Abstract
Dantrolene is believed to stabilize interdomain interactions between the NH2-terminal and central regions of ryanodine receptors by binding to the NH2-terminal residues 590–609 in skeletal ryanodine receptor (RyR1) and residues 601–620 in cardiac ryanodine receptor (RyR2). To gain further insight into the structural basis of dantrolene action, we have attempted to localize the dantrolene-binding sequence in RyR1/RyR2 by using GFP as a structural marker and three-dimensional cryo-EM. We inserted GFP into RyR2 after residues Arg-626 and Tyr-846 to generate GFP-RyR2 fusion proteins, RyR2Arg-626-GFP and RyR2Tyr-846-GFP. Insertion of GFP after residue Arg-626 abolished the binding of a bulky GST- or cyan fluorescent protein-tagged FKBP12.6 but not the binding of a smaller, nontagged FKBP12.6, suggesting that residue Arg-626 and the dantrolene-binding sequence are located near the FKBP12.6-binding site. Using cryo-EM, we have mapped the three-dimensional location of Tyr-846-GFP to domain 9, which is also adjacent to the FKBP12.6-binding site. To further map the three-dimensional location of the dantrolene-binding sequence, we generated 10 FRET pairs based on four known three-dimensional locations (FKBP12.6, Ser-437-GFP, Tyr-846-GFP, and Ser-2367-GFP). Based on the FRET efficiencies of these FRET pairs and the corresponding distance relationships, we mapped the three-dimensional location of Arg-626-GFP or -cyan fluorescent protein, hence the dantrolene-binding sequence, to domain 9 near the FKBP12.6-binding site but distant to the central region around residue Ser-2367. An allosteric mechanism by which dantrolene stabilizes interdomain interactions between the NH2-terminal and central regions is proposed.
Keywords: Calcium Intracellular Release, Fluorescence Resonance Energy Transfer (FRET), Receptor Structure-Function, Ryanodine, Site-directed Mutagenesis, Dantrolene, Malignant Hyperthermia, Ryanodine Receptor
Introduction
Ryanodine receptor (RyR)6 Ca2+ release channels are located in the sarco(endo)plasmic reticulum of muscle and some nonmuscle cells and play an essential role in muscle contraction and Ca2+ signaling (1, 2). There are three mammalian RyR isoforms, RyR1, RyR2, and RyR3, with distinct patterns of expression. RyR1 is predominantly expressed in skeletal muscle, whereas RyR2 is mainly expressed in cardiac muscle and the brain. RyR3 expression is widespread but at relatively low levels (3). RyRs also play a critical role in the pathogenesis of muscle disorders. Naturally occurring mutations in RyR1 are linked to malignant hyperthermia and central core disease (4, 5), whereas mutations in RyR2 are associated with ventricular arrhythmias and sudden cardiac death (6, 7). Interestingly, most of the disease-causing RyR1 and RyR2 mutations are located within the NH2-terminal, central, and COOH-terminal “hot spot” regions of the two channel isoforms (6, 7). This similar pattern of mutation distribution suggests that disease-causing RyR1 and RyR2 mutations are likely to affect the same aspects of channel structure and function. It has been proposed that interdomain interactions between the NH2-terminal and central regions of RyR1 or RyR2 are critical for stabilizing the closed state of the channel and that disease-causing RyR1 and RyR2 mutations in either the NH2-terminal or the central region weaken interdomain interactions and destabilize the closed state of the channel, leading to enhanced channel sensitivity and consequently uncontrolled Ca2+ release.
Stabilizing interdomain interactions within RyRs, therefore, represents a promising means to suppress uncontrolled Ca2+ release. Indeed, dantrolene, a skeletal muscle relaxant and currently the most effective treatment for malignant hyperthermia (8, 9), has been shown to inhibit uncontrolled Ca2+ release by stabilizing interdomain interactions between the NH2-terminal and central regions of RyR (10, 11). Affinity labeling studies using [3H]azido-dantrolene identified RyR1 as a specific target of dantrolene (12, 13). Further mapping studies revealed that dantrolene binds to residues 590–609 in the NH2-terminal region of RyR1 (14). Interestingly, dantrolene also binds to the corresponding sequence (residues 601–620) in RyR2 in a manner that is dependent on channel conformation (15). Furthermore, dantrolene suppresses the Ca2+ leak from the sarcoplasmic reticulum of failing cardiac myocytes or mutant myocytes expressing a disease-causing RyR2 mutation (11, 16). These observations suggest that RyR2 contains a dantrolene-binding sequence, although it is not accessible for some conformational states of the receptor. Despite its clear therapeutic relevance, the mechanism by which dantrolene may act to stabilize interdomain interactions has remained unclear. It has been suggested that the dantrolene-binding sequence (residues 590–609 in RyR1) constitutes part of the “domain switch” that is formed between the NH2-terminal (Cys-35 to Arg-614) and the central (Asp-2129 to Arg-2458) regions of RyR1 and that the binding of dantrolene to the domain switch stabilizes interdomain interactions between the NH2-terminal and central regions (10). However, direct structural evidence supporting these hypotheses is lacking.
To test this domain switch hypothesis, we have recently mapped the locations of residue Ser-437 in the NH2-terminal region and residue Ser-2367 in the central region in the three-dimensional structure of RyR2 and found that these two regions are indeed located in close proximity (17, 18). Although these findings generally support the domain switch hypothesis, it remains unclear whether the dantrolene-binding sequence is itself located within the proposed domain switch. To address this question, we now examine the location of the dantrolene-binding sequence identified in vitro in the three-dimensional structure of RyR2 using a combination of FRET and cryo-electron microscopy with single particle imaging. Our results show that the dantrolene-binding sequence is located to domain 9 adjacent to the FKBP12.6 (12.6-kDa FK506-binding protein)-binding site but is distant to the central region around residue Ser-2367, suggesting that dantrolene may stabilize interdomain interactions between the NH2-terminal and central regions by an allosteric mechanism. Because of the high sequence and structural similarity between RyR1 and RyR2 (1, 19), our findings should also apply to RyR1.
EXPERIMENTAL PROCEDURES
Materials
Restriction endonucleases and DNA modifying enzymes were purchased from New England BioLabs Inc (Ipswich, MA). The anti-RyR and anti-FKBP12 antibodies were obtained from Affinity BioReagents (Golden, CO). Soybean phosphatidylcholine was obtained from Avanti Polar Lipids, Inc (Alabaster, AL). CHAPS and other reagents were purchased from Sigma.
Construction of GFP-, YFP-, or CFP-tagged RyR2s
The cloning and construction of the 15-kb full-length cDNA encoding the mouse cardiac RyR2 has been described previously (NCBI reference sequence NP_076357.2, GI:124430578) (20, 21). The DNA encoding GFP, YFP, or CFP flanked by Gly-rich spacers and an AscI site was obtained by PCR as described previously (18). The AscI site was introduced into the RyR2 after specific residues by overlap extension using PCR (22). The AscI-AscI fragment containing GFP, YFP, or CFP and the spacers was then subcloned into the full-length RyR2 at the introduced AscI site. The sequences of all PCR fragments and the orientation of the inserted GFP, YFP, or CFP cDNA were verified by DNA sequencing analysis.
Cell Culture and DNA Transfection
HEK293 cells were maintained in Dulbecco's modified Eagle's medium as described previously (21). HEK293 cells grown in 100-mm tissue culture dishes for 18–20 h after subculture were transfected with 6–12 μg of RyR2 wild type (RyR2(WT)) or GFP-, YFP-, or CFP-tagged RyR2 cDNAs with or without 2 μg of CFP-FKBP12.6 or FKBP12.6 cDNA using Ca2+ phosphate precipitation (21).
Cryo-electron Microscopy and Image Processing
The expression and purification of RyR2(A4860G)Tyr-846-GFP was carried out as described previously (23). The purified RyR2(A4860G)Tyr-846-GFP was diluted 5–10-fold with EM dilution buffer (20 mm Na-PIPES (pH 7.2), 400 mm KCl, 3 mm EGTA, 0.5% CHAPS, 2 mm DTT, and 2 mg/ml leupeptin). Cryo-EM grids were prepared by an FEI Vitrobot computer-controlled freeze-plunging instrument (FEI Company, Hillsboro, OR). Micrographs were recorded using low dose protocols on an FEI Tecnai F20 field emission gun transmission electron microscope operated at 200 kV, equipped with an Oxford CT3500 cryo-transfer holder (Gatan, Inc., Warrendale, PA). The temperature of the grids was maintained at approximately −170 °C. The defocus of the micrographs ranged between 1.5 and 4.5 μm, at a magnification of 50,760× (±2%, as verified by a tobacco mosaic virus standard). To obtain sufficient orientational sampling of RyR molecules, we tilted the grids between 0° and 50° during data collection. Each exposure corresponded to an electron dose of ∼10 e−/Å2. Micrographs were checked for drift, astigmatism, and the presence of Thon rings by optical diffraction. Selected electron micrographs were digitized on a Zeiss/Imaging scanner (Z/I Imaging Corporation, Huntsville, AL) with a step size of 14 μm. The images were processed using the SPIDER/WEB software package (24), and three-dimensional reconstructions were obtained through use of the projection matching procedure (25). The final three-dimensional reconstructions of RyR2(A4860G) and RyR2(A4860G)Tyr-846-GFP were computed from 7,591 and 11,901 particles, respectively. Four-fold symmetry was enforced in both three-dimensional reconstructions. The final resolution of both reconstructions was estimated to be 27 Å, as determined by Fourier shell correlation with a cut-off value of 0.5 (26). The difference map was calculated by subtracting the three-dimensional volume of the RyR2(A4860G) from the RyR2(A4860G)Tyr-846-GFP volume.
Ca2+ Release Assay
Measurements of free cytosolic Ca2+ concentrations in the transfected HEK293 cells using fluorescence Ca2+ indicator dye fluo-3 were done as described previously (27).
GST-FKBP12.6 Pulldown, Immunoprecipitation, Immunostaining, Immunoblotting, and [3H]Ryanodine Binding Analyses
GST-FKBP12.6 pull-down, immunoprecipitation, immunostaining, immunoblotting, and [3H]ryanodine binding were carried out as described previously (27, 28).
FRET Measurements
HEK293 cells grown in 35-mm glass-bottomed culture dishes for 20–24 h after subculture were transfected with 1.8 μg of cDNA of various RyR2 or FKBP12.6 cDNA constructs using Ca2+ phosphate precipitation as described previously (29). 24–48 h after transfection, the cells were washed three times with KRH buffer without MgCl2 or CaCl2 and monitored on a Leica TCS SP5 confocal laser scanning microscope with a 63×/NA1.4 oil immersion objective lens. Cells were kept at 37 °C using a water-heated stage incubator. We utilized the acceptor photobleaching approach to detect and measure FRET signals in the live cells. For CFP/YFP pairs, CFP and YFP were excited with separate laser channels of 458 and 514 nm, respectively. Emission fluorescence intensity was obtained at 465–495 nm for CFP and 520–550 nm for YFP. For GFP/Alexa Fluor 555 pairs, GFP and Alexa Fluor 555 were excited at 476 and 543 nm, and fluorescent emission was obtained at 495–525 and 565–595 nm, respectively. We used a 700-Hz line frequency scan speed with bidirectional scan mode in combination with an image format of 1024 × 1024 pixels, which can record one image every 754 milliseconds. Repeated scans (30–60 in 23–45 s) with maximum laser intensity at 514 nm or 543 nm were used to photobleach YFP or Alexa Fluor 555, respectively. The FRET efficiency was calculated according to the equation: E = [(Idonor-post − Idonor-pre)/Idonor-post] × 100%, where Idonor-pre and Idonor-post are the respective background-corrected donor fluorescence intensities before and after photobleaching of the acceptor fluorescence (30). The photobleaching, fluorescence intensity measurements, and FRET efficiency calculation were controlled automatically by the software Leica Application Suite Advanced Fluorescence (LAS SF).
Fluorescence Recovery after Photobleaching (FRAP)
FRAP experiments were performed to investigate the possibility that diffusion of nonbleached RyRs into bleached areas interferes with the FRET analysis in live HEK293 cells. Photobleaching was performed in the same way as in FRET experiments, and fluorescent intensity of YFP was continuously monitored at 520–550 nm. The FRAP protocol was also controlled by LAS SF. FRAP was performed in 10 separate cells and then averaged for analysis.
Data Analysis
All of the data are presented as the means ± S.E. The data analysis was performed with the unpaired Student's t test. A p value below 0.05 is considered statistically significant.
RESULTS
Insertion of GFP near the Dantrolene-binding Sequence Abolishes RyR2/GST-FKBP12.6 Interaction
We have previously mapped the locations of a number of residues in the three-dimensional structure of RyR2 using GFP as a structural marker (31). In an attempt to map the three-dimensional location of the dantrolene-binding sequence (residues 601–620) in RyR2, we constructed and expressed in HEK293 cells (Fig. 1A) a GFP-RyR2 fusion protein, RyR2Arg-626-GFP, in which GFP was inserted into RyR2 after residue Arg-626 near the dantrolene-binding sequence. To ensure that the inserted GFP does not grossly disrupt the structure and function of RyR2, we determined the response of RyR2Arg-626-GFP to caffeine and ryanodine. As shown in Fig. 1B, like cells transfected with RyR2(WT), HEK293 cells transfected with RyR2Arg-626-GFP responded to both caffeine and ryanodine (Fig. 1B, panels a and b), whereas HEK293 cells transfected with no DNA showed no response to either caffeine or ryanodine (n = 3–4) (Fig. 1B, panel d). Ryanodine caused a slow release of Ca2+ (Fig. 1B, panels a and b), which was not seen in cells without ryanodine treatment (n = 3) (Fig. 1B, panel c). These data indicate that RyR2Arg-626-GFP forms a functional intact channel.
FIGURE 1.
Expression and characterization of RyR2Arg-626-GFP. A, HEK293 cells grown on glass coverslips were transfected with RyR2Arg-626-GFP cDNA. Bright field (panel a) and green fluorescence images (panel b) of HEK293 cells expressing RyR2Arg-626-GFP were taken under the fluorescence microscope at 40× magnification. B, HEK293 cells were transfected with RyR2(WT), RyR2Arg-626-GFP, or no cDNA. Fluorescence intensity of the fluo-3-loaded transfected cells was monitored continuously before and after addition of caffeine or ryanodine. The sharp decreases in fluorescence intensity immediately after the second and third additions of caffeine were due to fluorescence quenching by caffeine. Similar results were obtained from three or four separate experiments. C, RyR2(WT) or RyR2Arg-626-GFP expressed in HEK293 cells was pulled down using GST-FKBP12.6 (panel a), and RyR2(WT) or RyR2Arg-626-GFP co-expressed with FKBP12.6 or CFP-FKBP12.6 in HEK293 cells were co-immunoprecipitated using anti-RyR antibody 34c (panel b). The pulled down and immunoprecipitated proteins were separated in 6% SDS-PAGE and transferred to nitrocellulose membranes. The membrane was immunoblotted with either anti-RyR antibody (34c) or anti-FKBP12 antibody (n = 3). WB, Western blot; R626, Arg-626.
We found that RyR2Arg-626-GFP cannot be pulled down by the GST-FKBP12.6 affinity column (n = 3) (Fig. 1C, panel a). This result is in apparent contrast with the ability of RyR2Arg-626-GFP to form a functional channel. Furthermore, this result is in contrast to all other GFP-RyR2 fusion proteins reported previously (31), suggesting that the insertion of GFP at this particular site may block high affinity binding to the FKBP12.6 affinity column. To further investigate the impact of the inserted GFP on the FKBP12.6-RyR2 interaction, we co-transfected HEK293 cells with RyR2(WT) or RyR2Arg-626-GFP and FKBP12.6 or a bulky, CFP-tagged FKBP12.6 (CFP-FKBP12.6). The interactions of FKBP12.6 or CFP-FKBP12.6 with RyR2(WT) or RyR2Arg-626-GFP were then assessed by immunoprecipitation using an anti-RyR2 antibody, followed by immunoblotting. As shown in Fig. 1C (panel b), the bulky CFP-FKBP12.6 was co-immunoprecipitated with RyR2(WT), but not with RyR2Arg-626-GFP (n = 3), which is consistent with the inability of GST-FKBP12.6 to pull down RyR2Arg-626-GFP. However, RyR2Arg-626-GFP was able to interact with the smaller, non-GST, non-CFP-tagged FKBP12.6 (Fig. 1C, panel b), although the level of FKBP12.6 co-immunoprecipitated with RyR2Arg-626-GFP was reduced compared with that co-immunoprecipitated with RyR2(WT) (n = 3). These data suggest that the inserted GFP sterically hinders the binding of the bulky GST- or CFP-tagged FKBP12.6 to RyR2. These observations also suggest that the inserted GFP, and thus the dantrolene-binding sequence, is likely to lie near the FKBP12.6-binding site in the three-dimensional structure of RyR2.
Localization of GFP Inserted after Residue Tyr-846 in the Three-dimensional Structure of RyR2
Because the insertion of GFP after Arg-626 prevented RyR2 purification by the GST-FKBP12.6 affinity column, we reasoned that some further insight into the location of the dantrolene-binding sequence might be gained by mapping the locations of nearby residues. We have previously mapped the location of GFP inserted after residue Ser-437 (18), upstream of the dantrolene-binding sequence. Here we sought to map the location of GFP inserted downstream of the dantrolene-binding sequence. To this end, we inserted GFP into RyR2 after residue Tyr-846 to generate the GFP fusion protein RyR2Tyr-846-GFP and expressed it in HEK293 cells (Fig. 2A). As with RyR2(WT)-expressing cells, HEK293 cells expressing RyR2Tyr-846-GFP responded to both caffeine and ryanodine (n = 3) (Fig. 2B), indicating that the inserted GFP did not grossly disrupt the structure and function of RyR2. Furthermore, RyR2Tyr-846-GFP is able to bind both FKBP12.6 and CFP-FKBP12.6 (n = 3) (Fig. 2C).
FIGURE 2.
Expression and characterization of RyR2Tyr-846-GFP. A, bright field (panel a) and green fluorescence images (panel b) of HEK293 cells expressing RyR2Tyr-846-GFP. B, Ca2+ release measurement of RyR2Tyr-846-GFP following the addition of caffeine and ryanodine. C, RyR2(WT) or RyR2Tyr-846-GFP co-expressed with FKBP12.6 or CFP-FKBP12.6 in HEK293 cells were co-immunoprecipitated using anti-RyR antibody (34c) and immunoblotted with either anti-RyR antibody (34c) or anti-FKBP12 antibody. Similar results were obtained from three separate experiments. Y846, Tyr-846.
We next expressed and purified the GFP-RyR2 fusion protein for three-dimensional reconstruction by cryo-EM. We have previously found that the A4860G mutation, located in the inner pore helix of RyR2, results in a more structurally homogeneous cryo-EM preparation than RyR2(WT) (32, 33). Accordingly, this A4860G mutant was used as a background for the three-dimensional mapping of GFP insertions. To obtain sufficient purified protein for cryo-EM, we transfected large quantities of HEK293 cells (80 plates, 10-cm diameter) with the RyR2(A4860G)Tyr-846-GFP cDNA. The RyR2(A4860G)Tyr-846-GFP protein was purified from cell lysate by a single step affinity chromatography using GST-FKBP12.6 as the affinity ligand. The purified proteins remained functional, because they displayed Ca2+- and caffeine-sensitive [3H]ryanodine binding (Fig. 3A). The purified RyR2(A4860G)Tyr-846-GFP proteins were preserved into a thin layer of vitreous ice and imaged by cryo-EM (34, 35).
FIGURE 3.
Cryo-electron microscopy of RyR2(A4860G)Tyr-846-GFP and two-dimensional averages of RyR2(A4860G)Tyr-846-GFP and RyR2(A4860G). A, shows the effect of Ca2+ and caffeine on [3H]ryanodine binding to purified RyR2(A4860G)Tyr-846-GFP. The amounts of bound [3H]ryanodine under each condition are presented as percentages of the maximum binding (100%) determined at 100 μm Ca2+. The data shown are the means ± S.E. (n = 3). B, a portion of a cryo-EM micrograph of the purified RyR2(A4860G)Tyr-846-GFP proteins embedded in a thin layer of vitreous ice is shown. Several individual RyR2(A4860G)Tyr-846-GFP particles are marked with white circles. The scale bar represents 500 Å. C, panel a, two-dimensional average of RyR2(A4860G)Tyr-846-GFP (n = 304 particle images) top view; panel b, top view of the two-dimensional average of RyR2(A4860G) (n = 285 particle images); panel c, difference map obtained by the subtraction of panel b from panel a. The top view represents the projection of the channel as seen from the cytoplasmic side. The largest differences shown in panel c, corresponding to the additional masses caused by the insertion of GFP, are seen as bright white areas, one of which is circled. Panel d, map of statistically significant regions of difference obtained by t test; the map is displayed at >99.9% confidence level. The width of each frame is 544 Å.
A typical electron micrograph of frozen-hydrated RyR2(A4860G)Tyr-846-GFP is shown in Fig. 3B, and a two-dimensional analysis is shown in Fig. 3C. The two-dimensional average of RyR2(A4860G)Tyr-846-GFP (Fig. 3C, panel a) correlated closely with that of RyR2(A4860G) reported previously (Fig. 3C, panel b) (32), apart from the presence of additional small regions of density. To identify the subtle differences between the two-dimensional averages of RyR2(A4860G)Tyr-846-GFP and RyR2(A4860G), a difference map was generated by subtracting the two-dimensional average of RyR2(A4860G) from that of RyR2(A4860G)Tyr-846-GFP (Fig. 3C, panel c). The white areas shown in Fig. 3C (panel d) represent significant differences in protein mass between RyR2(A4860G)Tyr-846-GFP and RyR2(A4860G). These areas of extra mass were located in a region corresponding to domain 9 in the three-dimensional structure of RyR2.
To obtain a more detailed structure of RyR2(A4860G)Tyr-846-GFP, we carried out three-dimensional reconstructions. Fig. 4A shows a surface representation of the three-dimensional reconstruction of RyR2(A4860G)Tyr-846-GFP in the top, side, and bottom views. There are two major components in the reconstructed RyR2(A4860G)Tyr-846-GFP structure: a large cytoplasmic assembly (290 × 290 × 130 Å) encompassing 10 distinct domains (labeled with numbers) and a smaller transmembrane assembly (120 × 120 × 70 Å, labeled with TA). Comparing the three-dimensional reconstruction of RyR2(A4860G)Tyr-846-GFP with that of the RyR2(A4860G) obtained previously (32) revealed a major difference in domain 9, one of the domains located within the “clamp” structures at the corners of the cytoplasmic assembly (Fig. 4B). To more precisely determine the differences, we generated a three-dimensional difference map by subtracting the three-dimensional volume of RyR2(A4860G) from that of RyR2(A4860G)Tyr-846-GFP. The difference regions are displayed in green and superimposed on the three-dimensional reconstruction of RyR2(A4860G) (shown in blue) (Fig. 4C). The difference map clearly shows four significant differences located on the top side (i.e. facing the t-tubule) of domain 9 in the cytoplasmic assembly. Assuming a protein density of 1.37 g/cm3 (34), each of the four differences corresponds to a molecular mass of ∼28 kDa. This estimate agrees well with the molecular mass of GFP. Thus, the inserted GFP after residue Tyr-846 is located in domain 9 near the FKBP12.6-binding site.
FIGURE 4.
Three-dimensional reconstructions and difference map of RyR2(A4860G)Tyr-846-GFP and RyR2(A4860G). The three-dimensional reconstruction of RyR2(A4860G) is shown in A (blue), and RyR2(A4860G)Tyr-846-GFP is shown in B (green). The difference map (RyR2(A4860G)Tyr-846-GFP minus RyR2(A4860G)) shown in green is superimposed on the three-dimensional reconstruction of RyR2(A4860G) in blue (C). The asterisk indicates the location of the FKBP12.6-binding site. The three-dimensional reconstructions are shown in three views: left panel, top view from the cytoplasmic surface, which in situ would face the transverse tubule; middle panel, side view; right panel, bottom of the channel facing the lumen of the sarcoplasmic reticulum. The numbers on the cytoplasmic assembly indicate the distinguishable domains.
Localization of the Dantrolene-binding Sequence in the Three-dimensional Structure of RyR2 Based on FRET Analysis
We next sought to map the location of the dantrolene-binding sequence by comparing the efficiency of FRET between a fluorescent tag (YFP, GFP, or CFP) inserted after residue Arg-626 and each of the three known locations (FKBP12.6, Ser-437-GFP, and Tyr-846-GFP) in the three-dimensional structure of RyR2. We first used the location of FKBP12.6 as a reference point. We inserted YFP after residues Ser-437, Arg-626, and Tyr-846 to generate RyR2Ser-437-YFP, RyR2Arg-626-YFP, and RyR2Tyr-846-YFP and CFP into the NH2 terminus of FKBP12.6 to form CFP-FKBP12.6. We then co-transfected HEK293 cells with CFP-FKBP12.6 and RyR2Ser-437-YFP, RyR2Arg-626-YFP, or RyR2Tyr-846-YFP to form three FRET pairs: RyR2Ser-437-YFP/CFP-FKBP12.6, RyR2Arg-626-YFP/CFP-FKBP12.6, and RyR2Tyr-846-YFP/CFP-FKBP12.6. The CFP (donor) fluorescence signal was recorded using confocal microscopy before and after photobleaching of the YFP (acceptor) signal (Fig. 5A). The differences in the CFP fluorescence intensity before and after photobleaching were used to calculate the FRET efficiency as described under “Experimental Procedures.”
FIGURE 5.
FRET between CFP-FKBP12.6 and RyR2Ser-437-YFP, RyR2Arg-626-YFP, or RyR2Tyr-846-YFP. A, confocal images showing cyan and yellow fluorescence before and after photobleaching of HEK293 cells that co-express CFP-FKBP12.6 and RyR2Tyr-846-YFP. The ellipse in each panel demarcates the area selected for photobleaching. Scale bar, 10 μm. B, averaged FRET efficiencies of the RyR2Ser-437-YFP/CFP-FKBP12.6, RyR2Arg-626-YFP/CFP-FKBP12.6, and the RyR2Tyr-846-YFP/CFP-FKBP12.6 FRET pairs. The data are shown as the means ± S.E. with the number of cells indicated. C, FRAP of RyR2Ser-437-YFP. HEK293 cells expressing RyR2Ser-437-YFP were imaged with confocal laser scanning microscopy. YFP was photobleached at maximum laser intensity at 514 nm. Fluorescence intensity in the bleached region was monitored continuously during and after bleaching. D, three-dimensional locations of Ser-437-YFP, Tyr-846-YFP, FKBP12.6 (red), and the CFP inserted into the NH2 terminus of FKBP12.6.
There is a potential concern in measuring FRET in live cells using the photobleaching approach: the recovery of fluorescence through diffusion of acceptor RyR2s into the bleached field may lower the actual donor emission intensity after photobleaching, which would result in an underestimated FRET efficiency. To rule out the possibility that diffusion of RyR2s may contribute to changes in FRET, we performed a FRAP study. We continually monitored the recovery of acceptor fluorescence after applying the same photobleaching that was used for FRET study. Our data showed that the fluorescence intensity of photobleached acceptor recovered less than 3% after 50 s (same time frame in which we performed our photobleaching FRET experiments) and less than 5% after 3 min (Fig. 5C). The results from FRAP demonstrate that diffusion, both laterally and axially, of RyR2s in the live cells would not significantly interfere with FRET efficiency determined in our experiments.
Based on their three-dimensional locations (18, 19, 36, 37), CFP-FKBP12.6 and Ser-437-YFP are separated by 35 Å and should thus be situated close enough for FRET. Accordingly, the results in Fig. 5B demonstrate significant FRET between CFP-FKBP12.6 and Ser-437-YFP (7.8 ± 0.7%, n = 35, p < 0.001). Tyr-846-YFP is also situated close to CFP-FKBP12.6 in the three-dimensional structures (31-Å separation; Fig. 5D). Accordingly, the efficiency of FRET between CFP-FKBP12.6 and Tyr-846-YFP was even greater (12.2 ± 0.6%, n = 32, p < 0.05; Fig. 5B). In contrast, HEK293 cells co-transfected with CFP-FKBP12.6 and RyR2Arg-626-YFP displayed no significant FRET (0.6 ± 0.2%, n = 40), which is consistent with our observation that CFP-FKBP12.6 does not bind to RyR2Arg-626-GFP (Fig. 1).
Because FKBP12.6 can still bind to RyR2Arg-626-GFP, although YFP-FKBP12.6 or GST-FKBP12.6 cannot, we also employed a recombinant FKBP12.6 protein labeled with a small Alexa Fluor dye (AF555) at residue 14 of FKBP12.6 (a yellow dot in Fig. 6B) (38). Cells transfected with RyR2Ser-437-GFP, RyR2Arg-626-GFP, or RyR2Tyr-846-GFP were permeabilized by saponin and incubated with AF555-FKBP12.6. After removing the unbound AF555-FKBP12.6, the GFP (donor) fluorescence signals were recorded before and after photobleaching of AF555 (acceptor) signal and were used to calculate the FRET efficiency as described under “Experimental Procedures.” Based on their known three-dimensional locations, the distance between AF555-FKBP12.6 and Tyr-846-GFP is 43 Å, whereas the distance between AF555-FKBP12.6 and Ser-437-GFP is 55 Å. Consistent with these distance relationships, the efficiency of FRET between AF555-FKBP12.6 and Tyr-846-GFP is 18.5 ± 1.6% (n = 22), which is greater than that between AF555-FKBP12.6 and Ser-437-GFP (8.1 ± 0.8%, n = 20, p < 0.05). On the other hand, the FRET efficiency between AF555-FKBP12.6 and Arg-626-GFP is 10.8 ± 1.0% (n = 25), which is significantly greater than that between AF555-FKBP12.6 and Ser-437-GFP (p < 0.05) but significantly less than that between AF555-FKBP12.6 and Tyr-846-GFP (p < 0.05). Based on these data, we conclude that as with Tyr-846-GFP, Arg-626-GFP is located near to AF555-FKBP12.6 and overlaps with CFP-FKBP12.6 (Fig. 6B).
FIGURE 6.
FRET between AF555-labeled FKBP12.6 and RyR2Ser-437-GFP, RyR2Arg-626-GFP, or RyR2Tyr-846-GFP. A, averaged FRET efficiencies of the RyR2Ser-437-GFP/AF555-FKBP12.6, RyR2Arg-626-GFP/AF555-FKBP12.6, and the RyR2Tyr-846-GFP/AF555-FKBP12.6 FRET pairs. B, three-dimensional locations of Ser-437-GFP, Tyr-846-GFP, FKBP12.6 (red), AF555 (yellow dot) labeled at position 14 in FKBP12.6, and the CFP inserted into the NH2 terminus of FKBP12.6. The red dashed curved line depicts the possible location of Arg-626-GFP that overlaps the CFP-FKBP12.6.
We next used the other two known locations (residues Ser-437 and Tyr-846) as reference points to estimate the location of residue Arg-626. To this end, we generated three dual YFP- and CFP-labeled RyR2 constructs: 1) RyR2Ser-437-YFP/Arg-626-CFP (in which YFP was inserted into RyR2 after residue Ser-437 and CFP was inserted after residue Arg-626), 2) RyR2Tyr-846-YFP/Arg-626-CFP, and 3) RyR2Ser-437-YFP/Tyr-846-CFP. To ensure that the dual insertion of YFP and CFP does not grossly disrupt the structure and function of the RyR2 channel, we determined the response of these RyR2 constructs to caffeine and ryanodine as described above. As shown in Fig. 7A, HEK293 cells transfected with any of these dual YFP- and CFP-labeled RyR2 constructs responded to both caffeine and ryanodine, indicating that they are functional. We then measured the CFP (donor) fluorescence signals before and after photobleaching the YFP (acceptor) fluorescence signal and used these values to calculate the FRET efficiency. As shown in Fig. 7B, the FRET efficiencies between Ser-437-YFP and Arg-626-CFP (19.0 ± 1.6%, n = 25) and between Ser-437-YFP and Tyr-846-CFP (19.5 ± 1.5%, n = 25) are similar but are slightly greater than that between Tyr-846-YFP and Arg-626-CFP (16.5 ± 1.7%, n = 22). Based on these results, we conclude that Ser-437-YFP, Arg-626-CFP, and Tyr-846-YFP are located roughly equidistant from each other (Fig. 7C).
FIGURE 7.
FRET in dual CFP- and YFP-tagged RyR2s. A, HEK293 cells were transfected with RyR2Ser-437-YFP/Arg-626-CFP (panel a), RyR2Tyr-846-YFP/Arg-626-CFP (panel b), RyR2Ser-437-YFP/Tyr-846-CFP (panel c), or RyR2Ser-2367-YFP/Arg-626-GFP (panel d). Fluorescence intensity of the fluo-3-loaded transfected cells was monitored continuously before and after the addition of caffeine or ryanodine. Similar results were obtained from three to five separate experiments. B, averaged FRET efficiencies of RyR2Ser-437-YFP/Arg-626-CFP, RyR2Tyr-846-YFP/Arg-626-CFP, or RyR2Ser-437-YFP/Tyr-846-CFP, co-expressed with or without FKBP12.6 in HEK293 cells with or without permeabilization in the absence or presence of FK506 are shown. The averaged FRET efficiencies of the CFP-FKBP12.6/RyR2Tyr-846-YFP FRET pair after being treated with FK506 and the RyR2Ser-2367-YFP/Arg-626-CFP FRET pair are also shown. C, proposed location of Arg-626-CFP in relation to the locations of Ser-437-YFP, Tyr-846-YFP, and Ser-2367-YFP based on cryo-EM and FRET. S437, Ser-437; R626, Arg-626; Y846, Tyr-846; S2367, Ser-2367.
Because the locations of Ser-437-YFP, Tyr-846-YFP, and Arg-626-CFP are in close proximity to that of FKBP12.6, it is possible that the binding or unbinding of FKBP12.6 may alter the conformation of RyR2 and thus the relative distances between these locations. To test this possibility, we co-expressed each of the dual YFP- and CFP-labeled RyR2 FRET pairs with FKBP12.6 in HEK293 cells and determined the FRET efficiencies before and after membrane permeabilization, followed by the treatment of FK506 to dissociate FKBP12.6 from RyR2. We found that none of these conditions significantly altered the FRET efficiency of any of these FRET pairs (Fig. 7B), suggesting that the binding or unbinding of FKBP12.6 does not perturb the relative distances among Ser-437-YFP, Tyr-846-YFP, and Arg-626-CFP. It should be noted that treating HEK293 cells expressing RyR2Tyr-846-YFP and CFP-FKBP12.6 with FK506 completely abolished the FRET signal (Fig. 7B) (n = 20), indicating that under these conditions, FK506 is effective in dissociating FKBP12.6.
We have previously mapped the three-dimensional location of Ser-2367-GFP (17), which is 64 Å away from the estimated location of Arg-626-CFP (Fig. 7C). Based on this distance, one would expect that the FRET efficiency between Arg-626-CFP and Ser-2367-YFP would be much smaller than that between Arg-626-CFP and Ser-437-YFP (19.0%). Indeed, the FRET efficiency between Arg-626-CFP and Ser-2367-YFP is only 2.6 ± 0.4% (n = 21). These observations suggest that residue Arg-626, which is near the dantrolene-binding sequence, is not physically adjacent to residue Ser-2367 in the central region of the amino acid sequence of RyR2.
DISCUSSION
Dantrolene is currently the most effective treatment for malignant hyperthermia (8). It has also been used to treat other disorders such as muscle spasticity and neuroleptic malignant syndrome (39). The major target of dantrolene is the skeletal muscle ryanodine receptor (RyR1) that is responsible for Ca2+ release from the sarcoplasmic reticulum (12–14). Some Ca2+ influx pathways have also been shown to be a target of dantrolene (40, 41). Recently, dantrolene has also been shown to target the cardiac ryanodine receptor (RyR2) and improve intracellular Ca2+ handling in failing cardiomyocytes from a canine model of heart failure (11, 15, 16). Despite its remarkable effectiveness in suppressing malignant hyperthermia and potential clinical significance in treating heart failure, the molecular mechanism of dantrolene action remains largely undefined.
It has been proposed that the NH2-terminal and central regions, although separated by ∼2000 residues in the linear sequence, are co-localized and interact with each other in the three-dimensional structure of RyR to form a domain switch that is critical in stabilizing the closed state of the channel (42). Disease-causing RyR mutations in either the NH2-terminal or central region weaken or “unzip” the normal domain-domain interactions between these two regions and thus destabilize the closed state of the channel, leading to an enhanced sensitivity of the channel to activation by various stimuli. In contrast, dantrolene is believed to directly bind to this domain switch and stabilize the channel and thus suppress the impact of disease-causing mutations (10). In the absence of high resolution structures of RyR, the precise nature of this domain switch and its interaction with dantrolene is largely unknown.
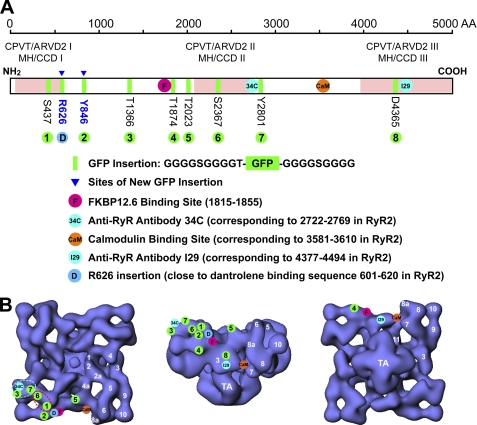
An alternative approach to gaining insight into domain-domain interactions in RyR is to map the domain topology of RyR by correlating the linear sequence of the channel with the characteristic domains identified in the low resolution three-dimensional structures that are available from cryo-EM (31). Using this approach, we have mapped various sites in the first ∼3000 amino acid sequence of RyR onto the three-dimensional structure of the channel by inserting GFP into specific sites and determining the three-dimensional locations of the inserted GFP using cryo-EM and single particle image processing (17, 18, 28, 31, 32, 43). For example, we have determined the three-dimensional locations of GFP inserted into the RyR2 sequence after residues Ser-437 and Ser-2367. These mapping studies revealed that residue Ser-437 and residue Ser-2367 are co-localized in the three-dimensional structure of RyR (Fig. 8, dashed red oval). Thus, these observations suggest that the NH2-terminal region around residue 437 may interact with the central region around residue Ser-2367, which is consistent with the domain switch hypothesis.
FIGURE 8.
Locations of specific sites and sequences in the three-dimensional structure of RyR2. A, schematic illustration of GFP insertions into various sites in the RyR2 amino acid sequence. The bar represents the ∼5000-amino acid sequence of RyR. Disease-linked RyR1 and RyR2 mutations are largely clustered in three regions (pink shaded areas). In this study, GFP flanked by two glycine-rich spacers was inserted after residues Arg-626 and Tyr-846 (marked by triangle pointed green bands). Other GFP insertions and sites mapped previously are also shown. B, three-dimensional structure of RyR2 with top view (T-tubule face) (left panel), side view (middle panel), and bottom view (sarcoplasmic reticulum face) (right panel). The three-dimensional locations of eight GFP insertions, and GFP inserted after Arg-626 (close to the dantrolene-binding sequence), the FKBP12/12.6-binding site (residues 1815–1855), an anti-RyR antibody (34c) epitope (residues 2722–2769), the CaM-binding site (residues 3614–3643 in RyR2), and an anti-RyR antibody epitope (residues 4377–4494) were shown. The numbers in white indicate subdomains of RyR2. The dashed ellipses indicate regions that potentially contain domain-domain contacts. S437, Ser-437; R626, Arg-626; Y846, Tyr-846; T1366, Thr-1366; T1874, Thr-1874; T2023, Thr-2023; S2367, Ser-2367; T2801, Thr-2801; D4365, Asp-4365.
In those cases where an alternative to GFP labeling was available (e.g. an antibody) to confirm the GFP localization, we have generally found good agreement of the results (23, 28, 32, 44, 45), indicating that the accuracy of GFP localization by three-dimensional cryo-EM is probably a few nanometers. However, if the GFP is inserted into a surface residue within an unusually flexible and extendable loop or if some physical attribute of the insertion site causes the polyglycine linkers that bridge the insertion site to the GFP to become fully extended, then deviations of the GFP from the insertion site of more than a few nanometers are possible. Such a situation might have occurred for our previously reported localization of Ser-437-GFP (see the following paragraph). However, it should be appreciated that regardless of the distance of GFP from residue Ser-437, it still serves as a legitimate FRET probe for investigating spatial relationships to other sites on the surface of RyR.
Tung et al. (46) have recently reported the crystal structure of a RyR1 fragment comprising the first 559 residues of the channel and further showed that this structure may be docked in cryo-EM density maps to a region surrounding the central pore of the cytoplasmic assembly of the channel. The dantrolene-binding sequence lies outside the crystal structure of Tung et al. (46). Similarly, the GFP insertions mapped here (Arg-626-GFP and Tyr-846-GFP) are also outside that crystal structure but may be assumed to identify subsequent structural domains formed by the RyR1 linear sequence. In this regard, our results indicate that these domains localize near the FK506-binding protein-binding site and the outer edge of the cytoplasmic assembly. Notably, the placement of Ser-422 in the docking model of Tung et al. is roughly 60 Å removed from the location predicted by our previous cryo-EM mapping of a GFP insertion at this site (Ser-437 in RyR2). The authors attribute this difference to the size of GFP itself, the use of extended polyglycine linkers, or the flexibility of the surface loop into which the insertion was made (46). Note, however, this localization for Ser-437 still places it nearby to Ser-2367, consistent with the domain switch hypothesis. Further clarification of structural relationships within the intact RyR complex will likely rely on the continued integration of low resolution cryo-EM structures and high resolution atomic structures of RyR fragments, as well as on biochemical and spectroscopic analysis.
An important question is whether dantrolene directly binds to this domain switch formed between the NH2-terminal (residues 1–437) and central (around residue Ser-2367) regions. To address this question, we attempted to localize the dantrolene-binding sequence (residues 601–620) in the three-dimensional structure of RyR2 using cryo-EM and single particle analysis. As we have done with other three-dimensional mappings, we used GFP as a structural marker and inserted it after residue Arg-626. We have successfully used GST-FKBP12.6 as an affinity ligand to purify a number of GFP-tagged RyR2 proteins for three-dimensional reconstruction in a single purification step (31). Surprisingly, we found that the insertion of GFP after residue Arg-626 abolished GST-FKBP12.6 binding. As a result, GST-FKBP12.6 cannot be used for the purification of RyR2Arg-626-GFP. A number of alternative affinity tags, including His, Strept, and GST tags, inserted into the NH2 terminus or internal sites of RyR2 were explored. Unfortunately, none of these alternative affinity purification methods was able to produce a sufficient amount of purified RyR2Arg-626-GFP protein suitable for three-dimensional reconstruction. Although RyR2Arg-626-GFP cannot be pulled down by GST-FKBP12.6, it does bind to a smaller, nontagged FKBP12.6, suggesting that the GFP inserted after Arg-626 is juxtaposed with the FKBP12.6-binding site. Consistent with this notion, we have previously shown that an NH2-terminal region (residues 305–784) encompassing residue Arg-626 and the dantrolene-binding sequence is required for FKBP12.6 binding (43, 47). We have also mapped the location of residue Ser-437, upstream of the dantrolene-binding sequence, to domain 5 near the FKBP12.6-binding site. To gain further structural insight into this NH2-terminal region, we inserted GFP after residue Tyr-846, downstream of the dantrolene-binding sequence, and mapped the three-dimensional location of the inserted GFP, and thus residue Tyr-846, to the top side of domain 9 in the cytoplasmic assembly also near the FKBP12.6-binding site (19, 36, 37). Taken together, these observations are consistent with the notion that the dantrolene-binding sequence is located in close proximity to the FKBP12.6-binding site.
To further map the three-dimensional location of the dantrolene-binding sequence, we used three known locations (the FKBP12.6-binding site, residue Ser-437, and residue Tyr-846) as reference points and measured the efficiency of FRET between fluorescence tags inserted into each of these sites. Based on the known distances and the FRET efficiencies measured between the three known sites and the FRET efficiencies measured between residue Arg-626 and each of the three known sites, we estimated the distances between Arg-626-GFP and AF555-FKBP12.6 to be in the range of 43–55Å, whereas we estimated the distance between Arg-626-CFP and Ser-437-YFP and that between Arg-626-CFP and Tyr-846-YFP to be ∼28 Å. On the basis of these estimations, we propose that the Arg-626-CFP or -GFP, and thus the dantrolene-binding sequence, is located to a region beside domain 5 and adjacent to the FKBP12.6-binding site (Fig. 7C). This estimated location of the dantrolene-binding sequence is far away (∼64 Å) from residue Ser-2367 in the central region. Consistent with this estimation, the FRET efficiency between Arg-626-CFP and Ser-2367-YFP is much less than that between Arg-626-CFP and Ser-437-YFP. The dantrolene-binding sequence represents an authentic dantrolene-binding site in intact RyR1 (14); the corresponding sequence in RyR2 likely binds dantrolene under certain environmental conditions (11). Therefore, these FRET analyses suggest that dantrolene is unlikely to bind directly to the domain switch that is formed between the NH2-terminal region around residue Ser-437 and the central region around residue Ser-2367.
How then does dantrolene stabilize the domain switch without directly binding to it? It is possible that the domain switch really represents several domain-domain contacts between the NH2-terminal and central regions and that dantrolene may bind near to the domain switch and allosterically stabilize it. A recent x-ray structure of RyR1 NH2-terminal residues 1–559 together with docking of the structure into three-dimensional reconstructions from cryo-EM of structurally intact RyR1 has been interpreted to indicate that multiple domain-domain interfaces are involved in disruption of calcium regulation by the various disease-causing mutations (46).
We have previously mapped residue Thr-1874 to domain 9 near the FKBP12.6-binding site (18, 43, 47). In the present study, we located residue Arg-626 to a region near domain 5 and adjacent to FKBP12.6-binding site. Consistent with these locations, we have previously shown that residues between 305 and 784 and residues between 1815 and 1855 are essential for FKBP12.6 binding (47). Thus, an NH2-terminal region around the dantrolene-binding sequence and a central region between 1815 and 1855 are co-localized and may form another domain-domain contact region. Dantrolene may stabilize interdomain interaction(s) in this region, which in turn may influence the stability of the domain switch. Additional domain-domain contacts may exist between an NH2-terminal region around residue Thr-1366 and a central region around residue Tyr-2801 and the anti-RyR antibody (34c) epitope (corresponding to residues 2722–2769 in RyR2), because these residues are also co-localized in the three-dimensional structure of RyR2 (Fig. 8, blue dashed circle) (17, 18, 28, 31, 32, 43). Therefore, there are multiple domain-domain contacts between the NH2-terminal and central regions. These interdomain interactions may interdependently stabilize the overall structure of the RyR channel.
Acknowledgments
We thank Jeff Bolstad for excellent technical assistance; the Wadsworth Center's Molecular Genetics, Advanced Light Microscopy and Image Analysis, Electron Microscopy Core Facilities; and the Resource for Visualization of Biological Complexity.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 HL075210 (to S. R. W. C.), R01HL095541 (to Z. L.), R01AR040615 (to T. W.), and R01HL076433 (to B. R. F.). This work was also supported by research grants from the Canadian Institutes of Health Research and the Heart and Stroke Foundation of Canada (to S. R. W. C.) and by National Institutes of Health Biotechnological Resource Grant P41RR01219.
- RyR
- ryanodine receptor
- CFP
- cyan fluorescent protein
- YFP
- yellow fluorescent protein
- CHAPS
- 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid
- PIPES
- 1,4-piperazinediethanesulfonic acid
- FRAP
- fluorescence recovery after photobleaching.
REFERENCES
- 1. Fill M., Copello J. A. (2002) Physiol. Rev. 82, 893–922 [DOI] [PubMed] [Google Scholar]
- 2. Bers D. M. (2002) Nature 415, 198–205 [DOI] [PubMed] [Google Scholar]
- 3. Sutko J. L., Airey J. A. (1996) Physiol. Rev. 76, 1027–1071 [DOI] [PubMed] [Google Scholar]
- 4. MacLennan D. H. (2000) Eur. J. Biochem. 267, 5291–5527 [DOI] [PubMed] [Google Scholar]
- 5. Robinson R., Carpenter D., Shaw M. A., Halsall J., Hopkins P. (2006) Hum. Mutat. 27, 977–989 [DOI] [PubMed] [Google Scholar]
- 6. Priori S. G., Napolitano C. (2005) J. Clin. Invest. 115, 2033–2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. George C. H., Jundi H., Thomas N. L., Fry D. L., Lai F. A. (2007) J. Mol. Cell. Cardiol. 42, 34–50 [DOI] [PubMed] [Google Scholar]
- 8. Krause T., Gerbershagen M. U., Fiege M., Weisshorn R., Wappler F. (2004) Anaesthesia 59, 364–373 [DOI] [PubMed] [Google Scholar]
- 9. Rosenberg H., Davis M., James D., Pollock N., Stowell K. (2007) Orphanet J. Rare Dis. 2, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kobayashi S., Bannister M. L., Gangopadhyay J. P., Hamada T., Parness J., Ikemoto N. (2005) J. Biol. Chem. 280, 6580–6587 [DOI] [PubMed] [Google Scholar]
- 11. Kobayashi S., Yano M., Suetomi T., Ono M., Tateishi H., Mochizuki M., Xu X., Uchinoumi H., Okuda S., Yamamoto T., Koseki N., Kyushiki H., Ikemoto N., Matsuzaki M. (2009) J. Am. Coll. Cardiol. 53, 1993–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Palnitkar S. S., Bin B., Jimenez L. S., Morimoto H., Williams P. G., Paul-Pletzer K., Parness J. (1999) J. Med. Chem. 42, 1872–1880 [DOI] [PubMed] [Google Scholar]
- 13. Paul-Pletzer K., Palnitkar S. S., Jimenez L. S., Morimoto H., Parness J. (2001) Biochemistry 40, 531–542 [DOI] [PubMed] [Google Scholar]
- 14. Paul-Pletzer K., Yamamoto T., Bhat M. B., Ma J., Ikemoto N., Jimenez L. S., Morimoto H., Williams P. G., Parness J. (2002) J. Biol. Chem. 277, 34918–34923 [DOI] [PubMed] [Google Scholar]
- 15. Paul-Pletzer K., Yamamoto T., Ikemoto N., Jimenez L. S., Morimoto H., Williams P. G., Ma J., Parness J. (2005) Biochem. J. 387, 905–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Uchinoumi H., Yano M., Suetomi T., Ono M., Xu X., Tateishi H., Oda T., Okuda S., Doi M., Kobayashi S., Yamamoto T., Ikeda Y., Ohkusa T., Ikemoto N., Matsuzaki M. (2010) Circ. Res. 106, 1413–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu Z., Wang R., Zhang J., Chen S. R., Wagenknecht T. (2005) J. Biol. Chem. 280, 37941–37947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang R., Chen W., Cai S., Zhang J., Bolstad J., Wagenknecht T., Liu Z., Chen S. R. (2007) J. Biol. Chem. 282, 17785–17793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wagenknecht T., Radermacher M., Grassucci R., Berkowitz J., Xin H. B., Fleischer S. (1997) J. Biol. Chem. 272, 32463–32471 [DOI] [PubMed] [Google Scholar]
- 20. Zhao M., Li P., Li X., Zhang L., Winkfein R. J., Chen S. R. (1999) J. Biol. Chem. 274, 25971–25974 [DOI] [PubMed] [Google Scholar]
- 21. Li P., Chen S. R. (2001) J. Gen. Physiol. 118, 33–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. (1989) Gene 77, 51–59 [DOI] [PubMed] [Google Scholar]
- 23. Liu Z., Zhang J., Li P., Chen S. R., Wagenknecht T. (2002) J. Biol. Chem. 277, 46712–46719 [DOI] [PubMed] [Google Scholar]
- 24. Frank J. (2006) Three-dimensional Electron Microscopy of Macromolecular Assemblies, 2nd Ed., pp. 71–142, Oxford University Press, New York [Google Scholar]
- 25. Penczek P. A., Grassucci R. A., Frank J. (1994) Ultramicroscopy 53, 251–270 [DOI] [PubMed] [Google Scholar]
- 26. Malhotra A., Penczek P., Agrawal R. K., Gabashvili I. S., Grassucci R. A., Jünemann R., Burkhardt N., Nierhaus K. H., Frank J. (1998) J. Mol. Biol. 280, 103–116 [DOI] [PubMed] [Google Scholar]
- 27. Jiang D., Wang R., Xiao B., Kong H., Hunt D. J., Choi P., Zhang L., Chen S. R. W. (2005) Circ. Res. 97, 1173–1181 [DOI] [PubMed] [Google Scholar]
- 28. Liu Z., Zhang J., Wang R., Wayne Chen S. R., Wagenknecht T. (2004) J. Mol. Biol. 338, 533–545 [DOI] [PubMed] [Google Scholar]
- 29. Liu Z., Wang R., Tian X., Zhong X., Gangopadhyay J., Cole R., Ikemoto N., Chen S. R., Wagenknecht T. (2010) J. Cell Sci. 123, 1775–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Papadopoulos S., Leuranguer V., Bannister R. A., Beam K. G. (2004) J. Biol. Chem. 279, 44046–44056 [DOI] [PubMed] [Google Scholar]
- 31. Jones P. P., Meng X., Xiao B., Cai S., Bolstad J., Wagenknecht T., Liu Z., Chen S. R. (2008) Biochem. J. 410, 261–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Meng X., Xiao B., Cai S., Huang X., Li F., Bolstad J., Trujillo R., Airey J., Chen S. R., Wagenknecht T., Liu Z. (2007) J. Biol. Chem. 282, 25929–25939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang R., Bolstad J., Kong H., Zhang L., Brown C., Chen S. R. W. (2004) J. Biol. Chem. 279, 3635–3642 [DOI] [PubMed] [Google Scholar]
- 34. Radermacher M., Rao V., Grassucci R., Frank J., Timerman A. P., Fleischer S., Wagenknecht T. (1994) J. Cell Biol. 127, 411–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sharma M. R., Penczek P., Grassucci R., Xin H. B., Fleischer S., Wagenknecht T. (1998) J. Biol. Chem. 273, 18429–18434 [DOI] [PubMed] [Google Scholar]
- 36. Sharma M. R., Jeyakumar L. H., Fleischer S., Wagenknecht T. (2006) Biophys. J. 90, 164–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Samsó M., Shen X., Allen P. D. (2006) J. Mol. Biol. 356, 917–927 [DOI] [PubMed] [Google Scholar]
- 38. Cornea R. L., Nitu F. R., Samsó M., Thomas D. D., Fruen B. R. (2010) J. Biol. Chem. 285, 19219–19226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Inan S., Wei H. (2010) Anesth. Analg. 111, 1400–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhao X., Weisleder N., Han X., Pan Z., Parness J., Brotto M., Ma J. (2006) J. Biol. Chem. 281, 33477–33486 [DOI] [PubMed] [Google Scholar]
- 41. Cherednichenko G., Ward C. W., Feng W., Cabrales E., Michaelson L., Samso M., Lopez J. R., Allen P. D., Pessah I. N. (2008) Mol. Pharmacol. 73, 1203–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ikemoto N., Yamamoto T. (2002) Front. Biosci. 7, d671–d683 [DOI] [PubMed] [Google Scholar]
- 43. Zhang J., Liu Z., Masumiya H., Wang R., Jiang D., Li F., Wagenknecht T., Chen S. R. W. (2003) J. Biol. Chem. 278, 14211–14218 [DOI] [PubMed] [Google Scholar]
- 44. Benacquista B. L., Sharma M. R., Samsó M., Zorzato F., Treves S., Wagenknecht T. (2000) Biophys. J. 78, 1349–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Murayama T., Oba T., Katayama E., Oyamada H., Oguchi K., Kobayashi M., Otsuka K., Ogawa Y. (1999) J. Biol. Chem. 274, 17297–17308 [DOI] [PubMed] [Google Scholar]
- 46. Tung C. C., Lobo P. A., Kimlicka L., Van Petegem F. (2010) Nature 468, 585–588 [DOI] [PubMed] [Google Scholar]
- 47. Masumiya H., Wang R., Zhang J., Xiao B., Chen S. R. W. (2003) J. Biol. Chem. 278, 3786–3792 [DOI] [PubMed] [Google Scholar]