminal peptide exerts autoinhibitory control over rat GTPCH and is required for GFRP binding on its own. Displacement of the autoinhibitory peptide provides a molecular mechanism for physiological up-regulation of GTPCH activity.
Keywords: Enzyme Inactivation, Enzyme Kinetics, Neurotransmitters, Nitric Oxide, Pterin, GFRP, GTP Cyclohydrolase, Tetrahydrobiopterin
Introduction
GTP cyclohydrolase I (GTPCH)2 (EC 3.5.4.16) catalyzes a ring expansion reaction, converting GTP to the pteridine biosynthetic precursor, dihydroneopterin triphosphate (H2NTP) (1, 2). Importantly, GTPCH is the first and rate-determining enzyme in the pathway for de novo synthesis of tetrahydrobiopterin (BH4), an essential cofactor that governs the activity of all three nitric-oxide (NO) synthases, the aromatic amino hydroxylases and glycerol ether monooxygenase (3). Because BH4 levels commonly restrict the activity of enzymes that rely on it as a cofactor, GTPCH activity can govern the synthesis rate of key neurotransmitters and cell signaling molecules, including NO, dopamine, norepinephrine, epinephrine, and serotonin. Genetic deficiencies in GTPCH activity have been identified as causative for 3,4-dihydroxyphenylalanine-responsive dystonia (4) and atypical phenylketonuria (5). BH4 insufficiency has also been implicated in more complex etiologies such as diabetic vasculopathy, hypertension, atherosclerosis, Parkinson and Alzheimer diseases (6, 7), as well as increased pain tolerance (8).
The best characterized mechanism for post-translational control of mammalian GTPCH utilizes an auxiliary protein, GTPCH feedback regulatory protein (GFRP), which mediates both end product inhibition by BH4 and reversal of this inhibition by phenylalanine (Phe) (9). Notably, in the presence of either BH4 or synthetic molecules that mimic feedback inhibition by BH4 (e.g. 2,4-diaminohydropyrimidine or DAHP (10)) GFRP triggers a noncompetitive attenuation of GTPCH activity, characterized by a diminished Vmax and increased KmGTP. In contrast, when the GTPCH·GFRP·DAHP inhibitory complex additionally binds the allosteric activator Phe, the complex is not disrupted, but the KmGTP of GTPCH is diminished to a lower level than observed in the absence of GFRP (9, 10).
Recent reports suggest that the N-terminal peptide of GTPCH may also contain regulatory elements that modulate catalytic activity. Notably, the Drosophila GTPCH protein sequence extends beyond the N terminus of mammalian orthologs with an amino acid sequence that apparently recapitulates the function of mammalian GFRP, i.e. conferring non-competitive feedback inhibition by BH4 (although this sequence lacks identifiable homology to GFRP itself). Additionally, N-terminal peptides in some Drosophila isoforms possess autoinhibitory properties in the absence of BH4 or BH4-mimetic agents. Indeed, when N-terminal residues were either deleted or phosphorylated in these isoforms, GTPCH activity was shown to increase (11).
High-resolution x-ray crystal structures of mammalian GTPCHs have been obtained; however, structure of the N-terminal 47 amino acids remains structurally undefined, presumably due to mobility of the N-terminal peptide (12, 13). The 47 amino acids of rat GTPCH correspond to 56 amino acids in the N terminus of human GTPCH and lack sequence homology to the N-terminal extension of Drosophila GTPCH isoforms. Nonetheless, deletion of the N-terminal peptide of human GTPCH was reported to elicit a modest increase in catalytic activity, relative to the full-length protein (14), suggesting that this peptide may also function as an autoinhibitory control element. Yeast two-hybrid analyses showed that the N-terminal peptide of human GTPCH may also mediate biologically relevant protein-protein interactions (15). In contrast, Escherichia coli GTPCH lacks a disordered N-terminal sequence and is completely unresponsive to inhibition by GFRP or small molecule allosteric effectors that modulate mammalian GTPCH activity (9). Taken together, these results suggest that the N-terminal peptide in mammalian GTPCHs does not contribute directly to catalysis, but may exert allosteric control over enzymatic activity on its own or via interactions with GFRP and perhaps other yet unrecognized regulatory factors.
Another post-translational mechanism for short-term regulation of GTPCH activity is phosphorylation. After several decades of study, a body of literature has accrued that ties phosphorylation of mammalian GTPCHs to in vitro increases or decreases in activity (11, 16–19). It is a possibility that GTPCH is phosphorylated within the N-terminal peptide, and that phosphorylation in this region can regulate activity (11, 16, 28).
The present enzymological study was performed to characterize the potential autoinhibitory function of the N-terminal peptide in rat GTPCH and to determine the extent to which this peptide is required for GFRP-mediated inhibition/stimulation of GTPCH activity. Possible influence of N-terminal peptide phosphorylation on its capacity for the GTPCH regulatory control was also evaluated.
EXPERIMENTAL PROCEDURES
Materials
Bacterial and mammalian cell culture supplies were purchased from Invitrogen. Oligonucleotides were synthesized by Invitrogen and peptide was synthesized by Genscript. Chemicals were purchased from Sigma in the best available grades.
Expression and Purification of Recombinant Wild Type GTP Cyclohydrolase
Recombinant rat GTP cyclohydrolase was expressed as a maltose-binding fusion protein as described previously (20), with some modifications. A His6 tag was added to the N terminus of the MBP tag and the Factor Xa cleavage site of pMAL-c2 (New England Biolabs) was replaced with a TEV protease consensus site using a Phusion Site-directed Mutagenesis Kit (New England Biolabs), according to the manufacturer instruction's (see supplemental Table S1 for primer sequences). The resulting vector was termed pHis-MBP-TEV-GTPCH. After introducing these and other changes to the GTPCH expression vector, the open reading frame was sequenced to ensure that no unintended mutations had been introduced into the amplicon.
The pHis-MBP-TEV-GTPCH vector was transfected into DH5αTM E. coli (Invitrogen) and expressed as described previously (10), with some modifications. Bacteria were lysed without sonication in Primary Amine-free BugBuster® Extraction Reagent (EMD Chemicals), containing 50 units/ml of benzonase nuclease (EMD Chemicals), 3 units/ml of rLysozyme (EMD Chemicals), and Complete Protease Inhibitor Mixture (Roche Applied Science). The lysate was cleared by centrifugation and diluted 1:5 with high salt TEN (20 mm Tris-HCl, pH 7.4, 400 mm NaCl, 1 mm EDTA) before affinity capture over 10 ml of amylose resin (New England Biolabs). The protein-bound resin was washed with 20 volumes of high salt TEN, followed by 5 volumes of low salt TEN (20 mm Tris-HCl, pH 7.4, 200 mm NaCl, 1 mm EDTA). Finally, His6-MBP-TEV-GTPCH was eluted from the resin with 10 mm maltose in low salt TEN. Purity of the eluted GTPCH protein was assessed using SDS-PAGE and GelCode Blue protein stain (Pierce). The purified fusion protein was quantified using Dc Protein Assay (Bio-Rad) and BSA as standard, then diluted to a final protein concentration of 5 μm using 10 mm Tris-HCl, 100 mm NaCl, 5 mm maltose, and 0.5 mm EDTA (pH 7.4). Diluted fusion protein (2–5 ml) was digested with 200–1000 units of AcTEV protease (Invitrogen) at 4 °C for 20–24 h. TEV-digested protein was used for assay within 72 h of digestion.
Construction and Expression of N-terminal Deletion and Phosphomimetic Mutant GTP Cyclohydrolase
Mutant rat GTP cyclohydrolase proteins were engineered using a Phusion Site-directed Mutagenesis kit (New England Biolabs) and pHis-MBP-TEV-GTPCH plasmid as template (see supplemental Table S1 for primer sequences). Products of the Phusion protocol were digested at restriction sites that flanked the target mutation, and this region was gel-purified (Qiaquick Gel Extraction Kit, Qiagen) and spliced back into the original unamplified template that had been digested at the same restriction sites to eliminate the possible introduction of unintended mutations in the vector backbone. Each mutant protein was expressed, purified, and TEV-digested in an identical manner to and in parallel with wild type GTPCH protein, as described above.
Expression and Purification of His6-GFRP
Recombinant rat GFRP protein was expressed and purified as described previously (10), with some modifications. Bacteria were lysed without sonication in Primary Amine-free BugBuster Extraction Reagent (Novagen) containing 50 units/ml of benzonase nuclease (Novagen) and Complete EDTA-free Protease Inhibitor Mixture (Roche Applied Science). Lysate was cleared by centrifugation and incubated with 5 ml of TALON metal affinity resin (Clontech) for 1 h at 4 °C. The resin was washed 3 times with 1 volume of wash buffer (50 mm HEPES, pH 7.8, 300 mm NaCl, 20 mm imidazole) and then loaded on Handee columns (Pierce). Columns were washed with 30 volumes of additional wash buffer, followed by elution of fusion protein with 50 mm HEPES (pH 7.8), 300 mm NaCl, 500 mm imidazole. The fusion protein was immediately exchanged into GFRP storage buffer (50 mm Tris-HCl, pH 7.8, 150 mm NaCl, 550 mm l-Arg) using Sephadex G-25 PD-10 columns (GE Healthcare).
Kinetic Assay of GTPCH
GTP cyclohydrolase activity was assayed spectrophotometrically, based on accumulation of the reaction product H2NTP, monitored by a progressive increase in A340, as described previously (10). A340 was recorded at 15-s intervals over a 1-h period at 37 °C using a Versamax microplate reader running SoftMax Pro (version 5.0.1). A 300-μl reaction mixture consisted of 100 mm Tris-HCl (pH 7.8), 0.1 μm GTPCH, and 0–1 mm GTP. Various combinations of the following were added to incubates, as desired: 1.5 mm DAHP, 3 mm l-Phe, 0.01–1 μm His6-GFRP. GFRP storage buffer was added in an equivalent volume to negative control samples in experiments that tested effects of His6-GFRP. In all experiments, at least three identical replicate reactions were analyzed for each experimental condition.
Blue Native (BN)-PAGE for Analysis of GTPCH·GFRP Complex Formation
GTPCH and GFRP were combined in 100 mm Tris-HCl (pH 7.8), with or without 1 mm GTP. Samples were incubated at 37 °C for 1 h, and then combined with Native PAGE Sample Buffer and Native PAGE 5% G-250 Sample Additive, according to the Native PAGE® system manual (Invitrogen). Indicated amounts of protein for Western blot were loaded from each incubation mixture onto Native PAGE 4–20% BisTris minigels, and non-denaturing gels were run in Light Blue Cathode Buffer. All reagents for the Native PAGE system were purchased from Invitrogen.
BN-PAGE Western Blot Analysis
After resolution on 4–20% BN-PAGE, proteins were electrotransferred to 0.2-μm pore size PVDF membranes (Invitrogen). Membranes were stained in 0.1% Ponceau S in 10% acetic acid for 15 min at room temperature to stain proteins and Native Mark (Invitrogen) protein standards. Positions of protein standards were marked with a pencil and membranes were air-dried. Dried membranes were pre-wet and destained in 100% methanol, then rinsed briefly in 1× TBS (Tris-buffered saline, pH 7.6) before incubation in blocking protein (5% skimmed milk; Difco) in TBS/T (Tris-buffered saline, pH 7.6, 0.1% Tween 20), followed by three washes in TBS. Membranes were incubated overnight at 4 °C or for 6 h at room temperature with primary antibodies diluted with 5% BSA in TBS/T, antibodies included rabbit anti-GTPCH antiserum (1:10,000) (see Ref. 20) and affinity-purified rabbit anti-GFRP antiserum (1:10,000) (see Ref. 10). After three TBS washes, membranes were incubated in a secondary antibody, horseradish peroxidase-conjugated goat anti-rabbit IgG (Invitrogen; diluted 3:10,000 with 5% skimmed milk in TBS/T) for 2 h at room temperature. Membranes were developed using ECL Western blotting Substrate (Pierce) and results were documented on Kodak Biomax Maximum Resolution (MR) Autoradiography Film. In some cases membranes were stripped using Restore PLUS (Thermo Fisher), and reprobed with additional antibodies. Western blot signals were quantified by densitometry, using NIH ImageJ version 10.2 software (21).
Tetrahydrobiopterin Assay by HPLC-ECD
Subconfluent HEK293 cells were transfected with 4 μg of plasmid DNA using Lipofectamine LTX (Invitrogen) following the manufacturer's protocol. Twenty-four hours after transfection, cells were washed twice in PBS, pelleted by centrifugation, and frozen at −80 °C until analyzed for BH4 content. For analysis, samples were extracted by addition of 400 μl of ice-cold homogenization buffer (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 20 mm CHAPS, 0.1 mm EDTA) and homogenized with a mini Tissue-Tearor on ice for 40 s, using a series of brief pulses. Ice-cold precipitation buffer (100 mm phosphoric acid, 230 mm TCA) was added to homogenates in a ratio of 3:1 (v/v) and then vortexed for 30 s. Samples were pelleted at 13,000 × g in a microcentrifuge at 4 °C for 1 min. Supernatants (50 μl) were analyzed by reverse phase HPLC with sequential electrochemical detection (Coularray ESA, Inc.) and fluorescence detection (PerkinElmer Life Sciences) for quantification of BH4 and more oxidized biopterins (22), with some modification. Pterins were resolved isocratically using a 100-mm C-18 column (Microsorb-MV, Varian, CA) and mobile phase composed of 90 mm sodium acetate, 35 mm citric acid, 130 μm EDTA, 460 μm sodium octane sulfonate (pH 4.35) at a flow rate of 0.75 ml/min. Biopterins that were not fully oxidized in the original sample were electrochemically oxidized using an electrode set to +700 mV. Biopterin produced from BH4 and BH2 by electrochemical oxidation, along with biopterin that was originally present in the sample, was quantified by fluorescence (excitation 350 nm/emission 480 nm) and compared with a standard curve generated using pure biopterin (Alexa Biochemicals) over a range of concentrations.
SDS-PAGE Western Blot Analysis
HEK293 cell homogenates (5 μl) were added to an equal volume of 2× SDS-PAGE loading buffer (Invitrogen), supplemented with freshly added 100 mm β-mercaptoethanol and then incubated at 95 °C for 10 min. Proteins in samples were resolved using 10–20% SDS-PAGE (Invitrogen), followed by electrotransfer of proteins to 0.2 μm pore size nitrocellulose membranes (Invitrogen). Protein transfer was confirmed by Ponceau S staining. Western blotting was performed in an identical manner to that described above for BN-PAGE, but with the membrane drying and methanol destaining steps omitted.
Statistical Analysis
Substrate-activity data were fit to sigmoidal curves with a variable slope, using Prism 4 for Macintosh (version 4.0c) according to the equation: activity = Vmin + (Vmax − Vmin)/(1 + 10 × ((Log(KmGTP) − Log[GTP]) × nH)). KmGTP, Vmax, and the Hill coefficient, nH, were derived from the fitted curves. Percent stimulation of wt-GTPCH and Δ45-GTPCH by His6-GFRP was determined by subtracting baseline values from GFRP-stimulated activity values at several concentrations of substrate (100–500 μm), dividing this difference by the baseline activity, and multiplying by 100 to calculate percent increase; mean ± S.E. were typically determined for 4 replicate analyses. In assays that quantified activity parameters of phosphomimetic mutant GTPCH constructs, or when activity was assayed at more than one concentration of His6-GFRP, percent stimulation of GTPCH by His6-GFRP was determined at a single concentration of substrate.
RESULTS
Deletion of the N-terminal Peptide in Rat GTPCH Increases Inherent Enzymatic Activity
To assess the effect of the N-terminal 45 amino acids on GTPCH activity, we engineered a rat GTPCH truncation mutant wherein GTPCH-(46–241) was linked at the N terminus to maltose-binding protein (MBP) via a TEV protease cleavage site. After expression in E. coli, the GTPCH fusion protein was purified on amylose resin, followed by TEV protease digestion to release Δ45-GTPCH, native GTPCH, but lacking 45 N-terminal amino acids. Ala-46 was chosen as the N terminus of Δ45-GTPCH, to provide this residue as the P1′ site of the consensus site of TEV and thus provide efficient cleavage by TEV protease (23). For comparative assessment of Δ45-GTPCH activities, wt-GTPCH was similarly expressed and purified, encoding the entire GTPCH ORF, commencing with Glu-2 (i.e. lacking the initial methionine residue, which is physiologically deleted). After TEV digestion of the wt-GTPCH fusion protein, no linker remains, save a single Ala residue at the TEV P1′ site (supplemental Fig. S1). Notably, the deleted N-terminal peptide in the Δ45-GTPCH construct is the predominant region of variability in GTPCH sequences across species (supplemental Table S2) and in x-ray crystal structures it was shown to be disordered (12, 13) in accord with its potential function as a regulatory control element.
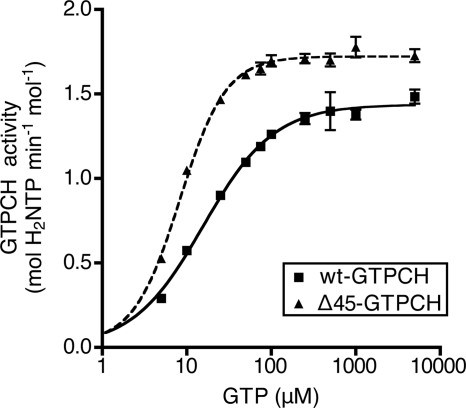
Enzymatic activity of wt-GTPCH and Δ45-GTPCH was characterized using a real time high-throughput kinetic microplate assay, quantifying the progressive increase in A340 (Fig. 1 and supplemental Table S3). wt-GTPCH displayed a Hill coefficient of 1.0 ± 0.2 for use of GTP as substrate with a mean value for KmGTP = 17.9 ± 4.0 μm and Vmax = 1.49 ± 0.08 mol of H2NTP min−1 mol−1 protein. In the case of Δ45-GTPCH, the Hill coefficient was significantly increased to 1.6 ± 0.2, KmGTP was diminished by >50% and Vmax increased by 20%. Thus, deletion of the N-terminal tail of wt-GTPCH apparently removes a barrier to maximal catalytic activity and results in enhanced substrate binding affinity.
FIGURE 1.
Comparison of enzymatic characteristics of recombinant rat wt-GTPCH and Δ45-GTPCH. Activity of 0.1 μm wt-GTPCH or Δ45-GTPCH was assayed in 100 mm Tris-HCl (pH 7.8) at 37 °C with 5–5000 μm GTP, using the kinetic microplate assay as described under “Experimental Procedures.” Shown is a typical plot of reaction rate versus substrate concentration from a single experiment. Values are mean ± S.E. of triplicate measurements. Nonlinear regression analysis was used to fit a curve to the data points; summary kinetic parameters from this and other experiments are provided under supplemental Table S3.
Differential Response of Δ45-GTPCH to His6-GFRP in the Absence of Small Molecule Allosteric Regulators
We next sought to determine whether the N-terminal peptide of GTPCH plays a role in regulation of activity by GFRP. Notably, in response to binding either BH4 or Phe, two GFRP homopentamers are thought to sandwich the GTPCH decamer, conferring inhibition or enhancement of GTPCH activity, respectively (9). Deletion of a portion of the human N-terminal peptide was reported to diminish the level of interaction between GTPCH and GFRP in a yeast two-hybrid analysis (15), whereas a similar N-terminal truncation mutant of recombinant human GTPCH was found to exhibit no change in regulation by GFRP (24). Given these seemingly contradictory reports, we sought to assess the ability of recombinant His6-tagged GFRP (His6-GFRP) to modulate the activity of Δ45-GTPCH versus wt-GTPCH, in the presence of inhibitory and stimulatory allosteric effector molecules.
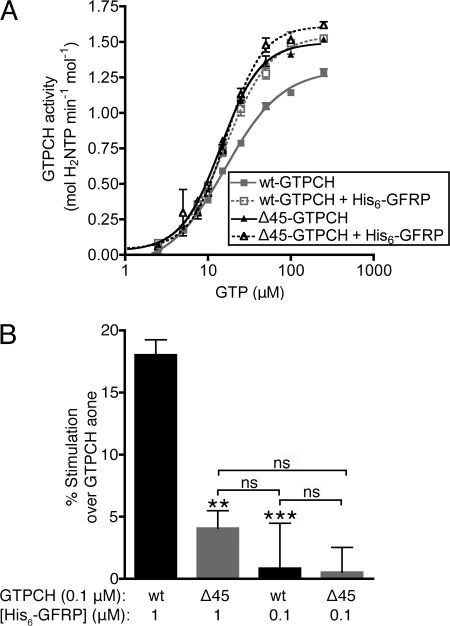
Pilot studies seeking to optimize conditions for these studies unexpectedly revealed that His6-GFRP stimulates GTPCH activity on its own (i.e. without addition of a small molecule allosteric modulator), a property that was not previously recognized (9). As shown in Fig. 2A, the Vmax for wt-GTPCH was increased by 28.1 ± 2.8 and 24.6 ± 5.9%, in two separate experiments, in the presence of a 10-fold molar excess of His6-GFRP. In contrast, the activity of Δ45-GTPCH increased to a much lesser degree by exposure to a 10-fold excess of His6-GFRP (8.3 ± 0.6 and 6.9 ± 0.3%; Fig. 2A). Notably, this stimulation of activity was observed when GFRP was in marked excess over GTPCH and was not detected when wt-GTPCH or Δ45-GTPCH was incubated with an equimolar amount of His6-GFRP (Fig. 2B).
FIGURE 2.
Comparison of the effects of His6-GFRP on wt-GTPCH and Δ45-GTPCH activity. GTPCH assays were conducted in the presence or absence of His6-GFRP, in 100 mm Tris-HCl (pH 7.8) at 37 °C, using the kinetic microplate assay described under “Experimental Procedures.” A, 0.1 μm wt-GTPCH or Δ45-GTPCH activity was assayed in the absence or presence of 1 μm His6-GFRP in reactions containing 5–500 μm GTP. Shown is a typical plot of the reaction rate versus substrate concentration from a single experiment. Values are mean ± S.E. of triplicate measurements. Nonlinear regression analysis was used to fit a curve to the data points. B, the activity of 0.1 μm wt-GTPCH and Δ45-GTPCH were assayed in the absence or presence of either 1.0 or 0.1 μm His6-GFRP, in reactions containing 1 mm GTP. Values are mean ± S.E. of quintuplicate measurements, and compared by analysis of variance. ***, p < 0.001; **, p < 0.01. ns, not significant.
Assembly of GTPCH·GFRP Complexes in the Absence of a Small Molecule Effector
Previous studies suggested that the interaction of GTPCH with GFRP in vitro requires one or more small molecule effectors: either the stimulatory allostere Phe, or an inhibitory allostere (e.g. BH4 or DAHP) (25). Opposing this view, the findings presented in Fig. 2 show that an allosteric effector is unnecessary for GFRP to influence GTPCH activity, suggesting that GFRP·GTPCH complexes can form in the absence of Phe or BH4/DAHP. To directly assess the assembly of GTPCH·GFRP complexes, experiments were performed using BN-PAGE, followed by visualization of GTPCH and GFRP complex formation using Western blot analysis.
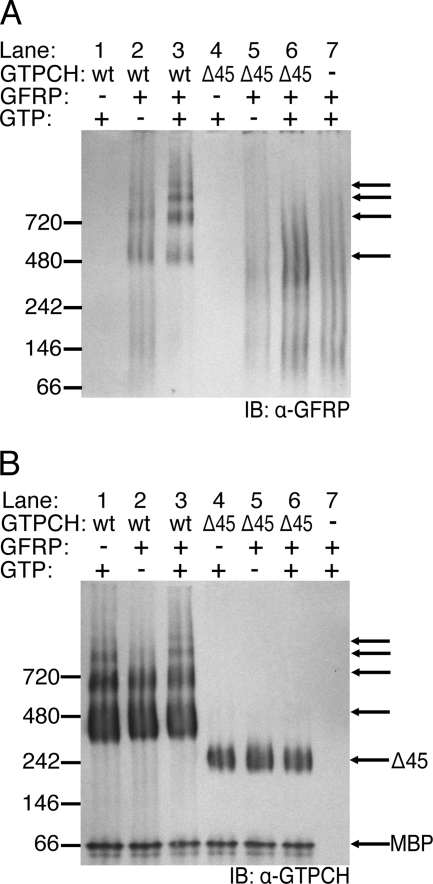
Multimeric assemblies of wt-GTPCH and Δ45-GTPCH on their own displayed markedly different banding patterns after BN-PAGE separation and detection by Western blotting (Fig. 3B). Notably, the majority of wt-GTPCH was contained in a band predicted to be of somewhat greater mass than 280 kDa, anticipated for a GTPCH homodecamer comprised of 28 kDa subunits. In contrast, the Δ45-GTPCH decamer appeared as a band at the predicted homodecameric mass of 220 kDa. Several higher molecular weight bands were also detected with wt-GTPCH, whereas no additional bands were seen with Δ45-GTPCH (Fig. 3B, compare lane 1 versus lane 4). Whether the higher molecular weight complexes observed with wt-GTPCH have functional relevance remains to be determined. In any case, following incubation with an equimolar concentration of His6-GFRP, there was no detectable change in the banding pattern of wt-GTPCH (Fig. 3B, lane 2 versus lane 1). Notwithstanding, Western blotting for His6-GFRP confirmed that GFRP does indeed associate with wt-GTPCH, even in the absence of GTP or other small molecule effectors, this is indicated by a His6-GFRP signal that conforms to the wt-GTPCH homodecamer banding pattern (Fig. 3A, lane 2 versus lane 7; black arrows in Fig. 3B indicate position of GFRP signal in relation to GTPCH signal). When 1 mm GTP was added to the wt-GTPCH/His6-GFRP incubate, a marked change in the oligomerization status of His6-GFRP was observed such that His6-GFRP engages to a much greater extent in high molecular weight complexes with wt-GTPCH (Fig. 3A, lane 3 versus lane 7). Although some complex formation may occur between His6-GFRP and Δ45-GTPCH, indicated by a slight increase in the GFRP signal near the mass of the Δ45-GTPCH decamer (Fig. 3A, lane 6 versus lane 7), Δ45-GTPCH does not induce His6-GFRP to significantly conform to its homodecameric banding pattern.
FIGURE 3.
Native complex formation between His6-GFRP and wt-GTPCH or Δ45-GTPCH. wt-GTPCH or Δ45-GTPCH (2.5 μm) were incubated in 100 mm Tris-HCl (pH 7.8) at 37 °C for 1 h. In some reactions, 1 mm GTP and/or 2.5 μm His6-GFRP were also included. A, mixtures containing wt-GTPCH (67.5 ng) or Δ45-GTPCH (55.3 ng) and His6-GFRP (29.5 ng) were resolved on a native PAGE BisTris 4–20% gel before transfer to PVDF membrane and Western blotting (IB) with affinity purified antiserum to GFRP. Black arrows highlight laddering complexes. B, the same PVDF membrane as in A, after stripping and reprobing with antiserum to GTPCH. Unlabeled arrows are as in A, to indicate the position of species identified with anti-GFRP antiserum.
BN-PAGE analysis was also performed on mixtures of GTPCH and a 10-fold molar excess of His6-GFRP. Although Δ45-GTPCH incubated with GTP and a ×10 excess of His6-GFRP resulted in a protein distribution essentially identical to that shown in Fig. 3A, a similar incubation with wt-GTPCH failed to detect GTPCH after electrophoresis by either Western blotting or Coomassie stain (data not shown). A likely explanation for the apparent loss of wt-GTPCH·His6-GFRP complexes is a diminished solubility of the high order complexes when formed at high molar ratios of His6-GFRP.
As shown by the results here, wt-GTPCH and His6-GFRP can interact in the absence of small allosteric effectors or substrate; however, the binding of substrate GTP substantially increases wt-GTPCH·His6-GFRP complex formation. In contrast to wt-GTPCH, Δ45-GTPCH exhibits a relative inability to engage in higher ordered complexes with His6-GFRP, both in the absence and presence of substrate. Thus, the N-terminal sequence of GTPCH appears to facilitate higher ordered complex assembly with His6-GFRP. Limited higher ordered assembly of Δ45-GTPCH with GFRP may explain the very minimal stimulation of Δ45-GTPCH activity following incubation with a 10-fold molar excess of GFRP.
GFRP-mediated Allosteric Regulation by Small Molecule Effectors Is Indistinguishable for Δ45-GTPCH and wt-GTPCH
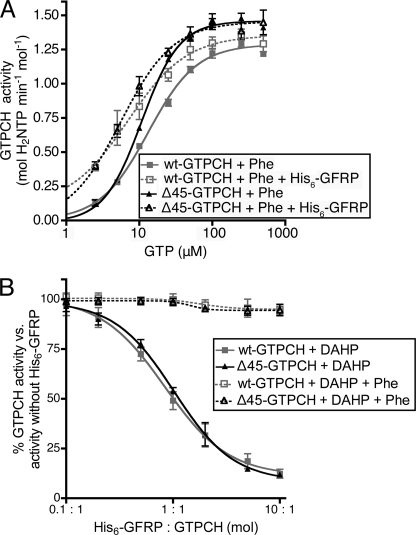
Having found that the N-terminal peptide sequence of GTPCH enhances the stimulation of catalytic activity by His6-GFRP and promotes physical interaction with His6-GFRP (in the absence of small molecule effectors), we next examined whether the N-terminal peptide influences GFRP-mediated regulation of GTPCH activity by small molecule effectors. Toward this end, the activity of wt-GTPCH and Δ45-GTPCH were assayed in the presence of excess His6-GFRP and Phe over a range of GTP substrate concentrations. As previously reported (9), Phe in the presence of His6-GFRP stimulated GTPCH activity at limiting substrate concentrations, reducing the KmGTP, whereas having no significant effect on Vmax. Notably, the catalytic activity of wt-GTPCH and Δ45-GTPCH were found to be similarly enhanced by incubation with His6-GFRP/Phe, after correction for the greater basal activity of Δ45-GTPCH (Fig. 4A).
FIGURE 4.
His6-GFRP-mediated allosteric regulation of GTPCH by small molecules. A, the activity of 0.1 μm wt-GTPCH or Δ45-GTPCH was assayed in the presence or absence of 1 μm His6-GFRP in 100 mm Tris-HCl and 3 mm Phe (pH 7.8) at 37 °C in reactions containing 5–500 μm GTP, using the kinetic microplate assay described under “Experimental Procedures.” Shown is a typical plot of reaction rate versus substrate concentration from one experiment. Values plotted are mean ± S.E. of triplicate measurements. B, the activity of 0.1 μm wt-GTPCH or Δ45-GTPCH was assayed in 1.5 mm DAHP or 1.5 mm DAHP and 3 mm Phe in 100 mm Tris-HCl (pH 7.8) at 37 °C, 1 mm GTP, in reactions containing 0.01 to 1 μm His6-GFRP, using the kinetic microplate assay described under “Experimental Procedures.” Values are plotted as a percentage of GTPCH activity, measured in the absence of His6-GFRP. Shown is a typical plot of reaction rate versus His6-GFRP concentration from a single experiment. Values are mean ± S.E. of triplicate measurements. Nonlinear regression analysis was used to fit the solid curve to the data points, whereas a dotted line simply connects all points.
The activity level of wt-GTPCH and Δ45-GTPCH was next studied for relative susceptibility to GFRP-dependent allosteric inhibition by the BH4-mimetic, DAHP. These studies were performed using a variable concentration of His6-GFRP in the presence of a maximal inhibitory concentration of DAHP (1.5 mm). As shown in Fig. 4B, His6-GFRP elicited a concentration-dependent unmasking of the ability of DAHP to inhibit GTPCH activity. When corrected for the increased basal activity of Δ45-GTPCH, the potency and efficacy of GFRP/DAHP was indistinguishable with wt-GTPCH and Δ45-GTPCH. Likewise, inhibition of both wt-GTPCH and Δ45-GTPCH was completely and equivalently reversed by the addition of excess Phe (3 mm) to DAHP/GFRP incubates (Fig. 4B, dotted line). Taken together with the observed GFRP-dependent allosteric stimulation by Phe, these results establish that small molecule allosteric effectors do not require the N-terminal peptide of GTPCH to confer GFRP-dependent stimulation or inhibition.
Assessment of the N-terminal Peptide of GTPCH as a Potential Inhibitor of GTPCH Catalytic Activity
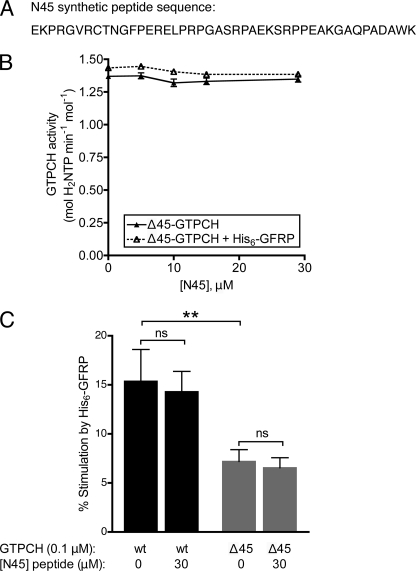
Given the higher inherent activity of Δ45-GTPCH, we hypothesized that the deleted N-terminal 45 residues may confer autoinhibition by interacting with some structural component of the GTPCH protein core. According to this view, deletion of this region would prevent the interaction and thus relieve autoinhibition, explaining the elevated activity of Δ45-GTPCH versus wt-GTPCH. Additionally, BN-PAGE results demonstrating more efficient binding of His6-GFRP to wt-GTPCH, relative to Δ45-GTPCH, indicated that the N-terminal peptide of GTPCH increases GTPCH affinity for His6-GFRP in the absence of small molecules. To test whether the N-terminal peptide could directly inhibit activity by interaction with a structural component of the GTPCH protein core, we tested whether inhibition could be reconstituted by exposure of Δ45-GTPCH to a 45-amino acid synthetic peptide corresponding to the deleted N-terminal sequence (termed N45; Fig. 5A). Conceivably, N45 peptide incubation could also increase the affinity of Δ45-GTPCH for His6-GFRP, so that the activity of Δ45-GTPCH would increase in response to His6-GFRP.
FIGURE 5.
Effect of a synthetic GTPCH-(2–45) N-terminal peptide (N45) on activity of GTPCH and regulation by His6-GFRP. The activity of 0.1 μm Δ45-GTPCH was assayed in 100 mm Tris-HCl (pH 7.8) at 37 °C, 1 mm GTP, in reactions containing 0–30 μm N45 peptide and 1 μm His6-GFRP. A, the N45 peptide corresponds to the N-terminal 44 amino acids of rat GTPCH (i.e. GTPCH-(1–45), minus the initial methionine residue). B, Δ45-GTPCH activity in the absence or presence of His6-GFRP, plotted as a function of peptide concentration. Values are mean ± S.E. of quintuplicate measurements. C, percent stimulation of 0.1 μm wt-GTPCH or Δ45-GTPCH activity by 1 μm His6-GFRP in the absence or presence of 30 μm N45 peptide. Values are mean ± S.E. of quintuplicate measurements. Analysis by one-way analysis of variance was used, **, p < 0.01. ns, not significant; p, > 0.05.
Δ45-GTPCH activity was assayed in the presence of excess GTP and progressively increasing concentrations of N45 peptide. Notably, increasing concentrations of N45, even to a 300-fold molar excess over Δ45-GTPCH, failed to influence Δ45-GTPCH activity (Fig. 5B, solid plot). Next, N45 was included in an assay combining Δ45-GTPCH and His6-GFRP, to determine whether the peptide would confer stimulation of Δ45-GTPCH by His6-GFRP to an extent similar to that observed following addition of His6-GFRP to wt-GTPCH, however, the peptide failed to act in this manner (Fig. 5, B and C). Likewise, incubation with N45 elicited no detectable effect on either the inherent activity of wt-GTPCH, or on the response of wt-GTPCH to His6-GFRP (Fig. 5C).
Taken together, the findings of Fig. 5 argue against a mechanism whereby autoinhibition of Δ45-GTPCH is mediated simply by docking of the N-terminal sequence to a site on the protein surface. Accordingly, autoinhibition conferred by the N-terminal sequence of GTPCH appears to require connection via a peptide bond for allosteric inhibition of GTPCH activity.
Phosphomimetic Modification of Sites within the N-terminal Peptide and Other Recognized Sites of Phosphorylation Do Not Elicit a Detectable Change in GTPCH Activity or Regulation by GFRP
Despite our conclusion that the N-terminal sequence requires direct connection to Δ45-GTPCH to confer autoinhibition and stimulation of activity by His6-GFRP, this did not eliminate the possibility that these actions of the N-terminal peptide also require docking with one or more sites on the surface of GTPCH. Deletion of the N-terminal sequence would eliminate these putative docking interactions, and post-translational amino acid modifications might similarly interfere with N-terminal peptide interactions and thereby offset autoinhibition. Unbiased studies of two yeast species have identified sites of phosphorylation within the variable N-terminal peptide of GTPCH (26), whereas functional studies in Drosophila have identified phosphorylation sites within the N-terminal peptide by protein kinase A (PKA) and protein kinase C (PKC) that affect GTPCH activity and protein-protein interactions (11, 16). Sequence dissimilarity within the N-terminal peptide between different species precludes a direct translation of phosphorylation sites identified in one species to another (supplemental Table S2). So phosphomimetic mutant variants at Ser and Thr residues in rat GTPCH were created via site-directed mutagenesis to determine the consequences for autoinhibition. Residues Thr-10, Ser-24, and Ser-30 comprise all potential Ser and Thr phosphorylation sites within the N-terminal 45 amino acids of rat GTPCH. Ser-51, just C-terminal to the region deleted for creation of Δ45-GTPCH, was also mutated based on previous experimental evidence of its phosphorylation and the observation that biopterin levels increased in endothelial cells transfected with an S51E mutant (18, 27). Notably, Ser-51 is positioned at the hinge between the N-terminal peptide of GTPCH and the globular protein core; bulky charge introduced by phosphorylation of Ser-51 could potentially alter positioning of the N-terminal peptide and influence catalytic activity. Each of the phosphomimetic mutants was assayed in parallel with a matched preparation of wt-GTPCH, purified in parallel to minimize purification and assay variation.
Surprisingly, the kinetic properties of each phosphomimetic enzyme in vitro closely resembled wt-GTPCH (supplemental Fig. S2 and Table S3). In the case of S51D, this observation contrasts with the findings of Du et al. (18), who observed increased levels of BH2 and BH4 when FLAG-S51E-GTPCH was overexpressed in bovine aortic endothelial cells, versus levels of these biopterins when wt-GTPCH was overexpressed. However, the study by Du et al. (18) consisted of an end point assay in bovine aortic endothelial cells that measures metabolites downstream of the GTPCH step in the reaction pathway, whereas our results derive from a real time kinetic assay that directly quantifies GTPCH activity in the absence of other cellular effectors. Intracellularly, phosphomimetic mutation of GTPCH may conceivably lead to interactions that modulate other biopterin pathway components and affect downstream products that require other enzymatic steps in BH4 biosynthesis. Results suggest that whereas the phosphomimetic mutations of GTPCH can lead to increased production of biopterins in cells, phosphomimetic modifications of the GTPCH N-terminal peptide on their own do not alter the H2NTP synthesis under our assay conditions.
When Expressed in Cells, Δ45-GTPCH Elicits a Markedly Greater Accumulation of Biopterins Versus wt-GTPCH
Although the activity of Δ45-GTPCH in vitro was increased relative to that of wt-GTPCH in the completely defined environment of an in vitro microplate assay, we wished to assess the functional relevance of the N-terminal peptide as a governor of GTPCH activity in vivo, especially in light of the contrasting results between our phosphomimetic data and that reported by Du et al. (18).
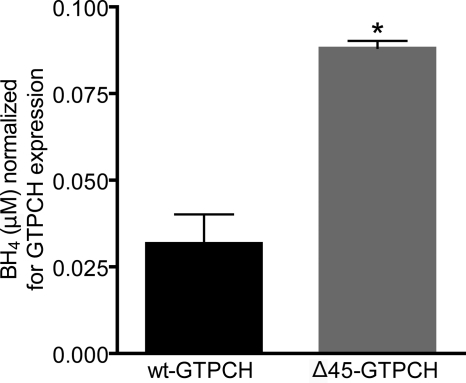
After correction for the expression level of recombinant GTPCH, Δ45-GTPCH-expressing HEK293 cells were found to accumulate 2–3-fold more pterin than wt-GTPCH-expressing cells (Fig. 6). Based on this apparent increase in the enzymatic activity of the N-terminal truncated GTPCH, relative to wt-GTPCH, we conclude that the N-terminal peptide of GTPCH confers substantial autoinhibition in vivo. Furthermore, the stimulation in GTPCH activity that results from deletion of the N-terminal peptide is amplified to a greater extent in vivo versus the observed stimulation by the identical truncation in vitro.
FIGURE 6.
Comparison of biopterin accumulation in HEK-293 cells after transfection with wt-GTPCH or Δ45-GTPCH, normalized for GTPCH expression levels. HEK293 cells were transiently transfected with pcDNA3-based plasmid vectors that express either wt-GTPCH or Δ45-GTPCH. Cells were harvested after 24 h of protein expression and levels of biopterin (BH2 + BH4) in cell lysates were quantified based on fluorescence, following HPLC separation and electrochemical oxidation (see “Experimental Procedures” for details). Enzymatic activity was normalized to the amount of wt-GTPCH or Δ45-GTPCH, as determined by quantitative densitometry after Western blotting. Values were compared by t test, *, p < 0.05.
DISCUSSION
GFRP on Its Own Stimulates wt-GTPCH, whereas Δ45-GTPCH Is Weakly Activated
Contrary to the prevailing view that GFRP regulates GTPCH by conferring only small molecule-mediated allosteric control, our results indicate that GFRP can itself stimulate GTPCH activity in the absence of a small molecule effector. Direct interaction of GTPCH with GFRP was further evidenced by results of BN-PAGE immunoblot studies, demonstrating that recombinant GTPCH and His6-GFRP form a complex in the absence of other proteins or small molecule allosteric effectors. Notwithstanding, the interaction between GTPCH and His6-GFRP was markedly enhanced by incubation with substrate GTP (under conditions that lead to the synthesis of H2NTP), suggesting the governance of the GTPCH/GFRP interaction by substrate binding, product formation, or both.
Prior literature failed to identify the association we observed between GTPCH and GFRP alone, instead reporting a requirement for either Phe or BH4 (or related pterins) to support the GTPCH·GFRP complex assembly (9, 25). Notably, these previous studies used GTPCH enzymes that may have had truncated N-terminal regions, either recombinant protein after cleavage from its affinity purification tag using Factor Xa (25), a protease known to cleave non-specifically at basic residues (28–30), or endogenous protein extracted from rat liver, which is post-translationally cleaved between Asn-11 and Gly-12 within the N-terminal peptide (31). Our results indicate that the GTPCH/GFRP interaction is modulated by the N-terminal sequence. The highly specific TEV protease used in the present study (32, 33) cleaves GTPCH from its affinity tag with negligible incidence of cleavage within the N-terminal sequence of the GTPCH. Thus, improved preservation of the complete N-terminal peptide may account for the ability of native wt-GTPCH protein in our hands to interact with His6-GFRP under the study conditions employed.
Autoinhibition by the N-terminal Peptide of GTPCH and Mechanism of Action
We observed that the N-terminal 45 amino acids of rat GTPCH is autoinhibitory, acting to increase the KmGTP, suppress the Vmax and lower the Hill coefficient to 1.0, from 1.6. Our finding that N-terminal peptide deletion increases the Hill coefficient by ≈50%, concomitant with a 50% reduction in KmGTP, is reconciled by the onset of positive cooperativity among the 10 active sites in the GTPCH homodecamer, such that occupancy by substrate on one or more subunits triggers an increase in binding or turnover of substrate at other subunits. Notably, each active site of the GTPCH decamer is formed by amino acids contributed by each of three distinct monomers; conversely, each GTPCH monomer contributes to the formation of three distinct active sites. This complex network of linkages between the 10 active sites of GTPCH via the protein backbone of all GTPCH subunits is the likely basis for positive cooperativity and the intricate system of allosteric control conferred by binding of substrate (GTP), GFRP, and diverse small molecule allosteric effectors. We hypothesize that interactions involving the N-terminal peptide of GTPCH and the protein body oppose movements among the GTPCH subunits that are driven by substrate binding, providing an explanation for the appearance of positive cooperativity among substrate sites when the N-terminal peptide is removed.
A common mechanism for enzyme autoinhibition is the transient obstruction of the active site by a mobile control element in the same protein. We attempted to oppose the action of deletion mutant GTPCH to wild type levels and restore its response to GFRP by adding back the deleted segment in the form of a synthetic peptide. However, the synthetic peptide failed to diminish Δ45-GTPCH activity on its own, nor did it enhance the ability of GFRP to stimulate activity. Accordingly, we conclude that simple binding of the N-terminal peptide to the active site or other regulatory site is not sufficient to confer autoinhibition or perturb GFRP binding, suggesting that the N-terminal peptide confers autoinhibition via a main chain effect. Indeed, the inhibition conferred on wt-GTPCH by the binding of the GFRP·DAHP complex from the “poles” of the GTPCH decamer to the equatorial active sites is apparently communicated by just such a main chain effect (12). To investigate whether the N-terminal peptide transitory interactions with the main body of the protein might also play a role in GTPCH regulation, phosphomimetic mutations within the N terminus were introduced via site-directed mutagenesis at every Ser and Thr residue in the N-terminal peptide, with the expectation that hindrance caused by the introduction of negative charges would disturb tail-core interactions. The activity of each phosphomimetic variant, however, was indistinguishable from that of its purification matched wt-GTPCH control. This finding provides further support that the N-terminal peptide acts on the inherent activity of GTPCH via an indirect main chain mechanism, and that solitary phosphomimetic interactions involving the N-terminal peptide and the protein body do not contribute significantly to autoinhibition by the N-terminal peptide.
The finding of a significant, albeit modest (20%) increase in Vmax following removal of the N-terminal peptide of GTPCH indicates the N-terminal 45 amino acids acts to slow the rate-determining step in GTPCH catalysis. GTPCH catalyzes a zinc-dependent complex multistep reaction, initiated by enzyme-catalyzed opening of the imidazole ring of GTP, followed by hydrolytic release of formate, rearrangement of the carbohydrate side chain, and then closure of the dihydropyrazine ring to yield the product dihydroneopterin triphosphate. Stopped-flow and quenched-flow studies have demonstrated that ring opening and formate release occur very rapidly, at least 10-fold more rapidly than the carbohydrate chain rearrangement, which is apparently rate-determining to product formation (34–36). Thus, the accelerated rate of GTPCH catalysis observed after deletion of the N-terminal peptide is best reconciled by accelerated carbohydrate rearrangements that form the incipient dihydropyrazine ring of dihydroneopterin triphosphate. N-terminal truncation-associated molecular movements responsible for the Vmax acceleration in GTPCH await specification, but are anticipated to be caused by those same allosteric movements that underlie the concomitant alterations in Km and Hill coefficient.
Mammalian N-terminal Peptide of GTPCH Contributes to Regulation by GFRP
Compared with wt-GTPCH, Δ45-GTPCH was markedly deficient in its ability to interact with His6-GFRP in the absence of small molecules, this was indicated by BN-PAGE immunoblot studies and the response to His6-GFRP alone in activity assays. Notwithstanding, Δ45-GTPCH activity responded to His6-GFRP in the presence of small molecule regulators in a manner that was not different from wt-GTPCH. Thus, in the presence of Phe and BH4 analogs, the truncation mutant apparently interacts normally with His6-GFRP.
The unperturbed response of Δ45-GTPCH to small molecule regulation mediated by His6-GFRP would appear to challenge previous findings demonstrating that the complete N-terminal peptide was required for normal interaction between GTPCH and GFRP in a yeast two-hybrid assay (15). However, because Saccharomyces cerevisiae lacks a complete BH4 pathway (37), no intracellular BH4 or BH2 is likely present in the yeast two-hybrid assay to support an interaction between the N-terminal deletion mutant and GFRP. Similarly, levels of Phe in S. cerevisiae were measured to be 1.9 ± 0.4 mm (38), so that Phe is likely present in yeast at a lower concentration than that used in our activity assays (3 mm), and may be sequestered away from GTPCH·GFRP complexes by intracellular components. Thus, the intracellular yeast environment may represent a setting in which GTPCH and GFRP are expressed in the absence of BH2 or BH4 and with relatively lower Phe levels. The failure of truncated GTPCH to interact normally with GFRP in S. cerevisiae (15) is in accord with our finding that the N-terminal peptide of GTPCH is crucial to the GTPCH/GFRP interaction in the absence of small molecule effectors.
That Δ45-GTPCH would respond in the same manner as wt-GTPCH to small molecule regulation mediated by His6-GFRP, although also being unresponsive to stimulation by His6-GFRP alone is even more reasonable when considering that GFRP interacts with distinct residues in GTPCH, depending on which small regulatory molecules are present. This has been experimentally established by reported x-ray crystal structures of the GTPCH·GFRP·Phe stimulatory complex (13) and the GTPCH·GFRP·BH2 inhibitory complex (12). Although the molar ratio of GTPCH:GFRP in these two complexes remains the same, as does the gross positioning of GFRP and GTPCH molecules in relationship to each other, subtle differences in the positioning of GFRP relative to GTPCH occur as a consequence of whether BH2 or Phe is the small molecule present, triggering substantial changes in the shape and size of the active site of GTPCH, far removed from the actual GFRP binding site. Furthermore, some GTPCH residues (e.g. Pro-190 of the inhibitory complex) interact with GFRP in only one of the complexes (12). In both stimulatory and inhibitory GTPCH/GFRP structures reported to date, the N-terminal peptide was found to be too disordered for structure specification, and no structure for a GTPCH/GFRP structure in the absence of small molecules has been solved. Based on our findings, it is predicted that such a structure would illustrate yet a third relationship between GTPCH and GFRP, perhaps with more order to the atoms of the N-terminal peptide.
As has been documented for phosphoregulation and GFRP-mediated allosteric regulation (18), the effect of the N-terminal peptide on GTPCH activity is likely to be integrated with other forms of regulation. Based on our observations that interference with the N-terminal peptide is amplified in an intracellular system, and previous evidence that certain GTPCH protein interactions depend exclusively on the N-terminal peptide (15), the regulation of GTPCH through protein-protein interactions and via conformation of the N-terminal peptide may each influence the state of the other.
Our detailed in vitro functional characterization of the N-terminal 45 amino acids in rat GTPCH indicates that this peptide functions as an important regulatory control element. Remarkably, the characteristics and degree of wt-GTPCH stimulation elicited by GFRP binding were phenocopied by Δ45-GTPCH in the absence of bound GFRP (in terms of the extent of increases in Vmax and Hill coefficient, and decrease in KmGTP). Moreover, the addition of GFRP failed to elicit any detectable complex formation or a substantial further increase in Δ45-GTPCH activity. Together these findings suggest that reversal of the N-terminal peptide-mediated autoinhibition, or disinhibition, is a likely molecular basis for stimulation of wt-GTPCH by binding GFRP (on its own), possibly because of GFRP-triggered allosteric displacement of inhibitory interactions that involve the N-terminal peptide of GTPCH. We hypothesize that via regulatory post-translational modifications and protein-protein interactions, the N-terminal peptide of mammalian GTPCHs may function as a physiological regulator of GTPCH activity, BH4 production, and consequently, modulate levels of the downstream products of BH4-dependent enzymes, including NO, catecholamines, and serotonin.
This work was supported, in whole or in part, by National Institutes of Health Grants R37 HL087062, PO1 HL046403 (to S. S. G.), and T32 HL007423 (to C. E. H.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental supplemental Figs. S1–S2 and Tables S1–S3.
- GTPCH
- GTP cyclohydrolase
- BH2
- 7,8-dihydro-l-biopterin
- BH4
- (6R)-5,6,7,8-tetrahydro-l-biopterin
- BN
- blue native
- DAHP
- 2,4-diamino-6-hydroxypyrimidine
- GFRP
- GTPCH feedback regulatory protein
- H2NTP
- dihydroneopterin triphosphate
- MBP
- maltose-binding protein
- TEV
- tobacco etch virus
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol.
REFERENCES
- 1. Burg A. W., Brown G. M. (1968) J. Biol. Chem. 243, 2349–2358 [PubMed] [Google Scholar]
- 2. Shiota T., Palumbo M. P., Tsai L. (1967) J. Biol. Chem. 242, 1961–1969 [PubMed] [Google Scholar]
- 3. Higgins C. E., Gross S. S. (2010) in Nitric Oxide: Biology and Pathobiology (Ignarro L. J. ed) pp. 169–209, MacMillan Publishing, London, United Kingdom [Google Scholar]
- 4. Ichinose H., Ohye T., Takahashi E., Seki N., Hori T., Segawa M., Nomura Y., Endo K., Tanaka H., Tsuji S. (1994) Nat. Genet. 8, 236–242 [DOI] [PubMed] [Google Scholar]
- 5. Niederwieser A., Blau N., Wang M., Joller P., Atarés M., Cardesa-Garcia J. (1984) Eur. J. Pediatr. 141, 208–214 [DOI] [PubMed] [Google Scholar]
- 6. Foxton R. H., Land J. M., Heales S. J. (2007) Neurochem. Res. 32, 751–756 [DOI] [PubMed] [Google Scholar]
- 7. Wu G., Meininger C. J. (2009) BioFactors 35, 21–27 [DOI] [PubMed] [Google Scholar]
- 8. Tegeder I., Costigan M., Griffin R. S., Abele A., Belfer I., Schmidt H., Ehnert C., Nejim J., Marian C., Scholz J., Wu T., Allchorne A., Diatchenko L., Binshtok A. M., Goldman D., Adolph J., Sama S., Atlas S. J., Carlezon W. A., Parsegian A., Lötsch J., Fillingim R. B., Maixner W., Geisslinger G., Max M. B., Woolf C. J. (2006) Nat. Med. 12, 1269–1277 [DOI] [PubMed] [Google Scholar]
- 9. Harada T., Kagamiyama H., Hatakeyama K. (1993) Science 260, 1507–1510 [DOI] [PubMed] [Google Scholar]
- 10. Kolinsky M. A., Gross S. S. (2004) J. Biol. Chem. 279, 40677–40682 [DOI] [PubMed] [Google Scholar]
- 11. Funderburk C. D., Bowling K. M., Xu D., Huang Z., O'Donnell J. M. (2006) J. Biol. Chem. 281, 33302–33312 [DOI] [PubMed] [Google Scholar]
- 12. Maita N., Hatakeyama K., Okada K., Hakoshima T. (2004) J. Biol. Chem. 279, 51534–51540 [DOI] [PubMed] [Google Scholar]
- 13. Maita N., Okada K., Hatakeyama K., Hakoshima T. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 1212–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Suzuki T., Ohye T., Inagaki H., Nagatsu T., Ichinose H. (1999) J. Neurochem. 73, 2510–2516 [DOI] [PubMed] [Google Scholar]
- 15. Swick L., Kapatos G. (2006) J. Neurochem. 97, 1447–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bowling K. M., Huang Z., Xu D., Ferdousy F., Funderburk C. D., Karnik N., Neckameyer W., O'Donnell J. M. (2008) J. Biol. Chem. 283, 31449–31459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lapize C., Plüss C., Werner E. R., Huwiler A., Pfeilschifter J. (1998) Biochem. Biophys. Res. Commun. 251, 802–805 [DOI] [PubMed] [Google Scholar]
- 18. Du J., Wei N., Xu H., Ge Y., Vásquez-Vivar J., Guan T., Oldham K. T., Pritchard K. A., Jr., Shi Y. (2009) Arterioscler. Thromb. Vasc. Biol. 29, 2161–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li L., Rezvan A., Salerno J. C., Husain A., Kwon K., Jo H., Harrison D. G., Chen W. (2010) Circ. Res. 106, 328–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xie L., Smith J. A., Gross S. S. (1998) J. Biol. Chem. 273, 21091–21098 [DOI] [PubMed] [Google Scholar]
- 21. Abramoff M. D., Magelhaes P. J., Ram S. J. (2004) Biophotonics Int. 11, 36–42 [Google Scholar]
- 22. Howells D. W., Hyland K. (1987) Clin. Chim. Acta 167, 23–30 [DOI] [PubMed] [Google Scholar]
- 23. Kapust R. B., Tözsér J., Copeland T. D., Waugh D. S. (2002) Biochem. Biophys. Res. Commun. 294, 949–955 [DOI] [PubMed] [Google Scholar]
- 24. Auerbach G., Herrmann A., Bracher A., Bader G., Gutlich M., Fischer M., Neukamm M., Garrido-Franco M., Richardson J., Nar H., Huber R., Bacher A. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 13567–13572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yoneyama T., Hatakeyama K. (1998) J. Biol. Chem. 273, 20102–20108 [DOI] [PubMed] [Google Scholar]
- 26. Wilson-Grady J. T., Villén J., Gygi S. P. (2008) J. Proteome Res. 7, 1088–1097 [DOI] [PubMed] [Google Scholar]
- 27. Villén J., Beausoleil S. A., Gerber S. A., Gygi S. P. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 1488–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wearne S. J. (1990) FEBS Lett. 263, 23–26 [DOI] [PubMed] [Google Scholar]
- 29. Quinlan R. A., Moir R. D., Stewart M. (1989) J. Cell Sci. 93, 71–83 [DOI] [PubMed] [Google Scholar]
- 30. Nagai K., Perutz M. F., Poyart C. (1985) Proc. Natl. Acad. Sci. U.S.A. 82, 7252–7255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hatakeyama K., Inoue Y., Harada T., Kagamiyama H. (1991) J. Biol. Chem. 266, 765–769 [PubMed] [Google Scholar]
- 32. Parks T. D., Dougherty W. G. (1991) Virology 182, 17–27 [DOI] [PubMed] [Google Scholar]
- 33. Parks T. D., Leuther K. K., Howard E. D., Johnston S. A., Dougherty W. G. (1994) Anal. Biochem. 216, 413–417 [DOI] [PubMed] [Google Scholar]
- 34. Rebelo J., Auerbach G., Bader G., Bracher A., Nar H., Hösl C., Schramek N., Kaiser J., Bacher A., Huber R., Fischer M. (2003) J. Mol. Biol. 326, 503–516 [DOI] [PubMed] [Google Scholar]
- 35. Schramek N., Bracher A., Fischer M., Auerbach G., Nar H., Huber R., Bacher A. (2002) J. Mol. Biol. 316, 829–837 [DOI] [PubMed] [Google Scholar]
- 36. Schramek N., Bracher A., Bacher A. (2001) J. Biol. Chem. 276, 44157–44162 [DOI] [PubMed] [Google Scholar]
- 37. Nardese V., Gütlich M., Brambilla A., Carbone M. L. (1996) Biochem. Biophys. Res. Commun. 218, 273–279 [DOI] [PubMed] [Google Scholar]
- 38. Luttik M. A., Vuralhan Z., Suir E., Braus G. H., Pronk J. T., Daran J. M. (2008) Metab. Eng. 10, 141–153 [DOI] [PubMed] [Google Scholar]