Abstract
The enzyme phosphofructokinase-1 (PFK-1) catalyzes the first committed step of glycolysis and is regulated by a complex array of allosteric effectors that integrate glycolytic flux with cellular bioenergetics. Here, we demonstrate the direct, potent, and reversible inhibition of purified rabbit muscle PFK-1 by low micromolar concentrations of long chain fatty acyl-CoAs (apparent Ki ∼1 μm). In sharp contrast, short chain acyl-CoAs, palmitoylcarnitine, and palmitic acid in the presence of CoASH were without effect. Remarkably, MgAMP and MgADP but not MgATP protected PFK-1 against inhibition by palmitoyl-CoA indicating that acyl-CoAs regulate PFK-1 activity in concert with cellular high energy phosphate status. Furthermore, incubation of PFK-1 with [1-14C]palmitoyl-CoA resulted in robust acylation of the enzyme that was reversible by incubation with acyl-protein thioesterase-1 (APT1). Importantly, APT1 reversed palmitoyl-CoA-mediated inhibition of PFK-1 activity. Mass spectrometric analyses of palmitoylated PFK-1 revealed four sites of acylation, including Cys-114, Cys-170, Cys-351, and Cys-577. PFK-1 in both skeletal muscle extracts and in purified form was inhibited by S-hexadecyl-CoA, a nonhydrolyzable palmitoyl-CoA analog, demonstrating that covalent acylation of PFK-1 was not required for inhibition. Tryptic footprinting suggested that S-hexadecyl-CoA induced a conformational change in PFK-1. Both palmitoyl-CoA and S-hexadecyl-CoA increased the association of PFK-1 with Ca2+/calmodulin, which attenuated the binding of palmitoylated PFK-1 to membrane vesicles. Collectively, these results demonstrate that fatty acyl-CoA modulates phosphofructokinase activity through both covalent and noncovalent interactions to regulate glycolytic flux and enzyme membrane localization via the branch point metabolic node that mediates lipid flux through anabolic and catabolic pathways.
Keywords: Calmodulin, Glycolysis, Lipid, Phosphofructokinase, Protein Palmitoylation, Acyl Coenzyme A, Acyl-protein Thioesterase
Introduction
Glycolysis occupies a central role in eukaryotic energy metabolism through the production of NADH and ATP to meet immediate cellular energy demands, the generation of glycerol 3-phosphate for the de novo synthesis of phospholipids and triglycerides, and the production of pyruvate that is converted to acetyl-CoA for subsequent utilization in the TCA cycle or for de novo fatty acid biosynthesis. Thus, glycolysis and lipid metabolic flux are interwoven metabolic networks that must be coordinately regulated to maintain cellular bioenergetic homeostasis. Although the ability of fatty acids to suppress glycolytic flux has been known for 50 years (1–4), the mechanisms by which metabolically active tissues undergo a rapid shift from glucose utilization to fatty acid oxidation during metabolic transitions remain incompletely understood. The increasing prevalence of obesity, diabetes, and the metabolic syndrome, in which there is increased reliance on fatty acid substrate at the expense of glucose (5–8), has renewed interest in the chemical mechanisms regulating substrate utilization during metabolic transitions.
Previous work has suggested that glycolysis and fatty acid β-oxidation are coordinately regulated by specific metabolites of each bioenergetic network (9–11). A prevailing hypothesis to explain the regulation of glycolysis by fatty acids originally put forth by Randle and co-workers (3, 4) states that the availability and subsequent β-oxidation of fatty acids results in the production of tricarboxylic acid cycle intermediates that down-regulate glycolysis. Specifically, acetyl-CoA generated from fatty acid β-oxidation leads to the accumulation of citrate that is a potent inhibitor of phosphofructokinase, which catalyzes the rate-determining step in glycolysis. However, substantial experimental evidence suggests that the regulation of glycolytic flux involves the integration of complex networks that coordinately integrate metabolic transitions.
Mammalian phosphofructokinase-1 (6-phosphofructo 1-kinase (PFK-1)3) catalyzes the first committed and rate-determining step of glycolysis and thus represents an essential metabolic control point or node for carbohydrate utilization. Accordingly, PFK-1 is subject to complex catalytic and allosteric regulation by multiple cellular metabolites, including AMP, ADP, ATP, fructose 2,6-bisphosphate, and citrate among others. Although the mechanisms by which PFK-1 is modulated have been extensively investigated through kinetic, biochemical, and biophysical studies for over 40 years (for reviews see Refs. 12–14), little information is available on the direct regulation of PFK-1 by fatty acids or acyl-CoAs. Because acyl-CoA is a branch point metabolite that serves as a regulatory node for fatty acid-mediated energy production (through β-oxidation) or for energy storage (through anabolic lipid synthesis), it seems evident that these networks must be coordinately regulated to effect physiologic metabolic transitions. However, among the few studies reporting the effects of fatty acids on PFK-1 activity, virtually all employed supramicellar (i.e. nonphysiologic) concentrations (>50 μm) to achieve inhibition (15, 16). Furthermore, to the best of our knowledge, no studies have directly examined the interaction of acyl-CoA with PFK-1, the effects of acyl-CoA on PFK-1 activity, the influence of acyl-CoA on PFK-1 protein-protein interactions, or the effects of acyl-CoA on the localization of PFK-1 to membrane bilayers. Because mammalian PFKs are known to contain an allosteric activation site (which binds ADP/AMP) and a catalytic site (which binds ATP), we considered the possibility that acyl-CoAs could bind to an adenine-based regulatory site and modulate PFK-1 activity to integrate glycolytic flux with fatty acid oxidation. Such a mechanism would be of particular relevance considering the well established accumulation of activated fatty acid derivatives (e.g. fatty acyl-CoA and acyl-carnitine) and their deleterious sequelae in lipid-related disease states such as diabetes, hepatic steatosis, hyperlipidemia, and related component parts of the metabolic syndrome.
In this study, we identified a direct mechanism through which the branch point metabolite in fatty acid anabolic and catabolic metabolism, acyl-CoA, regulates the rate-determining and first committed step of glycolysis, the phosphorylation of fructose 6-phosphate to fructose 1,6-bisphosphate by PFK-1. Both naturally occurring long chain fatty acyl-CoAs as well as a synthetic nonhydrolyzable analog (S-hexadecyl-CoA) resulted in potent inhibition of PFK-1 that was blocked by low concentrations of AMP and ADP (200 μm) but not ATP. Importantly, palmitoyl-CoA-mediated acylation of PFK-1 and inhibition of PFK-1 catalytic activity were reversible by acyl-protein thioesterase-1 (APT1) rendering this mechanism amenable to respond to rapid metabolic transitions. Finally, we demonstrated that incubation of PFK-1 with either palmitoyl-CoA or S-hexadecyl-CoA led to the increased association of the enzyme with both membrane vesicles and with one of its previously identified interacting regulatory proteins, calmodulin. Collectively, these results identify multiple mechanisms by which fatty acyl-CoAs promote alterations in PFK activity, PFK-membrane interactions, and PFK-regulatory protein associations that allow physiologic metabolic responses during health and likely compromise physiologic metabolic substrate transitions in lipid-related disease states in which fatty acyl-CoAs accumulate.
EXPERIMENTAL PROCEDURES
Reagents
Acyl-CoAs were obtained from Sigma, and their concentrations were determined using an extinction coefficient (ϵ257 = 15.4 mm−1 cm−1). [1-14C]Palmitoyl-CoA (PerkinElmer Life Sciences) was purified using an oligonucleotide purification cartridge (Applied Biosystems, Carlsbad, CA) as described previously before use (17). S-Hexadecyl-CoA was synthesized and purified as described previously (18). Briefly, 150 μmol (53 mg) of 1-iodohexadecane was dissolved in 5 ml of 5% benzene, 95% ethanol and added dropwise to 30 μmol (25 mg) of CoASH in 2.5 ml of degassed 40 mm aqueous Li2CO3. The reaction mixture was stirred overnight at ambient temperature under N2 in a sealed vial. S-Hexadecyl-CoA was precipitated by adjusting the pH to 1 with HCl, and the resultant solid was washed with diethyl ether followed by acetone. Purification of S-hexadecyl-CoA was accomplished using a C18 RP-HPLC column equilibrated with 25 mm KH2PO4, pH 5.0, using a linear gradient of acetonitrile. 1-Palmitoyl-2-hydroxyphosphorylcholine and 1-[12-[(7-nitro-2–1,3-benzoxadiazol-4-yl)amino]dodecanoyl]-2-hydroxy-sn-glycero-3-phosphocholine, 1-palmitoyl-2-oleoyl-sn-glycerophosphorylcholine, and phosphatidylethanolamine (porcine brain) were obtained from Avanti Polar Lipids (Alabaster, AL). Sequencing grade modified trypsin was purchased from Promega (Madison, WI). Rabbit polyclonal antibody (H-55) directed against residues 676–730 near the C terminus of PFK-1 was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Most other reagents and supplies were purchased from Sigma or Fisher.
Purification of PFK from Rabbit Skeletal Muscle
PFK was purified from rabbit skeletal muscle as described previously by Hussey et al. (19) with minor modifications. All procedures were performed at 4 °C. Briefly, skeletal muscle from New Zealand White rabbits was homogenized in 3× v/w 10 mm potassium phosphate buffer, pH 8.0, containing 30 mm KF and 3 mm EDTA utilizing a Waring blender (two times with 30-s intervals) prior to centrifugation at 10,000 × g for 30 min. Solid ammonium sulfate was added while stirring to 0.45 saturation while maintaining the pH of the solution at 7.5. After slowly stirring for 1 h, the suspension was centrifuged at 10,000 × g for 30 min, and the pellet was discarded. Additional solid ammonium sulfate was added to the supernatant while stirring to 0.58 saturation, and the resultant protein suspension was allowed to stand overnight. Precipitated protein was isolated by centrifugation at 10,000 × g for 30 min and resuspended in a minimal volume of 50 mm Tris-PO4, pH 8.0, containing 2.5 mm DTT and 0.2 mm fructose 1,6-bisphosphate. The concentrated protein was applied to a 2.5 × 40-cm Bio-Gel A-1.5m (fine) (Bio-Rad) column equilibrated with 50 mm Tris-PO4, pH 8.0, containing 1 m (NH4)2SO4, 2.5 mm DTT, and 1 mm EDTA. Peak fractions containing PFK-1 activity were pooled, concentrated by ammonium sulfate precipitation, redissolved in a minimal volume of 50 mm Tris-PO4, pH 8.0, containing 2.5 mm DTT, 1 mm EDTA, and 0.2 mm fructose 1,6-bisphosphate, and applied to the above column equilibrated with the same buffer. Active fractions containing PFK-1 in the void volume were concentrated using an Amicon ultracentrifugal filter device to >1 mg/ml and were used for all subsequent experiments. PFK-1 purified by this method was >95% pure as determined by silver staining following SDS-PAGE and was stable for several weeks when stored under nitrogen at 4 °C.
Cloning, Expression, and Purification of Human Acyl-protein Thioesterase (APT1)
The cDNA encoding human APT1 was amplified by PCR from a human heart cDNA library utilizing Pfu DNA polymerase in combination with a forward primer to introduce a 5′ EcoRI site followed by a Kozak consensus sequence and a reverse primer to introduce a poly-His tag (His6) at the C terminus followed by an XhoI site. The resultant 730-bp PCR fragment digested with EcoRI and XhoI was ligated into pFastBac1. Following sequence verification of the human APT1 coding sequence insert, recombinant baculovirus was produced and titered utilizing the Bac-to-Bac baculovirus expression system according to the manufacturer's instructions (Invitrogen). The baculovirus encoding human APT1 was used to infect Sf9 cells grown in suspension (5 × 100-ml culture volume at 1.5 × 106 cells/ml) at a multiplicity of infection of ∼1. Following incubation at 27 °C for 48 h, cells were harvested by centrifugation (900 × g for 10 min) and resuspended in 50 ml of 30 mm potassium phosphate buffer, pH 7.5, containing 20% glycerol, 10 mm 2-mercaptoethanol, and 10 μm 1-palmitoyl-sn-glycero-3-phosphocholine (Buffer A). The resuspended cells were sonicated (20 × 1-s bursts at 25% power) and centrifuged at 100,000 × g for 60 min, and the resultant cytosol was applied to a 15-ml DEAE-Sephacel column equilibrated with Buffer A. After washing with 25 ml of Buffer A, the flow-through fraction of the DEAE column containing APT1 was diluted 1:1 with Buffer A containing 500 mm NaCl (Buffer B) and applied to a 3-ml Co2+ His Select column (Sigma) equilibrated with Buffer B. Following washing with Buffer B, bound APT1 was eluted with Buffer B containing imidazole (200 mm), and the resultant enzyme was diluted 1:1 with Buffer A containing 500 mm NH4SO4 and applied to a 5-ml butyl-Sepharose column equilibrated with the same buffer. After washing with Buffer A containing 250 mm NH4SO4, bound APT1 was eluted with Buffer A. Active fractions were identified by measuring the hydrolysis of 1-[12-[(7-nitro-2–1,3-benzoxadiazol-4-yl)amino]dodecanoyl]-2-hydroxy-sn-glycero-3-phosphocholine as substrate in 100 mm potassium phosphate, pH 7.0, for 5 min at 37 °C. Released 12-[(7-nitro-2–1,3-benzoxadiazol-4-yl)amino]dodecanoic acid was extracted into butanol, separated by thin layer chromatography utilizing a solvent system of CHCl3/CH3OH/concentrated NH4OH (60:35:8), and visualized by fluorescence using a Kodak Image Station. Purified APT1 was concentrated using an Amicon ultracentrifugal filter device, diluted 1:1 with Buffer A containing 60% glycerol, and stored at −20 °C.
Coupled Spectrophotometric Assays for PFK-1 Activity
Phosphofructokinase activity was measured spectrophotometrically using a PerkinElmer Life Sciences Lambda 25 spectrophotometer at 22 °C as described previously. Briefly, PFK-1 (∼0.1 μm) was incubated in the presence or absence of selected concentrations of acyl-CoAs, S-hexadecyl-CoA, palmitic acid/CoASH, or palmitoylcarnitine for the indicated times at 30 °C prior to measuring PFK-1 activity. The preincubated PFK-1 (∼0.1 μg) was then added to 1 ml of 50 mm imidazole, pH 8.0, containing 50 mm KCl, 2 mm MgCl2, 2 mm fructose 6-phosphate, 1 mm ATP, 0.2 mm NADH, and 2 mm DTT in the presence of aldolase (1.25 units), glycerol-3-phosphate dehydrogenase (2 units), and triose-phosphate isomerase (2 units). The decrease in absorbance at 340 nm due to oxidation of NADH (ϵ = 6.22 mm−1 cm−1) was then measured for 90 s and quantified by comparison with external standards.
Radiometric Assay for PFK-1 Activity
PFK-1 activity was measured directly using a radiometric assay similar to that described previously (20) except that an improved thin layer chromatography system to separate [γ-32P]ATP from fructose-[1-32P]6-bisphosphate was employed (21). Briefly, PFK-1 was incubated with 1 mm fructose 6-phosphate and 0.1 mm [γ-32P]ATP (0.4 μCi/μmol) in the presence of 50 mm Tris-HCl, pH 7.4, containing 5 mm MgCl2 and 1 mm DTT. Reactions were terminated by addition of acetone (30% v/v final concentration), spotted on PEI-cellulose TLC plates (Selecto Scientific, Suwanee, GA), and developed using a mobile phase of 0.75 m Tris base, 0.45 m HCl, and 0.25 m (NH4)2SO4, pH 8. Plates were then dried and fructose 1,6-bisphosphate was quantified by autoradiography using a Kodak Image Station.
Covalent Acylation of PFK-1 by [14C]Palmitoyl-CoA and Analysis by SDS-PAGE and Autoradiography
PFK-1 (1 μm) purified from rabbit skeletal muscle was incubated with 10 μm [1-14C]palmitoyl-CoA in 25 mm Tris-HCl, pH 7.5, containing 50 mm KCl and 1 mm DTT for the indicated times at 35 °C. After addition of 2× SDS-PAGE loading buffer, samples were subjected to SDS-PAGE and fixed in the gel with 40% methanol, 10% acetic acid. Gels were then soaked in Amplify fluorographic reagent (Amersham Biosciences) for 45 min, dried, and exposed to film. In some experiments, bovine serum [methyl-14C]albumin (American Radiolabeled Chemicals, St. Louis, MO) was employed as standard to determine the stoichiometry of palmitoylation of PFK-1 using a Kodak Image Station.
Identification of the Palmitoylation Sites of PFK-1 by Mass Spectrometry
Purified rabbit muscle PFK-1 (10 μm) was incubated in the presence or absence of a stoichiometric amount of palmitoyl-CoA for 0–60 min. PFK-1 treated with palmitoyl-CoA was then precipitated as described previously (22). Briefly, following the addition of 4 volumes of CH3OH and 1 volume of CHCl3, the sample was vortexed and placed on ice. Three volumes of H2O were then added, and the mixture was centrifuged at 14,000 × g for 15 min. The upper phase was removed and replaced with 3 volumes of CH3OH. Proteins were pelleted following centrifugation as described above and solubilized in 0.1% RapiGestTM (Waters) in 25 mm NH4HCO3. Trypsin was added at a ratio of 1:30 w/w, and the samples were incubated at 37 °C for 2 h. Trifluoroacetic acid was then added to a final concentration of 0.5%. The samples were further incubated at 37 °C for 45 min and centrifuged at 13,000 rpm for 15 min. The supernatant was transferred to a new tube, and the pellet was resuspended into 70% isopropyl alcohol by vortexing and intermittent sonication. Both the supernatant and the pellet fractions were analyzed by nano-LC/MS/MS.
Processed samples were applied to a PepMap C4 precolumn (300 μm × 1 mm, Dionex, Sunnyvale, CA) using a Surveyor autosampler (ThermoFisher Scientific, Waltham, MA) and washed with 0.1% formic acid for 5 min. A nonlinear gradient from 90% mobile phase A (0.1% formic acid in water) and 10% mobile phase B (50% acetonitrile, 50% isopropyl alcohol, 0.1% formic acid) to 10% A and 90% B was applied to elute the tryptic peptides from the precolumn onto a reverse phase C4 analytical column (75 μm × 15 cm, Dionex, Sunnyvale, CA) at a flow rate of 250 nl/min. Peptides eluting from the C4 column were introduced into an LTQ-Orbitrap mass spectrometer (ThermoFisher Scientific, Waltham, MA) using a TriVersaTM Nanomate system (Advion, Ithaca, NY) with a spray voltage of 1.7 kV. The mass spectrometer was operated in a data-dependent acquisition mode with dynamic exclusion enabled allowing the five most intense ion peaks from the full mass scan to undergo product ion mass analysis. The instrument was calibrated externally following the manufacturer's instructions. Full mass scans were performed by the Orbitrap mass analyzer with a mass resolution of r = 30,000 at m/z 400 Thomson. The lock mass option was enabled to ensure a mass accuracy of <5 ppm (utilizing the m/z value of the protonated diisooctyl phthalate (391.2843) as the lock mass). The product ion mass spectra were acquired either by the ion trap or the Orbitrap mass analyzer with a mass resolution of 15,000 at m/z 400 Thomson. For the product ion analyses, a normalized collision energy of 30% was applied; the activation time was set at 30 ms with the activation parameter q = 0.250, and precursor ions were isolated within the range of 2–4 Thomson. The acquired data were searched against a customized protein sequence database using both SEQUEST (BioworksTM, ThermoFisher Scientific, Waltham, MA) and MASCOT algorithms (Matrix Science, London, UK). Positive search results were manually confirmed.
Generation of a Structural Model of Rabbit Muscle PFK-1
Molecular modeling of the N-terminal (residues 1–389) and C-terminal (residues 390–763) domains of rabbit muscle PFK-1 was performed by energy minimization algorithms available on the I-TASSER server based upon sequence alignments with prokaryotic PFK isoforms of known structure (23–25). Predictive models with the highest confidence score were chosen for illustrating the localization of the palmitoylated cysteine residues identified by mass spectrometry.
Deacylation of [14C]Palmitoyl PFK-1 by APT1 and Reversal of Palmitoyl-CoA Inhibited PFK-1 by APT1
PFK-1 (1 μm) was incubated with [14C]palmitoyl-CoA (10 μm) for 60 min at 35 °C in 25 mm Tris-HCl, pH 7.5, containing 20% glycerol, 50 mm KCl, and 1 mm DTT. Purified recombinant APT1 (15 μg) or buffer alone was then added, and the sample was incubated for the indicated times at 35 °C prior to SDS-PAGE and autoradiography as described above. For reconstitution of PFK-1 activity inhibited by palmitoyl-CoA, purified PFK-1 (1 μm) was incubated in the presence or absence (as control) of palmitoyl-CoA (10 μm) in 25 mm Tris-HCl, 20% glycerol, 50 mm KCl, and 1 mm DTT for 30 min at 30 °C. Purified APT1 or buffer alone was then added, and samples were incubated for the indicated times at 30 °C immediately before assaying PFK-1 activity utilizing either coupled spectrophotometric or radiometric assays.
Tryptic Footprinting of PFK-1
Purified PFK-1 (85 μg in 1 ml) was incubated in 25 mm Tris-HCl, pH 7.5, containing 50 mm KCl and 1 mm DTT in the presence or absence of MgAMP or S-hexadecyl-CoA for 15 min at 35 °C. Trypsin (2 μg) was added, and aliquots (50 μl) of the reaction were removed at the indicated time points. Trypsinolysis reactions were terminated by addition of an equal volume of SDS-PAGE loading buffer and vortexing. Tryptic fragments were resolved by SDS-PAGE and probed using a rabbit polyclonal antibody directed to the C terminus of PFK-1 (H-55, residues 676–730, Santa Cruz Biotechnology). Immunoreactive bands were detected by enhanced chemiluminescence using a protein A-horseradish peroxidase conjugate and visualized using a Kodak Image Station.
Binding of S-Hexadecyl-CoA- or Palmitoyl-CoA-treated PFK-1 to Calmodulin-agarose
Purified PFK (1 μm) was incubated in the presence or absence of 10 μm palmitoyl-CoA or 10 μm S-hexadecyl-CoA in 25 mm Tris-HCl, pH 7.5, containing 50 mm NaCl and 1 mm DTT. Calcium chloride was then added to a 1 mm final concentration prior to loading a calmodulin-agarose column (0.6 ml) equilibrated with the same buffer. The flow-through fraction was collected and after extensive washing with buffer containing 1 mm CaCl2, bound PFK-1 was eluted with buffer containing 10 mm EGTA. Load, flow-through, wash, and eluate fractions were then analyzed by SDS-PAGE and silver staining as described above.
Binding of PFK-1 to Large Unilamellar Vesicles
Purified PFK-1 was incubated in the presence or absence of palmitoyl-CoA or S-hexadecyl-CoA for the indicated times at 35 °C prior to addition to 10 mm HEPES buffer, pH 7.5, containing 150 mm KCl and 200 μm (final concentration) of the indicated lipid composition. Large unilamellar vesicles (LUVs) comprised of only 1-palmitoyl-2-oleoyl-sn-glycerophosphorylcholine or 1-palmitoyl-2-oleoyl-sn-glycerophosphorylcholine (50 mol %), phosphatidylethanolamine (porcine brain, 30 mol %), and cholesterol (20 mol %) were prepared by extrusion using a 0.8 μm filter in the presence of 10 mm HEPES buffer, pH 7.5, containing 1.25 m sucrose. Following incubation of PFK-1 with the LUVs for 15 min at room temperature, the samples were then centrifuged at 100,000 × g at 20 °C for 1 h. The supernatants were removed, and the pelleted LUVs were resuspended in 0.5 ml of buffer prior to analysis of PFK-1 distribution by SDS-PAGE and silver staining (26), or by immunoblot analysis as described above.
RESULTS
Inhibition of Purified Rabbit Muscle PFK-1 by Submicellar Concentrations of Long Chain Fatty Acyl-CoAs
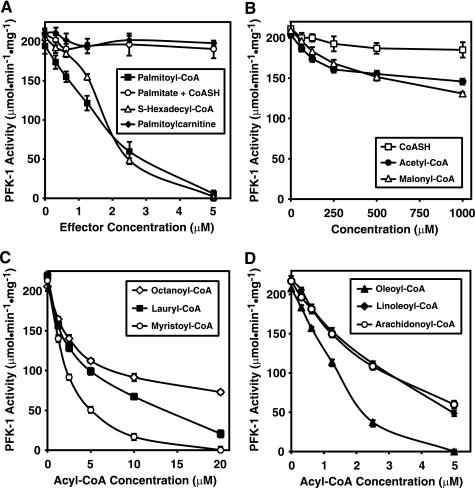
Although numerous nucleotide allosteric effectors of PFK-1 are known (e.g. AMP, ADP, ATP, cAMP, etc.), virtually nothing has been reported regarding the regulation of PFK-1 by CoA or its fatty acyl derivatives. To address the potential interactions of acyl-CoA with PFK-1, we purified rabbit skeletal muscle PFK to apparent homogeneity utilizing (NH4)2SO4 precipitation and sequential high and low salt gel filtration chromatographic steps as described under “Experimental Procedures.” Purified PFK-1 was inhibited by low micromolar concentrations (≤5 μm) of palmitoyl-CoA (Fig. 1A). These results demonstrate the potent inhibition of PFK-1 by acyl-CoA well below their known critical micelle concentrations (27, 28). Because it had been previously reported that high concentrations (supramicellar) of fatty acids inhibit PFK-1 (15, 29) and to examine the importance of the thioester linkage in palmitoyl-CoA-mediated inhibition of PFK-1, we performed identical incubations with free palmitic acid in the presence of equimolar amounts of CoASH at low micromolar concentrations. In addition, we examined the effect of another amphipathic intracellular carrier of conjugated fatty acid, palmitoyl carnitine, on PFK-1 catalytic activity. None of these conditions could recapitulate the inhibition obtained with palmitoyl-CoA (∼ IC50 = 1.5 μm) (Fig. 1A). We next sought to determine whether cleavage of the relatively labile thioester linkage of acyl-CoA was necessary for inhibition because fatty acyl-CoAs can acylate proteins in solution. To address this question, we synthesized and HPLC-purified S-hexadecyl-CoA, a nonhydrolyzable thioether analog of palmitoyl-CoA. Incubations of PFK-1 with S-hexadecyl-CoA demonstrated that it was equipotent as palmitoyl-CoA for inhibition of phosphofructokinase activity (Fig. 1A), indicating that covalent acylation of PFK-1 was not necessary for inhibition of the enzyme.
FIGURE 1.
Inhibition of PFK-1 by low micromolar concentrations of long chain fatty acyl-CoAs and the dependence of inhibition on acyl chain length. A, palmitoyl-CoA and the nonhydrolyzable analog S-hexadecyl-CoA, but not palmitic acid or CoASH alone or in combination, inhibit PFK-1 activity. Increasing concentrations of each reagent as indicated were incubated with purified rabbit skeletal muscle PFK-1 (0.1 μm) for 60 min at 30 °C in 100 mm Tris-HCl, pH 7.5, containing 1 mm DTT. Fructose-6-phosphate 1-kinase activity was measured at 22 °C using a coupled spectrophotometric assay as described under “Experimental Procedures.” B, effect of CoASH and short chain acyl-CoAs on PFK-1 activity. C, effect of medium chain length fatty acyl-CoAs on PFK-1 activity. D, effect of unsaturated fatty acyl-CoAs on phosphofructokinase activity. Increasing concentrations of fatty acyl-CoAs of varying chain length were incubated with purified PFK-1 and assayed as described above. Results are the mean ± S.E. from at least three separate experiments.
Examination of the effects of high concentrations (50–1000 μm) of CoASH, acetyl-CoA, or malonyl-CoA revealed that these metabolic intermediates did not significantly inhibit PFK-1 activity (Fig. 1B). To determine the importance of fatty acyl chain length and unsaturation on the potency of acyl-CoAs for inhibiting PFK-1 catalytic activity, purified PFK-1 was incubated with octanoyl-, lauryl-, myristoyl-, oleoyl-, linoleoyl-, and arachidonoyl-CoA molecular species. The apparent IC50 values of saturated acyl CoA molecular species of 8, 12, and 14 carbons were ∼7, 5, and 2 μm, respectively (Fig. 1C). Extension of the palmitoyl chain by 2 carbon units and introduction of a Δ9 double bond (i.e. oleoyl-CoA) did not significantly affect inhibition of PFK-1 (Fig. 1D), although additional unsaturation and/or extension of the acyl chain (i.e. linoleoyl-CoA or arachidonoyl-CoA) resulted in slightly less effective inhibition of the enzyme (Fig. 1D).
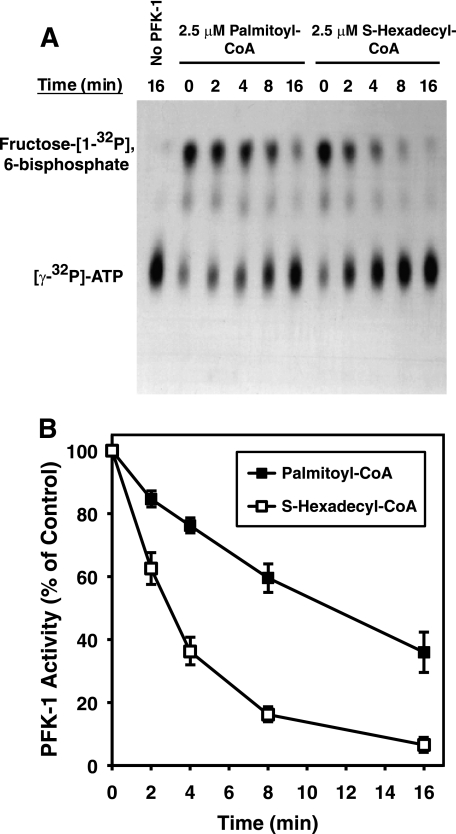
Conventional assays of PFK-1 activity routinely utilize a coupled spectrophotometric assay system (i.e. aldolase, glycerol-3-phosphate dehydrogenase, and triose-phosphate isomerase) that measures the decrease in NADH absorbance to quantify PFK-1 activity. To directly demonstrate the ability of acyl-CoA to inhibit PFK-1, the production of the enzymatic product, fructose 1,6-bisphosphate, was measured by quantifying the incorporation of 32P from [γ-32P]ATP into fructose 6-phosphate. Incubation of PFK-1 with either palmitoyl-CoA or S-hexadecyl-CoA resulted in marked inhibition of enzymatic activity under conditions of the radioisometric assay (Fig. 2). These results exclude the possibility of potential secondary effects of long chain acyl-CoAs on the enzymes of the coupled spectrophotometric assay system. Kinetic analysis demonstrated that low micromolar concentrations of S-hexadecyl-CoA inhibited PFK-1 activity more rapidly than palmitoyl-CoA (Fig. 2). Both palmitoyl-CoA and S-hexadecyl-CoA inhibited PFK-1 activity in a concentration-dependent manner similar to that observed with the coupled assay (data not shown). Thus, both the coupled spectrophotometric and the radiolabeled substrate assays demonstrated that long chain acyl-CoAs directly inhibit skeletal muscle PFK-1.
FIGURE 2.
Time course of palmitoyl-CoA- and S-hexadecyl-CoA-mediated inhibition of PFK-1 activity utilizing a direct radiometric assay employing [γ-32P]ATP. Purified rabbit muscle PFK-1 (0.1 μm) was incubated with palmitoyl-CoA (2.5 μm) in 25 mm Tris-HCl, pH 7.5, containing 50 mm NaCl and 1 mm DTT for the indicated times. PFK activity was then assayed in the presence of 1 mm fructose 6-phosphate and 100 μm [γ-32P]ATP (20 μCi/μmol) for 1 min at 25 °C. After termination of the reaction by addition of acetone, the products were resolved by TLC, detected by autoradiography, and quantified by densitometry as described under “Experimental Procedures.” A, representative TLC autoradiograph showing inhibition of fructose [1-32P]6-bisphosphate formation in the absence and presence of palmitoyl-CoA or S-hexadecyl-CoA. B, quantitation of palmitoyl-CoA and S-hexadecyl-CoA mediated inhibition of PFK-1 from three separate experiments (mean ± S.E.) performed as described above.
Inhibition of PFK-1 Activity in Rabbit Muscle Cytosol by S-Hexadecyl-CoA
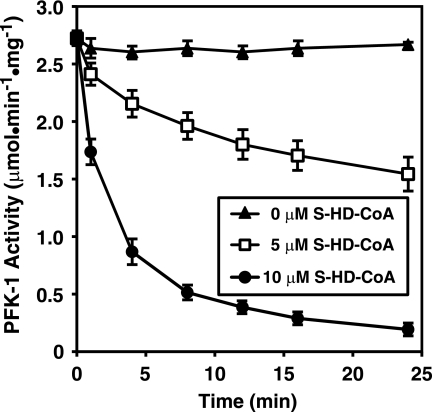
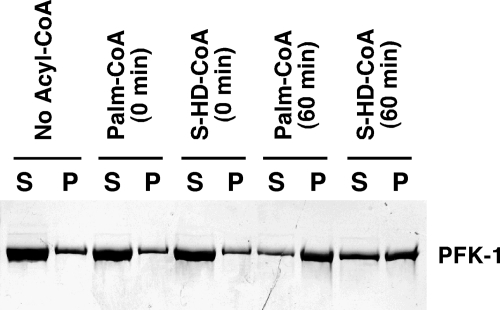
Considering the potent inhibition of purified PFK-1 by fatty acyl-CoAs, it next was of interest to determine whether similar inhibition of PFK-1 was manifest in a complex mixture of cytosolic proteins obtained from skeletal muscle homogenates. Incubation of the nonhydrolyzable analog of palmitoyl-CoA, S-hexadecyl-CoA, with crude skeletal muscle cytosol resulted in the inhibition of endogenous PFK-1 activity present in muscle cytosol in a time- and dose-dependent manner (Fig. 3). Similar inhibition of cytosolic PFK-1 was obtained with low micromolar (<10 μm) concentrations of palmitoyl-CoA (data not shown). These results indicate that endogenous PFK-1 activity in crude skeletal cytosol is modulated by long chain CoA derivatives.
FIGURE 3.
Time course of inhibition of PFK-1 activity by S-hexadecyl-CoA in rabbit muscle cytosol. Isolated rabbit muscle cytosol (0.5 mg/ml) in 100 mm Tris-HCl, pH 7.5, containing 1 mm DTT was incubated with either 0, 5, or 10 μm S-hexadecyl-CoA for the indicated times at 30 °C. PFK-1 catalytic activity was then determined using a coupled spectrophotometric assay measuring the rate of NADH oxidation employing aldolase, triose-phosphate isomerase, and glycerol-3-phosphate dehydrogenase as described under “Experimental Procedures.” Results presented are the mean ± S.E. from at least three separate experiments.
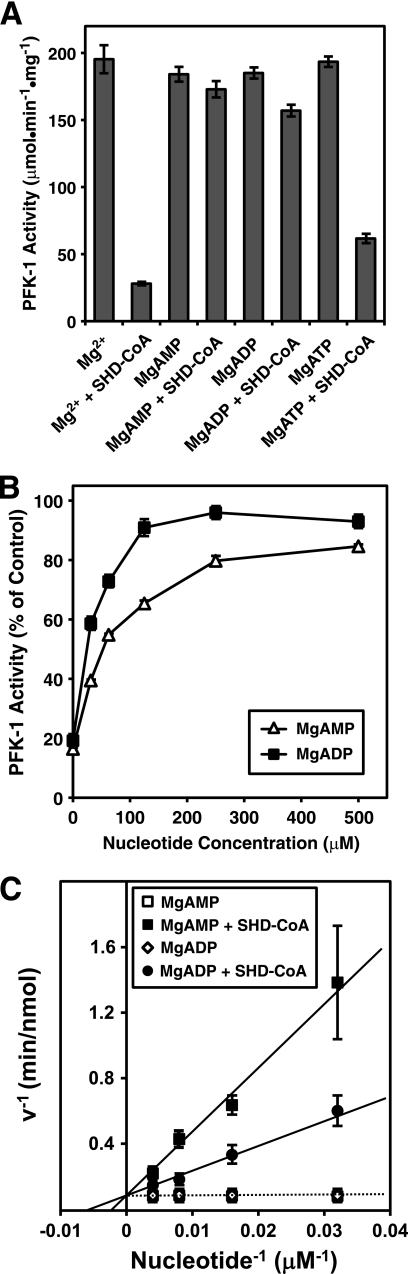
Allosteric Effectors AMP and ADP Protect PFK-1 from S-Hexadecyl-CoA-mediated Inhibition
Mammalian PFK-1 isoforms possess three kinetically distinct nucleotide-binding sites for MgATP (catalytic site), an “ATP inhibitory” site, and for MgAMP/MgADP (allosteric regulatory site). Incubation of PFK-1 in the presence of either MgAMP, MgADP, or MgATP demonstrated that MgAMP and MgADP, but not MgATP, could protect against S-hexadecyl-CoA-mediated inhibition of PFK-1 (Fig. 4A). Increased bioenergetic demand or metabolic stress raises the concentrations of these allosteric effectors to ∼100–200 μm, which were sufficient to preserve the majority of PFK-1 activity in the presence of S-hexadecyl-CoA (Fig. 4B). Additional kinetic experiments to examine the ability of MgAMP and MgADP to block S-hexadecyl-CoA-mediated inhibition of PFK-1 under initial binding conditions revealed that MgADP was more effective than MgAMP in abrogating the effects of S-hexadecyl-CoA (Fig. 4C). The results demonstrate AMP and ADP both provide partial protection of inhibition of PFK-1 by S-hexadecyl-CoA in a concentration-dependent manner consistent with a competitive mechanism (Fig. 4C). Collectively, these results indicate a selective interaction between fatty acyl-CoA and PFK-1 that is modulated by the high energy phosphate status of the cell.
FIGURE 4.
Protection of PFK-1 from S-hexadecyl-CoA-mediated inhibition by MgAMP and MgADP but not MgATP. A, purified rabbit muscle PFK-1 (0.1 μm) was preincubated for 5 min at 30 °C in the presence of either AMP (1 mm), ADP (1 mm), or ATP (1 mm) in 100 mm Tris-HCl, pH 7.5, buffer containing 1 mm DTT and 1 mm MgCl2. S-Hexadecyl-CoA (SHD-CoA) was then added to a 10 μm concentration, and the enzyme was incubated for 5 min at 30 °C before measuring phosphofructokinase activity as described under “Experimental Procedures.” B, purified rabbit muscle PFK-1 (0.1 μm) was preincubated with the indicated concentration of MgAMP or MgADP as described above before addition of 10 μm S-hexadecyl-CoA, incubation at 30 °C for 5 min, and measurement of PFK activity. C, double-reciprocal plot indicating competition between MgAMP or MgADP binding and S-hexadecyl-CoA (5 μm)-mediated inhibition of PFK-1 (0.1 μm) following incubation at 30 °C for 5 min as determined by kinetic rate analysis. Results are the mean ± S.E. from at least three separate experiments.
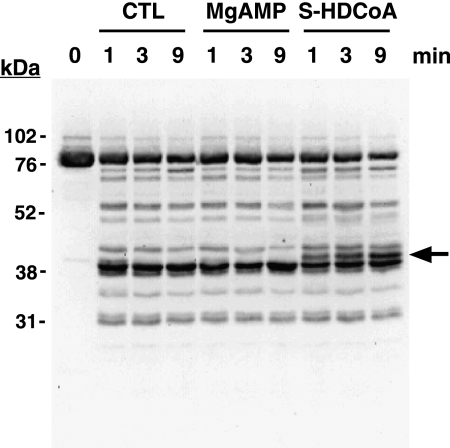
S-Hexadecyl-CoA Induces an Alteration in the Tryptic Footprint of PFK-1
To further determine the effects of fatty acyl-CoA on PFK-1 tertiary structure, we analyzed the tryptic footprint of PFK-1 in the presence or absence of 5 μm S-hexadecyl-CoA. Incubation of PFK-1 with trypsin led to the rapid (<1 min) production of a distinct pattern of tryptic peptides as detected by immunoblot analysis using an antibody directed against the C terminus (residues 676–730) of PFK-1. A major proteolytic product was a 40-kDa fragment likely indicating facile cleavage between the homologous N- and C-terminal halves of the PFK-1 holoenzyme (85 kDa). Kinetic analysis of the trypsinolysis products of PFK-1 in the presence of MgAMP did not generate significant differences in the resultant tryptic footprint. In sharp contrast, incubation of PFK-1 with low micromolar concentrations of S-hexadecyl-CoA (5 μm) and subsequent trypsinolysis resulted in the production of a novel 42-kDa tryptic peptide (Fig. 5). Thus, tryptic footprinting demonstrates that the binding of long chain acyl-CoAs to PFK-1 exposes a latent tryptic cleavage site at or near the molecular tether linking the N- and C-terminal domains of the enzyme.
FIGURE 5.
S-Hexadecyl-CoA alters the susceptibility of PFK-1 to proteolysis as determined by tryptic footprinting. Following preincubation of purified rabbit muscle PFK-1 (1 μm, 85 μg/ml) in the presence or absence of 0.25 mm MgAMP or 2.5 μm S-hexadecyl-CoA, sequencing grade modified trypsin was added at a 1:40 w/w ratio and incubated for the indicated times. Trypsinolysis reactions were terminated by addition of 2× SDS-PAGE loading buffer and vortexing. PFK-1 tryptic fragments were resolved by SDS-PAGE, transferred to PVDF membranes, and probed with an antibody directed against the C terminus of PFK-1. Immunoreactive bands were detected by enhanced chemiluminescence using a protein A-horseradish peroxidase conjugate and visualized using a Kodak Image Station as described under “Experimental Procedures.” A novel 42-kDa tryptic peptide generated only in the presence of S-hexadecyl-CoA is indicated by the arrow. Results are representative of three separate experiments. CTL, control.
Covalent Acylation of Rabbit Muscle PFK-1 by Palmitoyl-CoA
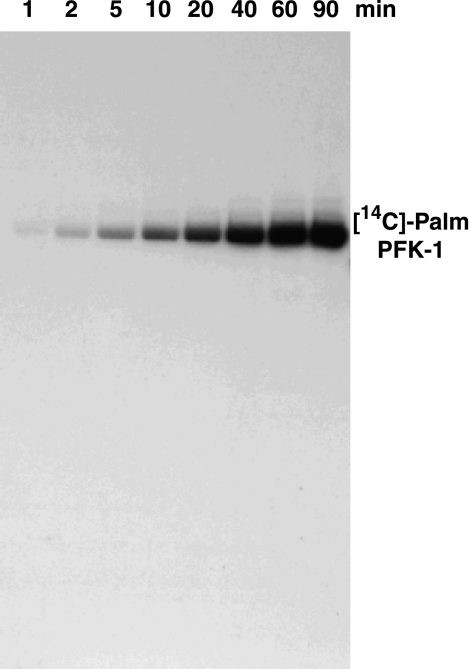
Although S-hexadecyl-CoA inhibited the fructose-6-phosphate 1-kinase activity of PFK-1, we next sought to determine whether the enzyme was covalently modified in the presence of low micromolar concentrations of radiolabeled acyl-CoA. Incubation of purified rabbit muscle PFK-1 with [14C]palmitoyl-CoA resulted in robust covalent palmitoylation of the enzyme (Fig. 6). Utilizing [14C]BSA as standard, we determined the stoichiometry of [14C]palmitate incorporation to be approximately two [14C]palmitate moieties per PFK-1 monomer following 60 min of incubation. These results demonstrate that palmitoyl-CoA-mediated acylation of PFK-1 occurs at submicellar concentrations of acyl-CoA in the absence of protein palmitoyltransferase enzymes.
FIGURE 6.
Time course of covalent acylation of PFK-1 with [14C]palmitoyl-CoA. Purified rabbit muscle PFK-1 (1 μm) was incubated with 10 μm [1-14C]palmitoyl-CoA at 35 °C in 25 mm Tris-HCl, pH 7.5, containing 50 mm KCl and 1 mm DTT for the indicated times. Samples were resolved by SDS-PAGE and visualized by autoradiography as described under “Experimental Procedures.” Results presented are representative of at least three separate experiments.
Determination of the Sites of Palmitoylation in PFK-1 by Mass Spectrometry
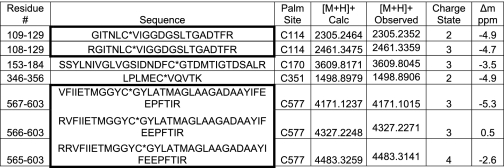
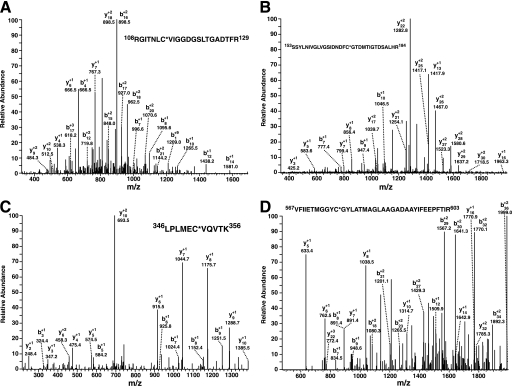
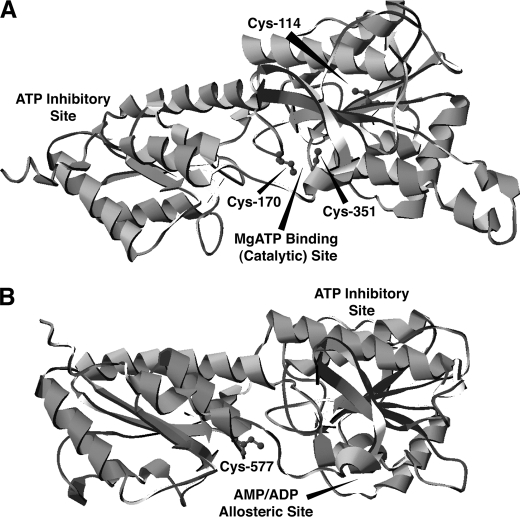
Next, the sites of PFK-1 acylated by palmitoyl-CoA were determined by mass spectrometry. Purified PFK-1 was incubated with stoichiometric amounts of palmitoyl-CoA, precipitated, and then digested with trypsin. Analysis of the resultant tryptic peptides revealed multiple sites of palmitoylation, including Cys-114, Cys-170, Cys-351, and Cys-577 as determined by the high mass accuracy identification of the palmitoylated peptides (Table 1) in conjunction with diagnostic fragmentation patterns (Fig. 7). Notably, Cys-351 is located in close spatial proximity to the predicted calmodulin-binding site of PFK-1 (residues 377–392, SFMNNWEVYKLLAHIR), which is composed of a signature 1–5-10 motif (indicating the position of hydrophobic residues interacting with calmodulin) localized within the molecular tether between the homologous N- and C-terminal domains of the enzyme.
TABLE 1.
Identification of the sites of palmitoylation of PFK-1 acylated with palmitoyl-CoA
Tryptic peptides were prepared from palmitoylated PFK-1, separated using reverse phase nanobore HPLC, and analyzed by an LTQ-Orbitrap mass spectrometer as described under “Experimental Procedures.” Palmitoylated peptides were identified using both SEQUEST and MASCOT algorithms. Identified peptides with missed cleavage sites are listed together with the completely trypsinized peptides in bold bordered boxes. Residue number (Residue #), amino acid sequence (Sequence), palmitoylation size (Palm, Site), calculated m/z value for the singly charged protonated ion ([M + H]+ Calc), observed m/z value for the singly charged protonated ion ([M + H]+ Observed), charge state, and mass difference in parts per million (Δm ppm) are as indicated.
FIGURE 7.
Identification of the sites of palmitoyl-CoA-mediated palmitoylation of phosphofructokinase by mass spectrometry. Purified rabbit skeletal muscle PFK-1 (10 μm) was incubated in the presence or absence of a stoichiometric amount of palmitoyl (Palm)-CoA in 25 mm Tris-HCl, pH 7.5, containing 50 mm KCl and 1 mm DTT for 60 min at 35 °C. Protein samples were precipitated, trypsinized, and processed as described under “Experimental Procedures.” Ion peaks corresponding to predicted palmitoylated tryptic peptides in the full mass scan were selected for product ion analysis. A, product ion mass spectrum of the ion at 2461.34 corresponding to the tryptic peptide (RGITNLC*VIGGDGSLTGADTFR) palmitoylated at Cys-114. B, product ion mass spectrum of the ion at 3609.80 corresponding to the tryptic peptide (SSYLNIVGLVGSIDNDFC*GTDMTIGTDSALR) palmitoylated at Cys-170. C, product ion mass spectrum of the ion at 1498.89 corresponding to the tryptic peptide (LPLMEC*VQVTK) palmitoylated at Cys-351. D, product ion mass spectrum of the ion at 4171.10 corresponding to the tryptic peptide (VFIIETMGGYC*GYLATMAGLAAGADAAYIFEEPFTIR) palmitoylated at Cys-577. Asterisks indicate the sites of palmitoylation. Mass to charge (m/z) ratios of the identified b and y fragment ions are as indicated.
To identify the proximity of the acylation sites identified by mass spectrometry to the nucleotide-binding sites in PFK-1, a three-dimensional model of mammalian PFK-1 was generated through energy minimization of the primary structure of rabbit muscle PFK-1 to known bacterial crystal structures. The primary sequences of the homologous N- and C-terminal domains (residues 1–389 and 390–763, respectively) of rabbit muscle PFK-1 were used for comparisons with the known crystal structures of three prokaryotic homologs of phosphofructokinase (Escherichia coli, Lactobacillus delbrueckii, and Bacillus stearothermophilus). Two of the palmitoylated cysteine residues (Cys-170 and Cys-351) are positioned ∼5 Å apart near the catalytic MgATP-binding site in the N-terminal domain (Fig. 8A). Notably, Cys-577 is positioned in an analogous location in the homologous C-terminal domain adjacent to the AMP/ADP allosteric site (Fig. 8B). These results suggest that fatty acyl-CoAs selectively modify cysteine residues within regions of PFK-1 near the nucleotide-binding sites of the enzyme.
FIGURE 8.
Localization of the palmitoylated cysteine residues identified by mass spectrometry in a molecular model of PFK-1. The primary sequence of rabbit muscle PFK-1 was used in combination with the known crystal structures of three bacterial phosphofructokinases for energy minimization modeling using I-TASSER as described under “Experimental Procedures.” A, molecular model of the N-terminal domain of rabbit muscle PFK-1 (residues 1–389) demonstrates the close spatial proximity of residues Cys-114, Cys-170, and Cys-351 identified as palmitoyl thioesters by mass spectrometry to the catalytic MgATP-binding site. B, model of the C-terminal domain of rabbit muscle PFK-1 (residues 390–763) identifies the homologous position of the acylated residue at Cys-577 in comparison with the identified palmitoylated residues in the N-terminal domain and its close localization to the AMP/ADP allosteric site.
Deacylation of Palmitoyl-PFK-1 by APT1
Previously, APT1 has been shown to catalyze the depalmitoylation of various cellular G protein subunits (including Gsα (30), Gα13 (31), and Ras isoforms (30, 32)), synaptosomal associated protein-23 (33), and endothelial nitric-oxide synthase (34), thereby regulating their membrane localization and/or interaction with protein partners within subcellular microdomains (e.g. lipid rafts). To determine whether the palmitoylation of PFK-1 was reversible through APT1-catalyzed hydrolysis of the acylated residues, we cloned and purified human APT1 as described under “Experimental Procedures.” Incubation of [14C]palmitoyl-PFK-1 with recombinant APT1 demonstrated that virtually all of the palmitoyl moieties were hydrolyzed by APT1 (Fig. 9A) indicating that palmitoylated PFK-1 is a substrate for this acyl-protein thioesterase. Thus, covalent palmitoylation of PFK-1 is reversible by APT1 and suggests that this PFK-1 acylation contributes to its known association with specific intracellular membrane domains in response to metabolic (35, 36) or signaling (37, 38) alterations similar to the roles of palmitoylation in other critical regulatory proteins (e.g. Ras (39)).
FIGURE 9.
Reversal of palmitoyl (Palm)-CoA inhibition of PFK-1 and deacylation of the enzyme by APT1. A, time course of [14C]palmitoyl-PFK deacylation by APT1. [14C]Palmitoyl-PFK-1 (1 μm) was incubated in the presence or absence of purified recombinant APT1 (15 μg) for the indicated times in 25 mm Tris-HCl, pH 7.5, containing 50 mm KCl, 20% glycerol, and 1 mm DTT. PFK-1 palmitoylation was then determined by SDS-PAGE and autoradiography as described under “Experimental Procedures.” Results are representative of three separate experiments. For APT1-mediated reconstitution of phosphofructokinase activity, PFK-1 was incubated in the presence or absence of palmitoyl-CoA as described above for 30 min at 30 °C. APT1 or buffer alone was then added, and samples were incubated for an additional 30 min prior to measuring PFK activity utilizing a coupled enzyme assay (B) (mean ± S.E., n = 4) or a radiometric assay (C) (representative of three separate experiments) as described under “Experimental Procedures.” D, time course of reconstitution of PFK-1 activity by APT1. PFK-1 was incubated in the presence or absence of palmitoyl-CoA for 30 min at 30 °C after which APT1 or buffer alone was then added and incubated for the indicated times. PFK activity (mean ± S.E., n = 3) was then measured using a coupled enzyme assay as described above.
APT1 Reverses Palmitoyl-CoA-mediated Inhibition of PFK-1
In order for the fatty acyl-CoA-mediated inhibition of PFK-1 to be a viable mechanism for the cellular regulation of glycolytic flux facilitating metabolic transitions, we reasoned that it must be a reversible process. Accordingly, we incubated PFK-1 (1 μm) in the presence of palmitoyl-CoA to result in inhibition of ∼50% enzymic activity. Next, APT1 was added, and PFK-1 activity was measured using both the spectrophotometric and autoradiographic assays. Addition of APT1 resulted in almost complete reconstitution of PFK-1 activity in both the coupled spectrophotometric as well as the direct radiometric assay (Fig. 9, B and C). The time dependence of reversal of palmitoyl-CoA-mediated inhibition of PFK-1 was consistent with an enzymic mechanism (Fig. 9D) capable of facilitating rapid metabolic transitions. Collectively, these results demonstrate that APT1 is able to protect and/or rescue PFK-1 from palmitoyl-CoA-mediated inhibition thereby implicating its importance (and/or those of other acyl-CoA/acyl-protein thioesterases) in the regulation of glycolytic flux.
Acyl-CoA and S-Hexadecyl-CoA Mediate Increased PFK-1 Binding to Large Unilamellar Vesicles
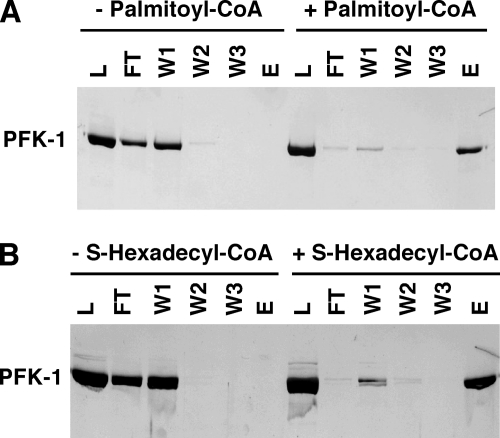
Previous work has shown that PFK-1 can associate with membrane fractions and is highly increased in the particulate fraction during myocardial ischemia, which is accompanied by large increases in palmitoyl-CoA content (40, 41) To determine whether palmitoylation or palmitoyl-CoA binding increased the association of PFK-1 with membrane vesicles, we incubated PFK-1 in the presence or absence of palmitoyl-CoA or S-hexadecyl-CoA and examined the binding of the treated enzyme with LUVs. PFK-1 bound to the LUVs was separated from the unbound enzyme by ultracentrifugation. The amount of native or treated PFK-1 membrane binding was determined by SDS-PAGE and silver staining. Although only 10% of untreated control PFK-1 associated with the LUV membrane bilayers, treatment with S-hexadecyl-CoA increased PFK-1 membrane association ∼5-fold in a time-dependent manner, whereas similar treatment with palmitoyl-CoA resulted in nearly complete binding of the treated enzyme to membrane vesicles (Fig 10). Thus, although covalent acylation facilitates the complete binding of PFK-1 to membrane bilayers, it is not obligatory for substantial increases in PFK-1 membrane association.
FIGURE 10.
Increased association of PFK-1 with large unilamellar vesicles following preincubation with either palmitoyl (Palm)-CoA or S-hexadecyl-CoA. Purified rabbit skeletal muscle PFK-1 was preincubated with the indicated concentrations of palmitoyl-CoA or S-hexadecyl-CoA for either 0 or 60 min at 35 °C. Unbound palmitoyl-CoA was removed by centrifugation of the incubated enzyme through a Bio-Spin column. The resultant enzyme was incubated with a suspension of 1-palmitoyl-2-oleoyl-sn-glycerophosphorylcholine in large unilamellar vesicles for 10 min at room temperature prior to centrifugation at 100,000 × g to separate supernatant (S) and pellet (P) fractions. Isolated membrane pellets were resuspended in a volume of buffer equal to that of the supernatant fraction. An aliquot (50 μl) of each fraction was subjected to SDS-PAGE and subsequent silver staining to determine the amount of PFK-1 in each fraction by densitometry as described under “Experimental Procedures.” Results are representative of three separate experiments.
Palmitoyl-CoA and S-Hexadecyl-CoA Mediate Enhanced Ca2+-dependent Binding of PFK-1 to Calmodulin
Previous work has demonstrated the complex hysteretic regulation of PFK-1 activity (42, 43), oligomeric state (44–46), and cytoskeletal association (47–49) by Ca2+-activated calmodulin. To determine whether palmitoyl-CoA or S-hexadecyl-CoA could influence the interaction of Ca2+/CaM with PFK-1, we incubated purified PFK-1 in the presence or absence of either palmitoyl-CoA or S-hexadecyl-CoA prior to calmodulin-agarose ternary complex affinity chromatography. As reported previously (44), we also found that purified PFK-1 very weakly binds to calmodulin-agarose in the presence of calcium ion (Fig. 11). Remarkably, the presence of either palmitoyl-CoA or S-hexadecyl-CoA dramatically increased the binding of PFK-1 to CaM-agarose in the presence of Ca2+ (Fig. 11, A and B). Importantly, PFK-1 bound to calmodulin-agarose under these conditions could be eluted with buffer containing EGTA indicating that this interaction was reversible and required calcium-induced activation of calmodulin and was not the result of nonspecific acyl-CoA-mediated effects.
FIGURE 11.
Enhanced binding of PFK-1 to calmodulin-agarose mediated by palmitoyl-CoA or S-hexadecyl-CoA. PFK-1 (1 μm) was incubated in the presence or absence of palmitoyl-CoA (10 μm) or S-hexadecyl-CoA (10 μm) for 1 h at 35 °C prior to application to a column of calmodulin-agarose (0.6 ml) equilibrated with buffer in the presence of 1 mm CaCl2. Following washing of the column with three 1-ml volumes (W1–W3) of equilibration buffer, bound PFK-1 was eluted with buffer containing EGTA. Samples (50 μl) of each fraction were subjected to SDS-PAGE and silver staining to visualize the amount of PFK-1 in each fraction by densitometry as described under “Experimental Procedures.” Results are representative of four separate experiments. Abbreviations used are as follows: L, load fraction; F, flow-through fraction; W, wash fraction; E, elution fraction.
Calcium-activated Calmodulin Displaces Acylated PFK-1 from LUV Membrane Bilayers
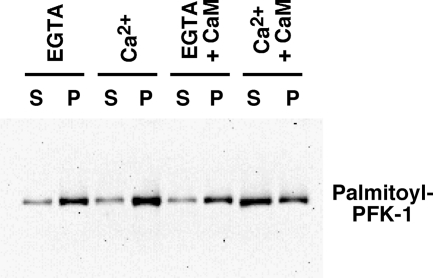
To further clarify the role of the increased interaction between acylated PFK-1 and Ca2+/CaM, we examined the effect of calcium-activated calmodulin on the binding of palmitoylated PFK-1 to LUV membrane bilayers. Remarkably, only Ca2+/CaM, but not Ca2+ alone or CaM in the presence of EGTA, mediated the release of palmitoylated PFK-1 bound to membrane vesicles to the soluble fraction (Fig. 12). Collectively, these results provide a mechanism by which Ca2+/CaM can modulate the binding of palmitoylated PFK-1 to cellular membranes.
FIGURE 12.
Ca2+-activated calmodulin attenuates the binding of palmitoylated PFK-1 to large unilamellar vesicles. PFK-1 acylated with palmitoyl-CoA was bound to a calmodulin-agarose column in the presence of 1 mm CaCl2, washed extensively with buffer containing 0.1 mm CaCl2, and eluted with buffer containing 1 mm EGTA as described under “Experimental Procedures.” Next, palmitoylated PFK was preincubated for 15 min at 22 °C with either EGTA (1 mm), CaCl2 (1 mm), CaM (10 μm) in the presence of EGTA (1 mm), or CaM (10 μm) in the presence of CaCl2 (1 mm). Following the addition of LUVs comprised of phosphatidylcholine (50 mol %), phosphatidylethanolamine (30 mol %), and cholesterol (20 mol %) to the palmitoylated PFK-1 and subsequent incubation at 22 °C for 15 min, samples were centrifuged at 100,000 × g for 1 h. Supernatant (S) and LUV pellet (P) fractions were separated and analyzed for PFK-1 content by immunoblot analysis as described under “Experimental Procedures.” Results are representative of three separate experiments.
DISCUSSION
Almost 50 years ago, Randle and co-workers (3, 4) proposed a hypothesis to explain the mechanism by which fatty acids suppress glucose utilization and oxidation in myocardial and diaphragm muscle tissues. According to this hypothesis, increased β-oxidation of fatty acids results in elevated intramitochondrial acetyl-CoA concentration and an increase in citrate, which is transported to the cytosol by the citrate carrier. Consequently, cytoplasmic citrate levels increase, which leads to direct inhibition of PFK-1 activity at the citrate regulatory site and accumulation of glucose 6-phosphate. The resultant elevation in the concentration of glucose 6-phosphate would then decrease hexokinase activity and thus decrease net glucose uptake. Subsequent in vivo studies to investigate the Randle hypothesis have yielded both supportive (9, 10, 50) and contradictory (51, 52) mechanisms. Accumulated evidence demonstrates that free fatty acids are inhibitory for both glucose uptake and utilization (53–55).
Numerous studies have investigated the complex allosteric regulation of phosphofructokinase. Notable examples of potent activators include sugar diphosphates such as fructose 2,6-bisphosphate and nucleotide mono- and diphosphates such as AMP and ADP (Fig. 13) (56–60). In contrast, inhibition of PFK-1 by citrate, glycerophosphate, phosphoenolpyruvate, high concentrations of ATP, or low pH has been well documented (Fig. 13) (14, 61–63). Supramicellar nonphysiologic concentrations (>100 μm) of short chain (e.g. octanoate) or medium chain (laurate) free fatty acids result in only modest inhibition (50%) of PFK-1 catalytic activity (15, 29). However, such high concentrations of free fatty acids do not exist in vivo and thus are unlikely to be relevant to PFK-1 regulation. Considering the known inhibition of glycolytic flux by fatty acids, it is surprising that no studies (to the best of our knowledge) have identified the direct regulation of phosphofructokinase by fatty acyl-CoAs.
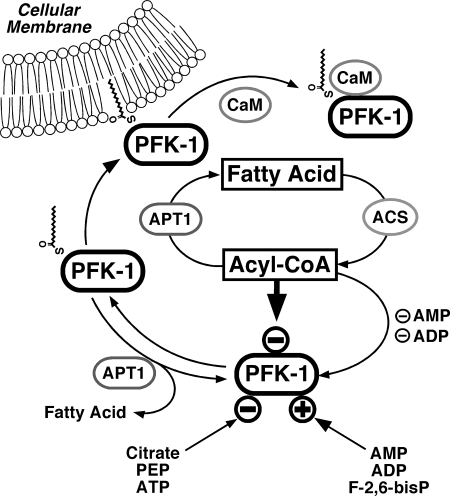
FIGURE 13.
Proposed regulation of glycolysis through fatty acyl-CoA mediated inhibition of PFK-1 and effect of Ca2+/CaM on the membrane compartmentation of acylated PFK-1. Fatty acyl-CoA generated from fatty acids through acyl-CoA synthetases (ACS) inhibit glycolytic flux through phosphofructokinase-1 (PFK-1). Previous work has identified activation of PFK-1 by AMP, ADP, and fructose 2,6-bisphosphate (F-2,6-bisP) and inhibition of PFK-1 through ATP, phosphoenolpyruvate (PEP), and citrate (generated from increased flux of acetyl-CoA through the tricarboxylic acid cycle from β-oxidation of fatty acids). Acyl-protein thioesterase (APT1) reverses fatty acyl-CoA mediated inhibition of PFK-1 either through hydrolysis of noncovalently bound fatty acyl-CoA or through deacylation. Palmitoylation of PFK markedly enhances the binding of calmodulin (CaM) to PFK in a Ca2+-dependent manner and attenuates the binding of palmitoylated PFK to membrane bilayers.
The results of this study demonstrate the potent and reversible inhibition of PFK-1 by long chain acyl-CoAs, the acylation of PFK-1 by palmitoyl-CoA at four cysteine residues (Cys-114, Cys-170, Cys-351, and Cys-577) and that covalent acylation of PFK-1 is reversible by APT1 (Fig. 13). Acylation of PFK is not obligatory for inhibition of enzymic activity, but it increases the affinity of the enzyme for membrane bilayers. Palmitoylation is a known mechanism for the binding of proteins to membranes, and the results indicate that PFK-1 membrane binding is regulated by acyl-CoA-mediated acylation of PFK-1. The concentrations of acyl-CoA in turn are modulated by fatty acid uptake, thioesterification, and cellular metabolic state. Moreover, acyl-CoA (as well as its nonhydrolyzable analog) induces a conformational change in PFK-1, which facilitates its association with calmodulin, a known regulator of PFK-1 (Fig. 13). Collectively, these results identify an integrated chemical network through which glycolytic flux is regulated by the central branch point intermediate of lipid anabolic and catabolic pathways.
Cellular long chain acyl-CoA concentrations are controlled by multiple interwoven metabolic pathways (i.e. β-oxidation versus esterification versus hydrolysis), which integrate the multiple diverse roles of acyl-CoA in cellular bioenergetic and signaling networks. Thus, the regulation of PFK-1 by its direct interaction with acyl-CoA likely has multiple downstream sequelae, including alterations in catalytic activity (glycolytic flux), subcellular localization, and interactions with cellular regulatory proteins such as calmodulin. Specifically, the present results demonstrate that fatty acyl-CoAs regulate PFK-1 activity through the nucleotide allosteric site of the enzyme because MgADP and MgAMP, but not MgATP, were found to significantly protect the enzyme from S-hexadecyl-CoA-mediated inhibition. Thus, long chain fatty acyl-CoAs inhibit PFK-1 under energy-replete (i.e. low AMP/ADP levels) but not deficient (i.e. high AMP/ADP) conditions, thereby protecting the cell from pathologic conditions such as ischemia or hypoxia where anaerobic glycolysis is accelerated and acyl-CoA levels markedly increase.
High fat feeding or lipid infusion results in increased intracellular fatty acyl-CoA concentrations in skeletal muscle (64, 65), and pharmacologic inhibition of lipolysis reduces serum-free fatty acid and intracellular fatty acyl-CoA concentrations (66). Increased utilization of fatty acids impairs multiple points of glucose metabolism, including inhibition of uptake (at the level of GLUT-4) and glucose phosphorylation (at the level of hexokinase). The effects of fatty acids on the levels of various metabolites of glucose have been controversial. Studies by Shulman and co-workers (55) utilizing 13C NMR demonstrate that fatty acids are likely acting primarily on glucose uptake and initial phosphorylation of glucose by hexokinase. However, more recent studies by Curi and co-workers (67) utilizing isolated rat soleus muscle have demonstrated a profound decrease in insulin-induced lactate production in the presence of palmitate. Importantly, levels of glucose 6-phosphate induced by insulin were also found to be increased in the presence of palmitate in that study. In addition, substantial increases in insulin-induced glycogen synthesis and pentose phosphate pathway flux were also observed, although palmitate did not alter insulin-stimulated 2-deoxyglucose uptake.
The inhibition of PFK-1 by fatty acyl-CoA and the subsequent reversal of inhibition by cellular thioesterases represent a previously unrecognized mechanism through which glycolysis and mitochondrial β-oxidation are coordinately regulated. The major cytosolic myocardial lysophospholipase was originally purified from rabbit myocardium and identified as both a lysophospholipase and acyl-CoA hydrolase (68, 69). Duncan and Gilman (30) demonstrated that this enzyme was also an acyl-protein thioesterase (APT1), which catalyzed the removal of palmitate esterified to G-protein cysteine residues. This study demonstrates that APT1 can remove palmitate groups from acylated PFK-1 and that PFK-1 activity could be rescued by APT1. Collectively, these results suggest a complex interrelationship between acyl-CoA-mediated covalent modifications and acyl-CoA-mediated noncovalent conformational alterations in PFK-1. Thus, APT1 likely coordinately regulates glycolytic and lipid flux in a spatio-temporal specific manner through the following: 1) control of cellular acyl-CoA and lysolipid levels (i.e. modulating fatty acyl storage into phospholipids and triglycerides); 2) reversal of acyl-CoA-mediated inhibition of PFK-1 activity; and 3) alteration of the palmitoylation state of PFK-1 thereby determining subcellular localization.
Recently, Martin and Cravatt (70) have utilized 17-octadecynoic acid metabolic labeling in combination with biotin-azide (i.e. “click chemistry”) to identify the presence of fatty acylated PFK in human Jurkat T cells by LC/MS/MS. The observed acylation of PFK-1 in that study was completely reversible by treatment with hydroxylamine, indicating that attachment of the fatty acyl moiety(ies) to cysteine residue(s) occurs through a thioester linkage. This result demonstrates the formation of stable fatty acylated PFK-1 by metabolic labeling in intact human cells. Palmitoylation of PFK-1 likely has many roles in the regulation of this enzyme through alterations in enzyme activity, subcellular localization, and in mediating protein-protein interactions. PFK-1 has been previously demonstrated to interact with multiple important cellular proteins such as calmodulin (43, 44, 71), neuronal nitric-oxide synthase (72), F-actin (73), as well as calcium-independent phospholipase A2 (74). Palmitoylation of PFK-1 results in increased partitioning to membrane vesicles (Fig. 10). Notably, PFK-1 has been shown to interact with caveolin-1 (38, 75) and caveolin-3 (37, 76) and may influence the localization of a glycolytic complex to lipid rafts. Whether PFK-1 requires acylation for its subcellular localization to caveolae or its interactions with protein partners is an intriguing area of future investigation.
Previous work has identified the importance of cysteine residues for the catalytic activity and allosteric regulation of phosphofructokinase (77–80). Quantitation of the stoichiometry of palmitoylation using [1-14C]palmitoyl-CoA revealed ∼2 mol of palmitoyl moieties incorporated per mol of PFK-1 monomer. Subsequent mass spectral analysis of palmitoylated PFK-1 determined that at least four cysteine residues (Cys-114, Cys-170, Cys-351, and Cys-577) were modified indicating multiple targets for thioesterification in the protein. Previously, Cys-170 in rabbit muscle PFK has been shown to be protected by either cAMP or fructose 6-phosphate from modification with iodoacetate (81). These results suggest that substrates/effectors of PFK likely modulate the nucleophilicity of its cysteine residues thereby altering the pattern of palmitoylation of these residues resulting in complex and reversible alterations in the subcellular distribution of PFK during metabolic stress (35, 40, 41).
In lipid-related disease states such as diabetes, there is increased reliance on the utilization of fatty acid substrate in comparison with glucose, which in conjunction with mitochondrial dysfunction leads to accumulation of multiple intermediates of fatty acid metabolism (e.g. fatty acyl-CoAs, acyl-carnitines, etc.) altering numerous cellular regulatory and signaling pathways (82, 83). In summary, this study identifies a novel direct mechanism through which PFK-1 is modulated by fatty acyl-CoA thereby contributing to the integration of glycolytic flux and cellular fatty acid utilization during physiologic metabolic transitions. It is hoped that pharmacologic manipulation of the dysfunctional and maladaptive regulation of PFK-1 by acyl-CoA in lipid-related disease states will lead to novel approaches for the treatment of the metabolic syndrome and related disease states in which acyl-CoAs are inappropriately increased leading to bioenergetic dysfunction.
This work was supported, in whole or in part, by National Institutes of Health Grants 5PO1HL57278 and RO1HL41250. R. W. G. has financial relationships with LipoSpectrum and Platomics.
- PFK
- phosphofructokinase
- CaM
- calmodulin
- LUV
- large unilamellar vesicle.
REFERENCES
- 1. Shipp J. C., Opie L. H., Challoner D. (1961) Nature 189, 1018–1019 [Google Scholar]
- 2. Newsholme E. A., Randle P. J., Manchester K. L. (1962) Nature 193, 270–271 [DOI] [PubMed] [Google Scholar]
- 3. Garland P. B., Randle P. J., Newsholme E. A. (1963) Nature 200, 169–170 [DOI] [PubMed] [Google Scholar]
- 4. Randle P. J., Garland P. B., Hales C. N., Newsholme E. A. (1963) Lancet 1, 785–789 [DOI] [PubMed] [Google Scholar]
- 5. Lopaschuk G. D. (2002) Heart Fail. Rev. 7, 149–159 [DOI] [PubMed] [Google Scholar]
- 6. Buchanan J., Mazumder P. K., Hu P., Chakrabarti G., Roberts M. W., Yun U. J., Cooksey R. C., Litwin S. E., Abel E. D. (2005) Endocrinology 146, 5341–5349 [DOI] [PubMed] [Google Scholar]
- 7. Poornima I. G., Parikh P., Shannon R. P. (2006) Circ. Res. 98, 596–605 [DOI] [PubMed] [Google Scholar]
- 8. Feuvray D., Darmellah A. (2008) Diabetes Metab. 34, S3–S9 [DOI] [PubMed] [Google Scholar]
- 9. Randle P. J., Priestman D. A., Mistry S. C., Halsall A. (1994) J. Cell Biochem. 55, Suppl. 1–11 [DOI] [PubMed] [Google Scholar]
- 10. Frayn K. N. (2003) Biochem. Soc. Trans. 31, 1115–1119 [DOI] [PubMed] [Google Scholar]
- 11. Hue L., Taegtmeyer H. (2009) Am. J. Physiol. Endocrinol. Metab. 297, E578–E591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mansour T. E. (1972) Curr. Top. Cell Regul. 5, 1–46 [PubMed] [Google Scholar]
- 13. Bloxham D. P., Lardy H. A. (1973) in The Enzymes (Boyer P. D. ed) 3rd Ed., pp. 240–274, Academic Press, New York [Google Scholar]
- 14. Kemp R. G., Foe L. G. (1983) Mol. Cell. Biochem. 57, 147–154 [DOI] [PubMed] [Google Scholar]
- 15. Lea M. A., Weber G. (1968) J. Biol. Chem. 243, 1096–1102 [PubMed] [Google Scholar]
- 16. Ramadoss C. S., Uyeda K., Johnston J. M. (1976) J. Biol. Chem. 251, 98–107 [PubMed] [Google Scholar]
- 17. Golovko M. Y., Murphy E. J. (2004) J. Lipid Res. 45, 1777–1782 [DOI] [PubMed] [Google Scholar]
- 18. Rosendal J., Ertbjerg P., Knudsen J. (1993) Biochem. J. 290, 321–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hussey C. R., Liddle P. F., Ardron D., Kellett G. L. (1977) Eur. J. Biochem. 80, 497–506 [DOI] [PubMed] [Google Scholar]
- 20. Sola-Penna M., dos Santos A. C., Alves G. G., El-Bacha T., Faber-Barata J., Pereira M. F., Serejo F. C., Da Poian A. T., Sorenson M. (2002) J. Biochem. Biophys. Methods 50, 129–140 [DOI] [PubMed] [Google Scholar]
- 21. Bochner B. R., Ames B. N. (1982) J. Biol. Chem. 257, 9759–9769 [PubMed] [Google Scholar]
- 22. Wessel D., Flügge U. I. (1984) Anal. Biochem. 138, 141–143 [DOI] [PubMed] [Google Scholar]
- 23. Zhang Y. (2008) BMC Bioinformatics 9, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang Y. (2009) Proteins 77, Suppl. 9, 100–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roy A., Kucukural A., Zhang Y. (2010) Nat. Protoc. 5, 725–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nesterenko M. V., Tilley M., Upton S. J. (1994) J. Biochem. Biophys. Methods 28, 239–242 [DOI] [PubMed] [Google Scholar]
- 27. Powell G. L., Grothusen J. R., Zimmerman J. K., Evans C. A., Fish W. W. (1981) J. Biol. Chem. 256, 12740–12747 [PubMed] [Google Scholar]
- 28. Constantinides P. P., Steim J. M. (1985) J. Biol. Chem. 260, 7573–7580 [PubMed] [Google Scholar]
- 29. Weber G., Convery H. J., Lea M. A., Stamm N. B. (1966) Science 154, 1357–1360 [DOI] [PubMed] [Google Scholar]
- 30. Duncan J. A., Gilman A. G. (1998) J. Biol. Chem. 273, 15830–15837 [DOI] [PubMed] [Google Scholar]
- 31. Siegel G., Obernosterer G., Fiore R., Oehmen M., Bicker S., Christensen M., Khudayberdiev S., Leuschner P. F., Busch C. J., Kane C., Hübel K., Dekker F., Hedberg C., Rengarajan B., Drepper C., Waldmann H., Kauppinen S., Greenberg M. E., Draguhn A., Rehmsmeier M., Martinez J., Schratt G. M. (2009) Nat. Cell Biol. 11, 705–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dekker F. J., Rocks O., Vartak N., Menninger S., Hedberg C., Balamurugan R., Wetzel S., Renner S., Gerauer M., Schölermann B., Rusch M., Kramer J. W., Rauh D., Coates G. W., Brunsveld L., Bastiaens P. I., Waldmann H. (2010) Nat. Chem. Biol. 6, 449–456 [DOI] [PubMed] [Google Scholar]
- 33. Flaumenhaft R., Rozenvayn N., Feng D., Dvorak A. M. (2007) Blood 110, 1492–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yeh D. C., Duncan J. A., Yamashita S., Michel T. (1999) J. Biol. Chem. 274, 33148–33154 [DOI] [PubMed] [Google Scholar]
- 35. Jenkins J. D., Madden D. P., Steck T. L. (1984) J. Biol. Chem. 259, 9374–9378 [PubMed] [Google Scholar]
- 36. Campanella M. E., Chu H., Low P. S. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 2402–2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Scherer P. E., Lisanti M. P. (1997) J. Biol. Chem. 272, 20698–20705 [DOI] [PubMed] [Google Scholar]
- 38. Vallejo J., Hardin C. D. (2004) Biochemistry 43, 16224–16232 [DOI] [PubMed] [Google Scholar]
- 39. Baekkeskov S., Kanaani J. (2009) Mol. Membr. Biol. 26, 42–54 [DOI] [PubMed] [Google Scholar]
- 40. Choate G. L., Lan L., Mansour T. E. (1985) J. Biol. Chem. 260, 4815–4822 [PubMed] [Google Scholar]
- 41. Hazen S. L., Wolf M. J., Ford D. A., Gross R. W. (1994) FEBS Lett. 339, 213–216 [DOI] [PubMed] [Google Scholar]
- 42. Mayr G. W. (1984) Eur. J. Biochem. 143, 521–529 [DOI] [PubMed] [Google Scholar]
- 43. Mayr G. W. (1984) Eur. J. Biochem. 143, 513–520 [DOI] [PubMed] [Google Scholar]
- 44. Mayr G. W., Heilmeyer L. M., Jr. (1983) FEBS Lett. 159, 51–57 [DOI] [PubMed] [Google Scholar]
- 45. Marinho-Carvalho M. M., Zancan P., Sola-Penna M. (2006) Mol. Genet. Metab. 87, 253–261 [DOI] [PubMed] [Google Scholar]
- 46. Marinho-Carvalho M. M., Costa-Mattos P. V., Spitz G. A., Zancan P., Sola-Penna M. (2009) Biochim. Biophys. Acta 1794, 1175–1180 [DOI] [PubMed] [Google Scholar]
- 47. Chen-Zion M., Bassukevitz Y., Beitner R. (1992) Int. J. Biochem. 24, 1661–1667 [DOI] [PubMed] [Google Scholar]
- 48. Glass-Marmor L., Beitner R. (1997) Eur. J. Pharmacol. 328, 241–248 [DOI] [PubMed] [Google Scholar]
- 49. Ashkenazy-Shahar M., Ben-Porat H., Beitner R. (1998) Mol. Genet. Metab. 65, 213–219 [DOI] [PubMed] [Google Scholar]
- 50. Rennie M. J., Winder W. W., Holloszy J. O. (1976) Biochem. J. 156, 647–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Berger M., Hagg S. A., Goodman M. N., Ruderman N. B. (1976) Biochem. J. 158, 191–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Boden G., Jadali F., White J., Liang Y., Mozzoli M., Chen X., Coleman E., Smith C. (1991) J. Clin. Invest. 88, 960–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kelley D. E., Mokan M., Simoneau J. A., Mandarino L. J. (1993) J. Clin. Invest. 92, 91–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Boden G., Chen X., Ruiz J., White J. V., Rossetti L. (1994) J. Clin. Invest. 93, 2438–2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Roden M., Price T. B., Perseghin G., Petersen K. F., Rothman D. L., Cline G. W., Shulman G. I. (1996) J. Clin. Invest. 97, 2859–2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hill D. E., Hammes G. G. (1975) Biochemistry 14, 203–213 [DOI] [PubMed] [Google Scholar]
- 57. Hood K., Hollaway M. R. (1976) FEBS Lett. 68, 8–14 [DOI] [PubMed] [Google Scholar]
- 58. Uyeda K., Furuya E., Luby L. J. (1981) J. Biol. Chem. 256, 8394–8399 [PubMed] [Google Scholar]
- 59. Pilkis S. J., El-Maghrabi M. R., Pilkis J., Claus T. H., Cumming D. A. (1981) J. Biol. Chem. 256, 3171–3174 [PubMed] [Google Scholar]
- 60. Van Schaftingen E., Jett M. F., Hue L., Hers H. G. (1981) Proc. Natl. Acad. Sci. U.S.A. 78, 3483–3486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Colombo G., Tate P. W., Girotti A. W., Kemp R. G. (1975) J. Biol. Chem. 250, 9404–9412 [PubMed] [Google Scholar]
- 62. Bock P. E., Frieden C. (1976) J. Biol. Chem. 251, 5637–5643 [PubMed] [Google Scholar]
- 63. Pettigrew D. W., Frieden C. (1979) J. Biol. Chem. 254, 1896–1901 [PubMed] [Google Scholar]
- 64. Chalkley S. M., Hettiarachchi M., Chisholm D. J., Kraegen E. W. (1998) Metabolism 47, 1121–1126 [DOI] [PubMed] [Google Scholar]
- 65. Ellis B. A., Poynten A., Lowy A. J., Furler S. M., Chisholm D. J., Kraegen E. W., Cooney G. J. (2000) Am. J. Physiol. Endocrinol. Metab. 279, E554–E560 [DOI] [PubMed] [Google Scholar]
- 66. Bajaj M., Suraamornkul S., Romanelli A., Cline G. W., Mandarino L. J., Shulman G. I., DeFronzo R. A. (2005) Diabetes 54, 3148–3153 [DOI] [PubMed] [Google Scholar]
- 67. Massao Hirabara S., de Oliveira Carvalho C. R., Mendonça J. R., Piltcher Haber E., Fernandes L. C., Curi R. (2003) FEBS Lett. 541, 109–114 [DOI] [PubMed] [Google Scholar]
- 68. Gross R. W., Sobel B. E. (1983) J. Biol. Chem. 258, 5221–5226 [PubMed] [Google Scholar]
- 69. Gross R. W. (1983) Biochemistry 22, 5641–5646 [DOI] [PubMed] [Google Scholar]
- 70. Martin B. R., Cravatt B. F. (2009) Nat. Methods 6, 135–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Buschmeier B., Meyer H. E., Mayr G. W. (1987) J. Biol. Chem. 262, 9454–9462 [PubMed] [Google Scholar]
- 72. Firestein B. L., Bredt D. S. (1999) J. Biol. Chem. 274, 10545–10550 [DOI] [PubMed] [Google Scholar]
- 73. Luther M. A., Lee J. C. (1986) J. Biol. Chem. 261, 1753–1759 [PubMed] [Google Scholar]
- 74. Hazen S. L., Gross R. W. (1993) J. Biol. Chem. 268, 9892–9900 [PubMed] [Google Scholar]
- 75. Vallejo J., Hardin C. D. (2004) Am. J. Physiol. Cell Physiol. 286, C43–C54 [DOI] [PubMed] [Google Scholar]
- 76. Sotgia F., Bonuccelli G., Minetti C., Woodman S. E., Capozza F., Kemp R. G., Scherer P. E., Lisanti M. P. (2003) Am. J. Pathol. 163, 2619–2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Froede H. C., Geraci G., Mansour T. E. (1968) J. Biol. Chem. 243, 6021–6029 [PubMed] [Google Scholar]
- 78. Kemp R. G., Forest P. B. (1968) Biochemistry 7, 2596–2602 [DOI] [PubMed] [Google Scholar]
- 79. Kemp R. G. (1969) Biochemistry 8, 4490–4496 [DOI] [PubMed] [Google Scholar]
- 80. Walters D. W., Gilbert H. F. (1986) J. Biol. Chem. 261, 15372–15377 [PubMed] [Google Scholar]
- 81. Latshaw S. P., Bazaes S., Randolph A., Poorman R. A., Heinrikson R. L., Kemp R. G. (1987) J. Biol. Chem. 262, 10672–10677 [PubMed] [Google Scholar]
- 82. Unger R. H., Clark G. O., Scherer P. E., Orci L. (2010) Biochim. Biophys. Acta 1801, 209–214 [DOI] [PubMed] [Google Scholar]
- 83. Wende A. R., Abel E. D. (2010) Biochim. Biophys. Acta 1801, 311–319 [DOI] [PMC free article] [PubMed] [Google Scholar]