Abstract
CTCF nuclear factor regulates many aspects of gene expression, largely as a transcriptional repressor or via insulator function. Its roles in cellular differentiation are not clear. Here we show an unexpected role for CTCF in myogenesis. Ctcf is expressed in myogenic structures during mouse and zebrafish development. Gain- and loss-of-function approaches in C2C12 cells revealed CTCF as a modulator of myogenesis by regulating muscle-specific gene expression. We addressed the functional connection between CTCF and myogenic regulatory factors (MRFs). CTCF enhances the myogenic potential of MyoD and myogenin and establishes direct interactions with MyoD, indicating that CTCF regulates MRF-mediated muscle differentiation. Indeed, CTCF modulates functional interactions between MyoD and myogenin in co-activation of muscle-specific gene expression and facilitates MyoD recruitment to a muscle-specific promoter. Finally, ctcf loss-of-function experiments in zebrafish embryos revealed a critical role of CTCF in myogenic development and linked CTCF to broader aspects of development via regulation of Wnt signaling. We conclude that CTCF modulates MRF functional interactions in the orchestration of myogenesis.
Keywords: Cell Differentiation, Gene Expression, Gene Regulation, Skeletal Muscle, Transcription Regulation, CTCF, MyoD
Introduction
CTCF (CCCTC-binding factor) is a widely expressed protein with 11 zinc fingers and multiple functions in regulating gene expression (1). CTCF, whose DNA-binding elements have been mapped throughout the human genome (2, 3), is probably best known for its function as a genomic insulator (1, 4). Among many ascribed roles, CTCF participates in gene imprinting, X chromosome inactivation (5–7), interchromosomal interactions (8), subnuclear spatial organization (9), demarcation of lamina-associated domains (10), long range chromatin looping (11), chromosome pairing and counting (12), and regulation of cohesins-mediated gene expression (13, 14). The functional versatility of CTCF might be due to different interactions with multiple co-regulators (15). CTCF is essential for early embryo development (7), but whether CTCF functions in cell differentiation programs or specific signaling pathways to control morphogenesis remains poorly understood.
Myogenesis is regulated by the MyoD family of transcription factors, including Myf5, MyoD, MRF4, and myogenin, which activate muscle-specific gene expression (16, 17). These proteins, also known as myogenic regulatory factors (MRFs),5 have different activities on muscle genes controlling myogenic lineage establishment and differentiation (18, 19). The complexity of MRF function during myogenesis is exemplified by MyoD-mediated up- and down-regulation of specific gene clusters during myogenic differentiation (20). Thus, MyoD exerts modulated gene-specific responses. The modulated regulatory influence of MyoD over its target promoters is largely dependent, as for many other transcription factors, on co-activator or co-repressor interactions (21, 22). For example, heterodimerization of MyoD with the ubiquitous basic helix-loop-helix E proteins E12 or E47 confers high DNA recognition affinity and enhanced transactivation potential (23–26). On the other hand, the interaction of MyoD with basic helix-loop-helix proteins Id and Twist results in transcriptionally incompetent complexes (27, 28). Similarly, MyoD-mediated recruitment of p300/CBP and PCAF to muscle promoters is required for maximal transcriptional activation (29, 30), but the presence of MyoD and HDAC1 at the Myogenin promoter correlates with transcriptionally inactive chromatin (31). These examples highlight the potential existence of unknown muscle-specific as well as ubiquitous MyoD partners that form part of regulatory modules controlling muscle gene expression and affecting myogenesis and muscle development.
MRFs integrate signaling from pathways coordinating myogenic induction and differentiation during development (19). The Wnt signaling pathway participates in the activation of myogenesis (32, 33) by regulating MRF function (34–37). In particular, Wnt11 controls the fiber patterning of the limb musculature (33) and determines the oriented elongation of myocytes (38). In addition, WNT11 activates the canonical WNT/β-catenin-dependent pathway (39), which is required for the expression of Myf5 (37) and for MyoD-mediated transactivation (40). Thus, proteins regulating the expression of Wnts are likely important for myogenesis and muscle development.
In this study we demonstrate that CTCF promotes myogenesis by functionally interacting with MRFs. Mechanistically, CTCF stimulates muscle gene expression by favoring MyoD recruitment. In line with a critical role of CTCF in myogenesis, ctcf loss of function in zebrafish results in defective muscle development. Furthermore, CTCF regulates Wnt signaling, linking CTCF to broad aspects of development.
EXPERIMENTAL PROCEDURES
In Situ Hybridization
RNA in situ hybridization in mouse and zebrafish embryos was performed as described (41, 42). Mouse Ctcf mRNA was detected with a human CTCF cDNA-derived probe, which has over 90% identity with its mouse counterpart (supplemental Fig. S1). The zebrafish ctcf antisense probe was synthesized from a 1054-bp DNA fragment corresponding to the 5′ end of the mRNA excluding the zinc fingers region (GenBankTM accession number BC097009).
Constructs and Plasmids
pCI-7.1 plasmid containing the full-length human CTCF was provided by Elena Klenova (University of Essex, Colchester, UK). pcDNA3-N-Myc, encoding a N-Myc tag, pcMyoD-N-Myc, and pcMyogenin-N-Myc, encoding a N-Myc-tagged MyoD and N-Myc-tagged Myogenin, respectively, were provided by Robin Meech (The Scripps Research Institute, La Jolla, CA). Constructs 1 and α-SGCP (43, 44), which include the α-SG full-length and core promoter (+4 to −76 relative to the transcription start site), respectively, were obtained from Ramón Coral-Vázquez (Instituto Mexicano del Seguro Social, Mexico City, Mexico). V5-tagged CTCF vectors were generated by PCR from constructs provided by Jeannie T. Lee (Harvard Medical School) (6). Probes for myod and myogenin were a gift from Eric S. Weinberg (University of Pennsylvania).
Cell Transfection
C2C12 and 10T1/2 cells were grown in DMEM supplemented with 10% fetal bovine serum. The α-SG promoter (1 μg) constructs were co-transfected with cDNAs encoding CTCF, MyoD, or Myogenin with Lipofectamine 2000 (Invitrogen). pRL/CMV vector, encoding Renilla reniformis luciferase, was co-transfected for normalization. 500,000 cells were seeded in six-well plates and transfected 24 h later. Luciferase activity was measured 48 h post-transfection. Luciferase activity was measured using the dual luciferase reporter assay system (Promega) in a TD-20/20 luminometer (Turner Designs). C2C12 cells stably expressing luciferase under the α-SG full-length promoter were as described (44). For overexpression of CTCF, 420,000 C2C12 cells were seeded in 6-well plates, and 24 h later, a CTCF encoding vector (5 μg) was transfected with FuGENE HD (Roche Applied Science), according to the manufacturer's instructions. The cells were harvested 1.5, 3, and 4 days post-transfection and used to isolate RNA and prepare cell lysates for RT-PCR and Western blot analyses, respectively.
RT-PCR and qPCR
RNA was isolated with TRIzol reagent (Invitrogen), according to the manufacturer's instructions. RNA (2 μg) was used to synthesize cDNA with a first strand synthesis kit (Invitrogen). One μl of the resulting reaction was used for PCR. Primer sequences are available upon request. qPCR values were normalized against β-actin, used as endogenous control.
siRNA and shRNA-mediated CTCF Knockdown
200,000 C2C12 cells stably expressing luciferase under the α-SG full-length promoter were seeded in six-well plates. 24 h later, the cells were transfected with 0, 1, 2, 5, and 10 pm of CTCF siRNA (Santa Cruz Biotechnology) using Lipofectamine 2000 (Invitrogen). After 24 h, the transfection was repeated. Luciferase assays and Western blot were performed 72 h post-transfection. Luciferase activity was normalized against protein concentration. The anti-CTCF shRNA vector was constructed with oligonucleotides targeting the sequence 5′-GGTGAGGCGGTTGAAGCCA-3′ corresponding to the exon 1-intron 1 boundary. C2C12 cells were stably transfected with a vector expressing the shRNA under control of the H1 promoter or a control vector. Stable transfectants were induced for differentiation and seeded on coverslips for immunofluorescence or lysed to obtain RNA.
Myogenic Conversion Assays
190,000 10T1/2 cells were seeded on coverslips in 12-well plates. After 24 h, 2 μg of either phCTCF, encoding CTCF cDNA, pcDNA3-N-Myc, encoding a N-Myc tag, pcMyoD-N-Myc, or pcMyogenin-N-Myc encoding a N-Myc-tagged MyoD and N-Myc-tagged Myogenin, respectively, were co-transfected in different combinations with FuGENE HD reagent (Roche Applied Science). Transfection efficiency was assessed by immunofluorescence against the Myc tag. Immunofluorescence against MHC was performed 7 days later. The percentage of positive cells, with respect to the total number of cells, was compared between conditions.
Immunofluorescence
Whole mount immunofluorescence in zebrafish was performed as described (42).
Co-immunoprecipitation
In vitro synthesized proteins were produced with the TnT quick coupled transcription/translation system (Promega). The synthesis products were mixed in the presence of immunoprecipitation buffer (20 mm Tris, pH 7.5, 150 mm NaCl, and 0.1% Triton X-100). Immunoprecipitation of TnT-produced proteins was done with ExactaCruz reagents (Santa Cruz Biotechnology). Antibodies for CTCF and MyoD were from Abcam and Santa Cruz Biotechnologies, respectively.
Chromatin Immunoprecipitation
C2C12 cells were transfected in 15-cm tissue culture dishes with 70 μg of either an empty vector or MyoD- or Myogenin-encoding cDNAs with FuGENE HD as recommended by the manufacturer. The cells were harvested 48 h after transfection and processed with the EZ-ChIP kit (Upstate). The analysis of immunoprecipitated chromatin was performed by qPCR using TaqMan (Applied Biosystems) probes recognizing the α-SG and serum response factor core promoters. Antibodies for CTCF and MyoD were from Abcam and Santa Cruz Biotechnologies, respectively. The anti-polymerase II antibody was from Upstate.
Morpholino Oligonucleotides Injection and Rescue Experiments
Morpholinos targeting the ATG (5′-CATGGAAGGGGGACCGACTGAGGCCG-3′) or the exon 1-intron 1 junction (5′-GCTTTCAGGTTTGTGATCTGTTTTG-3′) of the zebrafish ctcf gene were purchased from Gene Tools. Human Ctcf and zebrafish wnt11 mRNA was obtained using the MessageAmp II kit (Ambion). Zebrafish embryos at one cell stage were co-injected with 2 ng of ctcf ATG morpholino and 50 pg of CTCF mRNA. 4 ng of ctcf ATG morpholino were co-injected with 100 pg of wnt11 mRNA.
Microarray Data Analysis
c2 morphants were pooled from five independent injections and used for RNA purification. RNA was hybridized on Affymetrix GeneChip® zebrafish genome arrays following the manufacturer's recommendations. Linear models were fitted for each gene on morphant versus control embryos to derive morphant effect using the limma package in R/Bioconductor. Moderated t-statistics and the associated p values were calculated, adjusting for multiple testing by controlling for false discovery rate with the Benjamini-Hochberg method and for family-wise error rate using the Bonferroni correction (adjP). Genes with adjP<0.05 were considered as differentially expressed.
RESULTS
CTCF Is Expressed in Somites in Developing Mouse and Zebrafish
Although CTCF is often considered as a widely expressed factor (45, 46), it is down-regulated in differentiating cells in vitro (47), raising questions about the regulation of its expression during specific biological processes. Because the expression pattern of Ctcf in mammalian embryos is unknown, we observed its mRNA expression in the developing mouse by whole mount in situ hybridization. Ctcf mRNA was widely detected at embryonic days (E) 10.5 and E12, with predominant expression in the forming jaw at E10.5 (Fig. 1A). Ctcf expression in somites was observed at E10.5 and E12 (Fig. 1A). At E12, Ctcf was detected in limbs, jaw, brain, and facial muscles (Fig. 1A). Expression of Ctcf in the limbs is consistent with recent findings of a function of CTCF in mouse limb development (48). Later (E13), Ctcf presented a more restricted expression pattern; it was predominantly detected in brain and facial muscles, whereas its presence was barely observed in intercostal musculature. Ctcf decreased notably in E13 embryo forelimbs as compared with E12 embryos (Fig. 1A).
FIGURE 1.
Ctcf expression in developing mouse and zebrafish. A, Ctcf expression pattern in mouse. Whole mount in situ hybridization shows widely distributed Ctcf in mouse at E10.5. Ctcf was enriched in the first branchial arches (*) at E10.5. At E12, Ctcf was observed in brain (br), jaw, facial muscles, and limbs (black arrows). At E13, Ctcf localized preferentially in brain and was barely detected in intercostals (arrowhead) and limb musculature (arrow). Close-ups of boxed areas are presented. B, ctcf expression pattern in zebrafish. ctcf was present in somites of zebrafish embryos at 10–11, 14–15, and 16–17 hpf. In 26-hpf embryos, the levels of ctcf were greatly reduced, and by 36 hpf, ctcf was present only in distal somites (boxed area), whereas in 72-hfp embryos ctcf was restricted to brain and gut (arrow). The arrowheads indicate somites. C, flat whole mount zebrafish embryos showing expression of ctcf (upper panel) and myod (lower panel) in somites. D, Ctcf, MyoD, Mrf4, and Myogenin are similarly up-regulated during myogenic differentiation. qPCR on C2C12 myoblasts (MB) and at days 2–8 (D2–D8) of differentiation. The values were normalized against β-actin, which was used as endogenous control. The data are presented as the means ± S.D. of three biological replicates.
We extended our analysis to zebrafish embryos, in which ctcf was strongly expressed and widely distributed during early development (data not shown) in agreement with a previous report (49). We focused our attention on ctcf expression in somites, where myogenic precursors are determined. We used an anti-myod probe to identify somites in zebrafish (Fig. 1B). ctcf mRNA was detected in somites beginning at 10–11 to 16–17 h post-fertilization (hpf) (Fig. 1, B and C), stages at which myod is strongly expressed. However, the presence of ctcf was markedly reduced at 26 hpf (Fig. 1B), and by 36 hpf it was more diffused along the trunk and barely detected at the most distal somites (Fig. 1B). In 72-hpf embryos, ctcf mRNA was absent from somites, whereas it was clearly detected in the gut, forebrain, midbrain, and hindbrain. As expected, at this stage MyoD was only detected in the jaw and eye muscles (50) (Fig. 1B). Sense probes for Ctcf mRNA in mouse and zebrafish gave no detectable staining (supplemental Fig. S2 and data not shown).
To compare the expression patterns of Ctcf and MRFs during myogenic cell differentiation, we used reverse transcription followed by qPCR in differentiating C2C12 cells. Ctcf had a similar up-regulation pattern to that of MyoD, MRF4, and Myogenin (Fig. 1D). These results show that Ctcf is expressed in developing muscle in mouse and zebrafish and suggest that CTCF expression is developmentally regulated.
CTCF Overexpression Increases C2C12 Cell Myogenic Differentiation
The observation that Ctcf and the myogenic master regulator MyoD share expression domains during mouse and zebrafish development opened the possibility of CTCF involvement in myogenic differentiation. In a first attempt to address this issue, we overexpressed CTCF in C2C12 cells and analyzed the enrichment of myogenic differentiation marker genes. RT-PCR showed enrichment of Myf5, MyoD, and Myogenin and premature expression of Mrf4 and MHC (Fig. 2A) at day 1.5 post-transfection. Perinatal myosin heavy chain was not detected. α-SG (α-sarcoglycan, also known as Sgca), which is expressed in terminally differentiated muscle (51), and Myf5 were the only enriched markers at day 3 of differentiation (Fig. 2A). qPCR confirmed up-regulation of MyoD and Myogenin in CTCF-overexpressing cells at days 1.5 and 3 of differentiation (Fig. 2B). Accordingly, myogenic marker proteins were enriched in CTCF transfected cells at day 1.5 post-transfection, and precocious α-SG expression was detected at day 3 (Fig. 2A). Up-regulation of Myf5, whose expression normally decreases as differentiation proceeds (52), suggests that CTCF overexpression led to deregulation of the myogenic gene expression program. However, increased expression of terminal differentiation markers, such as MHC and α-SG, suggests that CTCF overexpression led to increased myogenic differentiation. This raised the possibility of a functional interaction between CTCF and MRFs.
FIGURE 2.
CTCF regulates myogenic differentiation. A, C2C12 cells were transiently transfected with an empty vector (−) or a CTCF cDNA (+). RT-PCR and Western blots (WB) were performed at 1.5 days post-transfection, except for the α-SG blot, which corresponds to cells at post-transfection day 3 (D3). Gapdh and RNA polymerase II are endogenous controls in the RT-PCR and Western blot, respectively. B, qPCR of MyoD and myogenin (Myog) on CTCF-overexpressing cells. *, p < 0.05. C, CTCF enhances the myogenic potential of MyoD. 10T1/2 cells were transfected with cDNAs encoding CTCF or Myc-MyoD. MHC-positive cells were detected by immunofluorescence after 7 days post-transfection. The nuclei were counterstained with DAPI, and MHC-positive cells were counted. The right panel shows the increase in MHC-positive cells transfected with MyoD plus CTCF versus MyoD. D, CTCF is required for myogenic gene expression. Upper panel, Western blot showing decreased expression of CTCF but not serum response factor (SRF). β-Actin is the loading control. Lower panel, CTCF, MyoD, and Myogenin levels in CTCF-deficient cells (siCTCF; red bars) as compared with control cells (blue bars). The analysis was performed at days 2–5 of differentiation (D2–D5). The data are presented as the means ± S.D. E, CTCF knockdown impairs C2C12 cells differentiation. C2C12 cells in which CTCF was knocked down by stable transfection of a shRNA (siCTCF) presented limited MHC expression as compared with cells transfected with a control shRNA. The right panel shows quantification of MHC signal in several fields across three independent differentiations by ImageJ software. *, p < 0.05 as determined by t test.
CTCF Enhances the Myogenic Potential of MRFs
Because CTCF overexpression affects muscle-specific gene expression (Fig. 2, A and B), which is orchestrated by MRFs, we determined whether CTCF affects the myogenic potential of MyoD and Myogenin. To this end, we performed myogenic conversion assays in 10T1/2 fibroblasts. Vectors encoding MyoD or myogenin fused to a Myc epitope tag were transiently transfected in absence or presence of a CTCF-encoding cDNA. Plasmid amounts were equaled for all conditions with empty vector. Myogenic converted cells were identified by the presence of MHC, which was revealed by immunofluorescence. The cell nuclei were stained with DAPI and counted, and the number of MHC-positive cells for each condition were compared (Fig. 2C). When CTCF was co-transfected with an empty vector, no MHC-positive cells were observed (Fig. 2C), indicating that CTCF does not induce myogenic differentiation. Similarly, CTCF did not increase the myogenic potential of myogenin (supplemental Fig. S3). In contrast, CTCF increased the myogenic potential of MyoD, because co-transfection of CTCF and MyoD resulted in more MHC-positive cells than did co-transfection of MyoD with an empty vector (Fig. 2C). CTCF also increased the myogenic efficiency of MyoD and Myogenin together, although to a lesser extent than MyoD alone (supplemental Fig. S3). Thus, CTCF likely cooperates with MyoD in myogenic induction.
Ctcf Is Indispensable for Myogenic Cell Differentiation
To address the requirement of CTCF in myogenic differentiation, we stably knocked down Ctcf in C2C12 cells. We transfected a vector encoding an siRNA directed against Ctcf exon 1. Ctcf was effectively knocked down, whereas the levels of serum response factor were not altered, as shown by Western blot and qRT-PCR (Fig. 2D). The cells were induced to differentiation by culturing under serum starvation, and their differentiation capacity was compared with that of cells transfected with a control siRNA by means of MHC immunofluorescence and qPCR. Progressive MHC abundance and formation of multinucleated myofibers was observed in control cells during a 5-day differentiation time course (Fig. 2E). In contrast, Ctcf-deficient cells presented limited differentiation potential as shown by drastically decreased MHC and fewer myogenic fibers. Quantification of the MHC signal confirmed reduced MHC levels in siRNA-transfected cells (Fig. 2E). Furthermore, expression of MyoD and Myogenin decreased notably in CTCF-deficient cells during myogenic differentiation (Fig. 2D). The expression of MyoD and Myogenin was less affected by CTCF knockdown at day 5 of differentiation (Fig. 2D). A potential explanation for this effect might be that factors stimulating expression of MyoD and Myogenin and not targeted by CTCF are up-regulated in differentiated myogenic cells. However, this possibility was not tested. Thus, Ctcf is indispensable for muscle-specific gene expression and myogenic cell differentiation.
CTCF Physically Interacts with MyoD
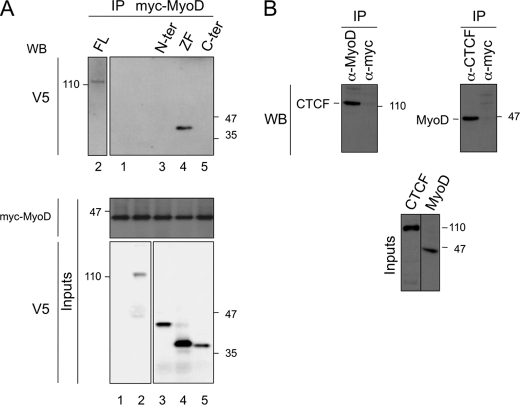
Our results suggest that Ctcf stimulates myogenic cell differentiation potentially by cooperating with MRFs. To test this hypothesis, we assessed the capacity of CTCF to physically interact with MyoD. We used co-immunoprecipitations with in vitro synthesized Myc-tagged MyoD and V5-tagged full-length CTCF. CTCF was detected in Myc-MyoD immunoprecipitates by Western blot (Fig. 3A, lane 2), indicating direct interaction between CTCF and MyoD. In a deletion series of Myc-tagged proteins, only the CTCF domain including the zinc fingers was co-immunoprecipitated with MyoD (Fig. 3A, lane 4), indicating that this domain mediates CTCF interaction with MyoD. In addition, endogenous CTCF and MyoD co-immunoprecipitated in C2C12 cells (Fig. 3B). Thus, CTCF might form part of a novel transcriptional regulatory module affecting muscle-specific gene expression.
FIGURE 3.
CTCF physically interacts with MyoD. A, extracts with in vitro synthesized V5-tagged full-length CTCF (FL), the CTCF amino-terminal (N-ter), zinc finger-containing (ZF), or the carboxyl-terminal (C-ter) domains were mixed with Myc-MyoD for co-immunoprecipitations (IP). The tagged CTCF versions were identified by Western blot (WB) using an anti-V5 antibody. Inputs for MyoD and the CTCF proteins are shown in the middle and bottom panels, respectively. The numbers indicate kDa. In lane 1, Myc-MyoD was added as negative control. B, endogenous CTCF and MyoD co-immunoprecipitate in C2C12 cell extracts. CTCF and MyoD were revealed in immunoprecipitates obtained with anti-MyoD and anti-CTCF antibodies (α-MyoD and α-CTCF, respectively) by Western blot (WB). The bottom panels show inputs. An anti-Myc (α-myc) antibody was used as a negative control.
Functional Interplay of CTCF and MRFs in Regulating Muscle Gene Expression
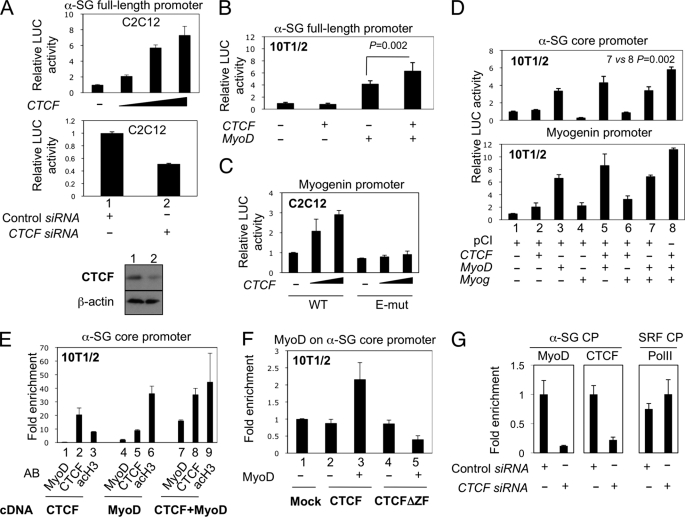
Because CTCF establishes physical and functional interactions with MRFs, we investigated whether CTCF cooperates in direct muscle-specific gene expression activation. First, we tested the activity of CTCF on the α-SG promoter in C2C12 myoblasts. We transiently transfected CTCF cDNA into C2C12 cells expressing luciferase under the full-length α-SG promoter (44), which contains multiple E-boxes and is activated by MyoD during myogenic differentiation (53). CTCF trans-activated the α-SG promoter in a concentration-dependent manner (Fig. 4A, top panel). To test whether endogenous CTCF regulates the α-SG promoter, we depleted CTCF with anti-Ctcf siRNA in C2C12 cells. CTCF was efficiently depleted, as shown by Western blot (Fig. 4A, bottom panel). CTCF depletion caused decreased α-SG promoter activity (Fig. 4A, middle panel). A control siRNA did not affect promoter activity. Therefore, CTCF activates the α-SG promoter in the myogenic environment in the presence of MRFs. Then we addressed whether CTCF interacts with the α-SG promoter. We performed EMSA with in vitro synthesized CTCF and a DNA probe corresponding to the α-SG core promoter, in which a CTCF-binding site was predicted (supplemental Fig. S4). Results from EMSAs indicate that CTCF is capable of binding the core promoter of the muscle-specific gene α-SG (supplemental Fig. S5).
FIGURE 4.
CTCF activates muscle-specific gene expression and modulates the transcriptional activity of MyoD and myogenin. A, CTCF regulates the α-SG full-length promoter. C2C12 cells stably expressing luciferase (LUC) under the full-length α-SG promoter were transfected with CTCF. Luciferase activity was assessed 48 h post-transfection (top panel). In this and further experiments, the values are given as the means ± S.D. from three independent experiments performed in duplicate. The same C2C12 cell line was transfected with anti-CTCF siRNA and induced to differentiation. The middle panel shows α-SG promoter activity in CTCF siRNA-transfected cells. Efficient depletion of CTCF was shown by Western blot (bottom panel). β-Actin was used as loading control. B, CTCF regulates the α-SG promoter in a MyoD-dependent manner. The full-length α-SG promoter was transiently co-transfected with CTCF with or without MyoD into 10T1/2 fibroblasts. The data represent the means ± S.D. The p values were obtained by t test (n = 3). C, CTCF-mediated activation of the Myogenin promoter depends on MyoD-binding sites. Luciferase reporter vectors containing the WT or a mutated version of the Myogenin promoter in which two E-boxes were ablated (E-mut) were transfected alone (−) or with increasing amounts of CTCF in C2C12 cells. D, CTCF is required for MyoD- and myogenin-coordinated activity on the α-SG core promoter. The α-SG core promoter or the Myogenin promoter were transiently co-transfected with an empty vector (pCI) or with the indicated combinations of cDNAs encoding CTCF, MyoD, or Myogenin (Myog) in 10T1/2 fibroblasts. The data represent the means ± S.D. The p values were obtained by t test (n = 3). E, CTCF facilitates MyoD binding to the α-SG core promoter in vivo. The α-SG core promoter was amplified by qPCR from chromatin immunoprecipitated with antibodies (AB) recognizing MyoD, CTCF, and acetylated histone H3 (acH3). Chromatin was obtained from 10T1/2 cells transfected with cDNAs encoding CTCF or MyoD, alone, or combined. F, CTCF without the zinc finger domain cannot recruit Myod to the α-SG core promoter. MyoD- and CTCF-associated chromatin from 10T1/2 cells transfected with the full-length CTCF (CTCF-WT) or a CTCF mutant lacking the zinc finger domain (CTCF-ΔZF) was analyzed by qPCR. G, chromatin immunoprecipitated with anti-MyoD, CTCF, or PollII was subjected to qPCR amplifying the α-SG, or the serum response factor (SRF) core promoters (CP). Chromatin was obtained from C2C12 cells transfected with a control siRNA or an anti-CTCF siRNA (CTCF siRNA). The data in E–G were normalized against inputs, are presented as relative enrichment over levels obtained with beads precipitation, and represent the means ± S.D. of at least two biological replicates.
Because CTCF increased the myogenic potential of MyoD, we tested the requirement of MRFs for CTCF-mediated muscle gene activation. We analyzed the activity of CTCF over the full-length α-SG promoter in 10T1/2 fibroblasts, which do not express MRFs. MyoD (44), but not CTCF, trans-activated the α-SG promoter (Fig. 4B). However, CTCF and MyoD induced a modest but statistically significant increase in promoter activity (Fig. 4B), suggesting that CTCF requires MyoD to stimulate muscle gene expression. In agreement with this notion, two E-boxes known to mediate MyoD responsiveness (54) were necessary for CTCF-mediated trans-activation of the Myogenin promoter in C2C12 cells (Fig. 4C). To further understand the functional interaction between CTCF and MRFs, we assessed the combinatorial activity of MyoD, myogenin, and CTCF on the α-SG and Myogenin promoters, both MyoD targets (44, 54), in 10T1/2 cells in which we can control for the presence of specific MRFs. For these experiments, we used the α-SG core promoter, which lacks E-boxes and is targeted by MyoD in differentiated myogenic cells via interaction with basal transcription factors (44). We used this promoter to eliminate activities mediated by multiple E-boxes present in the full-length promoter (44). Similar to the α-SG full-length promoter (Fig. 4B), CTCF weakly enhanced the activity of MyoD on both the α-SG core promoter and the myogenin promoter in 10T1/2 cells (Fig. 4D, lanes 3 and 5), suggesting that additional myogenic factors might be required for transactivation. In support of this notion, Ctcf enhanced the activity of MyoD and myogenin on both promoters (Fig. 4D, lanes 7 and 8). Thus, CTCF positively regulates muscle-specific gene expression through a MRF-dependent mechanism, perhaps as a modulator of MRF activity.
CTCF trans-activated muscle gene expression only in the presence of MyoD, raising the possibility that MyoD recruits CTCF to muscle genes. However, this possibility seemed unlikely because CTCF did not interact with a DNA probe containing two functional E-boxes (55) in the presence of MyoD, as revealed by EMSA (supplemental Fig. S6). Another possibility is that CTCF facilitates MRF recruitment to muscle promoters. To resolve this question, we examined the requirement of CTCF for MyoD recruitment to a muscle gene in the chromatin context (Fig. 4E). We performed chromatin immunoprecipitations on 10T1/2 cells transfected with CTCF alone or co-transfected with MyoD. We analyzed enrichment of the α-SG core promoter in chromatin immunoprecipitated with antibodies against MyoD, CTCF, or acetylated histone H3 (acH3) by qRT-PCR. CTCF was enriched in the α-SG promoter in the absence of MyoD (Fig. 4E, lane 2). However, this interaction is unlikely to be functional, because CTCF cannot stimulate α-SG promoter activity or promote myogenesis alone. In the absence of CTCF, MyoD interacted weakly with the α-SG core promoter in MyoD-transfected cells (Fig. 4E, lane 4), even though acH3 was enriched (Fig. 4E, lane 6), suggesting that the promoter was being remodeled for full gene expression activation. This finding agrees with robust interaction of MyoD with the α-SG core promoter only in C2C12 myotubes, as opposed to myoblasts, where the promoter is fully active (44). In contrast, in the presence of CTCF, the binding of MyoD to the α-SG core promoter increased, together with enrichment of CTCF and high acH3 (Fig. 4E, lanes 7–9), implying that CTCF promotes recruitment of MyoD to the α-SG core promoter. In agreement with this hypothesis, whereas MyoD was enriched on the α-SG core promoter in the presence of full-length CTCF (Fig. 4F, lane 3), MyoD did not bind the α-SG core promoter in the presence of a mutant CTCF lacking the zinc finger domain (Fig. 4F, lane 5), which mediates interaction with MyoD (Fig. 3A). Then we asked whether the endogenous CTCF is required for recruitment of MyoD to the α-SG promoter in the context of myogenic cells. We depleted CTCF in C2C12 cells by siRNA transfection and addressed the enrichment of MyoD and CTCF on the α-SG core promoter. qPCR on immunoprecipitated chromatin showed decreased MyoD and CTCF on the α-SG core promoter in cells transfected with CTCF siRNA, as compared with cells transfected with a control siRNA (Fig. 4G). Levels of polymerase II on the serum response factor were comparable between conditions (Fig. 4G).
These results suggest that CTCF is necessary for recruitment of MyoD to at least some of its target promoters and for muscle-specific gene expression activation. In addition, CTCF seems to regulate myogenic induction and differentiation by co-activating muscle-specific gene expression through interaction with MRFs, predicting a function for CTCF in muscle development.
ctcf Participates in Zebrafish Myogenesis
Next, we tested the relevance of CTCF in myogenesis in vivo. ctcf was knocked down in zebrafish by injection of morpholino oligonucleotides (MOs) complementary to the ctcf mRNA translation start site (ATG) or the boundary between exon and intron 1 (SP) in one-cell stage embryos. Depletion of ctcf in embryos injected with either MO was shown by Western blot (Fig. 5A). Injection of either MO led to consistent phenotypes in zebrafish embryos (Fig. 5 and data not shown), suggesting that the observed phenotypes are due specifically to ctcf knockdown. This was further confirmed by rescue with ctcf mRNA injection (Fig. 5, F–H). Injection of a scrambled morpholino at the same concentrations as ATG and SP MO yielded no obvious phenotype (supplemental Fig. S7). ctcf morphants obtained by injection of MO-ATG were grouped into class 1 (c1) and class 2 (c2), characterized by somite disorganization with different levels of severity (Fig. 5, B and C). c1 embryos presented a mild phenotype including loss of the characteristic chevron-like form of the somites (Fig. 5, B and C) and a curved tail, evident at 24 and 48 hpf, respectively (Fig. 5B). Injection of a control morpholino at the same concentration did not cause obvious morphological abnormalities (Fig. 5G). c1 and c2 morphants had abnormal somite morphology, as shown by actin staining and a graded loss of slow MHC correlated with the phenotype severity of c1 and c2 morphants at 24 hpf (Fig. 5C). Furthermore, electron microscopy showed reduced muscle fibers in c2 morphants (Fig. 5D).
FIGURE 5.
Ctcf knockdown affects myogenic development in zebrafish embryos. A, Western blot analyses show the absence of Ctcf in zebrafish embryos injected with morpholino oligonucleotides complementary to ctcf translation start codon (ATG) or the exon 1/intron 1 boundary (SP). B, lateral views of zebrafish embryos injected with morpholino oligonucleotides complementary to the ctcf translation start codon (ATG). The embryos were grouped in class 1 or class 2. c1 presented mild somite disorganization at 24 and 48 hpf as compared with noninjected control embryos (ct). Severe somite disorganization was observed at 24 hpf in c2 embryos. The scale bars represent 200 μm. C, confocal micrographs showing MHC and actin signals in somites from c1 and c2 morphants at 24 and 48 hpf. Scale bars, 50 μm. D, transmission electron microscopy images show decreased myofibers (arrowheads) in c2 morphants (ATG), as compared with control (ct) embryos. E, in situ hybridization shows decreased myogenin in c1 and c2 morphants at 24 hpf. F, injection of CTCF mRNA partially rescued the abnormal phenotype of ctcf morphants. The percentage of embryos with normal somite shape and absence of curved tail obtained after injection of ATG alone or with CTCF mRNA is shown. G, lateral views of 32 hpf embryos injected with a control morpholino (CM), ATG morpholino (ATG) alone, or co-injected with CTCF mRNA (ATG + CTCF mRNA). H, in situ hybridization against myogenin on 48-hpf embryos injected with a control morpholino (CM), ATG morpholino alone (ATG), or ATG co-injected with CTCF mRNA (ATG + CTCF mRNA). The left and right panels show lateral and dorsal views, respectively. Superior oblique, lateral rectus ocular, levator arcus palatini (white bracket), transversus ventralis (open arrowhead), lower jaw (white arrowhead), pectoral fin (arrow), and hypaxial musculature (black arrowhead) are indicated.
We examined the expression of MRFs in CTCF morphants. myogenin expression decreased in c2 morphants at 24 hpf (Fig. 5E). Decreased myod and myogenin mRNA levels were also detected by qPCR in ctcf morphants (Fig. 6B). In 48-hpf c1 morphants, myogenin was absent in specific muscles, including the superior oblique, lateral rectus ocular, levator arcus palatini, transversus ventralis, lower jaw, pectoral fin, and the hypaxial musculature. myogenin expression was lower also in pectoral fin muscles, but its expression in dorsal anterior myotomes was unaffected (Fig. 5E). Thus, Ctcf functions in muscle differentiation in the zebrafish embryo.
FIGURE 6.
CTCF acts upstream of wnt11 during zebrafish muscle development. A, heat map of the gene expression profiles of Ctcf morphants at 24 hpf. The numbers of down-regulated (green) and up-regulated (red) genes are shown on the left. Misregulated genes involved in hematopoiesis (black), muscle development and contraction (blue), as well as a putative Wnt receptor (pink) are shown. Color intensity reflects fold change in mRNA abundance as indicated in the reference bar. B, qPCR revealed down-regulation of myod and myogenin, but not mrf4, and up-regulation of myf5 in embryos injected with an ATG morpholino (ATG), as compared with control morpholino-injected (CM) embryos. C, number and percentage of genes enriched in the indicated gene ontologies. D, lateral views of whole embryos injected with CM and ATG alone or with ATG plus wnt11 mRNA. Injection of an ATG MO plus wnt11 mRNA re-established somite morphology and myogenin expression, as shown by in situ hybridization on 48-hpf embryos. E, the percentages of c1 and c2 embryos obtained after injection of a CM, an ATG, or the ATG morpholino plus wnt11 mRNA are shown.
Next, we determined whether counteracting ctcf knockdown rescues the abnormal phenotypes in ctcf morphants. In vitro transcribed human CTCF mRNA was co-injected with MO-ATG (Fig. 5, F–H). Rescued embryos were considered as those co-injected embryos with normal somite shape as well as absence of curved tail (Fig. 5G). Only 2% of embryos injected with 1 ng of ATG-MO presented normal somite morphology. In contrast, injection of 2 ng of ATG-MO plus 25 pg of CTCF mRNA rendered 41% of embryos with normal somite morphology (Fig. 5F). Moreover, in situ hybridization revealed re-established myogenin expression in rescued embryos (Fig. 5H). Because a human CTCF mRNA rescued the ctcf deficiency in zebrafish, CTCF might have conserved functions in zebrafish and human, consistent with the high conservation of both orthologs (49). The fact that co-injection of CTCF mRNA mediated partial rescue of ATG-morphants suggests an essential function of ctcf in early zebrafish development, consistent with its abundant expression in early embryos (49). Thus, ctcf is indispensable for zebrafish muscle development.
Identification of Potential Ctcf Target Genes
Early lethality from CTCF deficiency in the mouse has limited the study of the CTCF functions in vivo after preimplantation (55). To gain a broader view of the part of CTCF in developmental processes, we used microarrays to examine the global gene expression profile of 24-hpf Ctcf c2 morphants, which had the most severe phenotype. Of 187 genes that were differently expressed (Fig. 6A and supplemental Table S1), 100 were up-regulated, and 87 were down-regulated, indicating that ctcf-mediated transcriptional activation and repression function in zebrafish development. Expression of several genes involved in hematopoiesis decreased in ctcf morphants (Fig. 6, A and C), suggesting that ctcf is important in this process. As defined by gene ontology terms, muscle development was the third most-enriched category (Fig. 6C). Genes indispensable for skeletal muscle fiber development were down-regulated in ctcf morphants. These genes included nebulin (neb), sarcalumenin (srl), skeletal muscle α-actin (acta1), forkhead transcription factor c1.1 (foxc1a), and desmin (desm) (Fig. 6A and supplemental Table S1). Genes involved in muscle contraction were also affected, like sarcosin (Kbtbd10) and annexin (anxa6) (supplemental Table S1), which was among the most up-regulated genes. The microarray analysis did not detect misregulation of MRFs under the established cut-off; however, qPCR detected a significant reduction in myod and myogenin, but not mrf4, expression levels (Fig. 6B). In parallel, expression of myf5, which is expressed at high levels in muscle precursors and down-regulated as differentiation proceeds (52), was up-regulated in ctcf morphants (Fig. 6B), further supporting the requirement of CTCF for establishment of the myogenic gene expression program and muscle differentiation in vivo. Another down-regulated gene was wnt11 (wingless-type murine mammary tumor virus integration site family, member 11), which is important in muscle development (33, 38, 56). In addition, fzd8a (frizzled homolog 8a), which is a predicted Wnt signaling receptor, was also down-regulated (Fig. 6A). These findings support the involvement of CTCF in muscle development. Furthermore, CTCF might participate in a wide variety of differentiation and developmental programs that are, at least in part, regulated by Wnt signaling.
Ctcf Acts Upstream of wnt11
Wnt11 participates in broad aspects of development and regulates myogenic differentiation (32–40). wnt11 was down-regulated in ctcf morphants, raising the possibility that besides acting as MRF co-activator in myogenic differentiation, CTCF might act upstream Wnt11 in the regulation of some aspects of muscle development. We approached this issue by addressing whether exogenously provided wnt11 mRNA rescues at least some of the phenotypes caused by ctcf deficiency in developing zebrafish. We co-injected embryos with ATG-MO and wnt11 mRNA and scored for c1 and c2 morphants (with mild and severe phenotype, respectively) at 24 hpf. The percentage of c1 morphants obtained was less after injecting ATG-MO plus wnt11 mRNA (0%) than after injecting a ctcf ATG-MO alone (64%) (Fig. 6E; p < 0.001), although the percentage of c2 morphants decreased from 36 to 17% (Fig. 6E; p < 0.02). Accordingly, co-injection of an ATG-MO and wnt11 mRNA led to increased numbers of embryos with normal somite morphology and myogenin expression (Fig. 6, D and E). Injection of 100 or 200 pg of wnt11 mRNA did not cause any visible abnormality when injected alone in zebrafish embryos (supplemental Fig. S7). However, injection of 300 pg did cause abnormal morphology, consistent with a previous report (57). Exogenously supplied wnt11 partially counteracted Ctcf deficiency; thus, Ctcf likely acts upstream of wnt11 to regulate some aspects of muscle development and potentially other developmental processes.
DISCUSSION
CTCF regulates broad aspects of transcription (1) as a gene expression activator and repressor (15) and mediates local and long range chromatin organization (8, 9), and its activity as epigenetic modifier has deep implications in cancer (58). However, its role in developmental processes has only recently begun to be investigated (48) and remains poorly understood. In this work we show evidence of the involvement of CTCF in myogenic differentiation and development.
The physical and functional interactions between CTCF and MyoD implicate a mechanism in which CTCF co-activates muscle-specific gene expression. In in vitro experiments, CTCF could not activate the α-SG full-length and proximal promoter without MRFs. Myogenin alone did not activate the α-SG proximal promoter even with MyoD, but in the presence of MyoD and CTCF, myogenin contributed to promoter transactivation. This synergistic interaction also occurred on the Myogenin promoter. These results, in conjunction with the facts that CTCF promoted the interaction of MyoD with the α-SG core promoter and that it enhanced the myogenic conversion capacity of MyoD with myogenin but not myogenin alone, suggest that CTCF could act as mediator necessary for transactivation of common target genes (Fig. 7). MyoD and myogenin regulate an overlapping set of promoters during myogenic differentiation (59). MyoD induces the expression of early differentiation genes, and myogenin, which cannot activate such genes on its own, stimulates MyoD-activated genes later in differentiation (59). Thus, in at least a subset of promoters, myogenin activity depends on MyoD. Therefore, such dependence could lay in intermediary factors such as CTCF. Under this scenario, CTCF would be indispensable for the contribution of myogenin to muscle-specific gene expression in promoters previously contacted by MyoD (Fig. 7). We propose that CTCF functions in myogenic differentiation, at least in part, by stimulating MRFs and muscle structural gene expression by mediating functional interactions between MRFs (Fig. 7) and facilitating MRFs interaction with muscle-specific promoters. Although addressing the activity of CTCF in the presence of MRFs needs to be extended to other muscle-specific gene promoters, this hypothesis could explain the myogenic impairment caused by ctcf knockdown in zebrafish embryos, in which the expression of the myogenic regulator myogenin and the structural component slow-mhc was affected, highlighting the relevance of CTCF nuclear factor in muscle development.
FIGURE 7.
Model of CTCF involvement in myogenic differentiation. CTCF stimulates expression of Wnt11, which in turn favors MRF expression and muscle-specific gene activation. Downstream activation of Wnt11, CTCF stimulates muscle-specific gene expression (large red arrow) by interaction with MRFs, which reinforce myogenic differentiation by a feed forward loop (solid black arrow). Upon the absence of CTCF (represented as CTCF in dotted line), the interaction of MyoD with muscle promoters is disfavored, leading to inefficacy in the contribution of Myogenin (Myog) to the enhancement of MyoD-activated gene expression (MyoD separated from Myog). Therefore, the absence of CTCF results in defective myogenesis caused by deregulation of WNT signaling and disruption of the MRFs-mediated feed-forward loop (broken arrows) leading to altered muscle-specific transcription (small red arrow).
CTCF is essential for development (7) and likely controls the expression of key genes regulating several developmental programs, including myogenesis. In this regard, our finding that Ctcf acts upstream of wnt11 provides a potential explanation for the essentiality of CTCF in wide aspects of development. For instance, wnt11 plays an important role in driving convergent extension movements during zebrafish gastrulation (60). The early requirement of wnt11 raises the possibility that the muscular deficiency in Ctcf morphants is secondary to early morphogenesis defects and not only due to disruption of the muscle-specific transcriptional circuitry. However, Wnt11 is involved in myogenesis in the chicken embryo (33, 38), and although Wnt11 deficiency in zebrafish causes only a mild defect in somite morphology (56), it is an important component of a planar cell polarity pathway that does play a critical role in somite morphology and muscle differentiation (38). Therefore, the partial rescue of Ctcf morphants by exogenously provided wnt11 suggests that Wnt11 is relevant downstream of Ctcf but is only a part of the potentially important targets of CTCF in the regulation of myogenesis and development. Although we did not rule out the nonmyogenic component of the phenotype caused by Ctcf deficiency, our results in in vitro models point to a specific function of CTCF as a direct co-regulator of MRFs in myogenic differentiation, and accordingly, the results in zebrafish support a function of CTCF in muscle development. With this in mind, and considering that Wnts are necessary for MRF function and myogenesis in birds and mammals (34–38), we propose that CTCF might function upstream and downstream of a Wnt signaling pathway controlling at least some aspects of muscle development. Upstream, CTCF stimulates the expression of Wnt11 and thus MRF expression and activity, and downstream, CTCF modulates MRF functional interactions stimulating muscle-specific gene expression (Fig. 7). Our results reveal CTCF as a novel factor involved in myogenic regulation by modulating functional interactions between MRFs and link CTCF to broad aspects of development via Wnt signaling.
Acknowledgments
We thank Georgina Guerrero Avendaño for technical assistance, Inti A. De La Rosa-Velázquez for frequent discussions, J. A. García-Sainz and the Instituto de Fisiología Celular, Universidad Nacional Autónoma de México for support, Ru-Fang Yeh for microarray analysis, Jinny Wong from the Gladstone Electron Microscopy Core Facility, and Gary Howard for editorial assistance.
This work was supported, in whole or in part, by National Institutes of Health Grant HL54737 (to D. Y. S.). This work was also supported by Dirección General de Asuntos del Personal Académico, Universidad Nacional Autónoma de México Grants IX230104, IN209403, and IN214407; Consejo Nacional de Ciencia y Tecnología Grants 42653-Q and 58767; and Third World Academy of Sciences Grant 01-055 RG/BIO/LA (to F. R.-T.); and the J. David Gladstone Institutes (to B. G. B.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental references, Table S1, and Figs. S1–S7.
- MRF
- myogenic regulatory factor
- qPCR
- quantitative PCR
- E
- embryonic day
- hpf
- h post-fertilization
- MO
- morpholino oligonucleotide.
REFERENCES
- 1. Phillips J. E., Corces V. G. (2009) Cell 137, 1194–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barski A., Cuddapah S., Cui K., Roh T. Y., Schones D. E., Wang Z., Wei G., Chepelev I., Zhao K. (2007) Cell 129, 823–837 [DOI] [PubMed] [Google Scholar]
- 3. Kim T. H., Abdullaev Z. K., Smith A. D., Ching K. A., Loukinov D. I., Green R. D., Zhang M. Q., Lobanenkov V. V., Ren B. (2007) Cell 128, 1231–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bell A. C., West A. G., Felsenfeld G. (1999) Cell 98, 387–396 [DOI] [PubMed] [Google Scholar]
- 5. Chao W., Huynh K. D., Spencer R. J., Davidow L. S., Lee J. T. (2002) Science 295, 345–347 [DOI] [PubMed] [Google Scholar]
- 6. Donohoe M. E., Zhang L. F., Xu N., Shi Y., Lee J. T. (2007) Mol. Cell 25, 43–56 [DOI] [PubMed] [Google Scholar]
- 7. Fedoriw A. M., Stein P., Svoboda P., Schultz R. M., Bartolomei M. S. (2004) Science 303, 238–240 [DOI] [PubMed] [Google Scholar]
- 8. Ling J. Q., Li T., Hu J. F., Vu T. H., Chen H. L., Qiu X. W., Cherry A. M., Hoffman A. R. (2006) Science 312, 269–272 [DOI] [PubMed] [Google Scholar]
- 9. Yusufzai T. M., Tagami H., Nakatani Y., Felsenfeld G. (2004) Mol. Cell 13, 291–298 [DOI] [PubMed] [Google Scholar]
- 10. Guelen L., Pagie L., Brasset E., Meuleman W., Faza M. B., Talhout W., Eussen B. H., de Klein A., Wessels L., de Laat W., van Steensel B. (2008) Nature 453, 948–951 [DOI] [PubMed] [Google Scholar]
- 11. Splinter E., Heath H., Kooren J., Palstra R. J., Klous P., Grosveld F., Galjart N., de Laat W. (2006) Genes Dev. 20, 2349–2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Donohoe M. E., Silva S. S., Pinter S. F., Xu N., Lee J. T. (2009) Nature 460, 128–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wendt K. S., Yoshida K., Itoh T., Bando M., Koch B., Schirghuber E., Tsutsumi S., Nagae G., Ishihara K., Mishiro T., Yahata K., Imamoto F., Aburatani H., Nakao M., Imamoto N., Maeshima K., Shirahige K., Peters J. M. (2008) Nature 451, 796–801 [DOI] [PubMed] [Google Scholar]
- 14. Parelho V., Hadjur S., Spivakov M., Leleu M., Sauer S., Gregson H. C., Jarmuz A., Canzonetta C., Webster Z., Nesterova T., Cobb B. S., Yokomori K., Dillon N., Aragon L., Fisher A. G., Merkenschlager M. (2008) Cell 132, 422–433 [DOI] [PubMed] [Google Scholar]
- 15. Zlatanova J., Caiafa P. (2009) J. Cell. Sci. 122, 1275–1284 [DOI] [PubMed] [Google Scholar]
- 16. Davis R. L., Weintraub H., Lassar A. B. (1987) Cell 51, 987–1000 [DOI] [PubMed] [Google Scholar]
- 17. Weintraub H., Tapscott S. J., Davis R. L., Thayer M. J., Adam M. A., Lassar A. B., Miller A. D. (1989) Proc. Natl. Acad. Sci. U.S.A. 86, 5434–5438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tapscott S. J. (2005) Development 132, 2685–2695 [DOI] [PubMed] [Google Scholar]
- 19. Parker M. H., Seale P., Rudnicki M. A. (2003) Nat. Rev. Genet. 4, 497–507 [DOI] [PubMed] [Google Scholar]
- 20. Bergstrom D. A., Penn B. H., Strand A., Perry R. L., Rudnicki M. A., Tapscott S. J. (2002) Mol. Cell 9, 587–600 [DOI] [PubMed] [Google Scholar]
- 21. Puri P. L., Sartorelli V. (2000) J. Cell. Physiol. 185, 155–173 [DOI] [PubMed] [Google Scholar]
- 22. Sartorelli V., Caretti G. (2005) Curr. Opin. Genet. Dev. 15, 528–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Murre C., McCaw P. S., Baltimore D. (1989) Cell 56, 777–783 [DOI] [PubMed] [Google Scholar]
- 24. Blackwell T. K., Weintraub H. (1990) Science 250, 1104–1110 [DOI] [PubMed] [Google Scholar]
- 25. Davis R. L., Cheng P. F., Lassar A. B., Weintraub H. (1990) Cell 60, 733–746 [DOI] [PubMed] [Google Scholar]
- 26. Lassar A. B., Davis R. L., Wright W. E., Kadesch T., Murre C., Voronova A., Baltimore D., Weintraub H. (1991) Cell 66, 305–315 [DOI] [PubMed] [Google Scholar]
- 27. Benezra R., Davis R. L., Lockshon D., Turner D. L., Weintraub H. (1990) Cell 61, 49–59 [DOI] [PubMed] [Google Scholar]
- 28. Spicer D. B., Rhee J., Cheung W. L., Lassar A. B. (1996) Science 272, 1476–1480 [DOI] [PubMed] [Google Scholar]
- 29. Puri P. L., Sartorelli V., Yang X. J., Hamamori Y., Ogryzko V. V., Howard B. H., Kedes L., Wang J. Y., Graessmann A., Nakatani Y., Levrero M. (1997) Mol. Cell 1, 35–45 [DOI] [PubMed] [Google Scholar]
- 30. Dilworth F. J., Seaver K. J., Fishburn A. L., Htet S. L., Tapscott S. J. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 11593–11598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mal A., Harter M. L. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 1735–1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cossu G., Borello U. (1999) EMBO J. 18, 6867–6872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Anakwe K., Robson L., Hadley J., Buxton P., Church V., Allen S., Hartmann C., Harfe B., Nohno T., Brown A. M., Evans D. J., Francis-West P. (2003) Development 130, 3503–3514 [DOI] [PubMed] [Google Scholar]
- 34. Ridgeway A. G., Petropoulos H., Wilton S., Skerjanc I. S. (2000) J. Biol. Chem. 275, 32398–32405 [DOI] [PubMed] [Google Scholar]
- 35. Geetha-Loganathan P., Nimmagadda S., Pröls F., Patel K., Scaal M., Huang R., Christ B. (2005) Dev. Biol. 288, 221–233 [DOI] [PubMed] [Google Scholar]
- 36. Tajbakhsh S., Borello U., Vivarelli E., Kelly R., Papkoff J., Duprez D., Buckingham M., Cossu G. (1998) Development 125, 4155–4162 [DOI] [PubMed] [Google Scholar]
- 37. Borello U., Berarducci B., Murphy P., Bajard L., Buffa V., Piccolo S., Buckingham M., Cossu G. (2006) Development 133, 3723–3732 [DOI] [PubMed] [Google Scholar]
- 38. Gros J., Serralbo O., Marcelle C. (2009) Nature 457, 589–593 [DOI] [PubMed] [Google Scholar]
- 39. Tao Q., Yokota C., Puck H., Kofron M., Birsoy B., Yan D., Asashima M., Wylie C. C., Lin X., Heasman J. (2005) Cell 120, 857–871 [DOI] [PubMed] [Google Scholar]
- 40. Kim C. H., Neiswender H., Baik E. J., Xiong W. C., Mei L. (2008) Mol. Cell Biol. 28, 2941–2951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bruneau B. G., Bao Z. Z., Fatkin D., Xavier-Neto J., Georgakopoulos D., Maguire C. T., Berul C. I., Kass D. A., Kuroski-de Bold M. L., de Bold A. J., Conner D. A., Rosenthal N., Cepko C. L., Seidman C. E., Seidman J. G. (2001) Mol. Cell Biol. 21, 1730–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stainier D. Y., Gilbert W. (1990) Proc. Natl. Acad. Sci. U.S.A. 87, 923–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Delgado-Olguín P., Rosas-Vargas H., Recillas-Targa F., Zentella-Dehesa A., Bermúdez de León M., Cisneros B., Salamanca F., Coral-Vázquez R. (2004) Biochem. Biophys. Res. Commun. 319, 1032–1039 [DOI] [PubMed] [Google Scholar]
- 44. Delgado-Olguín P., Recillas-Targa F., Rosas-Vargas H., Salamanca F., Coral-Vázquez R. M. (2006) Biochim. Biophys. Acta 1759, 240–246 [DOI] [PubMed] [Google Scholar]
- 45. Filippova G. N., Fagerlie S., Klenova E. M., Myers C., Dehner Y., Goodwin G., Neiman P. E., Collins S. J., Lobanenkov V. V. (1996) Mol. Cell Biol. 16, 2802–2813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Klenova E. M., Nicolas R. H., Paterson H. F., Carne A. F., Heath C. M., Goodwin G. H., Neiman P. E., Lobanenkov V. V. (1993) Mol. Cell Biol. 13, 7612–7624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Delgado M. D., Chernukhin I. V., Bigas A., Klenova E. M., León J. (1999) FEBS Lett. 444, 5–10 [DOI] [PubMed] [Google Scholar]
- 48. Soshnikova N., Montavon T., Leleu M., Galjart N., Duboule D. (2010) Dev. Cell 19, 819–830 [DOI] [PubMed] [Google Scholar]
- 49. Pugacheva E. M., Kwon Y. W., Hukriede N. A., Pack S., Flanagan P. T., Ahn J. C., Park J. A., Choi K. S., Kim K. W., Loukinov D., Dawid I. B., Lobanenkov V. V. (2006) Gene 375, 26–36 [DOI] [PubMed] [Google Scholar]
- 50. Weinberg E. S., Allende M. L., Kelly C. S., Abdelhamid A., Murakami T., Andermann P., Doerre O. G., Grunwald D. J., Riggleman B. (1996) Development 122, 271–280 [DOI] [PubMed] [Google Scholar]
- 51. Roberds S. L., Leturcq F., Allamand V., Piccolo F., Jeanpierre M., Anderson R. D., Lim L. E., Lee J. C., Tomé F. M., Romero N. B. (1994) Cell 78, 625–633 [DOI] [PubMed] [Google Scholar]
- 52. Ott M. O., Bober E., Lyons G., Arnold H., Buckingham M. (1991) Development 111, 1097–2107 [DOI] [PubMed] [Google Scholar]
- 53. Heidt A. B., Rojas A., Harris I. S., Black B. L. (2007) Mol. Cell Biol. 27, 5910–5920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Delgado-Olguín P., Hernández-Hernández J. M., Salamanca F., Recillas-Targa F., Coral-Vázquez R. M. (2008) Biochim. Biophys. Acta. 1779, 74–80 [DOI] [PubMed] [Google Scholar]
- 55. Wan L. B., Pan H., Hannenhalli S., Cheng Y., Ma J., Fedoriw A., Lobanenkov V., Latham K. E., Schultz R. M., Bartolomei M. S. (2008) Development 135, 2729–2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yin C., Solnica-Krezel L. (2007) Dev. Biol. 304, 141–155 [DOI] [PubMed] [Google Scholar]
- 57. Seo J., Asaoka Y., Nagai Y., Hirayama J., Yamasaki T., Namae M., Ohata S., Shimizu N., Negishi T., Kitagawa D., Kondoh H., Furutani-Seiki M., Penninger J. M., Katada T., Nishina H. (2010) J. Cell. Biochem. 110, 1022–1037 [DOI] [PubMed] [Google Scholar]
- 58. Recillas-Targa F., De La Rosa-Velázquez I. A., Soto-Reyes E., Benítez-Bribiesca L. (2006) J. Cell. Mol. Med. 10, 554–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cao Y., Kumar R. M., Penn B. H., Berkes C. A., Kooperberg C., Boyer L. A., Young R. A., Tapscott S. J. (2006) EMBO J. 25, 502–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Heisenberg C. P., Tada M., Rauch G. J., Saúde L., Concha M. L., Geisler R., Stemple D. L., Smith J. C., Wilson S. W. (2000) Nature 405, 76–81 [DOI] [PubMed] [Google Scholar]