Abstract
The impermeant nature of the intestinal barrier is maintained by tight junctions (TJs) formed between adjacent intestinal epithelial cells. Disruption of TJs and loss of barrier function are associated with a number of gastrointestinal diseases, including neonatal necrotizing enterocolitis (NEC), the leading cause of death from gastrointestinal diseases in preterm infants. Human milk is protective against NEC, and the human milk factor erythropoietin (Epo) has been shown to protect endothelial cell-cell and blood-brain barriers. We hypothesized that Epo may also protect intestinal epithelial barriers, thereby lowering the incidence of NEC. Our data demonstrate that Epo protects enterocyte barrier function by supporting expression of the TJ protein ZO-1. As immaturity is a key factor in NEC, Epo regulation of ZO-1 in the human fetal immature H4 intestinal epithelial cell line was examined and demonstrated Epo-stimulated ZO-1 expression in a dose-dependent manner through the PI3K/Akt pathway. In a rat NEC model, oral administration of Epo lowered the incidence of NEC from 45 to 23% with statistical significance. In addition, Epo treatment protected intestinal barrier function and prevented loss of ZO-1 at the TJs in vivo. These effects were associated with elevated Akt phosphorylation in the intestine. This study reveals a novel role of Epo in the regulation of intestinal epithelial TJs and barrier function and suggests the possible use of enteral Epo as a therapeutic agent for gut diseases.
Keywords: Akt PKB, Interferon, Intestine, Rat, Tight Junction, T84, ZO-1, Erythropoietin, Gastrointestinal, Necrotizing Enterocolitis
Introduction
Intestinal impermeability is regulated by tight junctions (TJs)2 formed between intestinal epithelial cells (IEC) at the most apical areas of the epithelium. Tight junctions serve to establish size- and charge-selective barriers between the gut lumen and the mucosa to control the diffusion of solutes, water, and electrolytes while also serving a key role in host defense to confine toxins, allergens, and pathogens to the intestinal lumen. Disruption of TJs is associated with a number of gastrointestinal diseases, including neonatal necrotizing enterocolitis (NEC). NEC is the leading cause of death from gastrointestinal diseases in premature newborns and is characterized by loss of intestinal barrier function (1–3). It has been suggested that impaired intestinal barrier may predispose preterm infants to luminal bacteria invasion and immune system activation in the pathogenesis of NEC.
Tight junctions are composed of trans-membrane proteins, including occludin, claudins, and junctional adhesion molecules, as well as cytoplasmic proteins such as zonula occludens (ZO-1, ZO-2, and ZO-3) (4). These proteins work in concert to form physical connections between epithelial cells and confer basic barrier properties. Zonula occludens-1 (ZO-1), the first TJ protein identified, contains protein-binding domains for interaction with other tight junction-associated proteins, including ZO-2, ZO-3, occludin, β-catenin, paxillin, and talin as well as with the peri-junctional actin ring (5, 6). Therefore, ZO-1 serves as an important linker between the TJ and the actin cytoskeleton and is thought to be a functionally critical tight junction component.
Proinflammatory cytokines are frequently found elevated in intestinal diseases and NEC (7, 8). In addition to immune activation, accumulating evidence indicates that proinflammatory cytokines can induce intestinal TJ barrier disruption. IFN-γ is one of the proinflammatory cytokines found elevated in NEC (7, 8), and its role in the alteration of the intestinal TJ barrier has been well established (9–11). Previous studies have shown that IFN-γ treatment impairs barrier function and decreases the TJ protein ZO-1 expression in polarized human T84 enterocytes (10). This suggests that intestinal integrity could be compromised in disease states through altered TJ protein expression. In fact, in animal models of NEC, those with disease had both impaired intestinal barrier function and aberrant TJ protein expression, and in NEC infants, the significantly increased intestinal permeability is also associated with altered TJ protein expression (12). Thus, agents that can reverse the adverse effects of proinflammatory cytokines on TJ proteins and barrier function are expected to minimize barrier disruption following pathological insults in gastrointestinal diseases.
Human milk is protective against NEC as human milk-fed preterm infants are less susceptible to NEC compared with formula-fed infants (13). Erythropoietin (Epo) is a human milk factor (14). Although first described as a major regulator of erythropoiesis, Epo exhibits additional biological activities, including protection of endothelial cell-cell and blood-brain barriers (15, 16). Functional Epo receptors are present on the luminal side of villi in fetal and neonatal human and rat intestines (17, 18), suggesting physiological roles of Epo in the developing gut. These findings and previous studies showing the importance of an intact intestinal barrier in limiting the progression of gut diseases collectively lead to our hypothesis that Epo may protect intestinal epithelial barrier function, thereby lowering the incidence of NEC.
Our in vitro studies demonstrate that Epo is able to reverse the effect of IFN-γ and protect both TJ protein ZO-1 expression and barrier function. In this study, we also report the protective effects of Epo and reduced NEC incidence in vivo in the immature intestine of an animal model of NEC. The underlying mechanism appears to be mediated through PI3K-dependent activation of Akt. These Epo effects via the PI3K/Akt pathway appear to be specific as oral administration of another human milk factor, transforming growth factor-β (TGF-β), neither activated Akt nor protected intestinal barrier function. This study demonstrates a novel biological function of Epo and suggests a potential use for Epo in gut diseases.
MATERIALS AND METHODS
Cell Cultures and Reagents
In the intestinal epithelial T84 cell line, cells were grown in a 1:1 (v/v) mixture of DMEM and F-12 medium (Invitrogen) supplemented with 10% heat-inactivated fetal calf serum, 1% glutamine, 50 units/ml penicillin, and 50 μg/ml streptomycin. Cells were routinely trypsinized when they reached 50–75% confluence, and cell passages 50–64 were used. H4 cells (a generous gift from Dr. W. Allan Walker, Massachusetts General Hospital, Harvard Medical School) are a human fetal nontransformed primary intestinal epithelial cell line used as a model of immature IEC (19). They were cultured in DMEM with 10% heat-inactivated fetal calf serum, 1% glutamine, 1% sodium pyruvate, 1% amino acids, 1% HEPES, 50 units/ml penicillin, 50 μg/ml streptomycin, and 0.2 unit/ml insulin. Cell passages 11–21 were used. All cells were grown at 37 °C in a 5% CO2 atmosphere. Recombinant human and rat erythropoietin were purchased from R & D Systems (Minneapolis, MN). Recombinant human IFN-γ was from PeproTech (Rocky Hill, NJ). Inhibitors used to inhibit activation of intracellular pathways (IKK inhibitor II and MG262 for the NF-κB pathway, JNK inhibitor II for the JNK pathway, PD 98059 for the ERK pathway, SB 236580 for the p38 pathway, and wortmannin and LY 294002 for the PI3K/Akt pathway) were from Calbiochem.
In Vitro Barrier Function/Paracellular Permeability Assay
T84 human enterocytes (1 × 105 cells/200 μl) were plated on 0.4-μm pore size clear polystyrene Transwells (Costar, Cambridge, MA) in triplicate and fed twice a week. Cells were used when the transepithelial electrical resistance (TER), a measure of TJ integrity, was over 1,000 ohms·cm2 as measured with an epithelial voltohmmeter (World Precision Instruments, Sarasota, FL). IFN-γ was added to the basolateral side only, and Epo was added to both apical and basolateral sides of Transwells. Barrier function was determined at 72 h following treatments by apical to basolateral flux of 10-kDa fluorescein isothiocyanate-labeled dextran (FITC-dextran). FITC-dextran in culture media (1 mg/ml) was added to Transwell inserts (the apical side), and 5 h later, FITC-dextran concentrations in the lower compartment of the Transwell (the basolateral side) were determined using a Synergy 2 fluorometer (BioTek, Winooski, VT) (excitation, 485 nm; emission, 528 nm). An apparent permeability coefficient (Papp) was used as a barrier function index and calculated by the following formula: Papp (cm/s) = P/(A × C0), where P is the permeability rate (mol/s), C0 is the initial concentration (mol/ml or mol/cm3) in the upper chamber, and A is the surface area (cm2) of the monolayer.
RNA Interference
To perform ZO-1 silencing, 50% confluent cells were transiently transfected with 200 nm of a pool of four scrambled control or ZO-1 siRNAs (Dharmacon, Lafayette, CO) using Oligofectamine (Invitrogen) according to the manufacturer's guidelines. Cell lysates were harvested on days 4, 8, and 12 after transfection and subjected to immunoblotting for ZO-1 and β-actin. To determine TER and barrier function under a ZO-1-silencing condition, 5 × 105 T84 cells in 300 μl along with 200 nm control or ZO-1 siRNAs and Oligofectamine were plated in Transwells in triplicate. Media were changed the next morning and then twice a week. In vitro barrier function/paracellular permeability was measured when control siRNA-transfected cells reached a high TER (>1,000 ohms·cm2). Cell lysates were then collected for ZO-1 immunoblotting.
Immunoblot Analysis
Immunoblotting was conducted as described previously (20). For tissue lysate preparation, 2-cm distal intestine (ileum) was homogenized with a hand-held homogenizer (Pellet Pestle, Kimble/Kontes, Vineland, NJ) in lysis buffer (50 mm Tris·HCl (pH 7.4), 150 mm NaCl, 1 mm EDTA, 1% SDS, 50 mm DTT, 50 μg/ml aprotinin, 50 μg/ml leupeptin, and 5 mm PMSF). For cell culture lysate preparation, cells on plates were rinsed with cold PBS and lysed in the same lysis buffer used for tissue lysate preparation. Both tissue and cell lysates were sonicated and then centrifuged at 12,000 rpm for 10 min at 4 °C. Supernatants were collected, and the protein concentration was determined using the BCATM protein assay kit (Pierce). Lysates were resolved by SDS-PAGE and transferred to PVDF membranes. Membranes were blocked in 5% milk PBS-T (0.1% Tween 20 (v/v) in PBS) for 1 h at room temperature and then probed with primary and appropriate secondary antibodies in 5% milk PBS-T. Immunoreactive bands were visualized by chemiluminescence reaction using ECL reagents (Amersham Biosciences) followed by exposure of the membranes to autoradiography film (Midsci, St. Louis, MO). Protein levels were quantified by densitometry using ImageJ software and presented relative to untreated controls from three experiments. The β-catenin and E-cadherin antibodies were purchased from Transduction Laboratories, Inc. (San Diego). The ZO-1, ZO-2, occludin, claudin-1, and claudin-3 antibodies were obtained from Upstate Biotechnology, Inc. (Lake Placid, NY). The actin antibody was from Sigma.
Immunofluorescence Staining of Cells
Cells grown in chamber slides were fixed with 3.7% paraformaldehyde/PBS for 10 min at room temperature and permeabilized with 0.1% Triton X-100 in 1% BSA/PBS for 30 min at room temperature. Cells were blocked with 10% BSA/PBS for 1 h at room temperature and incubated with primary antibodies in 2.5% BSA/PBS overnight at 4 °C and then with an Alexa Fluor 488-conjugated second antibody in 2.5% BSA/PBS for 1 h at room temperature. Nuclei were stained with DAPI (100 ng/ml), and images were acquired by confocal microscopy with Leica SP2.
Immunofluorescence Staining of Tissues
After deparaffinization and rehydration, intestinal sections were blocked in 5% BSA for 1 h at room temperature and then incubated with ZO-1 and occludin antibodies (Zymed Laboratories Inc.) at 1:200 dilution, followed with an Alexa Fluor 488-conjugated second antibody (Molecular Probes, Eugene, OR). Nuclei were labeled with DAPI (100 ng/ml). Images were acquired by confocal microscopy using Leica SP2.
Neonatal Rat NEC Model
All animal studies were reviewed and approved by the Institutional Animal Care and Use Committee. Animal experiments were conducted following a well described rat NEC animal model (21). Neonatal rats from time-dated pregnant Sprague-Dawley dams were delivered by cesarean section at E20 following isoflurane anesthesia. Pups were then stabilized, dried, and maintained in a humidified incubator at 37 °C, and bowel/bladder function was stimulated by a soft cotton-tip applicator. Pups were fed with Esbilac puppy formula every 3 h via an orogastric feeding catheter and stressed under 5% O2 + 95% N2 for 10 min after feeding three times a day to induce NEC. The feeding volume began at 0.1 ml and was increased incrementally up to 0.25 ml. Naturally born and neonatal rats fed from dams were included in experiments as healthy controls. Animals were sacrificed when ill or at the end of the experiment on day 5, and mortality was not used as an end point. Intestine was collected and fixed in 10% buffered formalin overnight for tissue section preparation. H&E-stained intestinal sections were assessed histologically for ileal damage by a pathologist using a previously published NEC scoring system to evaluate the degree of intestinal injury on a “0–4” scale as follows: 0 no histological damage; 1 (mild), slight submucosal and/or lamina propria separation; 2 (moderate), moderate separation of the submucosa and/or lamina propria and/or edema in the submucosa and muscular layers; 3 (severe), severe separation of the submucosa and/or lamina propria and/or severe edema in the submucosa and muscular layers with regional villous sloughing; and 4 (necrosis), loss of villi and necrosis. Scores ≥2 are defined as NEC (22).
In Vivo Intestinal Barrier Function Assay
To investigate intestinal lumen-to-blood permeability/barrier function in the NEC model, an in vivo permeability assay was performed using FITC-dextran as described previously (23). Previous publications have used both 4- and 10-kDa FITC-dextran to determine in vivo gut permeability (24–27). Immature intestine is considered leakier than mature intestine (28), and a small molecule tracer does not allow for detection of subtle alterations in barrier function. After optimization, a 10-kDa rather than a 4-kDa FITC-dextran was used in our study to better distinguish improved barrier function of leaky immature intestine. Briefly, all surviving rat pups at the end of the experiments were gavaged with 10 mg/ml 10-kDa FITC-dextran (40 mg/100 g body weight). Four hours later, whole blood was collected and centrifuged at 3,000 rpm for 10 min. Twenty five μl of serum was then obtained from the supernatant, diluted in 100 μl of PBS, and added in a 96-well black wall microplate in duplicate (60 μl per well) for measurement of fluorescence intensity using a fluorometer. Standards were included to determine the concentrations of FITC-dextran in the serum. High concentrations of FITC-dextran in the serum indicate greater transmucosal transport of FITC-dextran across the intestinal barrier to blood. At the end of our 5-day NEC model, surviving pups grossly have histologically normal intestine (score 0). These pups were used to evaluate barrier function to remove the confounding effect of intestinal necrosis associated with disease state.
Statistical Analysis
All data are presented as means ± S.E. or S.E. from multiple independent experiments. Statistical analysis was performed using the χ2 test for NEC incidence, Student's t test for paired data, or one-way analysis of variance (ANOVA) with a Bonferroni correction for multiple comparisons with GraphPad InState software. Difference was considered to be significant with p values <0.05.
RESULTS
ZO-1 Silencing Impaired TER and Barrier Function in Enterocytes
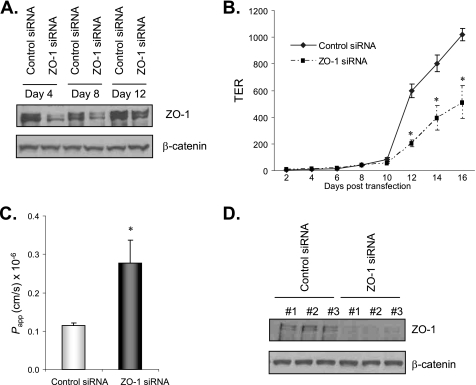
Inflammatory cytokines are elevated in a number of gastrointestinal diseases and are known to induce barrier dysfunction (7–10). T84 enterocytes (ATCC CCL-248) form a polarized and impermeable monolayer that possesses many characteristics of IEC in vivo, enabling the study of barrier function in vitro. Incubation of these cells with the pro-inflammatory cytokine IFN-γ (20 ng/ml) impairs barrier properties as well as expression of the TJ protein ZO-1 (9, 10). Although it is believed that ZO-1 mediates the effect of IFN-γ on barrier disruption, it is still unclear whether loss of ZO-1 alone is sufficient to disrupt TJ integrity. To address the question, we examined transepithelial electrical resistance (TER) and barrier function in ZO-1 knockdown T84 cells using a genetic silencing approach by siRNA transfection. Immunoblotting results show suppressed ZO-1 protein expression in cells on days 4, 8, and 12 following ZO-1 siRNA transfection compared with control (scrambled) siRNA transfection (Fig. 1A). Immunoblotting for β-catenin on the same membrane shows equivalent β-catenin levels in both ZO-1 and control siRNA-transfected cells, demonstrating specific RNA silencing and equal sample loading (Fig. 1A). When grown in Transwells, control siRNA-transfected T84 cells started to show increased TER, a measure of TJ integrity, on day 12 and continued to reach high TER (>1000 ohms·cm2) on day 16, whereas ZO-1 knockdown cells showed consistently lower TER compared with controls from day 12 to 16 (Fig. 1B). In vitro barrier function determined by a FITC-dextran flux experiment on day 16 when control siRNA-transfected cells reached high TER shows impaired barrier function/increased permeability in ZO-1 knockdown cells compared with controls (Fig. 1C). These results demonstrate that ZO-1 down-regulation significantly impairs TER and barrier function and that the IFN-γ effect on barrier disruption can be mediated in part through ZO-1 inhibition in T84 cells. ZO-1 immunoblotting with lysates harvested from Transwells confirms suppressed expression of ZO-1 in ZO-1 siRNA-transfected cells on day 16 when TER and barrier function were measured (Fig. 1D).
FIGURE 1.
ZO-1 silencing impaired TER and barrier function in enterocytes. A, ZO-1 siRNA transfection decreased ZO-1 protein expression. T84 cells at 50% confluence were transfected with 200 nm of a pool of four ZO-1 or scrambled control siRNAs. Cell lysates were collected on days 4, 8, and 12 after transfection for immunoblotting for ZO-1 and β-catenin. B, ZO-1 silencing decreased TER. Cells were plated in Transwells in triplicate along with transfection reagents and 200 nm ZO-1 or control siRNAs. Medium was changed the next day and then twice a week. TER was monitored over time using a voltohmmeter. C, ZO-1 silencing impaired barrier function. Cells were grown and transfected as in B and barrier function was determined as described under “Materials and Methods” on day 16 when control siRNA-transfected cells reached high TER (>1,000 ohms·cm2). Cell lysates from each Transwell were then collected for ZO-1 immunoblotting in D. Representative immunoblots from three experiments with similar results are shown. Data from three experiments are presented as mean ± S.E., and * depicts statistical significance with a p value < 0.05 by t test.
Epo Protected Enterocyte Barrier Function and ZO-1 Localization from Inflammatory Cytokine-induced Damages
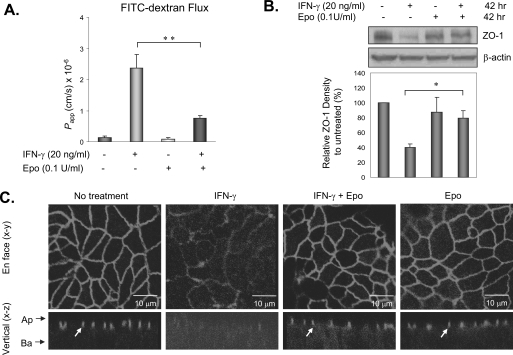
Human milk is protective against NEC, and Epo is a human milk factor that has been shown to protect cell-cell barrier in endothelial cells and the blood-brain barrier in animals (15, 16). We hypothesized that Epo may similarly protect intestinal epithelial barrier function. This was first assessed in vitro in T84 enterocytes where IFN-γ induces barrier disruption by down-regulating the TJ ZO-1 expression (10). T84 cells grown on Transwell inserts with high TER were treated with 20 ng/ml IFN-γ (10) to the basolateral side, where cellular IFN-γ receptors are known to be present and with recombinant human Epo at 0.1 unit/ml to the apical and basolateral sides. Epo was used within the physiological concentration range of Epo in human milk (29, 30). Following treatment, TER was monitored at 24, 48, and 72 h, and paracellular permeability/barrier function was determined at 72 h. Consistent with previous findings, IFN-γ treatment increased apical to basolateral FITC-dextran flux (Fig. 2A). Combined IFN-γ and Epo treatment significantly blunted the IFN-γ-induced increase in FITC-dextran flux (Fig. 2A), indicating that Epo attenuated the IFN-γ-induced barrier disruption. This Epo effect was not due to increased cell growth (data not shown) as a parallel cell proliferation assay showed little difference in cell proliferation among treatments. In T84 cells, IFN-γ disruption of barrier function is associated with down-regulation of the tight junction protein ZO-1 (Fig. 2) (10), and decreased ZO-1 expression alone was sufficient to cause barrier dysfunction (Fig. 1). In addition to barrier protection, Epo treatment prevented IFN-γ-induced down-regulation of ZO-1 (Fig. 2B) and subcellular localization (Fig. 2C). In untreated cells, confocal microscopy demonstrates well characterized ZO-1 localization at the cell boundaries in the en face (x-y) sections and at the TJs in apical areas in vertical (x-z) sections, which is in contrast to fainter and more diffuse ZO-1 staining at both places in IFN-γ-treated cells (Fig. 2C). These results show that Epo was able to protect TJ protein ZO-1 expression as well as subcellular distribution from IFN-γ-induced damages in T84 enterocytes.
FIGURE 2.
Epo protected enterocyte barrier function and ZO-1 localization from inflammatory cytokine-induced damage. A, Epo attenuated IFN-γ-induced barrier disruption. T84 human enterocytes were plated in Transwells in triplicate to reach a high TER and treated with 20 ng/ml IFN-γ ± 0.1 unit/ml Epo for 72 h. Barrier function was then determined by apical to basolateral flux of 10-kDa FITC-dextran, and apparent permeability coefficients (Papp) were calculated as described under “Materials and Methods.” B, Epo rescued IFN-γ down-regulation of ZO-1. T84 cells were grown and treated as in A for 42 h and lysed. The lysates were used for immunoblotting for ZO-1 and β-actin. C, confocal microscopy of ZO-1 localization in T84 cells with or without 100 ng/ml IFN-γ and 0.1 unit/ml Epo treatments for 72 h. Images shown are the en face (x-y) and vertical (x-z) sections. TJs shown in the vertical sections are designated by arrows. Arrows by the x-z sections indicate the apical (Ap) and basal (Ba) aspects of the cells. Representative immunoblots from three experiments with similar results are shown. Data from three experiments are presented as mean ± S.E. relative to controls in percentage. * and ** depict statistical significance with p values < 0.05 and <0.01, respectively, by one-way ANOVA with Bonferroni correction.
Epo Increased TJ Protein ZO-1 Expression in Human Fetal Immature IEC
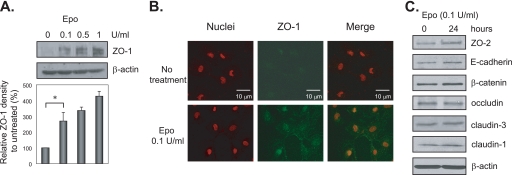
As 90% of NEC occurs in premature babies with immature intestines, the Epo regulation of the TJ protein ZO-1 results was confirmed in an immature IEC line. H4 cells are a human fetal nontransformed primary cell line used as a model of the preterm gut (a generous gift from Dr. W. Allan Walker, Massachusetts General Hospital, Harvard Medical School) (19). H4 cells were treated with 0.1, 0.5, and 1 units/ml recombinant human Epo for 24 h and then lysed. Immunoblotting of the lysates shows Epo stimulation of ZO-1 expression in a dose-dependent manner (Fig. 3A). H4 cells are undifferentiated and do not form TJs. We observed stronger ZO-1 staining both on the membrane and in the cytoplasm following Epo treatment by immunofluorescence microscopy (Fig. 3B). We also examined the effect of Epo on the expression of other TJ and adherens junctional proteins and found that Epo only slightly increased ZO-2 expression and had no effect on the expression of E-cadherin, β-catenin, occludin, claudin-1, or claudin-3 (Fig. 3C).
FIGURE 3.
Epo increased TJ protein ZO-1 expression in human fetal immature IEC. A and C, H4 immature IEC were grown on tissue culture plates and treated with 0, 0.1, 0.5, and 1 unit/ml recombinant human Epo for 24 h. Total cell lysates were collected and subjected to immunoblotting for ZO-1 and β-actin in A and ZO-2, E-cadherin, β-catenin, occludin, claudin-1, claudin-3, and β-actin in C. B, H4 cells grown on chamber slides were treated with or without 0.1 unit/ml Epo for 24 h and then subjected to immunofluorescence staining for ZO-1 and nuclei. Images were acquired by confocal microscopy. Representative immunoblots from three experiments with similar results are shown. Data from three experiments are presented as mean ± S.E. relative to controls in percentage. * depicts statistical significance with a p value <0.05 by one-way ANOVA with Bonferroni correction..
Epo Stimulated ZO-1 Expression via the PI3K/Akt Pathway in Human Fetal Immature IEC
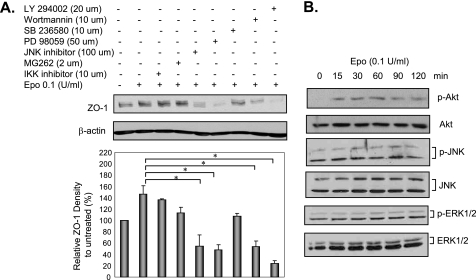
Following binding to preformed dimers or oligomers of Epo receptors, Epo elicits different cell responses by activating intracellular signaling pathways, including the NF-κB, JNK, ERK1/2, p38 MAPK, and PI3K/Akt pathways (31–34). Whether any of those pathways was activated and mediated Epo regulation of ZO-1 in H4 immature IEC was studied using specific inhibitors as follows: the IKK inhibitor (IKK inhibitor II) and the proteasome inhibitor (MG262) were used to suppress IκB-α phosphorylation and degradation, respectively, thereby suppressing activation of the NF-κB pathway. The JNK inhibitor (JNK inhibitor II) was used to inhibit the JNK pathway. The MEK inhibitor (PD 98059) was used to inhibit the ERK1/2 pathway. SB 236580 was used to inhibit the p38 pathway. The PI3K inhibitors (wortmannin and LY 294002) were used to suppress PI3K and downstream PI3K-dependent Akt activation. There was no inhibition of ZO-1 expression seen with IKK inhibitor II, MG262, or SB 236580 by immunoblotting. In contrast, Epo stimulation of ZO-1 expression was significantly inhibited in the presence of the JNK inhibitor II, PD 98059, wortmannin, and LY 294002 (Fig. 4A), suggesting that the JNK, ERK1/2, and PI3K/Akt pathways were involved in Epo regulation of ZO-1 expression. However, Epo only stimulated Akt, not JNK or ERK1/2 phosphorylation, as evaluated by immunoblotting using antibodies against p-Akt, p-JNK, and p-ERK1/2 (Fig. 4B). These results demonstrate that Akt activation by PI3K mediates Epo regulation of ZO-1 in immature IEC.
FIGURE 4.
Epo stimulated ZO-1 expression via the PI3K/Akt pathway in human fetal immature IEC. A, effect of inhibitors on Epo regulation of ZO-1 expression in immature IEC. H4 cells were left untreated or pretreated with inhibitors as indicated for 30 min and then together with 0.1 unit/ml Epo for another 24 h. Cell lysates were collected and subjected to immunoblotting for ZO-1 and β-actin. B, Epo activated the Akt pathway in immature IEC. H4 cells were treated with 0.1 unit/ml Epo for various periods of time as indicated and then lysed. Lysates were used for immunoblotting for p-Akt, total Akt, p-JNK, total JNK, p-ERK1/2, and total ERK1/2. Representative immunoblots from three experiments with similar results are shown. Data from three experiments are presented as mean ± S.E. relative to controls in percentage. * depicts statistical significance with a p value <0.05, by one-way ANOVA with Bonferroni correction
Epo Lowered the Incidence of NEC in an Animal Model
Loss of intestinal barrier function contributes to the pathogenesis of NEC (12). Because Epo protected enterocyte barrier function in vitro (Fig. 2A), we next assessed the physiological relevance of this finding by determining if enteral Epo can lower the incidence of NEC in a rat animal model (21). In this model, pups were subjected to three major risk factors for human NEC (prematurity, formula feeding, and hypoxia-ischemia), and many of the clinical and pathological changes in the immature rat pups were similar to those found in humans as follows: the abdomen was distended; blood was detected in the stool, and the ileum and proximal colon were the most affected parts of the intestine (35). Intestinal sections were H&E-stained and scored as described under “Materials and Methods.” Nonstressed naturally born pups fed by their mother (the dam-fed group) served as controls. Our results showed no NEC in the healthy mother-fed control group (n = 20) and 45% NEC incidence in the untreated experimental NEC group (n = 56). Interestingly, oral administration of Epo reduced NEC incidence from 45 to 23% (n = 56) (p < 0.05), demonstrating that enteral Epo can lower NEC incidence (Table 1). No difference was seen among groups in measured serum pH or hemoglobin levels (data not shown).
TABLE 1.
Epo lowered NEC incidence
A neonatal rat NEC model and NEC scoring were conducted as described under “Materials and Methods.” Experimental NEC rat pups were left untreated or treated with 0.1 unit/ml Epo. Naturally born and dam-fed pups were healthy controls.
| NEC (+) | NEC (−) | Total | |
|---|---|---|---|
| Experimental NEC − Epo | 25 (45%)a | 31 | 56 |
| Experimental NEC + Epo | 13 (23%) | 43 | 56 |
| Dam-fed | 0 (0%) | 20 | 20 |
a Data depict p < 0.05 between the experimental NEC groups by χ2 test.
Epo Protected Intestinal Barrier Function in the NEC Animal Model
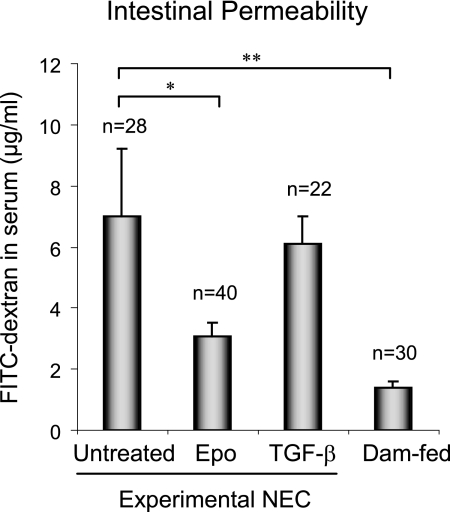
To determine whether Epo protection against NEC was associated with preservation of intestinal barrier function in the face of NEC stress, paracellular intestinal permeability was assessed by oral administration of the fluorescent tracer FITC-dextran (10 kDa) to all surviving pups at the end of the NEC animal experiment. The concentrations of fluorescent FITC-dextran in the blood were then measured as a reflection of intestinal permeability in vivo. As expected, the healthy dam-fed control pups (n = 30) had the lowest level of FITC-dextran in the blood (better barrier function), and the untreated NEC experimental preterm pups (n = 28) had the highest levels of FITC-dextran in the blood (poorer barrier function) (Fig. 5). Epo treatment in experimental NEC pups (n = 40) significantly decreased FITC-dextran flux to blood to near dam-fed control levels, indicating that the supplementation of Epo in the formula protected barrier function. TGF-β is a multifunctional cytokine and also a human milk factor used as a control for Epo treatment in the animal study. Both Epo (0.1 unit/ml) and TGF-β (30 ng/ml) were used within the physiological concentration range of Epo and TGF-β in human milk (29, 30). The effect of Epo on barrier function is specific as administration of TGF-β (n = 22) failed to protect intestinal barrier function. Histopathology of the intestine from animals used for the in vivo barrier function assay showed no evidence of NEC, suggesting that disruption of barrier function was not due to intestinal necrosis.
FIGURE 5.
Epo protected intestinal barrier function in an NEC animal model. Preterm neonatal rats delivered by cesarean section from Sprague-Dawley dams were fed with formula ± 0.1 unit/ml Epo or 30 ng/ml TGF-β (as a treatment control for Epo) and stressed to induce NEC. Naturally born, unstressed, and dam-fed pups were included as healthy controls. In vivo barrier function was determined in all surviving pups at the end of the animal experiment on day 5 as described under “Materials and Methods.” Data from seven animal experiments are presented as mean ± S.E. * and **depict statistical significance with p values <0.05 and 0.01, respectively, by one-way ANOVA with Bonferroni correction. Higher levels of the tracer FITC-dextran in blood indicate poorer intestinal barrier function.
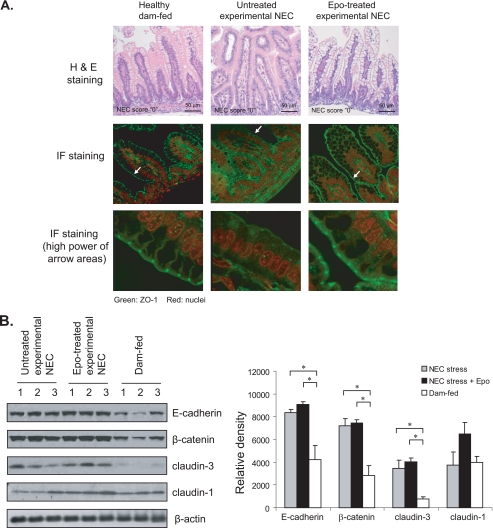
Epo Maintained ZO-1 at the Intestinal Tight Junction in the NEC Animal Model
Intestinal barrier function can be regulated by both TJ structure and proper TJ protein expression. Epo protected T84 enterocyte barrier function by maintaining ZO-1 expression (Fig. 2) as well as intestinal barrier function in experimental NEC animals (Fig. 5). Whether histological expression and localization of ZO-1 were altered during the development of NEC and whether Epo preserved ZO-1 in the ileum, the NEC-affected area, were evaluated in intestines from the same animals used for the in vivo barrier function assay. Immunofluorescence staining revealed normal TJ structure and ZO-1 protein expression predominantly at the TJs in mother-fed healthy control pups. In contrast, ZO-1 staining at TJs was lost in histologically normal (NEC score “0”) ileal villi from the pups exposed to NEC stress (Fig. 6A). This alteration in ZO-1 by NEC stress was prevented by feeding pups formula containing Epo supplementation. H&E staining confirmed no histological damage in the pups used for this experiment (Fig. 6, top panels), suggesting that the loss of ZO-1 at the TJ was not associated with intestinal necrosis. Although we were unable to directly demonstrate this, loss of ZO-1 at the TJ junction may be an early event that precedes histological alterations.
FIGURE 6.
Effect of Epo on the localization and expression of adherens and tight junction proteins in a NEC animal model. A, Epo maintained ZO-1 at the intestinal tight junction. Intestinal sections were collected from animals used for in vivo barrier function as in Fig. 5 and subjected to H&E staining and immunofluorescence (IF) staining for ZO-1 and nuclei. Images were acquired by confocal microscopy. Representative immunofluorescence staining shows expression and localization of ZO-1 at the TJs of healthy and Epo-treated experimental NEC intestines but not in the non-Epo-treated NEC stressed samples. H&E staining shows NEC score 0 for all sections. B, Epo did not alter the expression of E-cadherin, β-catenin, claudin-1, and claudin-3 in experimental NEC animals. Intestinal lysates were collected from animals used for in vivo barrier function as in Fig. 5 and subjected to immunoblotting for E-cadherin, β-catenin, claudin-1, claudin-3, and β-actin. Representative immunoblots from three experiments with similar results are shown. * depicts statistical significance with a p value < 0.05, by one-way ANOVA with Bonferroni correction.
Alterations of Adherens and Tight Junction Proteins during the Pathogenesis of NEC
To determine whether changes in intestinal permeability were associated with alterations in other TJ and adherens junction components, expression of claudin-1, claudin-3, E-cadherin, and β-catenin was evaluated by immunoblotting of intestinal lysates from rat pups sacrificed at the end of the animal experiments. Besides claudin-3, which is known to increase in NEC ilea (12), we also observed increases in the adherens junction proteins E-cadherin and β-catenin in the ilea from experimental NEC intestines compared with healthy controls (Fig. 6B). Claudin-1 expression was not altered between experimental NEC and healthy intestines. Consistent with our in vitro study, Epo treatment did not alter claudin-1, claudin-3, E-cadherin, or β-catenin levels in experimental NEC intestines.
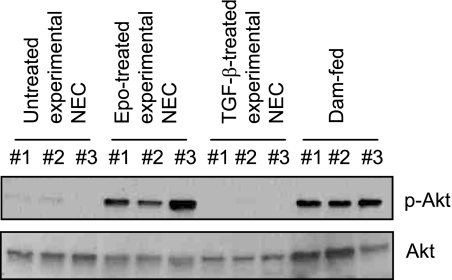
Epo Activated the Akt Pathway in Vivo in the NEC Animal Model
In the immature H4 IEC, the PI3K/Akt pathway mediated Epo stimulation of ZO-1 expression (Fig. 4). We studied whether Epo can activate the same pathway in vivo by p-Akt immunoblotting of intestinal lysates. In the intestines of healthy dam-fed pups, Akt activation is readily detected, which is in contrast to minimal Akt activation in non-Epo-treated experimental NEC intestines (Fig. 7). Epo administration in the experimental NEC animals maintained high levels of phospho-Akt to a similar degree as in the intestines of healthy dam-fed pups, whereas the TGF-β treatment, a control for Epo treatment, was unable to maintain sustained Akt activation.
FIGURE 7.
Epo activated the Akt pathway in vivo in the NEC animal model. Intestinal lysates were collected from animals used for in vivo barrier function as in Fig. 5 and subjected to immunoblotting for phospho- and total Akt. Representative immunoblots from three experiments with similar results are shown.
DISCUSSION
NEC is the most common cause of death from gastrointestinal diseases in preterm infants. Previous studies have revealed the protective effects of breast milk on both the incidence and the severity of NEC (36). Components in human milk responsible for this protective effect are expected to act on steps in the pathogenesis of NEC. Understanding the underlying mechanisms of biochemical cross-talk between human milk factors and the intestines of breastfed infants offers an opportunity for development of therapeutic agents for this disease.
Erythropoietin is found in human milk. Although first reported as a major stimulator of erythropoiesis in erythroid progenitor cells, several studies have indicated diverse effects of Epo in nonhematopoietic cells with Epo receptors, including cardiac muscle cells and neurons, where Epo acts as a protective cytokine (37–39). Functional Epo receptors are also present at the IEC brush border of fetal and postnatal intestines in humans and rats, suggesting a role of Epo in the developing gut (18). Our data demonstrate that Epo can specifically preserve intestinal barrier function under conditions of inflammatory stress. Our results thus reveal a novel protective effect of Epo on intestinal barrier integrity.
We found that Epo protected enterocyte barrier function via maintenance of ZO-1 levels and cellular localization in vitro in the cell line H4. As the immature H4 cells do not polarize or form sufficient tight junctions to measure barrier function, we measured the effect of Epo on ZO-1 and barrier function on immature intestinal epithelia under conditions of inflammatory stress in vivo. Our data in newborn rat pups demonstrated that Epo significantly decreased NEC incidence, attenuated intestinal barrier disruption, and maintained TJ structure and ZO-1 localization in vivo in an experimental NEC animal model.
NEC is known to be associated with overexpression of a number of proinflammatory cytokines. In addition to inducing an inflammatory response, proinflammatory cytokines are now known to induce barrier disruption. IFN-γ has been shown to be elevated in NEC (7, 40) and a mediator for inducing intestinal epithelial permeability both in vitro and in vivo (10, 11, 41). Correspondingly, NEC has been shown to involve structural and functional loss of the intestinal barrier as described in the study by Piena-Spoel et al. (42), in which human neonates diagnosed with severe cases of NEC had increased intestinal permeability compared with healthy controls. From our study, loss of the TJ protein ZO-1 important for barrier function was evident in animals exposed to NEC stress that did not yet have histological evidence of NEC. This suggests that the ZO-1 loss we saw was not just a consequence of necrosis associated with intestinal injury of the disease but instead could be an early event that preceded NEC.
Impaired intestinal barrier function in pathological conditions has been associated with altered expression of TJ as well as adherens junctional proteins. Interestingly, we found elevated E-cadherin and β-catenin in the experimental NEC compared with dam-fed pups. Claudins are other main TJ components playing an important role in the regulation of paracellular transport (43). Claudin-1 expression was comparable between experimental NEC and healthy intestines. In contrast, claudin-3 expression was up-regulated in experimental NEC intestines where increased intestinal permeability was detected. Interestingly, others have shown that claudin-3 can be used as a marker of intestinal injury (44). Our findings are consistent with the study by Clark et al. (12) in which increased claudin-3 was observed in NEC intestines and correlated with the degree of NEC injury. It is possible that intestinal epithelial cells further increase expression of some components important for maintaining barrier function to compensate for the barrier disruption as the intestine becomes more damaged. Furthermore, examination of mucosal expression of claudin-3 in the duodena in children with celiac disease also demonstrated the correlation between elevated claudin-3 and increased intestinal permeability (45). The claudins are thought to be the pore-forming proteins that regulate the size selectivity of the TJ barrier (46) and the increased claudin-3 expression may promote a “leakier” epithelium, allowing for overall increased intestinal permeability. Epo administration preserved ZO-1 but did not significantly affect expression of claudin-1, claudin-3, E-cadherin, or β-catenin in experimental NEC.
Our in vivo data show diminished Akt activation in experimental NEC intestine, which can be increased by Epo treatment to near healthy intestinal control levels, suggesting a role of Akt activation in mediating Epo modulation of ZO-1 distribution and intestinal barrier function in vivo. The Akt pathway has previously been shown to be involved in the regulation of barrier integrity in endothelial cells. A serum-borne bioactive lipid, sphingosine 1-phosphate, enhances endothelial barrier integrity by inducing ZO-1 redistribution to the cell-cell junctional contacts (47), and the effect of S1P was abrogated upon inhibition of PI3K/Akt activation. The Akt pathway is one of the known pathways downstream of Epo and was activated in the immature H4 IEC. In these cells, PI3K activation mediated Epo regulation of ZO-1 as treatments with LY 294002 and wortmannin, the inhibitors for the Akt upstream activator PI3K, abrogated Epo stimulation of ZO-1. Epo stimulation of ZO-1 expression was also significantly inhibited in the presence of the JNK inhibitor and PD 98059 (Fig. 4A), suggesting that the JNK and ERK1/2 pathways are also involved in regulation of ZO-1 expression. However, Epo stimulated only Akt but not JNK and ERK1/2 phosphorylation (Fig. 4B) as evaluated by immunoblotting using antibodies against p-Akt, p-JNK, and p-ERK1/2. It is possible that the JNK and ERK1/2 activation may be required for basal regulation of ZO-1 expression and that Akt activation via PI3K mediates Epo regulation of ZO-1 in immature IEC.
This is the first study to document the effects of enteral Epo directly on IEC and experimental NEC. Others have investigated the effect of Epo administered via injection. A retrospective cohort study of the subcutaneous injection of Epo administration for prevention and treatment of anemia of prematurity demonstrated lowered incidence of NEC in very low birth weight infants (500–1,250 g) (48). In separate animal studies, peritoneal injection of recombinant human Epo in a rat model of NEC appeared to reduce oxidative stress associated with the pathogenesis of NEC (49, 50). However, injection potentially carries risks of infection through skin disruption, and as multiple tissues have Epo receptors, systemic administration potentially carries a greater risk for systemic effects. Our study used immature rat pups obtained by cesarean section and oral administration of recombinant rat Epo to specifically address whether Epo has direct effects on IEC and whether Epo can protect an immature intestine from NEC through its intestinal receptors. In our model, NEC stage is a histological diagnosis and can only be determined after sacrifice; thus our study focused on prevention of disease rather than treatment. This is consistent with Epo as a component of protective human milk. Our study demonstrates that Epo is able to exert its activity against the pathophysiology of NEC within the gut following oral administration at physiological concentrations. Epo was used at a concentration within the physiological range of breast milk to mimic the protective role of breast milk and to specifically demonstrate that protection could be achieved at this relatively low dose.
Our data suggest that enteral administration of recombinant Epo at concentrations comparable with those found in human milk is sufficient for maintenance of ZO-1 expression in the context of inflammatory stress, leading to preservation of barrier function and reduced incidence of NEC in an animal model. As Epo is an Food and Drug Administration-approved agent and has been used to treat anemia in infants, this study not only represents an important first step toward understanding the actions of an isolated factor in breast milk in protecting infants from NEC but also potentially suggests broader application for the novel therapeutic use of Epo in intestinal diseases.
Acknowledgments
We thank Drs. Jerrold Turner and Le Shen from the Department of Pathology for project suggestions and technical support on intestinal ZO-1 staining, Dr. Amy Noffsinger from the Department of Pathology for scoring of the NEC experiment tissues, and Dr. Christine Labno from the Integrated Microscopy Core Facility for confocal microscopy. The Digestive Disease Research Core Center of the University of Chicago (supported by National Institutes of Health Grant DK42086) provided core facilities and services used for this study.
This work was supported, in whole or in part, by National Institutes of Health Grants R21 AT004044 (to E. C. C. and E. O. P.) and HD055237 and R01 HD059123 (to E. C. C.) from NICHD. This work was also supported by Children Research Foundation Grant and Kirschstein-NRSA Fellowship Award HD062144-01A1 (to S.-R. S.).
- TJ
- tight junction
- NEC
- neonatal necrotizing enterocolitis
- Epo
- erythropoietin
- IEC
- intestinal epithelial cells
- ANOVA
- analysis of variance
- TER
- transepithelial electrical resistance.
REFERENCES
- 1. Kosloske A. M. (1994) Acta Paediatr. Suppl. 396, 2–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Holman R. C., Stehr-Green J. K., Zelasky M. T. (1989) Am. J. Public Health 79, 987–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anand R. J., Leaphart C. L., Mollen K. P., Hackam D. J. (2007) Shock 27, 124–133 [DOI] [PubMed] [Google Scholar]
- 4. Anderson J. M., Balda M. S., Fanning A. S. (1993) Curr. Opin. Cell Biol. 5, 772–778 [DOI] [PubMed] [Google Scholar]
- 5. Stevenson B. R., Siliciano J. D., Mooseker M. S., Goodenough D. A. (1986) J. Cell Biol. 103, 755–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schneeberger E. E., Lynch R. D. (2004) Am. J. Physiol. Cell Physiol. 286, C1213–C1228 [DOI] [PubMed] [Google Scholar]
- 7. Ford H., Watkins S., Reblock K., Rowe M. (1997) J. Pediatr. Surg. 32, 275–282 [DOI] [PubMed] [Google Scholar]
- 8. Viscardi R. M., Lyon N. H., Sun C. C., Hebel J. R., Hasday J. D. (1997) Pediatr. Pathol. Lab. Med. 17, 547–559 [PubMed] [Google Scholar]
- 9. Madara J. L., Stafford J. (1989) J. Clin. Invest. 83, 724–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Youakim A., Ahdieh M. (1999) Am. J. Physiol. 276, G1279–G1288 [DOI] [PubMed] [Google Scholar]
- 11. Yang H., Kiristioglu I., Fan Y., Forbush B., Bishop D. K., Antony P. A., Zhou H., Teitelbaum D. H. (2002) Ann. Surg. 236, 226–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clark J. A., Doelle S. M., Halpern M. D., Saunders T. A., Holubec H., Dvorak K., Boitano S. A., Dvorak B. (2006) Am. J. Physiol. Gastrointest. Liver Physiol. 291, G938–G949 [DOI] [PubMed] [Google Scholar]
- 13. Lucas A., Cole T. J. (1990) Lancet 336, 1519–1523 [DOI] [PubMed] [Google Scholar]
- 14. Kling P. J., Sullivan T. M., Roberts R. A., Philipps A. F., Koldovský O. (1998) Pediatr. Res. 43, 216–221 [DOI] [PubMed] [Google Scholar]
- 15. Martínez-Estrada O. M., Rodríguez-Millán E., González-De Vicente E., Reina M., Vilaró S., Fabre M. (2003) Eur. J. Neurosci. 18, 2538–2544 [DOI] [PubMed] [Google Scholar]
- 16. Li Y., Lu Z. Y., Ogle M., Wei L. (2007) Neurochem. Res. 32, 2132–2141 [DOI] [PubMed] [Google Scholar]
- 17. Juul S. E., Yachnis A. T., Christensen R. D. (1998) Early Hum. Dev. 52, 235–249 [DOI] [PubMed] [Google Scholar]
- 18. Juul S. E., Joyce A. E., Zhao Y., Ledbetter D. J. (1999) Pediatr. Res. 46, 263–268 [DOI] [PubMed] [Google Scholar]
- 19. Sanderson I. R., Ezzell R. M., Kedinger M., Erlanger M., Xu Z. X., Pringault E., Leon-Robine S., Louvard D., Walker W. A. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 7717–7722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shiou S. R., Datta P. K., Dhawan P., Law B. K., Yingling J. M., Dixon D. A., Beauchamp R. D. (2006) J. Biol. Chem. 281, 33971–33981 [DOI] [PubMed] [Google Scholar]
- 21. Caplan M. S., Hedlund E., Adler L., Hsueh W. (1994) Pediatr. Pathol. 14, 1017–1028 [DOI] [PubMed] [Google Scholar]
- 22. Halpern M. D., Holubec H., Saunders T. A., Dvorak K., Clark J. A., Doelle S. M., Ballatori N., Dvorak B. (2006) Gastroenterology 130, 359–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Napolitano L. M., Koruda M. J., Meyer A. A., Baker C. C. (1996) Shock 5, 202–207 [DOI] [PubMed] [Google Scholar]
- 24. Homma H., Hoy E., Xu D. Z., Lu Q., Feinman R., Deitch E. A. (2005) Am. J. Physiol. Gastrointest. Liver Physiol. 288, G466–G472 [DOI] [PubMed] [Google Scholar]
- 25. Thiagarajah J. R., Gourmelon P., Griffiths N. M., Lebrun F., Naftalin R. J., Pedley K. C. (2000) Gut 47, 675–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zufferey C., Erhart D., Saurer L., Mueller C. (2009) Immunology 128, 351–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Naftalin R. J., Pedley K. C. (1999) J. Physiol. 514, 211–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Foulkes E. C., Mort T. L., Buncher C. R. (1991) Proc. Soc. Exp. Biol. Med. 197, 477–481 [DOI] [PubMed] [Google Scholar]
- 29. Semba R. D., Juul S. E. (2002) J. Hum. Lact. 18, 252–261 [DOI] [PubMed] [Google Scholar]
- 30. Saito S., Yoshida M., Ichijo M., Ishizaka S., Tsujii T. (1993) Clin. Exp. Immunol. 94, 220–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bose C., Udupa K. B. (2008) Am. J. Physiol. Cell Physiol. 295, C394–C405 [DOI] [PubMed] [Google Scholar]
- 32. Kumar S. M., Yu H., Fong D., Acs G., Xu X. (2006) Melanoma Res. 16, 275–283 [DOI] [PubMed] [Google Scholar]
- 33. Buck I., Morceau F., Cristofanon S., Heintz C., Chateauvieux S., Reuter S., Dicato M., Diederich M. (2008) Biochem. Pharmacol. 76, 1229–1239 [DOI] [PubMed] [Google Scholar]
- 34. Lee S. M., Nguyen T. H., Park M. H., Kim K. S., Cho K. J., Moon D. C., Kim H. Y., Yoon D. Y., Hong J. T. (2004) Biochem. Biophys. Res. Commun. 320, 1087–1095 [DOI] [PubMed] [Google Scholar]
- 35. Israel E. J. (1994) Acta Paediatr. Suppl. 396, 27–32 [DOI] [PubMed] [Google Scholar]
- 36. Kurscheid T., Holschneider A. M. (1993) Eur. J. Pediatr. Surg. 3, 139–143 [DOI] [PubMed] [Google Scholar]
- 37. Madonna R., Shelat H., Xue Q., Willerson J. T., De Caterina R., Geng Y. J. (2009) Exp. Cell Res. 315, 2921–2928 [DOI] [PubMed] [Google Scholar]
- 38. Liu R., Suzuki A., Guo Z., Mizuno Y., Urabe T. (2006) J. Neurochem. 96, 1101–1110 [DOI] [PubMed] [Google Scholar]
- 39. Yoo J. Y., Won Y. J., Lee J. H., Kim J. U., Sung I. Y., Hwang S. J., Kim M. J., Hong H. N. (2009) J. Neurosci. Res. 87, 150–163 [DOI] [PubMed] [Google Scholar]
- 40. Caplan M. S., Sun X. M., Hseuh W., Hageman J. R. (1990) J. Pediatr. 116, 960–964 [DOI] [PubMed] [Google Scholar]
- 41. Ferrier L., Mazelin L., Cenac N., Desreumaux P., Janin A., Emilie D., Colombel J. F., Garcia-Villar R., Fioramonti J., Bueno L. (2003) Gastroenterology 125, 795–804 [DOI] [PubMed] [Google Scholar]
- 42. Piena-Spoel M., Albers M. J., ten Kate J., Tibboel D. (2001) J. Pediatr. Surg. 36, 587–592 [DOI] [PubMed] [Google Scholar]
- 43. Tsukita S., Furuse M. (2000) Ann. N.Y. Acad. Sci. 915, 129–135 [DOI] [PubMed] [Google Scholar]
- 44. Thuijls G., Derikx J. P., de Haan J. J., Grootjans J., de Bruïne A., Masclee A. A., Heineman E., Buurman W. A. (2010) J. Clin. Gastroenterol. 44, e14–e19 [DOI] [PubMed] [Google Scholar]
- 45. Szakál D. N., Gyorffy H., Arató A., Cseh A., Molnár K., Papp M., Dezsofi A., Veres G. (2010) Virchows Arch. 456, 245–250 [DOI] [PubMed] [Google Scholar]
- 46. Tsukita S., Furuse M. (2000) J. Cell Biol. 149, 13–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lee J. F., Zeng Q., Ozaki H., Wang L., Hand A. R., Hla T., Wang E., Lee M. J. (2006) J. Biol. Chem. 281, 29190–29200 [DOI] [PubMed] [Google Scholar]
- 48. Ledbetter D. J., Juul S. E. (2000) J. Pediatr. Surg. 35, 178–182 [DOI] [PubMed] [Google Scholar]
- 49. Akisu M., Küllahçioðlu, Girgin F., Baka M., Hüsseyinov A., Kültürsay N. (2001) Eur. J. Pediatr. Surg. 11, 167–172 [DOI] [PubMed] [Google Scholar]
- 50. Kumral A., Baskin H., Duman N., Yilmaz O., Tatli M., Ozer E., Gökmen N., Genc S., Ozkan H. (2003) Biol. Neonate 84, 325–329 [DOI] [PubMed] [Google Scholar]