Abstract
Mammalian cells accumulate Ca2+ into agonist-sensitive acidic organelles, vesicles that possess a vacuolar proton- ATPase. Acidic Ca2+ stores include secretory granules and lysosome-related organelles. Current evidence clearly indicates that acidic Ca2+ stores participate in cell signaling and function, including the activation of store-operated Ca2+ entry in human platelets upon depletion of the acidic stores, although the mechanism underlying the activation of store-operated Ca2+ entry controlled by the acidic stores remains unclear. STIM1 has been presented as the endoplasmic reticulum Ca2+ sensor, but its role sensing intraluminal Ca2+ concentration in the acidic stores has not been investigated. Here we report that STIM1 and STIM2 are expressed in the lysosome-related organelles and dense granules in human platelets isolated by immunomagnetic sorting. Depletion of the acidic Ca2+ stores using the specific vacuolar proton-ATPase inhibitor, bafilomycin A1, enhanced the association between STIM1 and STIM2 as well as between these proteins and the plasma membrane channel Orai1. Depletion of the acidic Ca2+ stores also induces time-dependent co-immunoprecipitation of STIM1 with the TRPC proteins hTRPC1 and hTRPC6, as well as between Orai1 and both TRPC proteins. In addition, bafilomycin A1 enhanced the association between STIM2 and SERCA3. These findings demonstrate the location of STIM1 and STIM2 in the acidic Ca2+ stores and their association with Ca2+ channels and ATPases upon acidic stores discharge.
Keywords: Calcium Channels, Calcium Intracellular Release, Platelet, Protein-Protein Interactions, Signal Transduction, Orai1, STIM1, STIM2, hTRPC1, hTRPC6, Calcium Entry, Acidic Ca2+ Stores, Human Platelets
Introduction
Store-operated calcium entry (SOCE),4 a Ca2+ influx mechanism regulated by the filling state of the intracellular Ca2+ stores, is a major mechanism for Ca2+ influx in non-excitable cells (1). It has long been known that Ca2+ is stored in the endoplasmic reticulum (ER), which is the best-studied agonist-releasable Ca2+ store; however, a number of studies have revealed that Ca2+ is also dynamically accumulated in a number of acidic organelles (2), including secretory granules, lysosomes and lysosome-related organelles, endosomes, and vesicles of the Golgi complex (2–6). ER Ca2+ store depletion has been reported to be sensed by STIM1 (stromal interaction molecule-1) (7–9). Although STIM1 was originally identified as a surface membrane protein in stromal cells (10), STIM1 is found predominantly in the ER membrane, where the Ca2+ binding domain (the EF-hand motif) is located within the ER lumen. When the intraluminal Ca2+ concentration is reduced in the ER, STIM1 relocalizes within the ER membrane to puncta at ER-plasma membrane junctions, allowing its association with members of the Orai and TRPC families (11–14). Therefore, STIM1 acts as the ER Ca2+ sensor that directly activates the store-operated calcium channels until the ER Ca2+ levels are replenished. On the other hand, the STIM1 homologue STIM2 has been reported to play a relevant role in intracellular Ca2+ homeostasis as a feedback regulator that stabilizes resting cytosolic and ER Ca2+ concentrations (15). Unlike STIM1, STIM2 has been reported to be active at basal ER Ca2+ concentrations and might activate SOCE by interaction with Orai1 upon smaller decreases in ER Ca2+ concentration (15, 16).
In mammalian cells, Ca2+ uptake is mediated by either sarco/endoplasmic reticulum Ca2+-ATPases (SERCAs) or secretory pathway Ca2+-ATPases, located in the Golgi complex and secretory granules (17, 18). In addition, acidic organelles have a large concentration gradient of protons that has been reported to be necessary to maintain Ca2+ accumulated (19, 20), probably by supporting a Ca2+/H+ exchange mechanism mediated by the cooperative activity of Na+/H+ and Na+/Ca2+ exchangers (21–22). The proton gradient in acidic organelles is maintained by a vacuolar proton-ATPase (V-ATPase) that drives acidification (23–24). Acidic Ca2+ stores also possess different Ca2+-permeable channels, responsible for agonist-stimulated Ca2+ efflux, such as two pore channels that are sensitive to the nicotinic acid adenine dinucleotide phosphate (NAADP) (25, 26); members of the transient receptor potential superfamily, such as TRPM2, also sensitive to NAADP (27); and TRPV2. The latter have been located in secretory granules and the Golgi apparatus and might mediate Ca2+ release from the ER as well as from acidic Ca2+ stores (2).
Human platelets possess two agonist-sensitive Ca2+ stores, the dense tubular system (DTS), the analog of the ER in other mammalian cells, and acidic Ca2+ stores. In these cells, two different SERCA isoforms, with molecular masses of 100 and 97 kDa, have been identified, which are distributed separately in the two distinct Ca2+ stores (28–30). The 100-kDa isoform, identified as SERCA2b, is highly sensitive to thapsigargin (TG) (28), whereas the 97-kDa isoform, identified as SERCA3, shows a low sensitivity to TG but, in contrast to SERCA2b, is sensitive to 2,5-di-(t-butyl)-1,4-hydroquinone (TBHQ). The TBHQ-sensitive and -insensitive stores have been identified as the acidic Ca2+ stores and the DTS, respectively, in human platelets (4, 31). In these cells, depletion of the DTS or the acidic Ca2+ stores results in the activation of two independent pathways for SOCE, differentially regulated by the actin cytoskeleton. Reorganization of the actin cytoskeleton allows the activation of SOCE via both mechanisms, but only SOCE controlled by depletion of the TBHQ-insensitive DTS pool requires new actin polymerization, probably to support membrane trafficking toward the plasma membrane (32). Although STIM1 has been identified as the ER Ca2+ sensor, there is no evidence of the Ca2+ sensor in the acidic Ca2+ stores.
In the present study, we have investigated the localization of STIM1 and STIM2 in the acidic stores of human platelets as well as its interaction with Ca2+-permeable plasma membrane channels upon specific discharge of the acidic Ca2+ stores. Our results indicate that STIM1 and STIM2 are expressed in dense granules as well as in lysosome-related organelles, and depletion of the acidic Ca2+ stores by the specific inhibitor of the V-ATPase, bafilomycin A, results in the activation of SOCE and promotes the interaction of STIM1 and STIM2 with Orai1 and solely STIM1 with hTRPC1 and hTRPC6 independently of changes in cytosolic free Ca2+ concentration ([Ca2+]i), which suggests a functional role for STIM1 and STIM2 located in the acidic Ca2+ stores.
EXPERIMENTAL PROCEDURES
Materials
Fura-2 acetoxymethyl ester (fura-2/AM), Lysosensor Green DND-189, calcein/AM, and Alexa Fluor® 350- and 647-conjugated secondary antibodies were from Invitrogen. Apyrase (grade VII), aspirin, thrombin, ADP, TG, leupeptin, benzamidine, phenylmethylsulfonyl fluoride (PMSF), SDS, rabbit anti-Orai antibody (C-terminal), bafilomycin A1, bovine serum albumin (BSA), 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester) (BAPTA-AM), and glycyl-1-phenylalanine 2-naphthylamide (GPN) were from Sigma (Madrid, Spain). TBHQ was form Axxora (San Diego, USA). Anti-STIM1 antibody was from BD Transduction Laboratories (Franklin Lakes, NJ). Anti-SERCA2b, anti-protein-disulfide isomerase (PDI), and anti-cathepsin D antibodies were from Abcam plc. (Cambridge, UK). Ionomycin was from Calbiochem (Madrid, Spain). Anti-hTRPC1 polyclonal antibody was obtained from Alomone Laboratories (Jerusalem, Israel). Dynabeads panmouse IgG were from Invitrogen. Anti-SERCA3 (PL/IM430) antibody, horseradish peroxidase-conjugated goat anti-rabbit IgG antibody, and anti-LAMP-2 antibody were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Horseradish peroxidase-conjugated ovine anti-mouse IgG antibody (NA931) and hyperfilm ECL were from Amersham Biosciences. Protein A-agarose was from Upstate Biotechnology Inc. (Madrid, Spain). Enhanced chemiluminescence detection reagents were from Pierce. All other reagents were of analytical grade.
Platelet Preparation
Platelet suspensions were prepared as described previously (33) as approved by local ethical committees and in accordance with the Declaration of Helsinki. Briefly, blood was obtained from healthy drug-free volunteers and mixed with one-sixth volume of acid/citrate dextrose anticoagulant containing 85 mm sodium citrate, 78 mm citric acid, and 111 mm d-glucose. Platelet-rich plasma was then prepared by centrifugation for 5 min at 700 × g, and aspirin (100 μm) and apyrase (40 μg/ml) were added. Platelets were then collected by centrifugation at 350 × g for 20 min and resuspended in HEPES-buffered saline (HBS), pH 7.45, containing 145 mm NaCl, 10 mm HEPES, 10 mm d-glucose, 5 mm KCl, 1 mm MgSO4 and supplemented with 0.1% BSA and 40 μg/ml apyrase.
Cell viability was assessed using calcein and trypan blue. For calcein loading, platelets were incubated for 30 min with 5 μm calcein-AM at 37 °C and centrifuged, and the pellet was resuspended in fresh HBS. Fluorescence was recorded from 2-ml aliquots using a Cary Eclipse spectrophotometer (Varian Ltd., Madrid, Spain). Samples were excited at 494 nm, and the resulting fluorescence was measured at 535 nm. The results obtained with calcein were confirmed using the trypan blue exclusion technique. 95% of platelets were viable in our preparations.
Measurement of [Ca2+]i
Human platelets were loaded with fura-2 by incubation with 2 μm fura-2/AM for 45 min at 37 °C. Fluorescence was recorded from 2-ml aliquots of magnetically stirred cellular suspension (2 × 108 platelets/ml) at 37 °C using a Cary Eclipse spectrophotometer (Varian Ltd., Madrid, Spain) with excitation wavelengths of 340 and 380 nm and emission at 505 nm. Changes in [Ca2+]i were monitored using the fura-2 340/380 fluorescence ratio and calibrated according to an established method (34).
Ca2+ entry was estimated using the integral of the rise in [Ca2+]i for 2.5 min after the addition of CaCl2. Ca2+ release was estimated using the integral of the rise in [Ca2+]i for 3 min after the addition of the agent (35). Ca2+ entry and release are expressed as nm·s, as described previously (36).
Isolation of Platelet Lysosomes and Dense Granules
Platelet fractions were prepared by immunomagnetic sorting as described previously (37). Briefly, platelets (2 × 109 cells/ml) were homogenized, and the platelet homogenate was incubated for 1.5 h at 4 °C with anti-LAMP2 antibody in 1:100 dilution in PBS supplemented with 1× protease inhibitors. Cells were ultracentrifuged for 30 min at 100,000 × g at 4 °C and resuspended in 1 ml of PBS. 50 μl of Dynabeads panmouse IgG was added, and the mixture was incubated for 1.5 h at 4 °C to form the Dynabead-antibody-organelle complex. This complex was extracted by a magnet. Separation of the Dynabead-antibody-organelle complex was achieved by SDS elution as described (37).
Immunoprecipitation and Western Blotting
The immunoprecipitation and Western blotting were performed as described previously (38). Briefly, 500-μl aliquots of platelet suspension (2 × 109 cells/ml) were lysed with an equal volume of radioimmune precipitation assay buffer, pH 7.2, containing 316 mm NaCl, 20 mm Tris, 2 mm EGTA, 0.2% SDS, 2% sodium deoxycholate, 2% Triton X-100, 2 mm Na3VO4, 2 mm PMSF, 100 μg/ml leupeptin, and 10 mm benzamidine. Aliquots of platelet lysates (1 ml) were immunoprecipitated by incubation with 2 μg of anti-hTRPC1 antibody, anti-hTRPC6 antibody, anti-STIM1 antibody, anti-Orai1 antibody, or anti-SERCA3 antibody and 25 μl of protein A-agarose overnight at 4 °C on a rocking platform. For standardizing purposes, the protein content in the samples was determined by the Bradford method (39). The immunoprecipitates were resolved by 10% SDS-PAGE, and separated proteins were electrophoretically transferred onto nitrocellulose membranes for subsequent probing. Blots were incubated overnight with 10% (w/v) BSA in Tris-buffered saline with 0.1% Tween 20 (TBST) to block residual protein binding sites. Immunodetection of hTRPC1, hTRPC6, STIM1, Orai1, PDI, STIM2, and cathepsin D was achieved using the anti-hTRPC1 antibody diluted 1:200 in TBST for 1 h; the anti-hTRPC6 antibody diluted 1:200 in TBST for 2 h; the anti-STIM1, anti-STIM2, anti-SERCA3, and anti-cathepsin D antibody diluted 1:250 in TBST for 2 h; and the anti-Orai1 antibody diluted 1:500 in TBST for 2 h, respectively. The primary antibody was removed, and blots were washed six times for 5 min each with TBST. To detect the primary antibody, blots were incubated for 45 min with horseradish peroxidase-conjugated ovine anti-mouse IgG antibody or horseradish peroxidase-conjugated donkey anti-rabbit IgG antibody diluted 1:10,000 in TBST and then exposed to enhanced chemiluminiscence reagents for 5 min. Blots were then exposed to photographic films. The density of bands on the film was measured using scanning densitometry.
Reversible Electroporation
The platelet suspension was transferred to an electroporation chamber containing antibodies at a final concentration of 2 μg/ml, and the antibody was transjected according to published methods (40). Reversible electropermeabilization was performed at 4 kV/cm at a setting of 25-microfarad capacitance and was achieved by seven pulses using a Bio-Rad Gene Pulser Xcell electroporation system (Bio-Rad). Following electroporation, platelets were incubated with antibodies for an additional 60 min at 37 °C and centrifuged at 350 × g for 20 min and resuspended in HBS prior to the experiments.
Immunofluorescence
Cells were fixed using 3% paraformaldehyde (in PBS) for 10 min at room temperature. The cells were then permeabilized in PBS containing 0.025% (v/v) Nonidet P-40 detergent for 10 min at 4 °C. Samples were incubated with rabbit anti-Orai1 and mouse anti-STIM1 antibodies overnight at room temperature in PBS containing 0.5% BSA as blocking agent, followed by incubation with Alexa Fluor® 350- and 647-conjugated secondary antibodies for 1 h. The samples were examined using a Zeiss LSM 510 confocal microscope.
Statistical Analysis
Analysis of statistical significance was performed using Student's t test. For multiple comparisons, a one-way analysis of variance combined with the Tukey tests was used. p < 0.05 was considered to be significant for a difference.
RESULTS
TBHQ Releases Ca2+ from Agonist-sensitive Ca2+ Stores Independent of the DTS
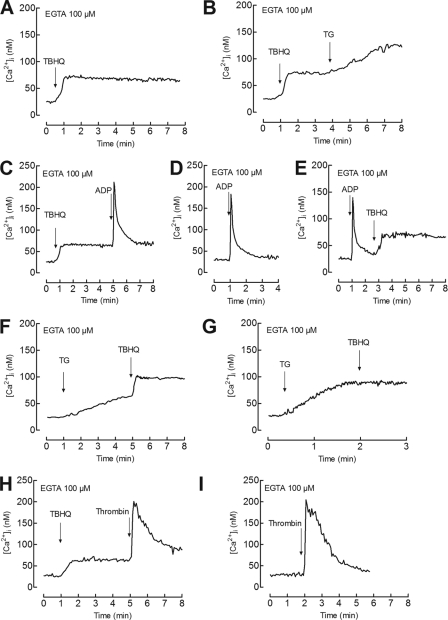
Human platelets have been reported to possess two independent Ca2+ stores on the basis of immunolocalization studies (29), different sensitivities to TG and TBHQ (41–43), and biochemical properties (4). In these cells, TBHQ has been reported to release Ca2+ from acidic organelles (4). Hence, we have further investigated whether TBHQ releases Ca2+ from an agonist-sensitive compartment separate from the DTS. We have reported that treatment of platelets with TBHQ induced a concentration-dependent Ca2+ release from the TBHQ-sensitive acidic stores, reaching a maximal effect at concentrations of 20 μm (32). As shown in Fig. 1A, treatment of human platelets with 20 μm TBHQ results in a sustained increase in [Ca2+]i. The subsequent addition of 10 nm TG induces a further increase in [Ca2+]i, indicative of Ca2+ efflux from a TBHQ-insensitive Ca2+ store (Fig. 1B). We have previously found that the physiological agonist ADP releases Ca2+ exclusively from the DTS (31). Our results indicate that platelet stimulation with ADP after treatment with TBHQ results in a transient rise in [Ca2+]i that was comparable with the response evoked in untreated cells (the integrals of the rise in [Ca2+]i for 3 min after the addition of 10 μm ADP were 1782 ± 217 nm·s and 1842 ± 195 nm·s in the absence and presence of TBHQ, respectively, Fig. 1, C versus D). Furthermore, platelet treatment with ADP did not significantly modify the response to TBHQ (the integrals of the rise in [Ca2+]i for 3 min after the addition of 20 μm TBHQ were 3226 ± 422 nm·s and 3297 ± 375 nm·s in cells prestimulated with ADP or HBS, respectively, Fig. 1, E versus C). These findings strongly suggest that ADP releases Ca2+ from a TBHQ-insensitive store, previously identified as the DTS (31). We have further explored the source of the TBHQ-evoked response. On the basis of the high sensitivity of SERCA2b to TG (28), platelets were treated with a low concentration of TG (10 nm), and 4 min later, 20 μm TBHQ was added to the cell suspension. As shown in Fig. 1F, Ca2+ mobilization induced by TBHQ in cells pretreated with TG was similar to that observed in control cells (the integral of the rise in [Ca2+]i for 3 min after the addition of TBHQ was 3159 ± 289 in TG-treated cells; Fig. 1, F versus A), thus suggesting that the TBHQ-sensitive store is insensitive to low concentrations of TG. Because both SERCA isoforms identified in platelets, SERCA2b and SERCA3, have been shown to be sensitive to high concentrations of TG, we repeated this protocol but using 1 μm TG. As depicted in Fig. 1G, TBHQ was without effect after treatment with a high concentration of TG, thus confirming that human platelets possess two separate Ca2+ stores: one pool is insensitive to TBHQ and shows a high sensitivity to TG, and the second compartment is sensitive to TBHQ and shows a low sensitivity to TG. Finally, treatment with TBHQ results in a significant reduction of the thrombin-induced rise in [Ca2+]i (the integrals of the rise in [Ca2+]i for 3 min after the addition of thrombin were 7215 ± 518 nm·s and 5367 ± 351 nm·s in the absence or presence of TBHQ, respectively; Fig. 1, H versus I), which further confirms that the TBHQ-sensitive store is an agonist-releasable compartment, as described previously (33).
FIGURE 1.
Ca2+ mobilization from TBHQ-sensitive and -insensitive stores in human platelets. Fura-2-loaded human platelets were suspended in HBS containing 100 μm EGTA and stimulated with TBHQ (20 μm) (A), TBHQ (20 μm) followed by the addition of TG (10 nm) 3 min later (B), TBHQ (20 μm) followed by the addition of ADP (10 μm) 4 min later (C), 10 μm ADP alone (D), ADP (10 μm) followed by the addition of TBHQ (20 μm) 2 min later (E), TG (10 nm) followed by the addition of TBHQ (20 μm) 4 min later (F), TG (1 μm) followed by the addition of TBHQ (20 μm) 1.5 min later (G), TBHQ (20 μm) followed by treatment with thrombin (1 unit/ml) 4 min later (H), or thrombin (1 unit/ml) alone (I). Changes in fura-2 fluorescence were monitored using the 340 nm/380 nm ratio and calibrated in terms of [Ca2+]i. Traces are representative of 5–7 independent experiments.
Expression of STIM1 in the Lysosomal Fraction and Dense Granules
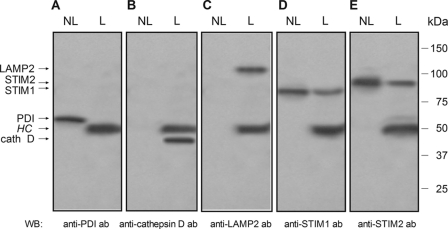
In mammalian cells, the acidic stores have been mostly identified in the secretory granules, lysosomes, and lysosome-related organelles. Therefore, we have investigated the location of STIM1 in the dense granules and the lysosomal fraction in human platelets by using the specific marker for dense granules and lysosomes, LAMP2 (44, 45). Specificity of the isolation of lysosomes and dense granules was supported by the lack of detection of the ER marker PDI in lysosomes and dense granules, the only fractions where cathepsin D and LAMP2 were detected (Fig. 2, A–C). As shown in Fig. 2, D and E, Western blotting reveals the expression of STIM1 and STIM2 in the dense granules and the lysosomal fraction, although the level of expression was found to be smaller than in non-lysosomal fractions.
FIGURE 2.
Expression of STIM1 and STIM2 in lysosome-related organelles and dense granules in human platelets. Lysosome-related organelles and dense granules from human platelets were isolated by immunomagnetic sorting using anti-LAMP2 antibody as described under “Experimental Procedures.” The lysosomal and dense granule fraction (L) and the non-lysosomal fraction (NL) were subjected to 10% SDS-PAGE (15 μg protein/lane) and subsequent Western blotting (WB) with specific anti-PDI (A), anti-cathepsin D (B), anti-LAMP2 (C), anti-STIM1 (D), or anti-STIM2 (E) antibody. Shown are results from one experiment representative of five others. Molecular masses indicated on the right were determined using molecular mass markers run on the same gel. HC, heavy chain of the immunoglobulin used for immunomagnetic sorting.
Discharge of the Acidic Ca2+ Stores Induces Association between STIM1 and SERCA3 in Human Platelets
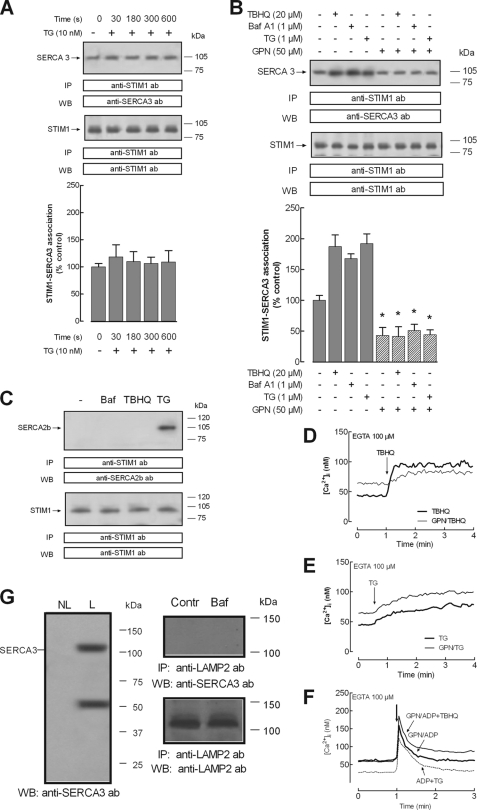
To further confirm the location of STIM1 in acidic stores, we investigated its association with the acidic store resident protein SERCA3. First of all, we confirmed the location of SERCA3 in the dense granules and the lysosomal fraction in human platelets by using the specific marker for dense granules and lysosomes, LAMP2. As shown in Fig. 3G (left), Western blotting reveals the expression of SERCA3 in the dense granules and the lysosomal fraction. The location of SERCA3 in the lysosomal fraction cannot be attributed to direct association with LAMP2, as depicted in Fig. 3G (right).
FIGURE 3.
Disruption of lysosomes inhibits STIM1-SERCA3 coimmunoprecipitation after discharge of the acidic Ca2+ stores in human platelets. Human platelets (2 × 109 cells/ml) were suspended in a Ca2+-free medium (100 μm EGTA added). A, cells were treated for various periods of time in the absence and presence of TG (10 nm) and lysed. B, cells were preincubated for 10 min in the presence of 50 μm GPN or the vehicle and then treated for 30 s with TBHQ (20 μm), bafilomycin A1 (Baf A1) (1 μm), or TG (1 μm), as indicated, and lysed. C, cells were treated for 30 s with bafilomycin A1 (Baf) (1 μm), TBHQ (20 μm), TG (10 nm), or the vehicle (−) and lysed. Whole cell lysates were immunoprecipitated (IP) with anti-STIM1 antibody (ab). Immunoprecipitates were subjected to 10% SDS-PAGE and subsequent Western blotting (WB) with a specific anti-SERCA3 antibody (A and B) or anti-SERCA2b antibody (C). Membranes were reprobed with the immunoprecipitating antibody for protein loading control. Shown are results from one experiment representative of five others. Molecular masses indicated on the right were estimated using molecular mass markers run on the same gel. Histograms represent the quantification of STIM1-SERCA3 association under the different experimental conditions. Results are presented as percentage of control and expressed as mean ± S.E. (error bars). *, p < 0.05 versus control (not treated with GPN). D–F, fura-2-loaded human platelets were suspended in Ca2+-free HBS (100 μm EGTA added) and incubated with GPN (50 μm) or DMSO (as control) for 10 min. Cells were then stimulated with TBHQ (20 μm) (D), TG (10 nm) (E), or ADP alone or in combination with 20 μm TBHQ or 10 nm TG, as indicated (F). Changes in fura-2 fluorescence were monitored using the 340 nm/380 nm ratio and calibrated in terms of [Ca2+]i. Traces are representative of six independent experiments. G, left, lysosome-related organelles and dense granules from human platelets were isolated by immunomagnetic sorting using anti-LAMP2 antibody as described under “Experimental Procedures.” The lysosomal and dense granule fraction (L) and the non-lysosomal fraction (NL) were subjected to 10% SDS-PAGE and subsequent Western blotting with specific anti-SERCA3 antibody. G, right, cells were stimulated with bafilomycin A1 (1 μm) or left untreated and lysed. Whole cell lysates were immunoprecipitated with anti-LAMP2 antibody, and immunoprecipitates were subjected to 10% SDS-PAGE and subsequent Western blotting with anti-SERCA3 antibody. Membranes were reprobed with the immunoprecipitating antibody for protein loading control. Shown are results from one experiment representative of five others. Molecular masses indicated on the right were determined using molecular mass markers run on the same gel.
We have investigated the association between STIM1 and SERCA3 by looking for co-immunoprecipitation from platelet lysates. Immunoprecipitation and subsequent SDS-PAGE and Western blotting were conducted using control platelets and platelets treated in a Ca2+-free medium for various periods of time with TG (10 nm) to induce specific depletion of the DTS or for 30 s with TBHQ (20 μm); bafilomycin A1 (1 μm), a widely used inhibitor of the V-ATPase (46) that dissipates the proton gradient into acidic organelles (19), which has been reported to induce leakage of Ca2+ accumulated into the acidic stores (4); or TG (1 μm) to induce depletion of both stores, respectively. After immunoprecipitation with anti-STIM1 antibody, Western blotting revealed the presence of SERCA3 in samples from resting platelets. We found that treatment with TG (10 nm) did not significantly increase the association between STIM1 and SERCA3 at least during 10 min of stimulation (Fig. 3A, top; n = 6). In contrast, treatment with TBHQ (20 μm), bafilomycin A1 (1 μm), or TG (1 μm) for 30 s significantly enhanced the co-immunoprecipitation between STIM1 and SERCA3 (Fig. 3B, top; n = 7). Western blotting of the same membranes with the antibody used for immunoprecipitation confirmed similar protein content in all lanes (Fig. 3B, bottom panels).
Furthermore, we have explored the effect of GPN, a lysosome-disrupting cathepsin C substrate that induces lysosome lysis (4, 47), on the association between STIM1 and SERCA3 evoked by acidic store depletion. Platelet treatment with 50 μm GPN resulted in a sustained elevation in [Ca2+]i, due to lysis of lysosome-related Ca2+ stores in these cells (4), and a significant attenuation of TBHQ-evoked Ca2+ release by 62 ± 15% (Fig. 3D). In contrast, GPN did not alter Ca2+ release induced by 10 nm TG (Fig. 3F). In order to test the source of the GPN-resistant TBHQ-induced Ca2+ release, we performed a series of experiments where the DTS was discharged by using 10 μm ADP, as described previously (31). If the GPN-resistant TBHQ response occurs by release from the DTS, no increase in the ADP-induced initial peak [Ca2+]i elevation might be expected, as observed when ADP was added in combination with 10 nm TG (Fig. 3F), where only a decrease in the return of [Ca2+]i to basal levels was found due to inhibition of Ca2+ reuptake by SERCA2b. As shown in Fig. 3F, stimulation of GPN-treated human platelets with 10 μm ADP in combination with 20 μm TBHQ enhanced the initial peak [Ca2+]i elevation induced by ADP from 157 ± 6 to 184 ± 8 nm, thus suggesting that the GPN-resistant TBHQ-evoked response was independent on the DTS, probably attributable to Ca2+ release from non-lysosomal acidic stores, such as the dense granules.
Our results indicate that treatment with 50 μm GPN for 10 min at 37 °C both reduced the association between STIM1 and SERCA3 in resting cells and abolished the response to TBHQ, bafilomycin A1, and 1 μm TG (Fig. 3B, top, n = 6). These findings indicate that the integrity of lysosome-related organelles is important for STIM1-SERCA3 co-immunoprecipitation, thus suggesting that STIM1 and SERCA3 co-localize in acidic stores and explaining the association between these proteins detected in resting cells.
We have further investigated the association of STIM1 with SERCA2b, which has been reported in the DTS in platelets (28, 31). As shown in Fig. 3C, discharge of the acidic stores with bafilomycin A1 or TBHQ was unable to induce association between SERCA2b and STIM1, whereas discharge of the DTS using a low concentration of TG (10 nm) resulted in co-immunoprecipitation of SERCA2b with STIM1 (Fig. 3C).
Depletion of the Acidic Stores Enhances Interaction between STIM1 and Orai1 in Human Platelets
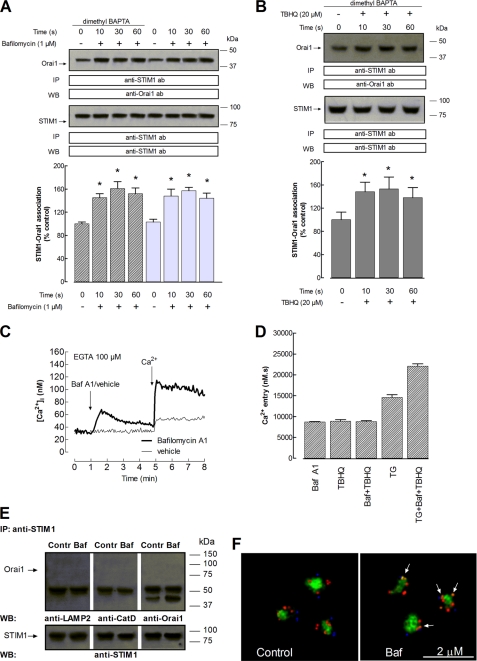
It is widely accepted that the interaction of STIM1 with Ca2+-permeable plasma membrane channels, such as Orai1 proteins, plays a key role in the activation of SOCE in a number of cells (48), including human platelets (49–50). Because we have found that acidic stores in human platelets express STIM1, we have further explored the interaction between STIM1 and Orai1 upon specific depletion of the acidic stores by looking for co-immunoprecipitation from platelet lysates. Immunoprecipitation and subsequent SDS-PAGE and Western blotting were conducted using dimethyl-BAPTA-loaded platelets to prevent Ca2+-dependent, but not store depletion-dependent, responses. Acidic stores are agonist-releasable Ca2+ stores characterized by the maintenance of a proton gradient across their membranes supported by the V-ATPase (4, 17, 46). We have investigated the effect of acidic store depletion on the association between STIM1 and Orai1 by using bafilomycin A1. As shown in Fig. 4C, treatment of human platelets in a Ca2+-free medium (100 μm EGTA added) with bafilomycin A1 (1 μm) evoked a transient elevation of [Ca2+]i, due to Ca2+ release from the acidic stores (the integral for 3 min of the rise in [Ca2+]i after the addition of bafilomycin A1 (1 μm) was 811 ± 73 nm·s; Fig. 4C). The subsequent addition of Ca2+ (300 μm) to the external medium induced a sustained increased in [Ca2+]i indicative of SOCE (the integral of the rise in [Ca2+]i after the addition of CaCl2 was 8685 ± 137 nm·s; Fig. 4, C and D). Similar results were obtained when SOCE was stimulated by TBHQ or a combination of bafilomycin A1 and TBHQ, thus suggesting that both agents discharge a common Ca2+ store (Fig. 4D). In contrast, the extent of SOCE induced by TG (10 nm) was greater and complementary to that induced by bafilomycin A1 and TBHQ, which indicates that 10 nm TG discharges a different Ca2+ store (Fig. 4D).
FIGURE 4.
Acidic stores depletion enhances interaction between STIM1 and Orai1 in human platelets. A and B, human platelets were loaded with dimethyl-BAPTA or left untreated, as indicated, and then resuspended (2 × 109 cells/ml) in a Ca2+-free medium (100 μm EGTA added). Cells were stimulated for various periods of time (10–60 s) with bafilomycin A1 (1 μm) (A) or TBHQ (20 μm) (B) and lysed. Whole cell lysates were immunoprecipitated (IP) with anti-STIM1 antibody, and immunoprecipitates were subjected to 10% SDS-PAGE and subsequent Western blotting (WB) with a specific anti-Orai1 antibody. Membranes were reprobed with the immunoprecipitating antibody for protein loading control. Shown are results from one experiment representative of 6–7 others. Molecular masses indicated on the right were determined using molecular mass markers run on the same gel. Histograms represent the quantification of the STIM1-Orai1 association in resting (control) and bafilomycin A1- or TBHQ-treated cells. Results are presented as a percentage of control and expressed as mean ± S.E. (error bars). *, p < 0.05 versus control. C, fura-2-loaded human platelets were suspended in Ca2+-free HBS (100 μm EGTA added), stimulated with bafilomycin A1 (1 μm), and 3 min later, 300 μm CaCl2 was added to the medium. Changes in fura-2 fluorescence were monitored using the 340 nm/380 nm ratio and calibrated in terms of [Ca2+]i. Traces are representative of eight independent experiments. D, histograms represent Ca2+ entry stimulated by treatment for 4 min with bafilomycin A1 (1 μm); TBHQ (20 μm); both agents; TG (10 nm); and a combination of TG, bafilomycin A1, and TBHQ and estimated as described under “Experimental Procedures.” E, platelets (2 × 109 cell/ml) suspended in a Ca2+-free medium (100 μm EGTA added) were stimulated for 30 s with bafilomycin A1 (Baf) (1 μm) or vehicle as control (Contr) and lysed. Whole cell lysates were immunoprecipitated with anti-STIM1 antibody, and immunoprecipitates were subjected to 10% SDS-PAGE and subsequent Western blotting with a specific anti-LAMP2, anti-cathepsin D (CatD), or anti-Orai1 antibody. Membranes were reprobed with the immunoprecipitating antibody for protein loading control. Shown are results from one experiment representative of four others. Molecular masses indicated on the right were determined using molecular mass markers run on the same gel. F, dimethyl-BAPTA-AM-loaded resting human platelets (Control) or platelets incubated for 30 s at 37 °C in the presence of 1 μm bafilomycin A1 following the protocol described in A, were loaded with Lysosensor Green DND-189 to stain the acidic organelles and immunostained with anti-Orai1 and anti-STIM1 antibodies, followed by Alexa Fluor 350- and 647-conjugated secondary antibodies, respectively, and fluorescence was detected using a confocal microscope as described under “Experimental Procedures.” Images shown are representative of six separate experiments. The arrows indicate colocalization of STIM1 (red) and Orai1 (blue).
In a Ca2+-free medium (100 μm EGTA was added), platelet treatment for various periods of time (10–60 s) with bafilomycin A1 (1 μm) resulted in a time-dependent increase in the association between STIM1 and Orai1, reaching a maximum after 30 s of treatment (Fig. 4A, top; n = 7). Similar results were observed when we repeated the experiments in cells not loaded with dimethyl-BAPTA (Fig. 4A, top; n = 6) and when BAPTA-loaded cells were stimulated with TBHQ (20 μm; Fig. 4B, top, n = 7). Western blotting of the same membranes with the antibody used for immunoprecipitation confirmed similar protein content in all lanes (Fig. 4, A and B, bottom panels). Treatment of platelets with bafilomycin A1 was unable to induce association between STIM1 and LAMP2 or cathepsin D, thus suggesting that the association of STIM1 with Orai1 cannot be attributed to bafilomycin A1-induced nonspecific protein aggregation in lysosomes (Fig. 4E).
We have also investigated the location of STIM1 in the acidic stores and colocalization with Orai1 by immunofluorescence. As shown in Fig. 4F, STIM1 was distributed in the acidic lysosomes and lysosome-like organelles but also outside of these organelles, presumably in the DTS, the ER analog in platelets. Orai1 was distributed in the cell periphery (Fig. 4F). Stimulation with bafilomycin A1 for 3 min resulted in colocalization of STIM1 with Orai1. We did not detect any translocation of lysosomes and lysosome-related organelles to the plasma membrane upon platelet activation, which is consistent with the organellar concentration in the platelet core reported upon platelet stimulation (51).
STIM1 and Orai1 Co-immunoprecipitate with hTRPC1 and hTRPC6 after Discharge of the Acidic Ca2+ Stores in Human Platelets
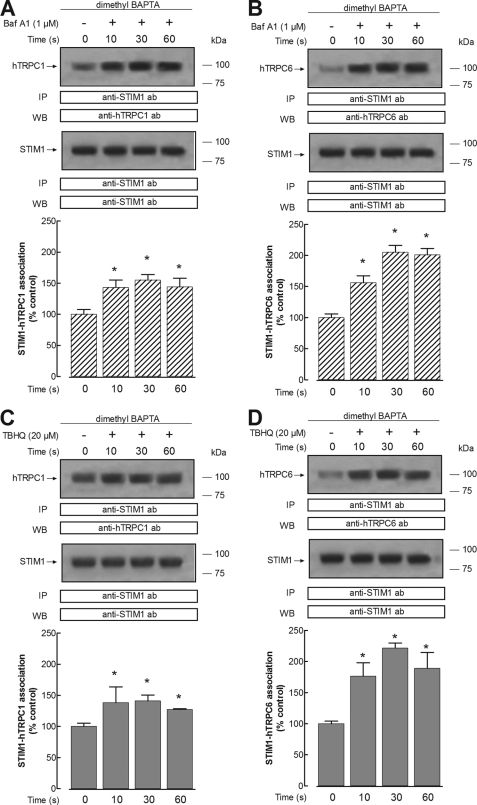
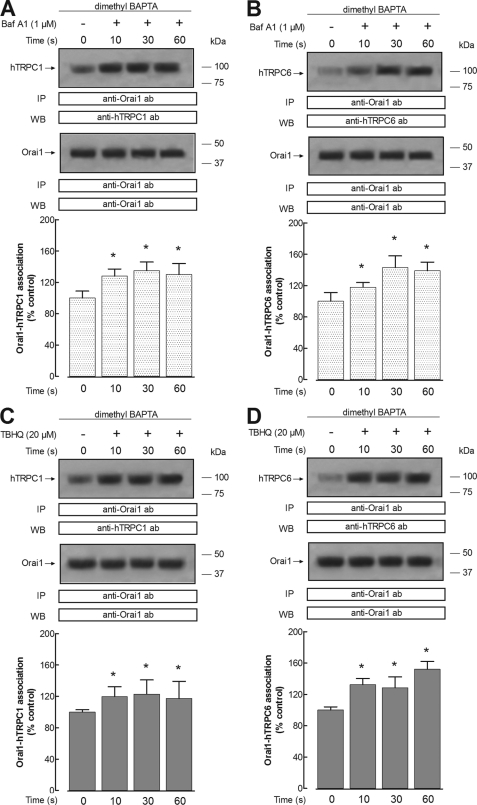
We have further investigated whether depletion of the acidic Ca2+ stores activates the interaction between STIM1 and Orai1 with hTRPC1 and hTRPC6 proteins, which have been shown to play an important role in agonist-evoked Ca2+ entry in human platelets (35, 52–53). Treatment of dimethyl-BAPTA-loaded human platelets with 1 μm bafilomycin A1 in a Ca2+-free medium resulted in a time-dependent increase in the association between hTRPC1 and STIM1, reaching a maximum after 30 s of treatment (Fig. 5A). Furthermore, our results indicate that bafilomycin A1 induces time-dependent interaction between hTRPC1 and Orai1, with a maximum achieved after 30 s of treatment (Fig. 6A). Similarly, platelet treatment with 1 μm bafilomycin A1 results in a time-dependent increase in the association between hTRPC6 and both STIM1 and Orai1 (Figs. 5B and 6B). We have noticed that the association between STIM1 and hTRPC6 stimulated by bafilomycin A1 is greater than that observed between STIM1 and hTRPC1 (Fig. 5, A versus B). The results observed with bafilomycin A1 were also reproduced by treatment with 20 μm TBHQ (Figs. 5 and 6, C and D).
FIGURE 5.
Acidic store depletion enhances interaction between STIM1 and hTRPC1 or hTRPC6 proteins in human platelets. Human platelets were loaded with dimethyl-BAPTA and then resuspended (2 × 109 cell/ml) in a Ca2+-free medium (100 μm EGTA added). Cells were then stimulated for various periods of time (10–60 s) with bafilomycin A1 (1 μm) (A and B) or TBHQ (20 μm) (C and D) and lysed. Whole cell lysates were immunoprecipitated (IP) with anti-STIM1 antibody. Immunoprecipitates were subjected to 10% SDS-PAGE and subsequent Western blotting (WB) with a specific anti-hTRPC1 antibody (A and C) or anti-hTRPC6 antibody (B and D). Membranes were reprobed with the antibody used for immunoprecipitation for protein loading control. Shown are results from one experiment representative of six others. Molecular masses indicated on the right were determined using molecular mass markers run on the same gel. Histograms represent the quantification of STIM1-hTRPC1 (A and C) or STIM1-hTRPC6 (B and D) association in resting (control) and TBHQ-treated cells. Results are presented as a percentage of control and expressed as mean ± S.E. (error bars). *, p < 0.05 versus control.
FIGURE 6.
Acidic store depletion enhances interaction between Orai1 and hTRPC1 or hTRPC6 proteins in human platelets. Human platelets were loaded with dimethyl-BAPTA and then resuspended (2 × 109 cell/ml) in a Ca2+-free medium (100 μm EGTA added). Cells were then stimulated for various periods of time (10–60 s) with bafilomycin A1 (1 μm; A and B) or TBHQ (20 μm; C and D) and lysed. Whole cell lysates were immunoprecipitated (IP) with anti-Orai1 antibody. Immunoprecipitates were subjected to 10% SDS-PAGE and subsequent Western blotting (WB) with a specific anti-hTRPC1 antibody (A and C) or anti-hTRPC6 antibody (B and D). Membranes were reprobed with the antibody used for immunoprecipitation for protein loading control. Shown are results from one experiment representative of seven others. Molecular masses indicated on the right were determined using molecular mass markers run on the same gel. Histograms represent the quantification of Orai1-hTRPC1 (A and C) or Orai1-hTRPC6 (B and D) association in resting (control) and TBHQ-treated cells. Results are presented as a percentage of control and expressed as mean ± S.E. (error bars). *, p < 0.05 versus control.
Inhibition of Acidic Store Depletion-evoked Interaction between STIM1 and Orai1 and SOCE by Electrotransjection with Anti-Orai1 C-terminal Antibody
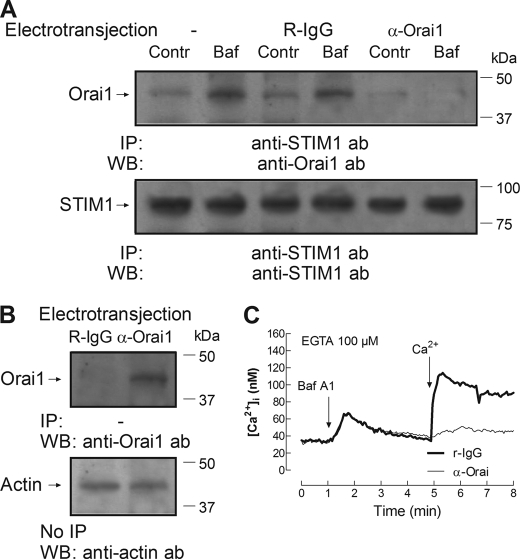
The amino acid sequence 288–301 of human Orai1 recognized by the anti-Orai1 antibody used is located in the cytosolic C-terminal region of Orai1, which is essential for the interaction with STIM1 (54). We have previously reported that introduction of this antibody into platelets prevents the association of STIM1 with Orai1 (35). We have now investigated whether introduction of the anti-Orai1 antibody impairs the interaction between STIM1 and Orai1 and SOCE stimulated by bafilomycin A1. To assess this possibility, the anti-Orai1 antibody was introduced into platelets by electropermeabilization, which has been successfully used for transferring antibodies into cells while maintaining the physiological integrity of the cells (35, 55, 56). Human platelets were reversibly electroporated as described under “Experimental Procedures.” The introduction of the antibody was confirmed in samples from platelets electropermeabilized and incubated with 1 μg/ml of either anti-Orai1 antibody or rabbit IgG, of the same nature of the anti-Orai1 antibody used, by immunoprecipitation without adding any additional antibody and subsequent Western blotting with the anti-Orai1 antibody. As shown in Fig. 7B, Orai1 was clearly detected in cells that had been previously electropermeabilized and incubated with anti-Orai1 antibody and not in cells incubated with rabbit IgG. Electropermeabilization allowed the anti-Orai1 antibody to enter the cells and to immunoprecipitate native Orai1, which was then detected by Western blotting, thus confirming the efficacy of the electrotransjection. As shown in Fig. 7A, electrotransjection of the anti-Orai1 antibody impairs the association between STIM1 and Orai1 stimulated by bafilomycin A1, whereas electrotransjection of a rabbit IgG did not significantly attenuate the response to bafilomycin A1. In addition, we have found that introduction of the anti-Orai1 antibody into cells abolished SOCE stimulated by bafilomycin A1, without having any effect on bafilomycin-induced Ca2+ release (Fig. 7C; p < 0.05). Introduction of a rabbit IgG did not significantly modify bafilomycin A1-evoked Ca2+ mobilization (Fig. 7C versus Fig. 4C).
FIGURE 7.
Inhibition of acidic store depletion-evoked Ca2+ entry and interaction between STIM1 and Orai1 by electrotransjection with anti-Orai1 C-terminal antibody. Human platelets were electropermeabilized in a Gene Pulser as described under “Experimental Procedures” and then incubated in the presence of 1 μg/ml anti-Orai1 antibody (α-Orai1) or 1 μg/ml rabbit IgG (R-IgG) for 60 min, as indicated. A, cells were then stimulated with 1 μm bafilomycin A1 (Baf) for 30 s or left unstimulated (Contr) and lysed. Whole cell lysates were immunoprecipitated (IP) with anti-STIM1 antibody followed by Western blotting (WB) using anti-Orai1 antibody (ab). B, whole cell lysates from samples electrotransjected with anti-Orai1 antibody or rabbit IgG were either immunoprecipitated in the absence of antibodies but adding protein A-agarose, followed by Western blotting (WB) using anti-Orai1 antibody (B, top) or subjected to Western blotting using anti-actin antibody for protein loading control (B, bottom). These results are representative of four independent experiments. Positions of molecular mass markers are shown on the right. C, human platelets (109 platelets/ml) were electropermeabilized in a Gene Pulser, as described under “Experimental Procedures.” Following electroporation, cells were incubated with 1 μg/ml rabbit IgG or with 1 μg/ml anti-Orai1 antibody for an additional 60 min at 37 °C, loaded with fura-2, centrifuged at 350 × g for 20 min, and resuspended in HBS containing 50 μm CaCl2. At the time of the experiment, 100 μm EGTA was added. Cells were stimulated with bafilomycin A1 (1 μm), and 3 min later, 300 μm CaCl2 was added to the medium. Changes in fura-2 fluorescence were monitored using the 340 nm/380 nm ratio and calibrated in terms of [Ca2+]i. Traces are representative of six independent experiments.
STIM2 Co-immunoprecipitates with Orai1 and SERCA3 but Not with hTRPC1 and hTRPC6 after Discharge of the Acidic Ca2+ Stores in Human Platelets
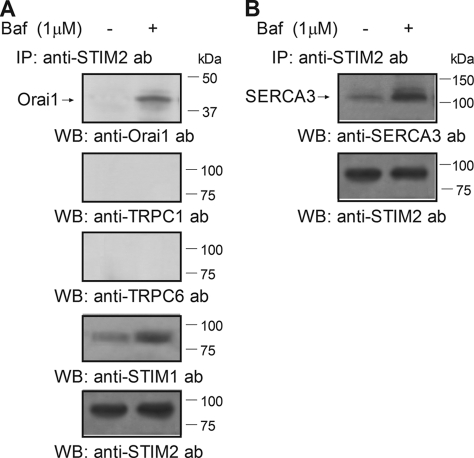
STIM2, as well as STIM1, has been reported to function as a Ca2+ sensor in the intracellular stores that interacts with and activates plasma membrane channels, including Orai1 subunits (57). Because we have found expression of STIM2 in acidic organelles (Fig. 2), we have further investigated its association with Ca2+-permeable channels. As shown in Fig. 8A, treatment of platelets with bafilomycin A1 results in association between STIM2 and both Orai1 and STIM1, as detected by immunoprecipitation with anti-STIM2 antibody followed by Western blotting with anti-Orai1 or anti-STIM1 antibody. In contrast, we found that bafilomycin A1 was unable to induce association of STIM2 with TRPC1 or TRPC6 (n = 6).
FIGURE 8.
STIM2 associates with Orai1 and SERCA3 upon discharge of the acidic Ca2+ stores in human platelets. Human platelets were loaded with dimethyl-BAPTA and then resuspended (2 × 109 cell/ml) in a Ca2+-free medium (100 μm EGTA added). Cells were then stimulated for 30 s with bafilomycin A1 (1 μm) and lysed. Whole cell lysates were immunoprecipitated (IP) with anti-STIM2 antibody. Immunoprecipitates were subjected to 10% SDS-PAGE and subsequent Western blotting (WB) with specific anti-Orai1, anti-hTRPC1, anti-hTRPC6, or anti-STIM1 antibodies (ab) (A) or with anti-SERCA3 antibody (B). Shown are results from one experiment representative of five others. Membranes were reprobed with the antibody used for immunoprecipitation for protein loading control (results shown in A are representative of the experiments performed to detect association of STIM2 with Orai1, hTRPC1, hTRPC6, or STIM1). Molecular masses indicated on the right were determined using molecular mass markers run on the same gel.
STIM2 has also been reported to play a relevant role, maintaining basal cytosolic and ER Ca2+ concentrations within tight limits (15). Hence, we have investigated the association of STIM2 with SERCA3, located in the acidic Ca2+ stores (see Fig. 3G). Co-immunoprecipitation between STIM2 and SERCA3 was detected in resting cells, but it was significantly enhanced by 318% upon platelet stimulation for 30 s with bafilomycin A1 (1 μm; Fig. 8B; p < 0.05; n = 6).
DISCUSSION
Acidic Ca2+ stores are agonist-sensitive Ca2+ pools that have been identified in a large number of cells, including human platelets. Our previous studies have reported that depletion of the acidic Ca2+ stores using TBHQ results in the activation of a SOCE pathway that is regulated differently from SOCE controlled by the DTS (32). Here, we report Ca2+ entry upon acidic store depletion by using a different tool to induce specific discharge of the acidic Ca2+ stores, the V-ATPase inhibitor bafilomycin A1. Our results show, for the first time, the expression of STIM1 in the acidic organelles, consisting of dense granules and lysosome-related organelles in human platelets. The expression of STIM1 in acidic organelles is also supported by co-immunoprecipitation of this protein with the SERCA3 isoform, which has been reported to be located in the acidic stores on the basis of its sensitivity to TBHQ (42) and the sensitivity of TBHQ-evoked Ca2+ release to bafilomycin A1 (4). We have found that STIM1 and SERCA3 co-localize in the lysosome-related organelles because lysis of lysosomes by osmotic swelling induced by treatment with GPN significantly attenuated both resting association between STIM1 and SERCA3 and interaction between SERCA3 and STIM1 stimulated by acidic store discharge using either TBHQ, bafilomycin A1, or a high concentration of TG. We have previously reported that treatment of platelets with GPN results in a dramatic reduction in the fluorescence of the lysosome tracker Lysosensor Green (4) and subsequent discharge of the Ca2+ accumulated in the cytosol, which explains the increased [Ca2+]i detected after 10 min of treatment with GPN in Fig. 3D. The fact that some STIM1-SERCA3 interaction as well as TBHQ-induced Ca2+ release was still found in cells treated with GPN might be attributed to the inability of GPN to completely lysate the lysosome-related organelles or to the existence of lysosome-unrelated acidic compartments, such as the dense granules, which might also contain these proteins. To confirm the specificity of our experimental maneuver, we have found that discharge of the acidic stores using bafilomycin A1 or TBHQ was unable to induce association of STIM1 with SERCA2b, located in the DTS in human platelets (28, 58), thus suggesting that the discharge of the acidic stores induces specific association of the pool of STIM1 located in the acidic compartments with SERCA3 but not with other SERCA isoforms located in different organelles. In support of this, discharge of the DTS using a low concentration of TG (10 nm) was unable to induce association between STIM1 and SERCA3 but resulted in co-immunoprecipitation of SERCA2b with STIM1. The latter is consistent with the presence of STIM1 also in the DTS, the analog of the ER in platelets (40), as shown in other cell types (8). The inability of TBHQ to induce association between STIM1 and SERCA2b also indicates that it specifically releases Ca2+ from the acidic stores because co-immunoprecipitation between STIM1 and SERCA2b was found upon discharge of the DTS with a low concentration of TG.
We have tested the relevance of STIM1 located in the acidic Ca2+ stores in the activation of SOCE by communicating information about the filling state of the stores to Ca2+ channels in the plasma membrane. Despite the fact that the special idiosyncrasy of human platelets does not allow the application of STIM1 silencing techniques, we have investigated the association of STIM1 with Orai1 and the channels hTRPC1 and hTRPC6 upon specific acidic store depletion. Our results indicate that passive discharge of the acidic Ca2+ stores by using the V-ATPase inhibitor bafilomycin A1 or TBHQ reported a significant and Ca2+-independent increase in the association between STIM1 and Orai1. We have also found that discharge of the acidic Ca2+ stores stimulates the association between STIM1 and Orai1 with both hTRPC1 and hTRPC6 channel subunits. Although we cannot rule out the possibility that Ca2+ released from the acidic stores triggers interaction between STIM1 located in the DTS and the plasma membrane channels, our results are more likely explained by the association between STIM1 located in the acidic Ca2+ stores and the channels in the plasma membrane because Ca2+-induced Ca2+ release from the DTS is absent in human platelets (59). To further investigate the role of the association between STIM1 and Orai1 stimulated by depletion of the acidic Ca2+ stores in SOCE, we have interfered with the interaction between both proteins by introduction of an anti-Orai1 C-terminal antibody that has been previously shown to block the association between STIM1 and Orai1 (35). Our results indicate that introduction of the anti-Orai1 C-terminal antibody prevents both the association of Orai1 with STIM1 and SOCE stimulated by discharge of the acidic Ca2+ stores. These findings strongly suggest that the association of STIM1 with Orai1 is essential for SOCE upon discharge of the acidic stores.
We have previously reported that bafilomycin A1 and TBHQ release Ca2+ from the same intracellular acidic Ca2+ compartment in human platelets (4). Our results indicate that this store is sensitive to agonists such as thrombin (31) and is independent on the DTS, which, in turn, is sensitive to low concentrations of TG and to the inositol 1,4,5-trisphosphate (IP3) generating physiological agonist ADP. Because ryanodine receptors have not been identified in human platelets, the acidic Ca2+ stores are likely to be sensitive to the intracellular second messenger NAADP, as reported in other cells (60, 61). Consistent with this, we have previously found that NAADP is able to induce Ca2+ release in human platelets, which is prevented by treatment with TBHQ (31). Thrombin is an IP3-generating agonist; however, we have found that the phospholipase C inhibitor, U-73122, only partially reduced thrombin-induced Ca2+ release, thus suggesting that IP3 is not the only Ca2+-mobilizing second messenger generated by activation of thrombin receptors. The generation of different second messengers by physiological agonists has previously been reported in pancreatic acinar cells, where acetylcholine leads to IP3 production, whereas cholecystokinin evokes cyclic ADP-ribose and NAADP synthesis (62). NAADP has been reported to release Ca2+ from lysosome-related organelles, which are insensitive to other Ca2+-mobilizing second messengers in different mammalian cells, including human platelets (3, 33). NAADP has been reported to act as a trigger, inducing primary Ca2+ release that subsequently recruits neighboring Ca2+-mobilizing second messenger receptors (63), the IP3 receptors in platelets, thus supporting the important physiological role of acidic Ca2+ stores in mammalian cells. In human platelets, activation of the high affinity thrombin receptor GPIb-IX-V releases Ca2+ exclusively from the acidic stores, whereas higher thrombin concentrations are necessary to induce discharge from the DTS (33). These findings suggest that acidic stores might be the primary source of Ca2+ release by thrombin, and, subsequently, SOCE regulated by the acidic stores might be the first thrombin-evoked capacitative Ca2+ entry mechanism in these cells, probably involving Orai1 and the TRPC proteins hTRPC1 and hTRPC6. The participation of TRPC subunits in the channel that conducts Ca2+ entry upon acidic store depletion, as suggested by their interaction with STIM1 and Orai1, explains the ability of TBHQ to induce divalent cation entry previously reported in human platelets (64).
Our results also report for the first time expression of STIM2 in human platelets. We have found that STIM2 is expressed in lysosomal and non-lysosomal fractions, and discharge of the acidic Ca2+ stores leads to association of STIM2 with the channel subunit Orai1, STIM1, and also SERCA3, which strongly suggests that STIM2 plays a relevant role in Ca2+ homeostasis in human platelets, probably regulating Ca2+ entry and sequestration into the acidic stores. These findings are in agreement with previous studies reporting that STIM2 finely regulates resting [Ca2+]i and intraluminal Ca2+ concentrations (15).
Our findings provide the first direct evidence for the expression of STIM1 and STIM2 in acidic organelles that function as agonist-sensitive Ca2+ stores. Depletion of the acidic Ca2+ stores results in the association between STIM1 and the Ca2+-permeable channel proteins Orai1, hTRPC1, and hTRPC6. In addition, we have found that discharge of the acidic stores results in the association between Orai1, hTRPC1, and hTRPC6. Impairment of the association of STIM1 and Orai1 attenuates SOCE controlled by the acidic Ca2+ stores, thus suggesting a functional role for this interaction in SOCE in human platelets. Discharge of the acidic Ca2+ stores also induces association of STIM2 with STIM1, Orai1, and SERCA3, thus suggesting a possible role of STIM2 in intracellular Ca2+ homeostasis in these cells.
This work was supported by Ministerio de Ciencia e Innovación Grant BFU2010-21043-C02-01, Ministerio de Asuntos Exteriores y de Cooperación Proyecto Conjunto de Investigación Grant A/023417/09, and Junta de Extremadura-Fondo Europeo de Desarrollo Regional Grant GR10010.
- SOCE
- store-operated calcium entry
- ER
- endoplasmic reticulum
- HBS
- HEPES-buffered saline
- hTRPC1 and hTRPC6
- human canonical TRP1 and TRP6, respectively
- IP3
- inositol 1,4,5-trisphosphate
- TBHQ
- 2,5-di-(tert-butyl)-1,4-hydroquinone
- TG
- thapsigargin
- SERCA
- sarco/endoplasmic reticulum Ca2+-ATPase
- V-ATPase
- vacuolar proton-ATPase
- NAADP
- nicotinic acid adenine dinucleotide phosphate
- DTS
- dense tubular system
- AM
- acetoxymethyl ester
- BAPTA-AM
- 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester)
- GPN
- glycyl-1-phenylalanine 2-naphthylamide
- PDI
- protein-disulfide isomerase.
REFERENCES
- 1. Putney J. W., Jr. (2007) Cell Calcium 42, 103–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Patel S., Docampo R. (2010) Trends Cell Biol. 20, 277–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kinnear N. P., Boittin F. X., Thomas J. M., Galione A., Evans A. M. (2004) J. Biol. Chem. 279, 54319–54326 [DOI] [PubMed] [Google Scholar]
- 4. López J. J., Camello-Almaraz C., Pariente J. A., Salido G. M., Rosado J. A. (2005) Biochem. J. 390, 243–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McGuinness L., Bardo S. J., Emptage N. J. (2007) Neuropharmacology 52, 126–135 [DOI] [PubMed] [Google Scholar]
- 6. Gerasimenko O., Gerasimenko J. (2010) Methods Mol. Biol. 591, 201–210 [DOI] [PubMed] [Google Scholar]
- 7. Liou J., Kim M. L., Heo W. D., Jones J. T., Myers J. W., Ferrell J. E., Jr., Meyer T. (2005) Curr. Biol. 15, 1235–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roos J., DiGregorio P. J., Yeromin A. V., Ohlsen K., Lioudyno M., Zhang S., Safrina O., Kozak J. A., Wagner S. L., Cahalan M. D., Veliçelebi G., Stauderman K. A. (2005) J. Cell Biol. 169, 435–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang S. L., Yu Y., Roos J., Kozak J. A., Deerinck T. J., Ellisman M. H., Stauderman K. A., Cahalan M. D. (2005) Nature 437, 902–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Manji S. S., Parker N. J., Williams R. T., van Stekelenburg L., Pearson R. B., Dziadek M., Smith P. J. (2000) Biochim. Biophys. Acta 1481, 147–155 [DOI] [PubMed] [Google Scholar]
- 11. Soboloff J., Spassova M. A., Tang X. D., Hewavitharana T., Xu W., Gill D. L. (2006) J. Biol. Chem. 281, 20661–20665 [DOI] [PubMed] [Google Scholar]
- 12. Schindl R., Muik M., Fahrner M., Derler I., Fritsch R., Bergsmann J., Romanin C. (2009) Cell Calcium 46, 227–232 [DOI] [PubMed] [Google Scholar]
- 13. Worley P. F., Zeng W., Huang G. N., Yuan J. P., Kim J. Y., Lee M. G., Muallem S. (2007) Cell Calcium 42, 205–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yuan J. P., Zeng W., Huang G. N., Worley P. F., Muallem S. (2007) Nat. Cell Biol. 9, 636–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brandman O., Liou J., Park W. S., Meyer T. (2007) Cell 131, 1327–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Potier M., Trebak M. (2008) Pflugers Arch. 457, 405–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mitchell K. J., Pinton P., Varadi A., Tacchetti C., Ainscow E. K., Pozzan T., Rizzuto R., Rutter G. A. (2001) J. Cell Biol. 155, 41–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Missiaen L., Dode L., Vanoevelen J., Raeymaekers L., Wuytack F. (2007) Cell Calcium 41, 405–416 [DOI] [PubMed] [Google Scholar]
- 19. Christensen K. A., Myers J. T., Swanson J. A. (2002) J. Cell Sci. 115, 599–607 [DOI] [PubMed] [Google Scholar]
- 20. Camello C., Pariente J. A., Salido G. M., Camello P. J. (2000) Curr. Biol. 10, 161–164 [DOI] [PubMed] [Google Scholar]
- 21. Krieger-Brauer H. I., Gratzl M. (1983) J. Neurochem. 41, 1269–1276 [DOI] [PubMed] [Google Scholar]
- 22. Ginger R. S., Askew S. E., Ogborne R. M., Wilson S., Ferdinando D., Dadd T., Smith A. M., Kazi S., Szerencsei R. T., Winkfein R. J., Schnetkamp P. P., Green M. R. (2008) J. Biol. Chem. 283, 5486–5495 [DOI] [PubMed] [Google Scholar]
- 23. Bode H., Himmen A., Göke B. (1996) Pflugers Arch. 432, 97–104 [DOI] [PubMed] [Google Scholar]
- 24. Camello-Almaraz C., Pariente J. A., Salido G., Camello P. J. (2000) Biochem. Biophys. Res. Commun. 271, 311–317 [DOI] [PubMed] [Google Scholar]
- 25. Brailoiu E., Hooper R., Cai X., Brailoiu G. C., Keebler M. V., Dun N. J., Marchant J. S., Patel S. (2010) J. Biol. Chem. 285, 2897–2901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhu M. X., Evans A. M., Ma J., Parrington J., Galione A. (2010) Commun. Integr. Biol. 3, 12–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Beck A., Kolisek M., Bagley L. A., Fleig A., Penner R. (2006) FASEB J. 20, 962–964 [DOI] [PubMed] [Google Scholar]
- 28. Cavallini L., Coassin M., Alexandre A. (1995) Biochem. J. 310, 449–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kovàcs T., Berger G., Corvazier E., Pàszty K., Brown A., Bobe R., Papp B., Wuytack F., Cramer E. M., Enouf J. (1997) Br. J. Haematol. 97, 192–203 [DOI] [PubMed] [Google Scholar]
- 30. Papp B., Enyedi A., Kovács T., Sarkadi B., Wuytack F., Thastrup O., Gárdos G., Bredoux R., Levy-Toledano S., Enouf J. (1991) J. Biol. Chem. 266, 14593–14596 [PubMed] [Google Scholar]
- 31. López J. J., Redondo P. C., Salido G. M., Pariente J. A., Rosado J. A. (2006) Cell Signal 18, 373–381 [DOI] [PubMed] [Google Scholar]
- 32. Rosado J. A., López J. J., Harper A. G., Harper M. T., Redondo P. C., Pariente J. A., Sage S. O., Salido G. M. (2004) J. Biol. Chem. 279, 29231–29235 [DOI] [PubMed] [Google Scholar]
- 33. Jardin I., Ben Amor N., Bartegi A., Pariente J. A., Salido G. M., Rosado J. A. (2007) Biochem. J. 401, 167–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grynkiewicz G., Poenie M., Tsien R. Y. (1985) J. Biol. Chem. 260, 3440–3450 [PubMed] [Google Scholar]
- 35. Jardin I., Lopez J. J., Salido G. M., Rosado J. A. (2008) J. Biol. Chem. 283, 25296–25304 [DOI] [PubMed] [Google Scholar]
- 36. Jardín I., López J. J., Redondo P. C., Salido G. M., Rosado J. A. (2009) Biochim. Biophys. Acta 1793, 1614–1622 [DOI] [PubMed] [Google Scholar]
- 37. Niessen J., Jedlitschky G., Greinacher A., Kroemer H. K. (2010) Curr. Protoc. Cell Biol. 46, 3.35.1–3.35.14 [DOI] [PubMed] [Google Scholar]
- 38. Redondo P. C., Jardin I., Lopez J. J., Salido G. M., Rosado J. A. (2008) Biochim. Biophys. Acta 1783, 1163–1176 [DOI] [PubMed] [Google Scholar]
- 39. Bradford M. M. (1976) Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 40. López J. J., Salido G. M., Pariente J. A., Rosado J. A. (2006) J. Biol. Chem. 281, 28254–28264 [DOI] [PubMed] [Google Scholar]
- 41. Bobe R., Bredoux R., Wuytack F., Quarck R., Kovàcs T., Papp B., Corvazier E., Magnier C., Enouf J. (1994) J. Biol. Chem. 269, 1417–1424 [PubMed] [Google Scholar]
- 42. Papp B., Enyedi A., Pászty K., Kovács T., Sarkadi B., Gárdos G., Magnier C., Wuytack F., Enouf J. (1992) Biochem. J. 288, 297–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wuytack F., Papp B., Verboomen H., Raeymaekers L., Dode L., Bobe R., Enouf J., Bokkala S., Authi K. S., Casteels R. (1994) J. Biol. Chem. 269, 1410–1416 [PubMed] [Google Scholar]
- 44. Niessen J., Jedlitschky G., Grube M., Bien S., Strobel U., Ritter C. A., Greinacher A., Kroemer H. K. (2007) J. Immunol. Methods 328, 89–96 [DOI] [PubMed] [Google Scholar]
- 45. Sehgal S., Storrie B. (2007) J. Thromb. Haemost. 5, 2009–2016 [DOI] [PubMed] [Google Scholar]
- 46. Bowman E. J., Siebers A., Altendorf K. (1988) Proc. Natl. Acad. Sci. U.S.A. 85, 7972–7976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Berg T. O., Strømhaug E., Løvdal T., Seglen O., Berg T. (1994) Biochem. J. 300, 229–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Feske S. (2009) Immunol. Rev. 231, 189–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Salido G. M., Sage S. O., Rosado J. A. (2009) Biochim. Biophys. Acta 1793, 223–230 [DOI] [PubMed] [Google Scholar]
- 50. Salido G. M., Sage S. O., Rosado J. A. (2009) Cell. Signal. 21, 457–461 [DOI] [PubMed] [Google Scholar]
- 51. Gear A. R. (1994) Can. J. Physiol. Pharmacol. 72, 285–294 [DOI] [PubMed] [Google Scholar]
- 52. Rosado J. A., Brownlow S. L., Sage S. O. (2002) J. Biol. Chem. 277, 42157–42163 [DOI] [PubMed] [Google Scholar]
- 53. Vaca L. (2010) Cell Calcium 47, 199–209 [DOI] [PubMed] [Google Scholar]
- 54. Muik M., Frischauf I., Derler I., Fahrner M., Bergsmann J., Eder P., Schindl R., Hesch C., Polzinger B., Fritsch R., Kahr H., Madl J., Gruber H., Groschner K., Romanin C. (2008) J. Biol. Chem. 283, 8014–8022 [DOI] [PubMed] [Google Scholar]
- 55. Dhar A., Shukla S. D. (1994) J. Biol. Chem. 269, 9123–9127 [PubMed] [Google Scholar]
- 56. Chakrabarti R., Wylie D. E., Schuster S. M. (1989) J. Biol. Chem. 264, 15494–15500 [PubMed] [Google Scholar]
- 57. Putney J. W. (2010) Mol. Interv. 10, 209–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rosado J. A., Pariente J. A., Salido G. M., Redondo P. C. (2010) Arterioscler. Thromb. Vasc. Biol. 30, 419–425 [DOI] [PubMed] [Google Scholar]
- 59. Adunyah S. E., Dean W. L. (1986) J. Biol. Chem. 261, 3122–3127 [PubMed] [Google Scholar]
- 60. Galione A. (2006) Biochem. Soc. Trans. 34, 922–926 [DOI] [PubMed] [Google Scholar]
- 61. Morgan A. J., Galione A. (2007) Biochem. J. 402, 301–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cancela J. M. (2001) Annu. Rev. Physiol. 63, 99–117 [DOI] [PubMed] [Google Scholar]
- 63. Cancela J. M., Charpentier G., Petersen O. H. (2003) Pflugers Arch. 446, 322–327 [DOI] [PubMed] [Google Scholar]
- 64. Jardín I., López J. J., Salido G. M., Rosado J. A. (2008) Cell. Signal. 20, 737–747 [DOI] [PubMed] [Google Scholar]