Abstract
Lymphocyte homing is regulated by the dynamic interaction between integrins and their ligands. Integrin α4β7 mediates both rolling and firm adhesion of lymphocytes by modulating its affinity to the ligand, mucosal addressin cell adhesion molecule-1 (MAdCAM-1). Although previous studies have revealed some mechanisms of α4β7-MAdCAM-1 binding, little is known about the different molecular bases of the low- and high-affinity α4β7-MAdCAM-1 interactions, which mediate rolling and firm adhesion of lymphocytes, respectively. Here, we found that two loops in immunoglobulin domains 1 and 2 (D1 and D2) of MAdCAM-1 played different roles in MAdCAM-1 binding to low-affinity (inactive) and high-affinity (activated) α4β7. The Asp-42 in the CC′ loop of D1 was indispensable for MAdCAM-1 binding to both low-affinity and high-affinity α4β7. The other CC′ loop residues except for Arg-39 and Ser-44 were essential for MAdCAM-1 binding to both inactive α4β7 and α4β7 activated by SDF-1α or talin, but not required for MAdCAM-1 binding to Mn2+-activated α4β7. Single amino acid substitution of the DE loop residues mildly decreased MAdCAM-1 binding to both inactive and activated α4β7. Notably, removal of the DE loop greatly impaired MAdCAM-1 binding to inactive and SDF-1α- or talin-activated α4β7, but only decreased 60% of MAdCAM-1 binding to Mn2+-activated α4β7. Moreover, DE loop residues were important for stabilizing the low-affinity α4β7-MAdCAM-1 interaction. Thus, our findings demonstrate the distinct roles of the CC′ and DE loops in the recognition of MAdCAM-1 by low- and high-affinity α4β7 and suggest that the inactive α4β7 and α4β7 activated by different stimuli have distinct conformations with different structural requirements for MAdCAM-1 binding.
Keywords: Cell Adhesion, Cell-Cell Interaction, Integrin, Lymphocyte, Protein Conformation, Protein-Protein Interactions
Introduction
Lymphocyte homing from circulation to lymphoid tissues and sites of inflammation is regulated by the dynamic interaction between lymphocyte integrin receptors and endothelial immunoglobulin superfamily cell adhesion molecules (1–3). Integrin α4β7 is an important lymphocyte homing receptor that can mediate both rolling and firm adhesion of lymphocytes, two of the critical steps in lymphocyte migration and tissue-specific homing (4). Its ligand, MAdCAM-1,2 is preferentially expressed on high endothelial venules of gut-associated lymphoid organs and on lamina propria venules, helping lymphocyte traffic to mucosal organs (5). The interaction between MAdCAM-1 and integrin α4β7 is the key step in lymphocyte homing to gut and plays vital roles in both gut mucosal immune homeostasis and intestinal inflammation (6, 7).
Human MAdCAM-1 is a multidomain molecule consisting of two Ig-like domains followed by mucin-like sequences (8). Domain swapping experiments with MAdCAM-1 and VCAM-1 have demonstrated the requirement of the two Ig domains of MAdCAM-1 for efficient integrin α4β7 binding (9). The crystal structure of MAdCAM-1 highlights two protruding loops from the two Ig domains, the CC′ loop in D1 and DE loop in D2, which are important for the interaction between MAdCAM-1 and α4β7 (10, 11). The essential integrin-binding motif (LDTS) resides in the CC′ loop of MAdCAM-1 D1, and the Asp-42 in the LDTS motif serves as the primary α4β7-binding site by directly interacting with the metal ion at the metal ion-dependent adhesion site (MIDAS) in the integrin β7 I domain (8, 12–14). Mutational studies suggest that some other residues in the CC′ loop of MAdCAM-1 are also involved in MAdCAM-1-α4β7 binding (9, 12, 13). The DE loop in D2 is predominated by negatively charged residues, which have been reported to play an important role in determining integrin binding specificity (15). Mutagenesis study has shown that some residues in the DE loop are important for MAdCAM-1 binding to integrin α4β7 (9).
Integrins are α/β heterodimeric cell adhesion molecules that mediate cell-cell, cell-extracellular matrix, and cell-pathogen interactions and transmit signals bidirectionally across the plasma membrane (16–18). Cell adhesion through integrin is dependent on the dynamic regulation of integrin affinity. The low-affinity integrin α4β7 mediates rolling adhesion of lymphocytes. Upon activation, α4β7 converts to a high-affinity state, which mediates firm cell adhesion. Early studies on integrin structure have revealed that integrin extracellular domains exist in at least three distinct global conformational states: bent with a closed headpiece, extended with a closed headpiece, and extended with an open headpiece, which correspond to the low-affinity, intermediate-affinity, and high-affinity states, respectively (19–21). The equilibrium among these different affinity states is regulated by integrin inside-out signaling and certain extracellular stimuli, such as divalent cations (22, 23). When compared with the low-affinity state in Ca2+ + Mg2+, removal of Ca2+ or the addition of Mn2+ strikingly increases ligand binding affinity and adhesiveness of almost all integrins (14, 24–26). Crystal structures of αVβ3 and αIIbβ3 integrins revealed three interlinked metal ion-binding sites in integrin β I domain (27, 28). The central MIDAS is flanked by two metal ion-binding sites, the adjacent to MIDAS (ADMIDAS) site and the synergistic metal ion-binding site. The divalent cation at MIDAS directly coordinates the acidic side chain shared by all integrin ligands and is essential for integrin-ligand binding (14, 29). The synergistic metal ion-binding site and ADMIDAS function as positive and negative regulatory sites, respectively (14, 25, 30–32). Upon activation, integrin with the bent conformation converts to the extended conformation coupled with a series of global and local conformational changes, including separation of cytoplasmic tails, extension of integrin ectodomains, swing-out of the hybrid domain, β I domain α7 helix downward movement, and conformational rearrangement at the integrin ligand-binding site around MIDAS and ADMIDAS.
Despite the above advances in understanding of α4β7-binding hotspots on MAdCAM-1 and integrin conformational rearrangement during activation, the molecular basis for the recognition of MAdCAM-1 by low- and high-affinity α4β7 remains elusive. The mechanism of rolling and firm adhesion of lymphocyte mediated by α4β7 on MAdCAM-1 is not well understood.
In this study, we found that the CC′ loop in D1 and DE loop in D2 of MAdCAM-1 exerted different functions in MAdCAM-1 binding to low- and high-affinity α4β7. In addition, we demonstrated that the inactive α4β7 and α4β7 activated by different stimuli might have distinct conformations with different structural requirements for MAdCAM-1 binding.
EXPERIMENTAL PROCEDURES
Cells and Reagents
cDNAs of human α4 and β7 were constructed in vector pcDNA3.1/Hygro(−) (Invitrogen). cDNA of mouse talin head domain (talin 1–435) with N-terminal-fused GFP (GFP-talin-head) in vector pcDNA3.1 was kindly provided by Dr. Minsoo Kim. All constructs were confirmed by DNA sequencing. Transient transfection of 293T cells was performed as described (14). 293T cells stably expressing integrin α4β7 were established by co-transfection of human α4/β7 cDNAs and selected by hygromycin (0.2 mg/ml). The expression level of integrin α4β7 was determined by immunofluorescence flow cytometry. Act-1 mAb specific for integrin α4β7 was as described previously (33). Recombinant human SDF-1α was from R&D Systems Inc.
Isolation of Peripheral Blood Lymphocytes (PBLs)
Peripheral venous blood from normal donors was collected using anticoagulant citrate dextrose as an anticoagulant. Human peripheral blood mononuclear cells were isolated from buffy coats using a Ficoll density gradient, washed, and suspended in RPMI 1640 (Invitrogen), supplemented with 100 μg/ml penicillin, 100 μg/ml streptomycin, 2 mm glutamine (all from Invitrogen), and 10% (v/v) FBS (Biocherom AG, Germany). Monocytes were depleted by incubation on tissue culture plastic for 30 min at 37 °C. The lymphocytes were rich in the supernatant fluid.
HuMAdCAM-1-Fc Cloning and Expression
Human MAdCAM-1 (from Val-1 to Pro-315) fused to the Fc1 and Fc2 regions of human IgG1 (huMAdCAM-1/Fc) was constructed in vector pcDNA3.0 (Invitrogen). Site-directed mutations were introduced into MAdCAM-1 cDNA using QuikChange (Stratagene). 293T cells were transiently transfected with huMAdCAM-1/Fc construct and cultured for 3–4 days in a humidified atmosphere of 5% (v/v) CO2 at 37 °C. The huMAdCAM-1/Fc was isolated from conditioned medium by protein A-Sepharose (Pierce) and further purified by gel filtration in 20 mm HEPES, 300 mm NaCl, pH 7.4 (HEPES-buffered saline).
Flow Chamber Assay
The flow chamber assay was performed as described (25). A polystyrene Petri dish was coated with a 5-mm diameter, 20-μl spot of 10 μg/ml purified huMAdCAM-1/Fc in coating buffer (PBS, 10 mm NaHCO3, pH 9.0) for 1 h at 37 °C followed by 2% BSA in coating buffer for 1 h at 37 °C to block nonspecific binding sites. Cells were washed twice with Ca2+- + Mg2+-free HEPES-buffered saline (20 mm Hepes, pH 7.4, 5 mm EDTA, 0.5% BSA), resuspended at 1 × 107/ml in buffer A (Ca2+- + Mg2+-free HEPES-buffered saline, 0.5% BSA), and kept at room temperature. Cells were diluted to 1 × 106/ml in buffer A containing different divalent cations immediately before infusion in the flow chamber using a Harvard apparatus programmable syringe pump. Cells were allowed to accumulate for 30 s at 0.3 dyne/cm2 and 10 s at 0.4 dyne/cm2. Then, shear stress was increased every 10 s from 1 dyne/cm2 up to 16 dynes/cm2 in 2-fold increments. The number of cells remaining bound at the end of each 10-s interval was determined. Rolling velocity at each shear stress was calculated from the average distance traveled by rolling cells in 3 s. To avoid confusing rolling with small amounts of movement due to tether stretching or measurement error, a velocity of 2 μm/s, which corresponds to a movement of one-half cell diameter during the 3-s measurement interval, was the minimum velocity required to define a cell as rolling instead of firmly adherent.
SDF-1α Stimulation Assay under Flow
A polystyrene Petri dish was coated with a 5-mm diameter, 20-μl spot of 10 μg/ml purified huMAdCAM-1/Fc with SDF-1α (2 μg/ml) or huMAdCAM-1/Fc alone in coating buffer for 1 h at 37 °C followed by 2% BSA in coating buffer for 1 h at 37 °C to block nonspecific binding sites. The PBLs (1 × 106/ml) were infused into the chamber, and then cells were allowed to settle for 2 min and to accumulate for 30 s at 0.3 dyne/cm2 and 10 s at 0.4 dyne/cm2. Then, shear stress was increased every 10 s from 1 dyne/cm2 up to 16 dynes/cm2, in 2-fold increments. Cells remaining bound under the wall shear stress of 1 dyne/cm2 were counted. The PBLs (1 × 106/ml) preincubated with α4β7-blocking mAb Act-1 (2 μg/ml) or treated with 5 mm EDTA were used as control.
RESULTS
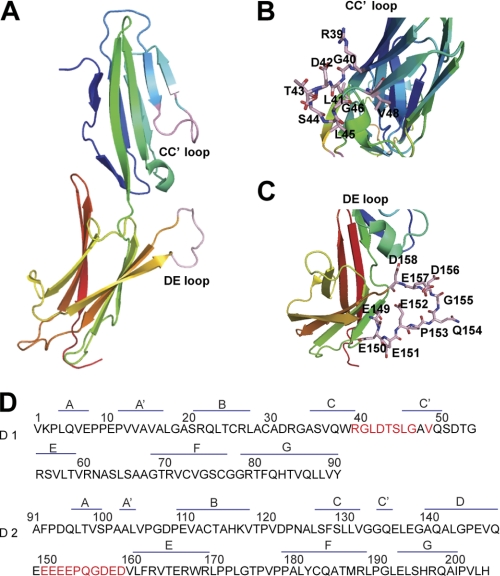
To study the molecular basis for the recognition of MAdCAM-1 by low- and high-affinity integrin α4β7, we generated soluble human MAdCAM-1 protein (from Val-1 to Pro-315), which contains domain 1, domain 2, and mucin-like domain with C-terminal fused Fc1 and two regions of human IgG1. Because the CC′ loop in D1 of MAdCAM-1 possesses the LDTS motif, which has been implicated as the primary integrin-binding site, we first investigated the function of the CC′ loop in the binding of low- and high-affinity integrin α4β7 to MAdCAM-1 by introducing a series of single point mutations in the CC′ loop based on the MAdCAM-1 crystal structure (Fig. 1) (10, 11).
FIGURE 1.
Structure and sequence of human MAdCAM-1 D1/D2. A, the N-terminal two-domain fragment of human MAdCAM-1 (Protein Data Bank (PDB) 1gsm) (11). The CC′ and DE loops reside in D1 and D2, respectively. B and C, the detailed view of the CC′ and DE loops. The amino acid residues in the CC′ and in the DE loops were shown in B and C, respectively. D, amino acid sequences of human MAdCAM-1 D1 and D2. The residues chosen for single amino acid substitution by Ala are highlighted in red.
Asp-42 in CC′ Loop of MAdCAM-1 D1 Is Essential for MAdCAM-1 Binding to Both Low-affinity and High-affinity α4β7
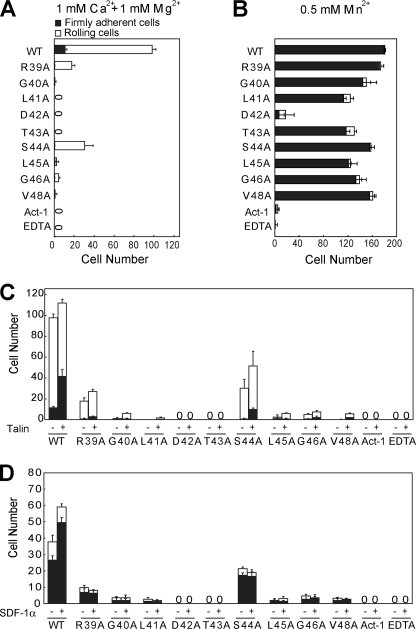
Adhesive behavior of 293T cells stably expressing human integrin α4β7 in shear flow was characterized in a parallel wall flow chamber by allowing them to adhere to MAdCAM-1 adsorbed to the lower wall. The shear stress was incrementally increased, and the velocity of the cells remaining bound at each increment was determined. Human α4β7 293T transfectants behaved as described previously for lymphoid cells expressing α4β7 (26). In 1 mm Ca2+ + 1 mm Mg2+, about 90% of the bound α4β7 transfectants rolled at the shear stress of 1 dyne/cm2 (Fig. 2A). By contrast, cells were firmly adherent in 0.5 mm Mn2+ (Fig. 2B). Rolling and firm adhesion represent the low- and high-affinity interactions of α4β7 with MAdCAM-1, respectively. As control, α4β7transfectants treated with α4β7 blocking antibody Act-1 or with 5 mm EDTA did not accumulate on MAdCAM-1 substrates (Fig. 2, A and B). In contrast to the robust cell adhesion on WT MAdCAM-1, substitution of Asp-42 with Ala abolished both rolling and firm adhesion on MAdCAM-1 mediated by the low-affinity α4β7 in 1 mm Ca2+ + 1 mm Mg2+ and high-affinity α4β7 in 0.5 mm Mn2+, suggesting its essential role in integrin-ligand binding (Fig. 2, A and B).
FIGURE 2.
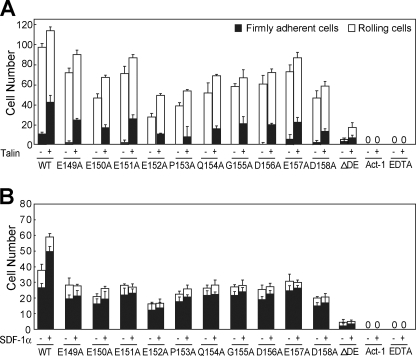
Effect of the CC′ loop point mutations on α4β7-mediated cell adhesion on MAdCAM-1 substrates in shear flow. A–C, rolling and firm adhesion on MAdCAM-1 substrates of α4β7 293T stable transfectants in 1 mm Ca2+ + 1 mm Mg2+ (A), in 0.5 mm Mn2+ (B), or before and after transfection with GFP-talin-head in 1 mm Ca2+ + 1 mm Mg2+ (C). D, adhesive modality of human PBLs on MAdCAM-1 substrates in 1 mm Ca2+ + 1 mm Mg2+ before and after stimulation with SDF-1α. The number of rolling and firmly adherent cells was measured in the indicated divalent cations at a wall shear stress of 1 dyne/cm2. The experiment was performed on the surface coated with WT or mutant MAdCAM-1 (10 μg/ml). The cells preincubated with the α4β7 blocking mAb Act-1 (2 μg/ml) at 37 °C for 5 min or treated with 5 mm EDTA were used as control. Error bars are ±S.D. (n = 3). Representative videos are shown as supplemental Videos S1–S5.
In Addition to Asp-42, other CC′ Loop Residues Except for Arg-39 and Ser-44 Are Essential for MAdCAM-1 Binding to Low-affinity α4β7
When compared with the efficient rolling cell adhesion on WT MAdCAM-1, single amino acid substitution of most residues in the MAdCAM-1 CC′ loop with Ala abolished the rolling cell adhesion on MAdCAM-1 mediated by low-affinity α4β7 in 1 mm Ca2+ + 1 mm Mg2+ (Fig. 2A). Arg-39 and Ser-44 to Ala mutations showed less effect, which led to 70 and 80% decrease of cell adhesion to MAdCAM-1, respectively.
CC′ Loop Residues Other than Asp-42 Are Not Important for MAdCAM-1 Binding to High-affinity α4β7 Activated by Mn2+
In contrast to rolling cell adhesion mediated by low-affinity α4β7, the firm cell adhesion mediated by Mn2+-activated α4β7 was only slightly affected by the same mutations, except for Asp-42 (Fig. 2B). More than 70% of cell adhesion was retained when Leu-41, Thr-43, Leu-45, and Gly-46 were mutated to Ala, respectively. The rest of the mutations only caused less than 10% loss of cell adhesion in Mn2+. Thus, except for the primary MAdCAM-1-α4β7-binding site Asp-42, the rest of the CC′ residues other than Arg-39 and Ser-44 in the CC′ loop are essential for the interaction between low-affinity α4β7 and MAdCAM-1, but not required for the binding of Mn2+-activated α4β7 to MAdCAM-1.
CC′ Loop Residues Other than Arg-39 and Ser-44 Are Crucial for MAdCAM-1 Interaction with High-affinity Integrin α4β7 Activated by Talin or SDF-1α
Besides the unphysiological strong activation by Mn2+, integrin can be activated by more physiological pathways such as overexpression of intracellular talin or SDF-1α stimulation (34). Talin is a cytoskeletal protein that can interact with the cytoplasmic tail of the integrin β subunit and activate integrin. To investigate the influence of the CC′ loop mutations on the interaction between MAdCAM-1 and talin-activated α4β7, we overexpressed the GFP-talin-head in α4β7 293T transfectants (supplemental Fig. S1) and examined the cell adhesion behavior to WT and mutant MAdCAM-1 under flow (Fig. 2C). The firmly adherent α4β7 transfectants on WT MAdCAM-1 increased from 11 to 37% of total bound cells under the shear stress of 1 dyne/cm2 after co-transfection with GFP-talin-head, suggesting the activation of integrin α4β7 by talin. Surprisingly, unlike the mild effects of the CC′ mutations on the cell adhesion mediated by Mn2+-activated α4β7 to MAdCAM-1, the cell adhesion mediated by talin-activated α4β7 was greatly disrupted by most CC′ loop mutations. GFP-talin-head overexpression only slightly increased cell adhesion on R39A, G40A, S44A, L45A, G46A, and V48A mutants. R39A and S44A showed less effect, which led to 76 and 54% decrease of cell adhesion mediated by talin-activated α4β7 to MAdCAM-1. Thus, the above results suggest that the CC′ loop residues other than Arg-39 and Ser-44 are crucial for the recognition of MAdCAM-1 by talin-activated α4β7 and that integrin α4β7 activated by Mn2+ and talin could have different conformations with different structural requirements for MAdCAM-1 binding.
Because the CC′ loop might play different roles in MAdCAM-1 interaction with Mn2+- and talin-activated α4β7, we next tested its function in the interaction between MAdCAM-1 and α4β7 activated by SDF-1α. SDF-1α can induce integrin activation through the PI3 kinase pathway by binding to CXCR4, the G protein-coupled receptor of SDF-1α (35, 36). Human PBLs were used that express high levels of integrin α4β7 (37) and CXCR4 (38). In contrast to the robust cell adhesion to WT MAdCAM-1 in 1 mm Ca2+ + 1 mm Mg2+, PBL adhered weakly to most CC′ loop mutants and did not adhere to D42A and T43A mutants at all (Fig. 2D). R39A and S44A mutations showed less effect, which led to 86 and 68% decrease of cell adhesion to MAdCAM-1, respectively. Activation of α4β7 by SDF1-α stimulation notably increased the number of PBLs bound to WT MAdCAM-1, but not to the MAdCAM-1 CC′ loop mutants, suggesting the importance of the CC′ loop residues in the interaction between MAdCAM-1 and α4β7 activated by SDF1-α (Fig. 2D). Interestingly, cell adhesion to the MAdCAM-1 CC′ loop mutants could be increased by talin but not SDF1-α, indicating the subtle difference between integrins activated by talin and SDF-1α (Fig. 2, C and D).
Taken together, the above data demonstrate that Asp-42 in the CC′ loop is essential for MAdCAM-1 binding to both low-affinity and high-affinity α4β7, and the rest of the CC′ loop residues other than Arg-39 and Ser-44 are crucial for MAdCAM-1 binding to α4β7 activated by physiological stimuli as SDF-1α or talin, but not required for MAdCAM-1 binding to α4β7 activated by Mn2+. Thus, integrins activated by distinct stimuli might have different high-affinity conformations with diverse structural requirements for MAdCAM-1 binding.
CC′ Loop Is Required for the Stable Interaction between MAdCAM-1 and Low-affinity Integrin α4β7
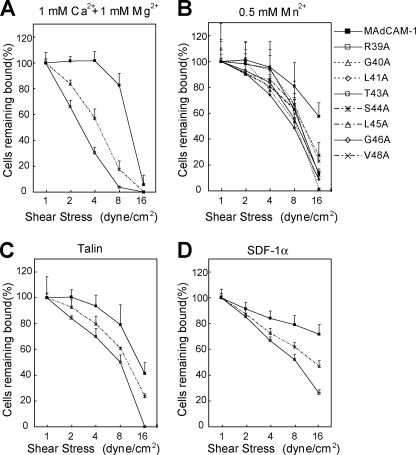
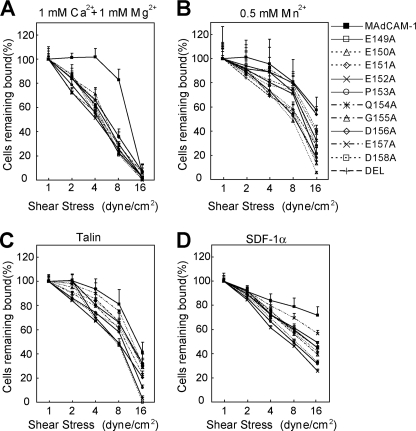
To test the effect of the CC′ loop on the strength of α4β7-mediated cell adhesion to MAdCAM-1, we examined the resistance of adherent cells to detachment by increasing shear stress (Fig. 3). MAdCAM-1 R39A and S44A mutants were chosen for studies on low-affinity α4β7 in Ca2+ + Mg2+ and high-affinity α4β7 activated by talin or SDF-1α because they were the only CC′ loop mutants supporting clearly detectable cell adhesion under those conditions. In 1 mm Ca2+ + 1 mm Mg2+, α4β7 293T transfectants detached more rapidly from the R39A and S44A mutants than from the WT MAdCAM-1 (Fig. 3A), suggesting the less stable interaction between integrin and the mutant ligands. In contrast, adhesion of cells bearing high-affinity α4β7 activated by Mn2+, talin, or SDF-1α to the MAdCAM-1 CC′ loop mutants was much less susceptible to the increased shear stress (Fig. 3, B–D).
FIGURE 3.
Effect of the CC′ loop mutation on the strength of α4β7-mediated adhesion to MAdCAM-1. A–C, resistance to detachment of α4β7 293T transfectants at increasing wall shear stresses in 1 mm Ca2+ + 1 mm Mg2+ (A), in 0.5 mm Mn2+ (B), or in 1 mm Ca2+ + 1 mm Mg2+ after transfection with GFP-talin-head (C). D, resistance to detachment of human PBL stimulated with SDF-1α in 1 mm Ca2+ + 1 mm Mg2+. The total number of cells remaining bound at each indicated wall shear stress was determined as a percentage of adherent cells at 1 dyne/cm2. The experiment was performed on the surface coated with WT or mutant MAdCAM-1 (10 μg/ml). Error bars are ±S.D. (n = 3).
The Intact DE Loop Is Essential for MAdCAM-1 Binding to Low-affinity α4β7, but Not for Its Binding to High-affinity α4β7 Activated by Mn2+
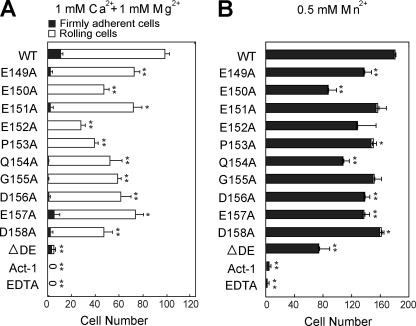
To further define the different structural requirements for MAdCAM-1 binding to inactive and activated integrin α4β7, we generated a series of single amino acid substitutions and a partial deletion (ΔDE, from Glu-152 to Asp-158) of the DE loop in MAdCAM-1 D2. MAdCAM-1 with deletion of the whole DE loop (from Glu-149 to Asp-158) could not be expressed. All of the single amino acid substitutions by Ala in the DE loop (residue 149–158) decreased the cell adhesion mediated by both low-affinity and high-affinity α4β7 on MAdCAM-1, but to a different extent (Fig. 4A). For the rolling cell adhesion mediated by low-affinity α4β7 on MAdCAM-1 in 1 mm Ca2+ + 1 mm Mg2+, substitution of Glu-152 and Glu-153 by Ala led to an ∼70 and 60% decrease of adherent cells, respectively. E150A, E154A, and D158A showed less effect, which resulted in an ∼50% decrease (Fig. 4A). Other point mutations had even milder impact, retaining from 60 to 80% of adherent cells. For the firm cell adhesion mediated by high-affinity α4β7 in Mn2+, most DE loop point mutations showed much milder effects, except for Glu-150 and Glu-154, which led to 50 and 40% decrease of bound cells, respectively (Fig. 4B). Notably, the DE loop residues that mostly affected MAdCAM-1 interaction with low-affinity and high-affinity α4β7 are different, suggesting the different roles of the DE loop residues in the recognition of integrin α4β7 before and after activation. Different from single amino acid substitutions, the partial deletion of the DE loop abolished the interaction between low-affinity α4β7 and MAdCAM-1, but only caused about 60% loss of cell adhesion to MAdCAM-1 mediated by the Mn2+-activated α4β7. The data demonstrate that the intact DE loop is essential to MAdCAM-1 binding to low-affinity α4β7, but not to high-affinity α4β7 activated by Mn2+.
FIGURE 4.
Effect of the DE loop point mutations on α4β7-mediated cell adhesion on MAdCAM-1 substrates in shear flow. A and B, rolling and firm adhesion on MAdCAM-1 substrates of α4β7 293T transfectants in 1 mm Ca2+ + 1 mm Mg2+ (A) and 0.5 mm Mn2+ (B). The number of rolling and firmly adherent α4β7 293T transfectants was measured in the indicated divalent cations at a wall shear stress of 1 dyne/cm2. The experiment was performed on the surface coated with WT or mutant MAdCAM-1 (10 μg/ml). The cells preincubated with the α4β7 blocking mAb Act-1 (2 μg/ml) at 37 °C for 5 min or treated with 5 mm EDTA were used as control. Error bars are ±S.D. (n = 3). *, p < 0.05, **, p < 0.01, when compared with MAdCAM-1 WT.
The Intact DE Loop Is Required for MAdCAM-1 Binding to Integrin α4β7 Activated by Talin and SDF-1α
To further study the function of the DE loop in MACAM-1 binding to activated α4β7, we examined the impact of DE loop deletion on the interaction between MAdCAM-1 and α4β7 activated by talin and SDF-1α. Opposite to the partial rescued cell adhesion to ΔDE MAdCAM-1 after α4β7 was activated by Mn2+, the activation of α4β7 by either talin or SDF-1α did not rescue the abolishment of cell adhesion by DE loop deletion in 1 mm Ca2+ + 1 mm Mg2+ (Fig. 5). Thus, the intact DE loop is important for MAdCAM-1 interaction with both low-affinity and high-affinity integrin α4β7 activated by talin or SDF-1α. On the other hand, α4β7 activated by Mn2+ could support decent cell adhesion to MAdCAM-1 in the absence of the intact DE loop, suggesting that the conformation of Mn2+-activated α4β7 may be different from those of the low-affinity and high-affinity α4β7 activated by more physiological stimuli. The overexpression of GFP-talin-head augmented the firm adhesion of α4β7 293T transfectants on both WT and DE loop single residue mutant MAdCAM-1 (Fig. 5A). In contrast, SDF-1α stimulation increased the PBL adhesion only to the WT MAdCAM-1, but not to the DE loop mutants (Fig. 5B). These data suggest that the residues in the DE loop might be involved in distinguishing the subtle difference between α4β7 activated by talin and α4β7 activated by SDF-1α.
FIGURE 5.
Effect of DE loop mutations on MAdCAM-1 binding to integrin α4β7 activated by talin or SDF-1α. A, rolling and firm adhesion on MAdCAM-1 substrates of α4β7 293T transfectants before and after transfection with GFP-talin-head. B, rolling and firm adhesion on MAdCAM-1 substrates of human PBLs before and after stimulation with SDF-1α. The number of rolling and firmly adherent cells was measured at a wall shear stress of 1 dyne/cm2. The experiment was performed on the surface coated with WT or mutant MAdCAM-1 (10 μg/ml). The cells preincubated with the α4β7 blocking mAb Act-1 (2 μg/ml) at 37 °C for 5 min or treated with 5 mm EDTA were used as control. Error bars are ±S.D. (n = 3).
The DE Loop Is Required for the Stable Interaction between MAdCAM-1 and Low-affinity Integrin α4β7
Next, we investigated the function of the DE loop in the strength of α4β7-mediated cell adhesion to MAdCAM-1 (Fig. 6). In 1 mm Ca2+ + 1 mm Mg2+, the DE loop mutations significantly decreased the shear resistance of adherent cells bearing low-affinity α4β7 (Fig. 6A). In contrast, the same mutations showed little effect on the stability of adhesion mediated by high-affinity α4β7 activated by Mn2+, talin, or SDF-1α (Fig. 6, B–D). Thus, the residues in the DE loop of MAdCAM-1 are important for stabilizing the interaction between low-affinity α4β7 and MAdCAM-1.
FIGURE 6.
Effect of the DE loop on the strength of α4β7-mediated adhesion to MAdCAM-1. A–C, resistance to detachment of α4β7 293T transfectants at increasing wall shear stresses in 1 mm Ca2+ + 1 mm Mg2+ (A), in 0.5 mm Mn2+ (B), or in 1 mm Ca2+ + 1 mm Mg2+ after transfection with GFP-talin-head (C). D, resistance to detachment of human PBL stimulated with SDF-1α. The total number of cells remaining rolling and firmly adherent at increasing wall shear stress was determined as a percentage of adherent cells at 1 dyne/cm2. The experiment was performed on the surface coated with WT or mutant MAdCAM-1 (10 μg/ml). Error bars are ±S.D. (n = 3).
DISCUSSION
Lymphocyte homing to gut is dependent on the interaction between integrin α4β7 and MAdCAM-1. The resting (low-affinity) and activated (high-affinity) integrin α4β7 can mediate rolling and firm adhesion of lymphocytes, respectively, which are two of the critical steps in lymphocyte homing. Previous studies have shown that integrin undergoes global and local conformational changes upon activation, resulting in the distinct conformations of low-affinity and high-affinity integrins. Thus, it is tempting to speculate that the low-affinity and high-affinity α4β7 binds MAdCAM-1 differently, which might play a fundamental role in supporting the rolling and firm cell adhesion. The integrin α4β7-MAdCAM-1 interaction is dependent on a conserved acidic peptide motif in the first Ig-like domain of MAdCAM-1, which is present as a surface-exposed structure. The Asp-42 in this motif forms the primary interaction with the divalent cation at β7 MIDAS. Because the primary interaction between Asp-42 and the MIDAS metal ion is shared by both low-affinity and high-affinity α4β7-MAdCAM-1 binding, there should be other interactions between MAdCAM-1 and α4β7 that determine rolling or firm adhesion.
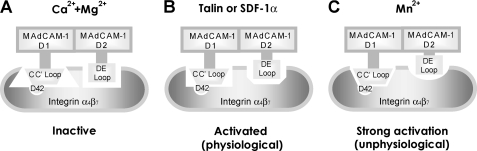
Although previous studies have revealed that some residues in MAdCAM-1 are important for MAdCAM-1-α4β7 binding (9, 12, 13, 15), the structural basis for supporting MAdCAM-1-α4β7-mediated rolling and firm cell adhesion remains elusive because the static cell adhesion assay used in those studies is unable to distinguish rolling and firm cell adhesion. In this study, we used a flow chamber assay to screen the critical residues in MAdCAM-1, which are important for supporting rolling and firm cell adhesions, respectively. Our results demonstrate that the CC′ and DE loops play distinct roles in the recognition of MAdCAM-1 by low- and high-affinity α4β7 and suggest that the inactive α4β7 and α4β7 activated by different stimuli have distinct conformations with different structural requirements for MAdCAM-1 binding (Fig. 7).
FIGURE 7.
Schematic illustration of distinct interactions between MAdCAM-1 and integrin α4β7 at different activation states. Integrin α4β7 at different activation states has distinct binding interfaces for the MAdCAM-1 CC′ and DE loops. A, binding of MAdCAM-1 to low-affinity (inactive) integrin α4β7. B, binding of MAdCAM-1 to high-affinity integrin α4β7 activated by physiological stimuli (talin or SDF-1α). C, binding of MAdCAM-1 to high-affinity integrin α4β7 activated by Mn2+.
The Asp-42 in the CC′ loop is required for both low-affinity α4β7-mediated rolling adhesion and high-affinity α4β7-mediated firm adhesion. In addition to Asp-42, most other CC′ loop residues other than Arg-39 and Ser-44 are essential for the low-affinity α4β7-MAdCAM-1 interaction, suggesting the potential binding sites at the CC′ loop for low-affinity α4β7. In contrast, the same CC′ loop mutations only slightly decreased cell adhesion mediated by Mn2+-activated α4β7, suggesting the different interactions between MAdCAM-1 and Mn2+-activated integrin other than with low-affinity integrin. The reasons that make the CC′ loop subsidiary in supporting α4β7-MAdCAM-1 binding could be due to the stronger interaction between Asp-42 and Mn2+ at MIDAS of β7, and/or there could be additional interactions formed between Mn2+-activated α4β7 and MAdCAM-1.
The DE loop in D2 of MAdCAM-1 is another α4β7 binding interface. Our results showed that the DE loop was less important than the CC′ loop in supporting MAdCAM-1 binding to low-affinity α4β7. Only the removal of the DE loop abolished the interaction between MAdCAM-1 and low-affinity α4β7. Thus, the major function of the DE loop could be to stabilize the interaction between MAdCAM-1 and α4β7, especially the low-affinity integrin. The DE loop is a long and flexible loop exposed on the MAdCAM-1 surface, and its orientation and conformation should be crucial to the MAdCAM-1-α4β7 interaction. Among all of the DE loop single amino acid substitution mutations tested, E152A resulted in the maximal decrease of MAdCAM-1 binding to the low-affinity or talin or SDF-1α-activated α4β7. The MAdCAM-1 crystal structure revealed that the side chain of Glu-152 faces inside the DE loop and protrudes to Glu-158 (9, 10). The Glu-152 side-chain oxygen could form a hydrogen bond with the Asp-158 main-chain oxygen, which might be important to maintain the proper conformation of the DE loop. E152A mutation could lead to loss of this hydrogen bond, which disrupts the optimal conformation of the DE loop for low-affinity α4β7 binding.
Asp-150 is another important residue in the DE loop. E150A mutation decreased the firm cell adhesion mediated by Mn2+-activated α4β7 to the similar level of the DE loop deletion mutant, suggesting its critical position in maintaining the DE loop function. The crystal structure of human MAdCAM-1 showed that Glu-150 could form a hydrogen bond with the Gln-147, which might be important for the control of correct DE loop orientation. E150A mutation could eliminate this hydrogen bond and lead to the rearrangement of DE loop orientation, thus affecting the MAdCAM-1-α4β7 interaction (11).
Another notable finding of our study is the distinct structural requirements for MAdCAM-1 binding to α4β7 activated by Mn2+ and more physiological stimuli, such as talin and SDF-1α. In our study, we found that the CC′ loop and the intact DE loop were crucial for MAdCAM-1 binding to integrin α4β7 activated by talin and SDF-1α; however, the MAdCAM-1 binding to α4β7 activated by Mn2+ was mostly dependent on Asp-42 and only partially affected by some CC′ and DE loop mutations. Thus, the high-affinity α4β7 activated by Mn2+ should have distinct conformation, at least at the ligand-binding interface, than α4β7 activated by talin or SDF-1α through the inside-out signaling.
Acknowledgments
We thank Dr. Minsoo Kim (University of Rochester Medical Center) for kindly providing GFP-talin-head and Dr. Jianping Ding and Dr. Xianchi Dong for discussion.
This work was supported by grants from the National Basic Research Program of China (Grant 2010CB529703), National Natural Science Foundation of China (Grants 30700119 and 30970604), the Chinese Academy of Sciences (Grant KSCX2-YW-R-67), and the Shanghai Pujiang Program (Grant 08PJ14106).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1 and supplemental Videos S1–S5.
- MAdCAM-1
- mucosal cell adhesion molecule-1
- MIDAS
- metal ion-dependent adhesion site
- ADMIDAS
- adjacent to MIDAS
- PBL
- peripheral blood lymphocyte
- hu
- human.
REFERENCES
- 1. Springer T. A. (1994) Cell 76, 301–314 [DOI] [PubMed] [Google Scholar]
- 2. Butcher E. C. (1991) Cell 67, 1033–1036 [DOI] [PubMed] [Google Scholar]
- 3. Butcher E. C., Picker L. J. (1996) Science 272, 60–66 [DOI] [PubMed] [Google Scholar]
- 4. Gorfu G., Rivera-Nieves J., Ley K. (2009) Curr. Mol. Med. 9, 836–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Briskin M. J., McEvoy L. M., Butcher E. C. (1993) Nature 363, 461–464 [DOI] [PubMed] [Google Scholar]
- 6. Adams D. H., Eksteen B. (2006) Nat. Rev. Immunol. 6, 244–251 [DOI] [PubMed] [Google Scholar]
- 7. Eksteen B., Liaskou E., Adams D. H. (2008) Inflamm. Bowel Dis. 14, 1298–1312 [DOI] [PubMed] [Google Scholar]
- 8. Shyjan A. M., Bertagnolli M., Kenney C. J., Briskin M. J. (1996) J. Immunol. 156, 2851–2857 [PubMed] [Google Scholar]
- 9. Green N., Rosebrook J., Cochran N., Tan K., Wang J. H., Springer T. A., Briskin M. J. (1999) Cell Adhes. Commun. 7, 167–181 [DOI] [PubMed] [Google Scholar]
- 10. Tan K., Casasnovas J. M., Liu J. H., Briskin M. J., Springer T. A., Wang J. H. (1998) Structure 6, 793–801 [DOI] [PubMed] [Google Scholar]
- 11. Dando J., Wilkinson K. W., Ortlepp S., King D. J., Brady R. L. (2002) Acta Crystallogr. D Biol. Crystallogr. 58, 233–241 [DOI] [PubMed] [Google Scholar]
- 12. Viney J. L., Jones S., Chiu H. H., Lagrimas B., Renz M. E., Presta L. G., Jackson D., Hillan K. J., Lew S., Fong S. (1996) J. Immunol. 157, 2488–2497 [PubMed] [Google Scholar]
- 13. Newham P., Craig S. E., Seddon G. N., Schofield N. R., Rees A., Edwards R. M., Jones E. Y., Humphries M. J. (1997) J. Biol. Chem. 272, 19429–19440 [DOI] [PubMed] [Google Scholar]
- 14. Chen J., Salas A., Springer T. A. (2003) Nat. Struct. Biol. 10, 995–1001 [DOI] [PubMed] [Google Scholar]
- 15. Briskin M. J., Rott L., Butcher E. C. (1996) J. Immunol. 156, 719–726 [PubMed] [Google Scholar]
- 16. Hynes R. O. (2002) Cell 110, 673–687 [DOI] [PubMed] [Google Scholar]
- 17. Luo B. H., Carman C. V., Springer T. A. (2007) Annu. Rev. Immunol. 25, 619–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Springer T. A., Wang J. H. (2004) Adv. Protein Chem. 68, 29–63 [DOI] [PubMed] [Google Scholar]
- 19. Arnaout M. A., Goodman S. L., Xiong J. P. (2007) Curr. Opin. Cell Biol. 19, 495–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xiao T., Takagi J., Coller B. S., Wang J. H., Springer T. A. (2004) Nature 432, 59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Luo B. H., Springer T. A. (2006) Curr. Opin. Cell Biol. 18, 579–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arnaout M. A., Mahalingam B., Xiong J. P. (2005) Annu. Rev. Cell Dev. Biol. 21, 381–410 [DOI] [PubMed] [Google Scholar]
- 23. Carman C. V., Springer T. A. (2003) Curr. Opin. Cell Biol. 15, 547–556 [DOI] [PubMed] [Google Scholar]
- 24. Leitinger B., McDowall A., Stanley P., Hogg N. (2000) Biochim. Biophys. Acta 1498, 91–98 [DOI] [PubMed] [Google Scholar]
- 25. Chen J., Takagi J., Xie C., Xiao T., Luo B. H., Springer T. A. (2004) J. Biol. Chem. 279, 55556–55561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. de Château M., Chen S., Salas A., Springer T. A. (2001) Biochemistry 40, 13972–13979 [DOI] [PubMed] [Google Scholar]
- 27. Zhu J., Luo B. H., Xiao T., Zhang C., Nishida N., Springer T. A. (2008) Mol Cell 32, 849–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xiong J. P., Mahalingham B., Alonso J. L., Borrelli L. A., Rui X., Anand S., Hyman B. T., Rysiok T., Müller-Pompalla D., Goodman S. L., Arnaout M. A. (2009) J. Cell Biol. 186, 589–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee J. O., Rieu P., Arnaout M. A., Liddington R. (1995) Cell 80, 631–638 [DOI] [PubMed] [Google Scholar]
- 30. Chen J., Yang W., Kim M., Carman C. V., Springer T. A. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 13062–13067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Valdramidou D., Humphries M. J., Mould A. P. (2008) J. Biol. Chem. 283, 32704–32714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pan Y., Zhang K., Qi J., Yue J., Springer T. A., Chen J. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 21388–21393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tidswell M., Pachynski R., Wu S. W., Qiu S. Q., Dunham E., Cochran N., Briskin M. J., Kilshaw P. J., Lazarovits A. I., Andrew D. P., Butcher E. C., Yednock T. A., Erle D. J. (1997) J. Immunol. 159, 1497–1505 [PubMed] [Google Scholar]
- 34. Calderwood D. A., Zent R., Grant R., Rees D. J., Hynes R. O., Ginsberg M. H. (1999) J. Biol. Chem. 274, 28071–28074 [DOI] [PubMed] [Google Scholar]
- 35. Kunkel E. J., Butcher E. C. (2002) Immunity 16, 1–4 [DOI] [PubMed] [Google Scholar]
- 36. Wright N., Hidalgo A., Rodríguez-Frade J. M., Soriano S. F., Mellado M., Parmo-Cabañas M., Briskin M. J., Teixidó J. (2002) J. Immunol. 168, 5268–5277 [DOI] [PubMed] [Google Scholar]
- 37. Erle D. J., Briskin M. J., Butcher E. C., Garcia-Pardo A., Lazarovits A. I., Tidswell M. (1994) J. Immunol. 153, 517–528 [PubMed] [Google Scholar]
- 38. Bleul C. C., Wu L., Hoxie J. A., Springer T. A., Mackay C. R. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 1925–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]