Abstract
Vascular calcification is a common complication in atherosclerosis. Bone morphogenetic protein-2 (BMP-2) plays an important role in atherosclerotic vascular calcification. The aim of this study was to determine the effect of oxidized low density lipoprotein (oxLDL) on BMP-2 protein expression in human coronary artery endothelial cells (CAECs), the roles of Toll-like receptor (TLR) 2 and TLR4 in oxLDL-induced BMP-2 expression, and the signaling pathways involved. Human CAECs were stimulated with oxLDL. The roles of TLR2 and TLR4 in oxLDL-induced BMP-2 expression were determined by pretreatment with neutralizing antibody, siRNA, and overexpression. Stimulation with oxLDL increased cellular BMP-2 protein levels in a dose-dependent manner (40–160 μg/ml). Pretreatment with neutralizing antibodies against TLR2 and TLR4 or silencing of these two receptors reduced oxLDL-induced BMP-2 expression. Overexpression of TLR2 and TLR4 enhanced the cellular BMP-2 response to oxLDL. Furthermore, oxLDL was co-localized with TLR2 and TLR4. BMP-2 expression was associated with activation of nuclear factor-κB (NF-κB), p38 mitogen-activated protein kinase (MAPK), and extracellular signal-regulated kinase (ERK)1/2. Inhibition of NF-κB and ERK1/2 reduced BMP-2 expression whereas inhibition of p38 MAPK had no effect. In conclusion, oxLDL induces BMP-2 expression through TLR2 and TLR4 in human CAECs. The NF-κB and ERK1/2 pathways are involved in the signaling mechanism. These findings underscore an important role for TLR2 and TLR4 in mediating the BMP-2 response to oxLDL in human CAECs and indicate that these two immunoreceptors contribute to the mechanisms underlying atherosclerotic vascular calcification.
Keywords: Bone Morphogenetic Protein (BMP), Cytokine, Endothelium, Lipoprotein, Signal Transduction, Toll-like Receptor (TLR)
Introduction
Calcification in intima is often associated with atherosclerosis. Atherosclerotic calcification of coronary artery is a significant risk for the unsuccessful coronary intervention and balloon-induced coronary artery dissection (1), and calcification is found to be an indicator of plaque instability (2). Although inhibition of atherosclerotic calcification might be a promising approach to stabilize atherosclerotic plaques, the molecular mechanism(s) underlying atherosclerotic vascular calcification remains incompletely understood.
It is known that oxLDL2 accumulation in the vascular wall provokes the development of atherosclerosis and vascular calcification (3). BMP-2 is an osteogenic factor. It is expressed in calcified human atherosclerotic plaque. Cells from the atherogenic aortic wall express BMP-2 in vitro and formed calcified nodules with prolonged culture (4). The BMP-2 mRNA level increased after oxLDL stimulation in human CAECs (5). However, it remains unclear whether oxLDL up-regulates BMP-2 protein levels in CAECs. Further, the signaling mechanism that regulates the cellular BMP-2 response to oxLDL is unknown.
Accumulating evidence indicates that atherosclerosis is an inflammatory process involving a network of vascular cells, monocytes, and T lymphocytes. Proinflammatory mediators, including cytokines, chemokines, and growth factors, play critical roles in the inflammatory process associated with atherosclerosis. Native LDL and electronegative LDL increase the expression and release of interleukin (IL)-8, monocyte chemoattractant protein-1, and IL-6 in human umbilical artery endothelial cells (6). TLR2 and TLR4 play important roles in the vascular inflammatory response and are involved in the initiation and progression of atherosclerosis (7–10). Increased endothelial expression of TLR2 at sites of disturbed blood flow exacerbates early atherogenic events (11). Increased levels of TLR4 are expressed by macrophages in murine and human lipid-rich atherosclerotic plaques (12, 13). Lack of TLR4 suppresses atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E (7). Several studies indicate that oxLDL modulates TLR4 expression or signaling (12, 14–16). In macrophages, oxLDL is found to up-regulate TLR4 expression (12). Further, TLR4 signaling is required for the induction of macrophage actin polymerization by oxLDL (14). Currently, the role of TLRs in the cellular BMP-2 response to oxLDL has not been determined.
We hypothesize that oxLDL up-regulates BMP-2 protein expression in human CAECs through TLR2 and TLR4. In this study, we determined the effect of oxLDL on BMP-2 protein levels in human CAECs, evaluated the roles of TLR2 and TLR4 in oxLDL-induced BMP-2 expression, and analyzed the signaling pathways involved.
EXPERIMENTAL PROCEDURES
Materials
Human CAECs and cell culture reagents were purchased from Lonza (Walkersville, MD). OxLDL (from human plasma, CuSO4-oxidized, endotoxin-free) was obtained from Biomedical Technologies (Stoughton, MA). Monoclonal neutralizing antibody to human lectin-like oxLDL receptor-1 (LOX-1) was obtained from HyCult Biotechnology (Montrouge, The Netherlands). Monoclonal neutralizing antibodies to human TLR2 and TLR4 were purchased from Imgenex (San Diego, CA). Antibodies against BMP-2, TLR2, and TLR4 for immunoblotting were purchased from ProSci (Poway, CA). Antibody against ICAM-1 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against phosphorylated p38 MAPK (Thr180/Tyr182), phosphorylated ERK1/2 (Thr202/Tyr204), phosphorylated NF-κB p65 (Ser536), and β-actin were purchased from Cell Signaling (Danvers, MA). Antibodies against oxLDL, TLR2, and TLR4 for immunostaining were purchased from Chemicon (Temecula, CA), Imgenex, and Santa Cruz Biotechnology, respectively. ON-TARGET plus SMART pool human TLR2 and TLR4 siRNA, scrambled siRNA, DharmaFECT1 transfection reagent, and other transfection-related reagents were purchased from Dharmacon (Lafayette, CO). Opti-MEM I reduced serum medium was purchased from Invitrogen. A Bio-Plex human cytokine kit was purchased from Bio-Rad. Lipopolysaccharide (LPS, Escherichia coli 0111:B4), peptidoglycan (PGN), and all other chemicals were purchased from Sigma.
Cells and Treatments
Human CAECs were cultured in complete medium (EGM-2 medium with 5% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin). Cells of passages 4–6 were used for the experiments. Unless otherwise specified, cultures at 90% confluence were used for treatment. Cells were stimulated with oxLDL (40, 80, or 160 μg/ml), LPS (200 ng/ml), or PGN (10 μg/ml). Neutralizing antibodies against LOX-1, TLR2, and TLR4, 10 μg/ml each, were added into culture medium 30 min before stimulation and were present during stimulation. NF-κB inhibitor SN50 (a cell-permeable peptide that blocks the nuclear localization signal on NF-κB, 40 μm) SN50M (an inactive peptide for control, 40 μm; from Enzo Life Sciences), and MAPK inhibitors (10 μm SB203580 or 25 μm PD98059) were added into culture medium 30 min before oxLDL stimulation and were present during stimulation.
Immunoblotting
Immunoblotting was performed to detect BMP-2, TLR2, TLR4, ICAM-1, phosphorylated p38 MAPK, phosphorylated ERK1/2, and phosphorylated NF-κB p65, with β-actin as a loading control. After treatment, cells were lysed with lysis buffer (PBS containing a protease inhibitor mixture and 1% Triton X-100, pH 7.4). Samples were separated on 4–20% SDS-polyacrylamide gels (Bio-Rad) and then transferred to nitrocellulose membranes. Membranes were rinsed with Tris-buffered saline solution (TBS), blocked for 1 h at room temperature with 5% dry milk in TBST (Tris-buffered saline solution containing 0.1% Tween 20), then incubated with primary antibodies (anti-BMP-2 and anti-TLR2 were diluted to 2.0 μg/ml, anti-TLR4 to 5.0 μg/ml, anti-ICAM-1 1:200, phosphorylated ERK1/2 antibody 1:500, and all others 1:1000) overnight at 4 °C. After washing with TBST, membranes were incubated with horseradish peroxidase (HRP)-linked secondary antibodies (1:5000 dilution with TBST containing 5% dry milk) at room temperature for 1 h. After washing with TBST, target bands were developed using the enhanced chemiluminescence technique and exposed on x-ray films. Band density was measured using National Institutes of Health ImageJ software.
Gene Knockdown
Silencing TLR2 and TLR4 with siRNA was performed as described previously (17, 18). Cells were cultured in antibiotic-free growth medium in 12-well plates to 60% confluence. A transfection mixture of TLR2 or TLR4 siRNA (60 nm) and DharmaFECT1 transfection reagent (0.5 μl/ml) was made in Opti-MEM I reduced serum medium. Cells were incubated with the transfection mixture in complete medium for 48 h. This protocol was optimal for silencing TLR2 and TLR4 and resulted in >95% cell survival. After transfection, cells were incubated in growth medium for 24 h and then stimulated with oxLDL, PGN, or LPS. Controls were treated with scrambled siRNA and transfection reagent. The sense and antisense siRNA oligonucleotides used to generate siRNA have been reported (18).
Gene Overexpression
Transfection was performed with Effectene reagent (Qiagen, Valencia, CA) according to the manufacturer's instructions, except that a ratio of 6.25 μl Effectene/μg of DNA was optimal for human CAECs. Cells were plated in 12-well plates at a density allowing for ∼2–3 cell doublings. 0.5–1.0 μg of DNA/well was used for transfection. Yellow fluorescent protein (YFP)-TLR fusion protein expression constructs in pcDNA3 were obtained from Addgene (Cambridge, MA; plasmids 13018 (YFP-TLR4) and 13016 (YFP-TLR2)). The functionality of these receptor constructs has been demonstrated (9). All plasmids were isolated with EndoFree Maxi kits (Qiagen).
Bio-Plex Cytokine Assay
Cell culture supernatant was collected and analyzed for cytokines by Luminex 100 (Luminex Corp., Austin, TX) using a Human Cytokine 27-Plex assay kit. The kit contains beads conjugated with antibodies specific for IL-1β, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12, IL-13, IL-15, IL-17, eotaxin, FGF, G-CSF, GM-CSF, IFN-γ, IFN-inducible protein-10, monocyte chemoattractant protein-1, macrophage inflammatory protein-1α and -1β, PDGF-BB, RANTES, TNF-α, and VEGF. For each cytokine, nine-points standards were constructed, and the minimum detectable levels were between 1 and 5 pg/ml. Standard curves and the concentration of cytokines in samples were calculated with the Bio-Plex Manager 3.0 software.
Immunofluorescent Staining
Immunofluorescent staining was performed as described previously to co-localize oxLDL and TLRs following oxLDL stimulation (19). Briefly, cells were cultured in chamber slides to 30–50% confluence. OxLDL (80 μg/ml) was added to cells for 30 min. After thoroughly washing with cold PBS, cells were incubated with a mixture of 30% methanol and 70% acetone at room temperature for 5 min and then air dried. After fixing in PBS-buffered 3.5% paraformaldehyde at room temperature for 10 min, cells were blocked with 10% donkey serum for 30 min. Then cells were incubated for 2 h with a rabbit polyclonal antibody against oxLDL together with a mouse monoclonal antibody against TLR2 or TLR4. Control cells were incubated with nonimmune rabbit and nonimmune mouse IgG. After washing with PBS, cells were incubated with Cy3-conjugated donkey anti-rabbit IgG, Alexa Fluor 488-conjugated donkey anti-mouse IgG (Molecular Probes, Eugene OR) for 1 h. Nuclei were counterstained with bis-benzimide. Photography was performed with a Leica DMRX microscope (Wetzlar, Germany).
Statistics
Statistical analysis was performed by ANOVA with Fisher post hoc test. Statistical significance was accepted within a 95% confidence limit.
RESULTS
OxLDL Induces a Pro-osteogenic Response in Human CAECs
We determined the effect of oxLDL on the expression of the osteogenic factor BMP-2. As shown in Fig. 1A, stimulation of human CAECs with oxLDL increased BMP-2 protein levels in a dose-dependent fashion. Following 24-h stimulation with 80 μg/ml oxLDL, BMP-2 levels increased 6-fold. This oxLDL concentration was used for subsequent experiments. Temporal analysis found that BMP-2 protein levels increased at 4 h, and accumulation of this protein occurred over time with 24-h stimulation (Fig. 1B).
FIGURE 1.
OxLDL induces BMP-2 expression in human CAECs with a minor effect on the inflammatory response. A, representative immunoblot of four separate experiments and densitometric data show that stimulation of cells with oxLDL for 24 h increased BMP-2 protein levels in human CAECs in a concentration-dependent fashion. Densitometric data are expressed as mean ± S.E. (error bars). n = 4; *, p < 0.05 versus control (no oxLDL). B, representative immunoblot of two separate experiments shows that BMP-2 protein increased at 4 h of oxLDL stimulation (80 μg/ml) and accumulated with prolonged stimulation. C, IL-8 level increased moderately after stimulation with oxLDL (80 μg/ml) for 24 h whereas PGN (10 μg/ml) and LPS (200 ng/ml) induced marked increases in multiple cytokines. Data are expressed as mean ± S.E. n = 4; *, p < 0.05 versus control. D, representative immunoblot of two separate experiments shows that oxLDL (160 μg/ml, 24 h) failed to induce ICAM-1 expression although LPS significantly increased the cellular ICAM-1 level.
To determine the effect of oxLDL on the proinflammatory response in human CAECs, we analyzed the levels of 27 cytokines in culture media. Among the 27 cytokines, only IL-8 levels increased significantly after stimulation with oxLDL for 24 h (shown in Fig. 1C). The IL-8 level in cell culture medium increased 2.1-, 2.8-, and 3.5-fold, respectively, after stimulation with oxLDL in concentrations of 40, 80, and 160 μg/ml. In contrast, PGN and LPS induced robust increases in the levels of most cytokines analyzed (a selected group of cytokines is shown in Fig. 1C for comparison with oxLDL). Apparently, oxLDL is different from TLR agonists PGN and LPS in the induction of the proinflammatory response in human CAECs.
Because endothelial cells are sensitive to LPS in the expression of ICAM-1, we examined ICAM-1 expression after oxLDL stimulation to address further the concern of LPS contamination. Although LPS significantly increased the cellular ICAM-1 level, oxLDL failed to induce ICAM-1 (Fig. 1D). These results confirm that the effect of oxLDL on IL-8 production is not due to LPS contamination.
Neutralization of TLR2 or TLR4 Reduces OxLDL-induced BMP-2 Expression in Human CAECs
Several studies have linked TLR2 and TLR4 to atherosclerosis (7–9). Interestingly, minimally modified LDL induces macrophage actin polymerization and cell spreading via a TLR4-dependent mechanism (14). Further, oxLDL induces cell death in ganglion culture in a TLR4-dependent fashion (16). These studies indicate a role for TLR4 in mediating the effect of oxLDL. We have found that activation of TLR2 or TLR4 induces BMP-2 expression in human aortic valve interstitial cells (18). We hypothesized that TLR2 and TLR4 regulate BMP-2 expression in human CAECs. We determined the effect of neutralizing antibodies against TLR2 and TLR4 on oxLDL-induced BMP-2 expression. Interestingly, neutralization of either TLR2 or TLR4 markedly reduced BMP-2 levels after oxLDL stimulation (Fig. 2, A and B). It appears that oxLDL activates these two receptors to induce BMP-2 expression in human CAECs. To confirm the effect of TLR2 and TLR4 activation on BMP-2 expression in human CAECs, we stimulated cells with the TLR2 agonist PGN and TLR4 agonist LPS and determined their effects on cellular BMP-2 levels. PGN and LPS induced BMP-2 expression, and their effects were markedly reduced in cells pretreated with the neutralizing antibodies (Fig. 2, C and D). Therefore, activation of TLR2 and TLR4 increases BMP-2 expression in human CAECs, and these two immunoreceptors play critical roles in mediating oxLDL-induced BMP-2 expression in this cell type.
FIGURE 2.
Neutralization of TLR2 or TLR4, but not LOX-1, reduces oxLDL-induced BMP-2 expression. A and B, representative immunoblots of three separate experiments and densitometric data show that neutralization of TLR2 or TLR4 markedly reduced BMP-2 levels after oxLDL stimulation (80 μg/ml, 24 h). C and D, representative immunoblots of three separate experiments and densitometric data show that PGN (10 μg/ml, 24 h) and LPS (200 ng/ml, 24 h) also induced BMP-2 expression and that their effects were greatly reduced by neutralizing antibodies against the receptors. E, representative immunoblot of three separate experiments and densitometric data show that neutralization of LOX-1 had no influence on oxLDL-induced BMP-2 expression (80 μg/ml oxLDL, 24 h). Densitometry data are expressed as mean ± S.E. (error bars). n = 3; *, p < 0.05 versus control; #, p < 0.05 versus correspondent treatment without receptor neutralization.
The scavenger receptor LOX-1 mediates cellular uptake of oxLDL and oxLDL signaling (20, 21). We examined whether LOX-1 plays a role in oxLDL-induced BMP-2 expression in human CAECs using LOX-1 antibody to neutralize this receptor. Interestingly, neutralization of LOX-1 had no influence on oxLDL-induced BMP-2 expression (Fig. 2E). It appears that oxLDL induces BMP-2 protein expression independently of LOX-1 in human CAECs.
Silencing of TLR2 or TLR4 Also Reduces OxLDL-induced BMP-2 Expression
We used siRNA to knock down cellular TLR2 and TLR4 to further determine the roles of these two innate immunoreceptors in mediating oxLDL-induced BMP-2 expression. Treatment of cells with specific siRNA reduced cellular TLR2 and TLR4 levels to 21 and 22%, respectively (Fig. 3, A and B) and resulted in significant reductions in the cellular response to PGN and LPS for BMP-2 expression (Fig. 3, C and D). Similarly, silencing TLR2 and TLR4 reduced oxLDL-induced BMP-2 expression by 60 and 63%, respectively (Fig. 3, E and F). These results further confirmed that TLR2 and TLR4 play a critical role in mediating the BMP-2 response to oxLDL in human CAECs.
FIGURE 3.
Silencing of TLR2 or TLR4 reduces BMP-2 expression induced by oxLDL. A and B, representative immunoblots and densitometric data show that treatment with specific siRNA for 48 h reduced cellular TLR2 and TLR4 levels. C and D, silencing TLR2 or TLR4 reduced cellular BMP-2 levels following stimulation with a receptor agonist for 24 h. E and F, BMP-2 levels were reduced in cells treated with siRNA specific to TLR2 or TLR4 before oxLDL stimulation (80 μg/ml, 24 h). Densitometry data are expressed as mean ± S.E. (error bars). n = 3; *, p < 0.05 versus control; #, p < 0.05 versus correspondent treatment without silencing TLR2 or TLR4.
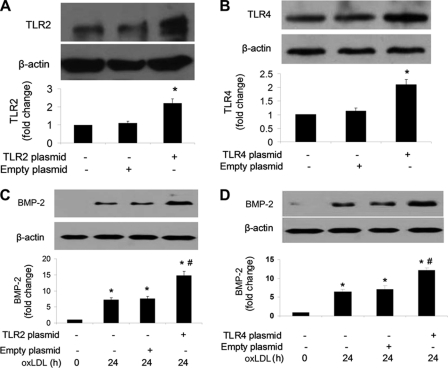
Overexpression of TLR2 or TLR4 Enhances OxLDL-induced BMP-2 Expression
We tested whether the cellular BMP-2 response to oxLDL is enhanced by increasing TLR2 and TLR4 levels. We overexpressed TLR2 and TLR4 in human CAECs by transfection of cells with DNA constructs. As shown in Fig. 4, A and B, overexpression increased cellular TLR2 and TLR4 by 121 and 108%, respectively. Overexpression of TLR2 and TLR4 enhanced the cellular response to oxLDL for BMP-2 expression (Fig. 4, C and D). Thus, overexpression of TLR2 and TLR4 had opposite effects on oxLDL-induced BMP-2 expression compared with those resulted from neutralizing and silencing these two receptors.
FIGURE 4.
Overexpression of TLR2 or TLR4 enhances oxLDL-induced BMP-2 expression. A and B, human CAECs were transfected with plasmids to overexpress TLR2 or TLR4. Representative immunoblots and densitometric data show increased TLR2 and TLR4 levels after transfection. C and D, BMP-2 levels after oxLDL stimulation (80 μg/ml, 24 h) were increased in cells overexpressing TLR2 or TLR4. Densitometry data are expressed as mean ± S.E. (error bars). n = 3; *, p < 0.05 versus control; #, p < 0.05 versus correspondent treatment without overexpressing TLR2 or TLR4.
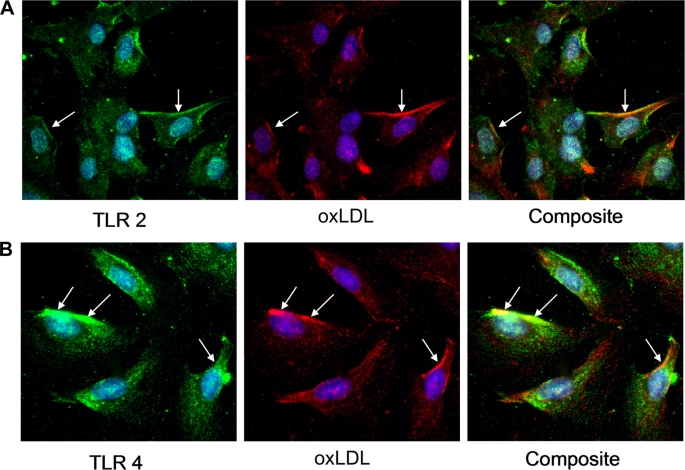
OxLDL Is Co-localized with TLR2 and TLR4 in Human CAECs
We examined whether oxLDL interacts with TLR2 and TLR4. We used dual immunofluorescent staining to co-localize oxLDL and TLR2 or TLR4. The results show that oxLDL is localized on the cell surfaces and in cytoplasm. Co-localization with TLR2 and TLR4 on cell membranes is evident (Fig. 5).
FIGURE 5.
OxLDL is co-localized with TLR2 and TLR4 in human CAECs. Cells were treated with oxLDL (80 μg/ml, 30 min). Cells were fixed immediately after the treatment. TLR2 or TLR4 was stained green, and oxLDL was stained red by indirect immunostaining. Nuclei were counterstained blue with bis-benzimide. Representative images show that oxLDL is co-localized with TLR2 (A) and TLR4 (B) on cell surfaces (yellow in composite images, arrows).
ERK1/2 and NF-κB Are Independently Involved in the Mechanisms Underlying OxLDL-induced BMP-2 Expression
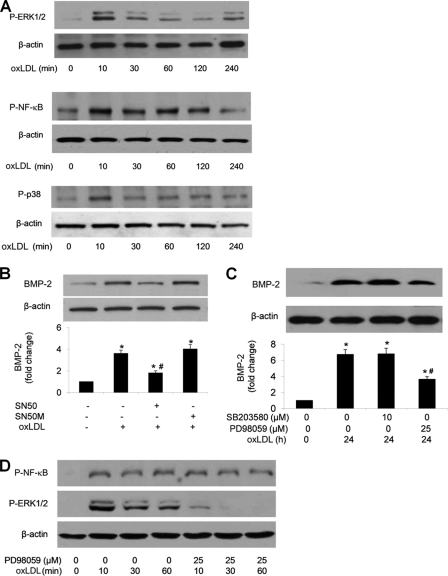
NF-κB, p38 MAPK, and ERK1/2 are downstream of TLR2 and TLR4 in the proinflammatory signaling. We determined the effect of oxLDL on the phosphorylation of NF-κB, p38 MAPK, and ERK1/2 and the role of these signaling molecules in oxLDL-induced BMP-2 expression. As shown in Fig. 6A, oxLDL stimulation resulted in rapid phosphorylation of NF-κB, p38 MAPK, and ERK1/2. Inhibition of NF-κB or ERK1/2 reduced BMP-2 expression (Fig. 6, B and C). However, inhibition of p38 MAPK had no effect (Fig. 6C). To examine whether ERK1/2 activation is linked to NF-κB phosphorylation, we examined NF-κB phosphorylation in the presence of ERK1/2 inhibitor. Inhibition of ERK1/2 had no effect on NF-κB phosphorylation induced by oxLDL (Fig. 6D). These results indicate that NF-κB and ERK1/2 are involved in mediating BMP-2 expression in response to oxLDL and that ERK1/2 modulates oxLDL-induced BMP-2 expression independent of NF-κB.
FIGURE 6.
ERK1/2 and NF-κB pathways independently modulate oxLDL-induced BMP-2 expression. A, representative immunoblots show that oxLDL (80 μg/ml) induced rapid phosphorylation of ERK1/2, p38 MAPK, and NF-κB. B, inhibition of NF-κB with SN50 (40 μm) reduced BMP-2 levels following oxLDL stimulation, but SN50M (a control peptide for SN50, 40 μm) had no effect. C, inhibition of p38 MAPK had no effect, but inhibition of ERK1/2 reduced oxLDL-induced BMP-2 expression. D, inhibition of ERK1/2 did not influence NF-κB phosphorylation. Densitometry data are expressed as mean ± S.E. (error bars). n = 3; *, p < 0.05 versus untreated control; #, p < 0.05 versus oxLDL alone.
DISCUSSION
The results of this study demonstrate that oxLDL up-regulates BMP-2 protein levels in human CAECs through TLR2 and TLR4. The ERK1/2 and NF-κB pathways are involved in the signaling mechanism for oxLDL-induced BMP-2 expression. These novel findings underscore an important role for TLR2 and TLR4 in mediating the BMP-2 response to oxLDL and indicate that these two innate immunoreceptors contribute to the mechanisms underlying atherosclerotic calcification.
Molecules that play a key role in the bone calcification, such as matrix γ-carboxyglutamic acid protein and BMP-2, have been identified in calcified atherosclerotic plaques (22). Accumulated evidence has suggested that vascular calcification is a process actively regulated by osteogenic factors locally expressed in blood vessels (23–25). However, the mechanisms underlying the up-regulation of pro-osteogenic factors are incompletely understood.
OxLDL Up-regulates Pro-osteogenic Protein BMP-2 in Human CAECs
MBP-2 is a potent osteogenic factor and plays an important role in vascular calcification (26–28). We found that stimulation of human CAECs with oxLDL increased BMP-2 protein levels. An increase in cellular BMP-2 levels is observed at 4 h of oxLDL stimulation and becomes evident at 24 h. Further, oxLDL induces BMP-2 expression in a dose-dependent fashion. These observations indicate that endothelial BMP-2 production induced by oxLDL may induce vascular osteogenic changes associated with atherosclerosis.
BMP-2 could be a component of the inflammatory response. To determine the effect of oxLDL on the proinflammatory response in human CAECs, we analyzed the levels of 27 cytokines in culture media. Among the 27 cytokines, only IL-8 levels significantly increased after stimulation with oxLDL for 24 h. In contrast, stimulation of human CAECs with proinflammatory stimuli, PGN (TLR2 agonist) and LPS (TLR4 agonist), induced robust increases in the levels of most cytokines analyzed. Apparently, oxLDL induces BMP-2 production without elaborating a generalized inflammatory response in human CAECs. Thus, oxLDL is potent to induce BMP-2 but has a minimal effect on the inflammatory response in human CAECs. The selective effect of oxLDL on BMP-2 production indicates that the observed effect of the human oxLDL preparation is not due to LPS contamination. We addressed this issue further by examining ICAM-1 production after oxLDL stimulation. Although LPS significantly increased the cellular ICAM-1 level, oxLDL in a high concentration failed to induce ICAM-1. This result excludes the possibility that LPS contamination is responsible for the effect of the human oxLDL preparation.
Both TLR2 and TLR4 Are Involved in Induction of BMP-2 by OxLDL
The scavenger receptor LOX-1 mediates oxLDL uptake and signaling in macrophages and vascular cells (21). To determine whether LOX-1 plays a role in oxLDL-induced BMP-2 expression in human CAECs, we treated cells with a LOX-1-neutralizing antibody prior to oxLDL stimulation. Unexpectedly, neutralization of LOX-1 had no influence on oxLDL-induced BMP-2 expression. It appears that oxLDL induces BMP-2 protein expression independently of LOX-1 in human CAECs.
TLRs are pattern recognition receptors that sense the presence of numerous pathogen-associated molecular patterns. TLR2 and TLR4 have been implicated in the signaling mechanisms that contribute to chronic inflammatory diseases, including atherosclerosis (7–9). A number of studies found that TLRs, particularly TLR2 and TLR4, also respond to endogenous agents, such as heat shock proteins (29–32). Previous studies by Miller's Laboratory have demonstrated that minimally modified LDL binds to CD14, and activates macrophages via TLR4/MD-2 (14). In contrast to LPS stimulation, the most robust TLR4-mediated effect of minimally modified LDL in macrophages is cytoskeleton remodeling (14, 33).
The results of the present study provided three lines of evidence to support the notion that oxLDL induces BMP-2 production in human CAECs through TLR2 and TLR4. First, up-regulation of BMP-2 levels by oxLDL is markedly suppressed by neutralization or silencing of TLR2 and TLR4. Second, overexpression of TLR2 and TLR4 enhances the cellular BMP-2 response to oxLDL. Finally, TLR2 and TLR4 agonists are potent to induce BMP-2 production in human CAECs. Together, these results demonstrate that oxLDL up-regulates BMP-2 production in human CAECs through a mechanism dependent on TLR2 and TLR4. In this regard, cell death in dorsal root ganglion cell culture induced by oxLDL is attributed to TLR4, not LOX-1 (16). Our further experiments using immunostaining found that oxLDL is co-localized with TLR2 and TLR4. It appears that oxLDL interacts with these two innate immunoreceptors. OxLDL may function as an endogenous molecular pattern to activate TLR2 and TLR4 although it is not as potent as pathogen molecular patterns and other molecular patterns, such as heat shock proteins, in elaborating the inflammatory response. Interaction of oxLDL with TLR2 and TLR4 preferentially induces BMP-2 production in human CAECs. Further studies are needed to determine whether the MyD88 pathway is required for the induction of BMP-2 expression by oxLDL as TLR2 and TLR4 utilize this shared signaling pathway (34).
NF-κB and ERK1/2 Pathways Contribute to the Signaling Mechanism
Activation of NF-κB is critical for induction of BMP-2 expression in endothelial cells by TNF-α (35). Further, NF-κB has been reported to activate BMP-2 expression in osteoblasts as well as in chondrocytes (36). We found that oxLDL induces rapid and sustained NF-κB phosphorylation in human CAECs, and inhibition of NF-κB reduces BMP-2 expression. In addition, BMP-2 expression is associated with p38 MAPK and ERK1/2 phosphorylation. Interestingly, inhibition of p38 MAPK does not affect BMP-2 expression. Although inhibition of ERK1/2 attenuates BMP-2 expression, it has no effect on NF-κB phosphorylation. Thus, both NF-κB and ERK1/2 play a role in oxLDL-induced BMP-2 expression in human CAECs. NF-κB activation by oxLDL does not require ERK1/2 activation although ERK1/2 has a role in regulating oxLDL-induced BMP-2 expression. The NF-κB and ERK1/2 pathways contribute independently to the molecular mechanism underlying oxLDL-induced BMP-2 expression in human CAECs. As ERK1/2 is also involved in BMP-2 signaling in many cell types (37), this pathway could be important in atherosclerotic coronary artery calcification.
Taken together, we demonstrated that oxLDL up-regulates the levels of pro-osteogenic protein BMP-2 in human CAECs through TLR2 and TLR4. Activation of NF-κB and ERK1/2 is required for BMP-2 expression induced by oxLDL, and the NF-κB and ERK1/2 pathways contribute independently to the mechanism underlying oxLDL-induced BMP-2 expression. Considering the important role of BMP-2 in vascular calcification, down-regulation BMP-2 expression, up-regulation of the expression of its antagonists, or inhibition of BMP-2 intracellular signaling might be feasible for prevention of atherosclerotic vascular calcification.
This work was supported, in whole or in part, by National Institutes of Health Grant HL079051. This work was also supported by American Heart Association Grant 0850148Z.
- oxLDL
- oxidized low density lipoprotein
- BMP-2
- bone morphogenetic protein-2
- CAEC
- coronary artery endothelial cell
- ICAM-1
- intercellular adhesion molecule 1
- LOX-1
- lectin-like oxLDL receptor-1
- PGN
- peptidoglycan
- TBS
- Tris-buffered saline solution
- TBST
- TBS containing Tween 20.
REFERENCES
- 1. Fitzgerald P. J., Ports T. A., Yock P. G. (1992) Circulation 86, 64–70 [DOI] [PubMed] [Google Scholar]
- 2. Speer M. Y., McKee M. D., Guldberg R. E., Liaw L., Yang H. Y., Tung E., Karsenty G., Giachelli C. M. (2002) J. Exp. Med. 196, 1047–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Galle J., Hansen-Hagge T., Wanner C., Seibold S. (2006) Atherosclerosis 185, 219–226 [DOI] [PubMed] [Google Scholar]
- 4. Boström K., Watson K. E., Horn S., Wortham C., Herman I. M., Demer L. L. (1993) J. Clin. Invest. 91, 1800–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cola C., Almeida M., Li D., Romeo F., Mehta J. L. (2004) Biochem. Biophys. Res. Commun. 320, 424–427 [DOI] [PubMed] [Google Scholar]
- 6. de Castellarnau C., Bancells C., Benítez S., Reina M., Ordóñez-Llanos J., Sánchez-Quesada J. L. (2007) Clin. Chim. Acta 376, 233–236 [DOI] [PubMed] [Google Scholar]
- 7. Michelsen K. S., Wong M. H., Shah P. K., Zhang W., Yano J., Doherty T. M., Akira S., Rajavashisth T. B., Arditi M. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 10679–10684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mullick A. E., Tobias P. S., Curtiss L. K. (2005) J. Clin. Invest. 115, 3149–3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu X., Ukai T., Yumoto H., Davey M., Goswami S., Gibson F. C., 3rd, Genco C. A. (2008) Atherosclerosis 196, 146–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Monaco C., Gregan S. M., Navin T. J., Foxwell B. M., Davies A. H., Feldmann M. (2009) Circulation 120, 2462–2469 [DOI] [PubMed] [Google Scholar]
- 11. Mullick A. E., Soldau K., Kiosses W. B., Bell T. A., 3rd, Tobias P. S., Curtiss L. K. (2008) J. Exp. Med. 205, 373–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xu X. H., Shah P. K., Faure E., Equils O., Thomas L., Fishbein M. C., Luthringer D., Xu X. P., Rajavashisth T. B., Yano J., Kaul S., Arditi M. (2001) Circulation 104, 3103–3108 [DOI] [PubMed] [Google Scholar]
- 13. Katsargyris A., Tsiodras S., Theocharis S., Giaginis K., Vasileiou I., Bakoyiannis C., Georgopoulos S., Bastounis E., Klonaris C. (2011) Cerebrovasc. Dis. 31, 29–36 [DOI] [PubMed] [Google Scholar]
- 14. Miller Y. I., Viriyakosol S., Binder C. J., Feramisco J. R., Kirkland T. N., Witztum J. L. (2003) J. Biol. Chem. 278, 1561–1568 [DOI] [PubMed] [Google Scholar]
- 15. Miller Y. I., Viriyakosol S., Worrall D. S., Boullier A., Butler S., Witztum J. L. (2005) Arterioscler. Thromb. Vasc. Biol. 25, 1213–1219 [DOI] [PubMed] [Google Scholar]
- 16. Nowicki M., Müller K., Serke H., Kosacka J., Vilser C., Ricken A., Spanel-Borowski K. (2010) J. Neurosci. Res. 88, 403–412 [DOI] [PubMed] [Google Scholar]
- 17. Su X., Ao L., Zou N., Song Y., Yang X., Cai G. Y., Fullerton D. A., Meng X. (2008) Biochim. Biophys. Acta 1783, 1623–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang X., Fullerton D. A., Su X., Ao L., Cleveland J. C., Jr., Meng X. (2009) J. Am. Coll. Cardiol. 53, 491–500 [DOI] [PubMed] [Google Scholar]
- 19. Ao L., Zou N., Cleveland J. C., Jr., Fullerton D. A., Meng X. (2009) Am. J. Physiol. Heart Circ. Physiol. 297, H21–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen M., Masaki T., Sawamura T. (2002) Pharmacol. Ther. 95, 89–100 [DOI] [PubMed] [Google Scholar]
- 21. Mehta J. L., Chen J., Hermonat P. L., Romeo F., Novelli G. (2006) Cardiovasc. Res. 69, 36–45 [DOI] [PubMed] [Google Scholar]
- 22. Dhore C. R., Cleutjens J. P., Lutgens E., Cleutjens K. B., Geusens P. P., Kitslaar P. J., Tordoir J. H., Spronk H. M., Vermeer C., Daemen M. J. (2001) Arterioscler. Thromb. Vasc. Biol. 21, 1998–2003 [DOI] [PubMed] [Google Scholar]
- 23. Hruska K. A., Mathew S., Saab G. (2005) Circ. Res. 97, 105–114 [DOI] [PubMed] [Google Scholar]
- 24. Shao J. S., Cai J., Towler D. A. (2006) Arterioscler. Thromb. Vasc. Biol. 26, 1423–1430 [DOI] [PubMed] [Google Scholar]
- 25. Shao J. S., Cheng S. L., Sadhu J., Towler D. A. (2010) Hypertension 55, 579–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Willette R. N., Gu J. L., Lysko P. G., Anderson K. M., Minehart H., Yue T. (1999) J. Vasc. Res. 36, 120–125 [DOI] [PubMed] [Google Scholar]
- 27. Li X., Yang H. Y., Giachelli C. M. (2008) Atherosclerosis 199, 271–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nakagawa Y., Ikeda K., Akakabe Y., Koide M., Uraoka M., Yutaka K. T., Kurimoto-Nakano R., Takahashi T., Matoba S., Yamada H., Okigaki M., Matsubara H. (2010) Arterioscler. Thromb. Vasc. Biol. 30, 1908–1915 [DOI] [PubMed] [Google Scholar]
- 29. Asea A., Rehli M., Kabingu E., Boch J. A., Bare O., Auron P. E., Stevenson M. A., Calderwood S. K. (2002) J. Biol. Chem. 277, 15028–15034 [DOI] [PubMed] [Google Scholar]
- 30. de Graaf R., Kloppenburg G., Kitslaar P. J., Bruggeman C. A., Stassen F. (2006) Microbes Infect. 8, 1859–1865 [DOI] [PubMed] [Google Scholar]
- 31. Zou N., Ao L., Cleveland J. C., Jr., Yang X., Su X., Cai G. Y., Banerjee A., Fullerton D. A., Meng X. (2008) Am. J. Physiol. Heart Circ. Physiol. 294, H2805–2813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Su X., Sykes J. B., Ao L., Raeburn C. D., Fullerton D. A., Meng X. (2010) Cytokine 51, 60–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Choi S. H., Harkewicz R., Lee J. H., Boullier A., Almazan F., Li A. C., Witztum J. L., Bae Y. S., Miller Y. I. (2009) Circ. Res. 104, 1355–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. O'Neill L. A., Bryant C. E., Doyle S. L. (2009) Pharmacol. Rev. 61, 177–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Csiszar A., Smith K. E., Koller A., Kaley G., Edwards J. G., Ungvari Z. (2005) Circulation 111, 2364–2372 [DOI] [PubMed] [Google Scholar]
- 36. Feng J. Q., Xing L., Zhang J. H., Zhao M., Horn D., Chan J., Boyce B. F., Harris S. E., Mundy G. R., Chen D. (2003) J. Biol. Chem. 278, 29130–29135 [DOI] [PubMed] [Google Scholar]
- 37. Yang X., Meng X., Su X., Mauchley D. C., Ao L., Cleveland J. C., Jr., Fullerton D. A. (2009) J. Thorac. Cardiovasc. Surg. 138, 1008–1015 [DOI] [PubMed] [Google Scholar]