Abstract
Recent studies implicate a role for WD repeat domain, phosphoinositide-interacting 1 (WIPI1) in the biogenesis of melanosomes, cell type-specific lysosome-related organelles. In this study, we determined that WIPI1, an ATG18 homologue that is shown to localize to both autophagosomes and early endosomes, inhibited mammalian target of rapamycin (MTOR) signaling, leading to increased transcription of melanogenic enzymes and the formation of mature melanosomes. WIPI1 suppressed the target of rapamycin complex 1 (TORC1) activity, resulting in glycogen synthase kinase 3β inhibition, β-Catenin stabilization, and increased transcription of microphthalmia transcription factor and its target genes. WIPI1-depleted cells accumulated stage I melanosomes but lacked stage III-IV melanosomes. Inhibition of TORC1 by rapamycin treatment resulted in the accumulation of stage IV melanosomes but not autophagosomes, whereas starvation resulted in the formation of autophagosomes but not melanin accumulation. Taken together, our studies define a distinct role for WIPI1 and TORC1 signaling in controlling the transcription of melanogenic enzymes and melanosome maturation, a process that is distinct from starvation-induced autophagy.
Keywords: Autophagy, Endosomes, Intracellular Trafficking, Signal Transduction, TOR Complex (TORC), Melanosomes
Introduction
Melanin protects the skin and eye from UV irradiation (1, 2), the ear from traumatic noise (3), and neural cells from heavy metal toxicity (4, 5). Abnormal melanogenesis is a feature of many human skin diseases including, but not limited to, acquired pigmentary disorders (vitiligo and melasma) (6), genetic pigmentary disorders (Hermansky-Pudlak and oculocutaneous albinism syndromes) (7), and melanoma (8). Melanin is synthesized within the melanosome, a lysosome-related organelle whose biogenesis is regulated by specific transport pathways through four distinct stages (9). Stage I melanosomes, vesicles derived from early endosomal membranes, contain the amyloid protein PMEL17 (10) and MART1 (11). During stage I melanosome maturation, PMEL17 forms the lumenal fibrillar striations that characterize stage II melanosomes (12). Premelanosomes mature to stage III and IV melanosomes by the delivery of melanogenic components from early endosomes via vesicular transport (13). Once these proteins are transported, melanin pigment is synthesized and deposited onto the PMEL17 striations (stage III melanosome), eventually giving rise to the stage IV melanosome, which appears opaque on electron microscopy (14–16). Melanocyte-specific microphthalmia-associated transcription factor (MITF-M)3 is known to be the master regulator for the transcription of enzymes and proteins that are core components of the melanosome: tyrosinase (TYR) (the rate-limiting step in melanin synthesis), tyrosinase-related protein 1 (TYRP1), PMEL17, and MART1 (17). The expression of MITF is controlled by at least five transcription factors: LEF1, cAMP response element-binding protein (CREBP), SOX10, OC2, and PAX3, which directly bind to the MITF-M core promoter (18). Multiple different intracellular signaling pathways regulate the activity of these transcription factors (19). In summary, mammalian cells have evolved complex mechanisms to regulate melanin production both at the level of transcription and cellular compartmentalization.
Although extensive studies have identified more than 150 genes that regulate melanin production (16), genetic studies suggest that many other genes influencing human pigment variation remain unidentified (20). To identify additional genes that regulate melanogenesis, our laboratory completed a genome wide RNAi screen (21). Our screen identified novel genes that regulate the expression of MITF and TYR and also a number of putative regulators of autophagy (21). Functional validation studies revealed that autophagy regulators impact melanogenesis both in vitro and in vivo (21). Intriguingly, depletion of one regulator of vesicle trafficking, WIPI1, resulted in both decreased pigment accumulation and decreased accumulation of MITF, the master regulator for the transcription of enzymes and proteins that are components of the melanosome (17).
WIPI1, a human homologue of the yeast protein ATG18, is composed of multiple WD40 repeat domains, which allows it to bind to phosphatidylinositol 3-phosphate and phosphatidylinositol 3,5-bisphosphate (22, 23). Preliminary studies suggest that although WIPI1 localizes to multiple different vesicular compartments under normal nutritional conditions (22) it localizes to the autophagosome under conditions of starvation or TORC1 inhibition (24). In yeast, ATG18 functions to retrieve membrane from the amphisome after fusion of the autophagosome with the lysosome (25, 26) and also acts as a phosphatidylinositol 3,5-bisphosphate effector to remodel the membrane of vacuoles (27, 28). Yeast cells deficient in ATG18 fail to initiate autophagy correctly (29), leading to impaired maturation of proteins normally trafficked to the autophagosome (27). Recent evidence suggests that human cells contain multiple ATG18 homologues (WIPI1, WIPI2, WIPI3, and WIPI4) that form distinct subcomplexes with ATG2P (30) and may play different roles in autophagy. Although WIPI1 colocalizes with LC3B and binds phosphatidylinositol 3-phosphate located on the autophagosome membrane under starvation (23), it forms only weak associations with ATG2P homologues in human cells (30), suggesting that it may not carry out the same function in human cells as it does in yeast cells. In COS-7 cells, WIPI1 was shown to regulate trans-Golgi-endosomal protein trafficking (22). Although published studies (22, 23) suggest that WIPI1 controls endosome/autophagosome dynamics in other cell types, our RNAi screen determined that WIPI1 depletion also significantly inhibited the accumulation of MITF and TYR mRNA in MNT-1 cells (21) even though WIPI1 has no DNA binding domain or nuclear localization signal (22). In this study, we investigated the role of WIPI1 in melanogenesis and defined how this protein regulates the transcription of melanogenic enzymes and MTOR signaling. Our studies revealed that WIPI1 represses TORC1 signaling, leading to the increased transcription of MITF target genes and melanosome maturation. Although MTOR inhibition led to increased accumulation of mature melanosomes, starvation did not induce melanin accumulation but did induce autophagosome formation. Taken together, these studies define a role for WIPI1 and MTOR signaling in melanogenesis that is distinct from their role in autophagy.
EXPERIMENTAL PROCEDURES
Antibodies and Other Reagents
MITF (C5) and TYR (T311) antibodies were purchased from Santa Cruz Biotechnology Inc. or Lab Vision Co. We used an LC3B (4E12) antibody purchased from MBL International Corp. for Western blotting and a polyclonal LC3B antibody from Invitrogen for immunostaining. PMEL17 (HMB45) and TYRP1 (TA99) antibodies produced from mice were purchased from Santa Cruz Biotechnology Inc. PMEL17 antibody produced from rabbit were purchased from Sigma. TYRP1 antibody produced from rabbit and LAMP2 antibody were purchased from Abcam. AKT, phospho-AKT, GSK3β, phospho-GSK3β, β-Catenin, phospho-β-Catenin, MTOR, phospho-MTOR, p70S6K, phospho-p70S6K, and EEA1 antibodies were purchased form Cell Signaling Technology, Inc. Rapamycin was also purchased from Cell Signaling Technology, Inc. 2′Z,3′E)-6-Bromoindirubin-3′-oxime (BIO) was purchased from Sigma.
Cell Culture
Human MNT-1 melanoma cells were cultured in DMEM supplemented with 10% fetal bovine serum. Human deeply pigmented neonatal epidermal melanocytes were cultured in Medium 254 supplemented with phorbol 12-myristate 13-acetate-free Human Melanocyte Growth Supplement (Cascade Biologics) and 0.1 mm 3-isobutyl-1-methylxanthine.
RNA Interference
For our siRNA experiments, MNT-1 cells were reverse transfected with 50 nm pooled siRNAs toward human genes using established protocols (21). We used non-targeting control (SMARTpool), WIPI1 (oligo ID D-018205-01, D-018205-02, D-018205-03, and D-018205-04), and BECN1 (oligo ID D-010552-02-0005 and D-010552-17-0005) siRNAs purchased from Dharmacon and the following oligos pooled for each gene from Ambion: MTOR (oligo ID s602 and s603), GBL (oligo ID s34578 and s227014), RAPTOR (oligo ID s33214 and s33215), KRT7 (oligo ID s7965, s7966, and s7967), VPS34 (oligo ID s10517, s10518, and s10519), VPS15 (oligo ID s103, s104, and s105), ATG2B (oligo ID s30171, s30172, and s30173), ATG14 (oligo ID s22526, s22527, and s22528), ULK1 (oligo ID s15963, s15964, and s15965), ATG9B (oligo ID s50067, s50068, and s50069), ATG12 (oligo ID s17464, s17465, and s17466), ATG5 (oligo ID s18158, s18159, and s18169), LC3B (oligo ID s22385, s22387, and s223228), ATG8L (oligo ID s24331, s24332, and s24333), ATG7 (oligo ID s20650, s20651, and s20652), and ATG16L (oligo ID s30069, s30070, and s30071). For our shRNA experiments, we utilized two different lentiviral based shRNA expression systems (pGIPZ and pLKO.1, Open Biosystems). MNT-1 cells and melanocyte shRNA-expressing lines were established using the manufacturer's protocol. We utilized the following shRNA constructs purchased from Open Biosystems: pGIPZ-WIPI1-1, RHS4430-98513807; pGIPZ-WIPI1-2, RHS4430-98853022; pGIPZ-non-targeting control, RHS4346; pLKO.1-WIPI1-1, RHS3979-98832433; pLKO.1-WIPI1-2, RHS3979-98832494; pLKO.1-WIPI1-3, RHS3979-98832500; and pLKO.1-non-targeting control. Gene knockdown was quantified using quantitative RT-PCR and also measured via immunoblotting. A two-tailed Student's t test was used to measure statistical significance.
Luciferase Assays
MNT-1 cells were transfected with a firefly luciferase reporter driven by MITF-M promoter (pGL3-MITF/M) (31) or a firefly luciferase reporter driven by TYR promoter (pHTL12) (32). These cells were also co-transfected with a Renilla luciferase reporter driven by an SV40 promoter as an internal control using a Calcium Phosphate Transfection kit (Invitrogen). 48 h after plasmids transfection, cells were lysed and assayed using a Dual-Glo luciferase assay system (Promega) according to the manufacturer's protocol. Firefly luminescence values were normalized to Renilla luminescence. The presented data were normalized to control. For experiments involving both plasmids and siRNA transfection, plasmid transfection was performed 24 h post-siRNA transfection. A Student's t test was utilized to determine statistical significance. The WIPI1 overexpression construct was in the pcDNA3.1/nV5-DEST backbone (Invitrogen), and the control plasmid was the pre-recombined vector.
Real Time Quantitative PCR for mRNA
A Cells-to-Ct kit was utilized for RT-qPCR (Applied Biosystems). TaqMan Gene Expression Assays for MITF-M (Hs00165156), TYR (Hs01099964), TYRP1 (Hs00167051), PMEL17 (Hs00173854), MART1 (Hs00194133), WIPI1 (Hs00215872), and β-actin loading control were obtained from Applied Biosystems. A 7900HT Fast Real-Time PCR System (Applied Biosystems) and SDS 2.4 (Applied Biosystems) were utilized to determine Ct values. Values were normalized using actin and analyzed using the relative quantification mathematical model (Pfaffl). A Student's t test was utilized to determine statistical significance, and significantly different values are indicated.
Pigment Measurement
Relative pigment accumulation was measured as described (21). Briefly, MNT-1 cells were transfected with the indicated siRNAs or incubated in the presence of the indicated concentration of drug in 96-well plates. Cells were lysed by adding 15 μl of Cell-Titer Glo reagent (Promega). ATP accumulation was measured using a Cell-Titer Glo luminescence assay, and relative melanin accumulation was determined by measuring the absorbance of each well at 405 nm. 405-nm absorbance values were divided by Cell-Titer Glo luminescence and then were normalized to control, called pigment index (PI): Pigment induction (%) = (PI of test − PI of control) × 100%. Each experiment was performed in triplicate, and a two-tailed Student's t test was utilized to determine statistical significance.
Chromatin Immunoprecipitation
We adapted the protocol from Rabinovich et al. (33) with the following modifications. Briefly, MNT-1 cells expressing control or WIPI1 shRNA (pLKO backbone) were fixed in 2% paraformaldehyde for 10 min and incubated with 0.125 m glycine for 10 min. Cells were then scraped off plates (2 × 6-cm dish for one immunoprecipitation) and washed with PBS before sonication. After sonication shearing in 0.4 ml of Lysis Buffer (50 nm Tris, 10 mm EDTA, 1% SDS, 1 mm PMSF, protease inhibitors), 0.8 ml of Immunoprecipitation Dilution Buffer (0.01% SDS, 1.1% Triton X-100, 1.2 mm EDTA, 16.7 mm Tris, pH 8.0, 167 mm NaCl, 1 mm PMSF, protease inhibitors) plus β-Catenin antibody (BD Bioscience) or control IgG was added to the cell lysates. After a 4 °C overnight incubation, lysates were incubated with blocked Staph A cells for 15 min at room temperature. After centrifugation, the pellets were washed twice with Low Salt Buffer (0.1% SDS, 1% Triton X-100, 2 mm EDTA, 20 mm Tris-HCl, pH 8.1, 150 mm NaCl), High Salt Buffer (0.1% SDS, 1% Triton X-100, 2 mm EDTA, 20 mm Tris-HCl, pH 8.1, 500 mm NaCl), LiCl Buffer (0.25 m LiCl, 1% IGEPAL CA-630, 1% deoxycholic acid, 1 mm EDTA, 10 mm Tris, pH 8.1), and TE Buffer (10 mm Tris-HCl, 1 mm EDTA, pH 8.0). DNA was eluted in 1% SDS, 5 mm NaHCO3 by vortexing. Cross-links were reversed in 10% of the input collected from IgG immunoprecipitation samples and eluted by incubating these samples with 0.2 m NaCl at 67 °C overnight. Eluted DNA samples were purified using a GenElute PCR cleanup kit (Sigma) and amplified by the following primers centered on LEF1/β-Catenin binding sites: MITF-342F (5′-TAGATGATGTCTCCTCCAAAGG-3′) and MITF-102R (5′-GAAGCCCTACGAGTTTGGTC-3′). For the negative control, we used the primers GAPDH F (5′-TACTAGCGGTTTTACGGGCG-3′) and GAPDH R (5′-TCGAACAGGAGGAGCAGAGAGCGA-3′) using a SYBR Green assay system (Applied Biosystems). A Student's t test was utilized to determine statistical significance.
Immunofluorescence Microscopy
Control and WIPI1 shRNA (pGIPZ)-expressing human melanocytes were fixed in 2% paraformaldehyde for 1 h. Coverslips were rinsed with PBS, and cells were permeabilized with 0.4% saponin (primary melanocytes) and blocked in 1% BSA with 0.1% Tween 20 for 1 h. Cells were incubated with anti-LC3B antibody (Invitrogen) and then labeled with secondary antibodies conjugated with Alexa Fluor 594 (Invitrogen). For each clone, the cytosolic fluorescence intensity per unit area in 10 cells was quantified using ImageJ, and the data were then normalized to control shRNA-expressing melanocytes. Statistical significance was determined using a Student's t test. MNT-1 cells expressing control and WIPI1 shRNAs (pLKO) were immunostained by anti-β-Catenin (Cell Signaling Technology, Inc.), EEA1 (Cell Signaling Technology, Inc.), LAMP2 (Abcam), and Alexa Fluor 488 (Invitrogen) using the same method described above except that 0.2% Triton X-100 was used for permeabilization. For β-Catenin, the nuclear fluorescence intensities of 100 cells per unit area in each treatment was quantified using ImageJ, and the data were then normalized to vehicle-treated control shRNA-expressing MNT-1 cells. Statistical significance was determined using a Student's t test. For EEA1 and LAMP2, total fluorescence intensities per unit area in 10 cells was quantified using ImageJ, and the data were then normalized to the value of control shRNA-expressing MNT-1 cells, and statistical significance was determined using a Student's t test. Colocalization studies were performed in MNT-1 parent cells using rabbit LC3B antibody (Invitrogen) with mouse PMEL17 or TYRP1 (Santa Cruz Biotechnology Inc.) or using mouse p62-SQSTM1 antibody (BD Biosciences) and rabbit PMEL17 (Sigma) or TYRP1 (Abcam) followed by secondary antibodies conjugated to Alexa Flour 594 or 488 (Invitrogen). Coverslips were mounted in a solution containing DAPI. Confocal images were acquired using a LSM 510 META confocal multiphoton microscope or a Nikon Ti fluorescence microscope as indicated. The three-dimensional projected images were processed as 12-bit images by LSM Image Browser (v4.0, Zeiss).
Transmission Electron Microscopy
Human deeply pigmented neonatal melanocytes expressing control or WIPI1 shRNA (pGIPZ) and MNT-1 cells expressing control or WIPI1 shRNA (pLKO) treated with either vehicle or 100 nm rapamycin for 5 days were fixed in 1% glutaraldehyde and pelleted by centrifugation. For experiments involving melanocytes, control or WIPI1 shRNA-expressing (GFP+) melanocytes were sorted by flow cytometry, then fixed in 1% glutaraldehyde, and embedded in 1.5% low melt agarose containing 0.1 m sodium cacodylate. Fixed cells or agarose blocks were placed in 1% osmium tetroxide for 1 h and dehydrated by ethanol and propylene oxide immersion. A flat embedding procedure was used after which each tissue block was trimmed using a single edged razor blade under a dissecting microscope. Ultrathin (60–80-nm) sections from each block were cut with an ultramicrotome (UltraCut E, Reichert-Jung, Wetzlar, Germany), and sequential sections were collected on mesh and Formvar-coated slot grids. The sections were stained with uranyl acetate and lead citrate to enhance contrast. Sections were examined with a Philips CM-10 transmission electron microscope. Images of cells were captured with a Gatan Ultrascan digital camera. The number of stage I–IV melanosomes were quantified in a minimum of 10 cells from each shRNA and used to calculate the percentages of stage I–IV melanosomes as described previously (34). These values were normalized to control shRNA-expressing cells to determine the relative percentage of melanosomes in shRNA-expressing cells.
Starvation-induced Autophagy
MNT-1 cells were rinsed twice with prewarmed Earle's balanced salt solution for 1–3 h before being subjected to transmission electron microscopy (TEM), pigment measurement, and RT-qPCR as described above. Starvation conditions used in these experiments were identical to those in a previous study using melanoma cells (24).
RESULTS
WIPI1 Controls Expression of MITF-M- and MITF-M-regulated Proteins
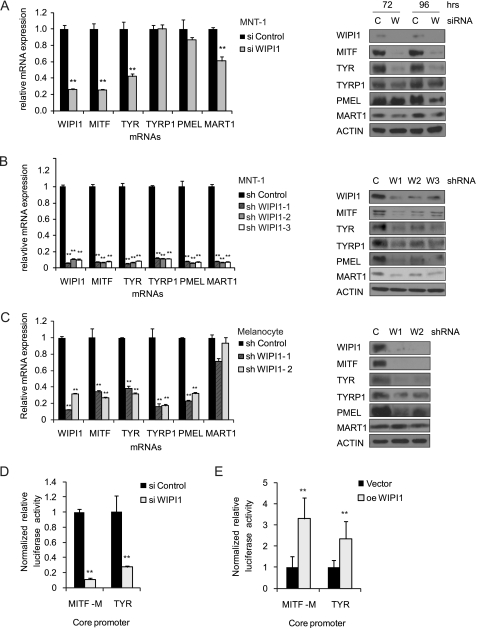
Although immunolocalization studies suggest that WIPI1 regulates vesicle trafficking, published studies from our group suggest that WIPI1 also supports the expression of MITF and TYR at both the protein and mRNA levels (21) despite its lack of a DNA binding domain. Transient suppression of WIPI1 expression using pooled siRNAs inhibited the accumulation of MITF-M, TYR, and MART1 mRNAs and proteins (Fig. 1A) in MNT-1 melanoma cells. Prolonged suppression of WIPI1 expression using lentivirally expressed short hairpin RNAs inhibited the accumulation of a wider spectrum of MITF-M target genes and proteins (MITF-M, TYR, TYRP1, PMEL17, and MART1) (Fig. 1B) in MNT-1 cells, consistent with the sustained inhibition of gene expression observed with shRNAs. Similarly, shRNA-mediated suppression of WIPI1 expression in deeply pigmented human foreskin melanocytes also inhibited the accumulation of MITF-M, TYR, TYRP1, and PMEL17 mRNA and proteins (Fig. 1C). However, WIPI1 depletion did not inhibit the accumulation of MART1 mRNA, suggesting that there might be subtle differences in the regulation of MART1 between melanocytes and MNT-1 cells. Nevertheless, our results indicate that WIPI1 regulates the mRNA accumulation of MITF-M and several MITF-M target genes in normal melanocytes and MNT-1 melanoma cells.
FIGURE 1.
WIPI1 regulates expression of MITF-M and MITF-M target genes. A, MNT-1 cells were transiently transfected with the indicated pooled siRNAs. WIPI1, MITF-M, and MITF-M target gene (TYR, TYRP1, PMEL17, and MART1) mRNA expression was measured 72 h after transfection by quantitative RT-PCR, and the protein levels were measured by immunoblotting 72 and 96 h post-transfection. MNT-1 cells (B) or human deeply pigmented foreskin melanocytes (C) were transduced with lentiviruses expressing independent WIPI1 (W) shRNAs or control (C) shRNA and selected with puromycin. The expression of the indicated genes was measured in shRNA-expressing cells via quantitative RT-PCR, and the protein levels were measured by immunoblotting. MNT-1 cells were transfected with WIPI1 siRNA (D) or with pCMV-driven WIPI1 overexpression (oe) construct or control plasmid (E) and assayed for MITF-M and TYR core promoter activities. For all experiments, data shown are mean ± S.D. (n = 3 as indicated by the error bars). **, p < 0.01 using a Student's t test versus control. Each experiment was performed three times in triplicate.
To examine whether WIPI1 directly regulates the transcription of MITF, we determined the effect of WIPI1 depletion or overexpression on the activity of an MITF-M promoter-luciferase reporter that has been validated in other studies (31). WIPI1 siRNA-transfected MNT-1 cells exhibited a 10-fold decrease in MITF-M core promoter activity (Fig. 1D). WIPI1-overexpressing cells exhibited a 3-fold induction of MITF promoter activity (Fig. 1E), consistent with a positive role for WIPI1 in regulating MITF expression. To further validate that WIPI1 stimulated the transcription of target genes regulated by MITF, we examined the impact of WIPI1 depletion or overexpression on a luciferase reporter mainly regulated by MITF-M, the TYR promoter (32). WIPI1 siRNA-transfected cells exhibited a 3.7-fold decrease in TYR core promoter activity compared with control siRNA-transfected cells (Fig. 1D), whereas WIPI1-overexpressing cells exhibited a 2-fold up-regulation in TYR promoter activity compared with control-transfected cells (Fig. 1E). Taken together, these results demonstrate that WIPI1 regulates melanogenesis by controlling the expression of MITF-M and MITF-M target genes. As WIPI1 lacks a DNA binding domain, WIPI1 presumably regulates the expression of MITF-M and its target genes via an indirect mechanism.
WIPI1 Regulates Melanogenic Protein Transcription by Repressing TORC1
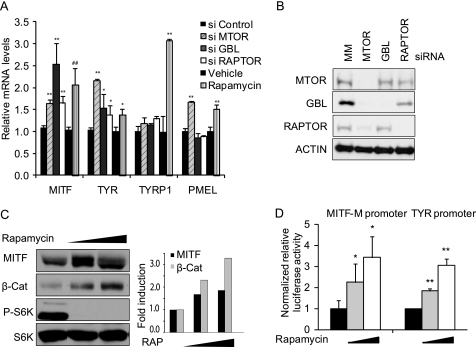
Published studies have shown that rapamycin increases the expression of the MITF-M-regulated genes TYR and TYRP1 in mouse melanoma cells (35, 36), suggesting that TORC1 may inhibit the expression of melanogenic enzymes via MITF. To determine whether TORC1 represses the expression of MITF and its target genes in human cells, we inhibited TORC1 signaling in MNT-1 cells by either depleting components of the MTOR complex (Fig. 2A) or by treating the cells with rapamycin (Fig. 2C). Depletion of TORC1 components (GBL, RAPTOR, and MTOR) using pooled siRNAs (Fig. 2B) had varying impacts on MITF and TYR mRNA accumulation (Fig. 2A), but all stimulated MITF and TYR mRNA accumulation to a certain degree. Rapamycin treatment also increased MITF protein levels (Fig. 2C) and inhibited the phosphorylation of p70S6K, a downstream target of TORC1 (Fig. 2C). To determine whether rapamycin increased MITF and TYR transcription, MNT-1 cells incubated in the presence of increasing doses of rapamycin were transfected with MITF-M- and TYR-luciferase reporter constructs. Rapamycin treatment increased the reporter activities of both MITF- and TYR-luciferase reporter constructs in a dose-dependent fashion, consistent with a direct role for MTOR in inhibiting the transcription of both MITF and TYR (Fig. 2D).
FIGURE 2.
TORC1 negatively regulates transcription of MITF and MITF target genes. A, MNT-1 cells treated with mismatch control (MM), MTOR, GBL, or RAPTOR siRNA for 96 h (*, p < 0.05 or **, p < 0.01 using a Student's t test versus si control) or 1 nm rapamycin (**, p < 0.01 using a Student's t test versus vehicle) were assayed by quantitative RT-PCR for MITF-M, TYR, TYRP1, and PMEL17 mRNA expression. Data are shown from a representative experiment as mean ± S.D. (n = 3). B, MNT-1 cells treated with control, MTOR, GBL, or RAPTOR siRNA for 96 h were immunoblotted with anti-MTOR, GBL, and RAPTOR antibodies. C, MNT-1 cells treated with 0, 0.4, or 2 nm rapamycin (RAP) for 6 h were lysed and subjected to immunoblotting with MITF, phospho-Thr-389-p70S6K, p70S6K, and β-Catenin antibodies. Right, quantitation of MITF and β-Catenin protein levels. D, MNT-1 cells were treated with 0, 0.5, or 5 nm rapamycin for 48 h. Cells were then transfected with reporter plasmids as indicated, and luciferase activity was measured using a Dual-Glo luciferase assay system and normalized by SV40 promoter-driven Renilla luminescence. Data shown are mean ± S.D. (n = 5). **, p < 0.01 using a Student's t test versus vehicle. Each experiment was performed three times in triplicate.
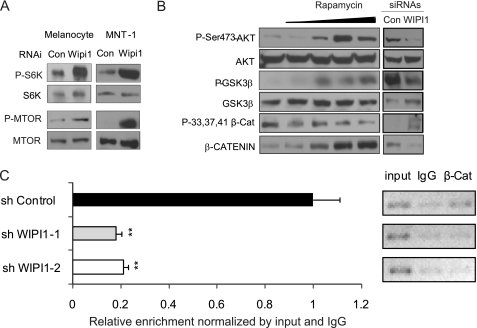
As WIPI1 depletion inhibited TYR and MITF expression whereas TORC1 inhibition increased MITF and TYR transcription, we hypothesized that WIPI1 controls melanogenic enzyme transcription by repressing TORC1. Consistent with our hypothesis, WIPI1 depletion stimulated the phosphorylation of p70S6K in both melanocytes and MNT-1 cells (Fig. 3A). WIPI1 depletion also stimulated the phosphorylation of MTOR itself at Ser-2448 (Fig. 3A), a site that is phosphorylated by p70S6K (37) and AKT (38), two TORC1 downstream targets. These results are consistent with a role for WIPI1 in suppressing TORC1 activity. When coupled with other data demonstrating that TORC1 inhibition increased the transcription of MITF and TYR, our results are consistent with the hypothesis that WIPI1 activates the transcription of melanogenic proteins by suppressing TORC1 activity.
FIGURE 3.
WIPI1 inhibits TORC1 activity, leading to β-Catenin stabilization and MITF transcription. A, MNT-1 cells treated with control or WIPI1 siRNA and human deeply pigmented foreskin melanocytes expressing control or WIPI1 shRNA were lysed and subjected to immunoblot analysis with phospho-Thr-389-p70S6K, p70S6K, phospho-Ser-2448-MTOR, or MTOR antibodies. B, MNT-1 cells treated with 0, 0.1, 1, 10, or 100 nm rapamycin for 5 days or transfected with WIPI1 and control (Con) siRNAs were lysed and analyzed by immunoblotting with phospho (P)-Ser-473-AKT, AKT, phospho-Ser-9-GSK3β, GSK3β, phospho-Ser-33,37/Thr-41-β-Catenin, and β-Catenin (β-Cat) antibodies. C, ChIP of β-Catenin was performed on MNT-1 cells expressing control or WIPI1 shRNA. Chromatin from formaldehyde-cross-linked MNT-1 cells was immunoprecipitated with monoclonal β-Catenin and isotypic IgG control antibodies. ChIP immunoprecipitates were analyzed by quantitative RT-PCR using primers that detect the LEF1-binding region on the MITF core promoter (−342 to −102). **, p < 0.01. Data shown are mean ± S.D. (n = 3). Representative gels for the same primer are shown. Each experiment was performed three times in triplicate.
WIPI1 Regulates MITF Expression by Modulating β-Catenin Stability
MTOR participates in two different signaling complexes, TORC1 and TORC2. Although TORC1 plays a direct role in regulating autophagy and translation, TORC2 is important in regulating cell survival and cytoskeletal dynamics (39). TORC2 phosphorylates AKT (40), which activates AKT, leading to the phosphorylation and degradation of GSK3β (41, 42). GSK3β constitutes part of the “destruction complex,” which can capture and phosphorylate β-Catenin, destabilizing β-Catenin and leading to its degradation (43). β-Catenin cooperates with LEF1 to activate the MITF promoter. β-Catenin is a central regulator of MITF expression in vivo (44). Published studies have determined that TORC1 negatively regulates TORC2 (39), leading us to hypothesize that WIPI1 functions to inhibit TORC1, leading to the activation of TORC2, decreased GSK3β levels, increased β-Catenin nuclear localization, and increased MITF transcription. To test our hypothesis, we first determined whether TORC1 inhibition led to TORC2 activation. Treatment of MNT-1 cells with a selective TORC1 inhibitor (rapamycin) induced a dose-dependent increase in the phosphorylation of AKT at Ser-473 (Fig. 3B), a TORC2-dependent site (40), consistent with a role for TORC1 in repressing TORC2 activity. Rapamycin also induced GSK3β phosphorylation, which then suppressed its ability to phosphorylate β-Catenin at Ser-33,37/Thr-41 (Fig. 3B). This ultimately led to the accumulation of β-Catenin protein (Figs. 2C and 3B). Chromatin immunoprecipitation (ChIP) analysis revealed that WIPI1-depleted cells have less β-Catenin bound to the MITF promoter (Fig. 3C). Together, these data are consistent with a model where TORC1 activates AKT, leading to the repression of melanogenic enzyme transcription via a mechanism involving GSK3β and β-Catenin.
Next, we utilized a loss of function approach to determine the impact of WIPI1 depletion on TORC2 activity. WIPI1 depletion inhibited the phosphorylation of AKT at Ser-473 and the phosphorylation of GSK3β (Fig. 3B). Because depletion of WIPI1 activated TORC1 (Fig. 3A), these results are consistent with a role for TORC1 in suppressing TORC2 activity. Suppression of GSK3β phosphorylation resulted in the increased phosphorylation of β-Catenin at Ser-33,37/Thr-41, leading to decreased β-Catenin total protein levels (Fig. 3B) and decreased β-Catenin nuclear localization (Fig. 4A). Together, these results demonstrate that WIPI1 normally functions to inhibit TORC1 and activate TORC2, sustaining β-Catenin levels that lead to increased MITF transcription.
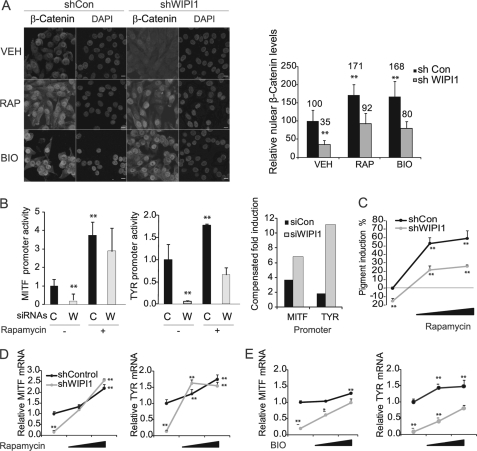
FIGURE 4.
WIPI1 regulates MITF transcription by suppressing TORC1 and GSK3β. A, MNT-1 cells expressing control or WIPI1 shRNA were treated with vehicle, 5 μm BIO, or 10 nm rapamycin (RAP) for 24 h. Cells were stained with β-Catenin antibody and examined by confocal microscopy. Right, relative nuclear β-Catenin levels were quantified using ImageJ. Data shown are mean ± S.D. (n = 100). Scale bar, 10 μm. B, MNT-1 cells transfected with WIPI1 (W) or control (C) siRNA were incubated in the presence and absence of rapamycin for 48 h. Cells were subsequently transfected with MITF-M and TYR promoter constructs, and relative luciferase activity was determined. Data shown are mean ± S.D. (n = 5). Compensated -fold induction is the ratio of treated to untreated samples for each individual siRNA transfection. C, MNT-1 cells expressing control or WIPI1 shRNA were treated with 0, 1, or 10 nm rapamycin for 5 days. Relative melanin accumulation was measured spectrophotometrically as described. Data shown are mean ± S.D. (n = 5). MNT-1 cells were treated with 0, 0.1, or 0.5 nm rapamycin for 5 days (D) or 0, 1,or 5 μm BIO for 24 h (E), and the relative accumulation of MITF-M and TYR mRNA levels was measured by RT-qPCR. Data shown are mean ± S.D. (n = 3). For all subfigures, *, p < 0.05 or **, p < 0.01 using a Student's t test versus control (Con) RNAi treated with vehicle (VEH). Each experiment was performed three times in triplicate.
To verify that WIPI1 regulates MITF via a mechanism involving both TORC1 and β-Catenin, we sought to complement the phenotypes induced by WIPI1 depletion by the addition of TORC1 inhibitors (rapamycin) or by the addition of pharmacologic agents that induce β-Catenin accumulation (GSK3 inhibitor). TORC1 inhibition (rapamycin) was sufficient to induce β-Catenin nuclear accumulation (Fig. 4A), MITF and TYR promoter activity (Fig. 4B), and mRNA accumulation (Fig. 4C) in the context of WIPI1 depletion. Rapamycin treatment also complemented the impact of WIPI1 depletion on melanin accumulation (Fig. 4D). Although we were able to observe that rapamycin regulated the expression of MITF targets and β-Catenin at early time points, we were unable to assess the impact of rapamycin on MITF target proteins and β-Catenin nuclear localization at later time points secondary to the growth-suppressing effects of rapamycin on melanocytes.
Our data demonstrated that WIPI1 activates TORC1 and inhibits TORC2 (Fig. 3, A and B), leading to increased GSK3β activity (Fig. 3B) and decreased β-Catenin nuclear localization (Fig. 4A). To examine whether WIPI1 regulates β-Catenin via GSK3β, we determined whether a GSK3β inhibitor, BIO (45), was able to complement the impact of WIPI1 depletion on MITF-M and TYR mRNA accumulation. BIO treatment complemented the impact of WIPI1 depletion on β-Catenin nuclear localization (Fig. 4A) and TYR and MITF mRNA accumulation (Fig. 4E). These impacts were less robust than that observed with rapamycin, likely secondary to the different potencies of the inhibitors used in these studies. Nevertheless, our results do demonstrate that WIPI1 suppresses TORC1 signaling, leading to TORC2 activation, β-Catenin stabilization, and increased transcription of melanogenic enzymes.
WIPI1 Depletion Inhibits Normal Melanosome Maturation
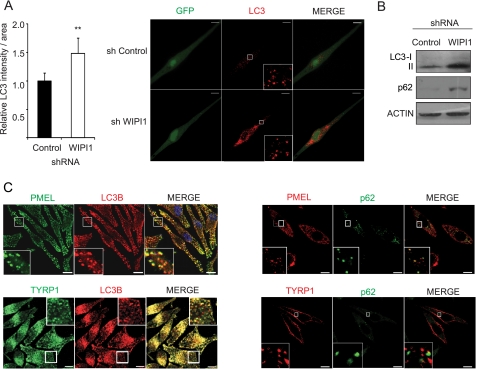
Our results revealed that WIPI1 modulates MTOR signaling, resulting in decreased transcription of MITF target genes. Published studies have determined that melanosome components are delivered to the developing melanosome from the early endosome by vesicular transport (34). Many of the key melanosome components are MITF target genes whose transcription is regulated by WIPI1. To determine whether WIPI1 depletion also interrupted melanosome maturation, we established human MNT-1 melanoma cells and primary melanocytes expressing WIPI1 shRNAs and demonstrated that WIPI1-depleted melanocytes (Fig. 5C) and MNT-1 cells (data not shown) accumulate less melanin. Electron microscopy revealed that WIPI1-depleted melanocytes exhibited greatly reduced numbers of mature stage III and IV melanosomes (Fig. 5, A and B) but accumulated both stage I and II melanosomes and atypical vesicle structures that contained fibrillar material (Fig. 5A). Similarly, WIPI-depleted MNT-1 cells also contained fewer stage III and IV melanosomes (supplemental Fig. S1B). Western blotting analysis revealed that WIPI1-depleted normal melanocytes accumulated the autophagy markers lipidated LC3 (LC3-II) and p62 (Fig. 6A). Published studies have determined that p62 is an autophagosome cargo protein that is degraded when the autophagosome fuses with the lysosome, whereas LC3-II is a marker of the autophagosome (46). Taken together, these results suggest that the stage I-II melanosome structures that accumulate in WIPI1-deficient cells are similar to the autophagosome in that they contain both LC3 and p62. Immunofluorescence analysis confirmed that WIPI1-depleted melanocytes accumulate more LC3 puncta when compared with control shRNA-expressing melanocytes (Fig. 6B), further suggesting that WIPI1-depleted cells accumulate vesicles expressing autophagy markers. Colocalization studies in MNT-1 cells revealed partial but not complete colocalization of LC3B and p62 with the stage I-II melanosome marker PMEL17 (Fig. 6C), suggesting that immature melanosomes contain both p62 and LC3-II (see Fig. 10). In contrast, the late stage melanosome marker TYRP1 colocalized with LC3 but did not colocalize with p62, suggesting that p62 is a marker of early but not late stage melanosomes (Fig. 6C and see Fig. 10). Immunofluorescence analysis revealed that WIPI1-depleted cells did not accumulate either the early endosome marker EEA1 (47) or the late endosome/lysosome marker LAMP2 (48) (supplemental Fig. S1), suggesting that the atypical vesicles that accumulate do not express these endosomal markers. Coupled together, our studies indicate that WIPI1-deficient melanocytic cells accumulate stage I-II melanosomes that contain both LC3 and p62.
FIGURE 5.
WIPI1-depleted cells accumulate early stage melanosomes and atypical endosomes. A, human deeply pigmented melanocytes expressing control or WIPI1 shRNAs were fixed and imaged by TEM. Scale bar, 2 μm. The lower panel demonstrates classic stage I–IV melanosomes and atypical endosome-like vesicles that accumulate in WIPI1 shRNA-expressing cells. Scale bar, 0.1 μm. B, quantification of stage I–IV melanosomes in control and WIPI1 shRNA-expressing melanocytes. Data shown are mean ± S.D. (n = 10). **, p < 0.01 using a Student's t test versus sh control. C, photographs were taken of cell pellets from shRNA-expressing melanocytes to capture the impact of each shRNA on pigment production. Con, control.
FIGURE 6.
WIPI1-depleted cells accumulate LC3 positive vesicles. A, human deeply pigmented melanocytes expressing control or WIPI1 shRNA (green cells) were stained with anti-LC3B (red) and imaged by confocal microscopy. Flattened three-dimensional reconstruction images are shown. Scale bar, 10 μm. Left, quantification of cytosolic LC3B signal per area in melanocytes expressing control or WIPI1 shRNA. Data shown are normalized to control shRNA (mean ± S.D.; n = 10). **, Student's t test p value <0.01. B, melanocytes used above were subjected to immunoblot with anti-LC3B, anti-p62 (SQSTM1), and anti-actin. C, MNT-1 cells stained with indicated antibodies were imaged by confocal microscope. Flattened 3D-projected images are shown. Right, LC3B (red) was stained in combination either with PMEL17 or with TYRP1 (green) in MNT-1 cells. Left, p62 (green) was stained in combination either with PMEL17 or with TYRP1 (red). Each experiment was performed three times in triplicate. Scale bar, 10 μm.
FIGURE 10.
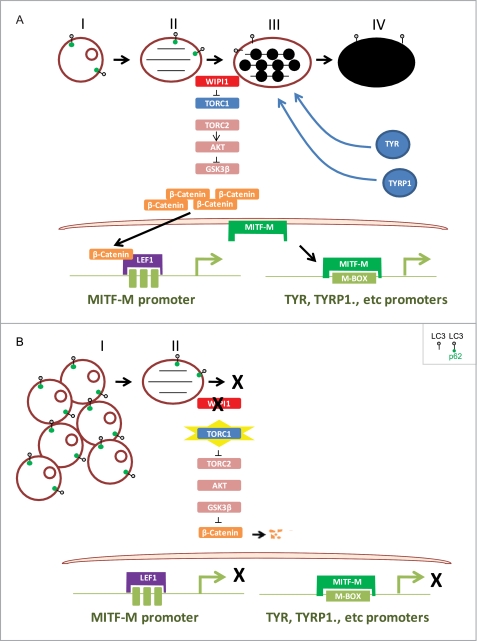
WIPI1 coordinates melanogenic protein transcription and melanosome maturation. A, WIPI1 regulates the maturation of stage III-IV melanosomes from early stage melanosomes by repressing TORC1 activity, which keeps the default TORC2 and AKT activity to inhibit GSK3β, resulting in β-Catenin stabilization and activated transcription of MITF-M and MITF-M target genes. B, in the absence of WIPI1, melanosome maturation is inhibited, and stage I melanosomes accumulate. TORC1 is no longer suppressed by WIPI1, leading to TORC2 and subsequent AKT inactivation, which causes the activation of GSK3β and degradation of β-Catenin. Without β-Catenin, MITF-M and MITF-M target gene expression are suppressed.
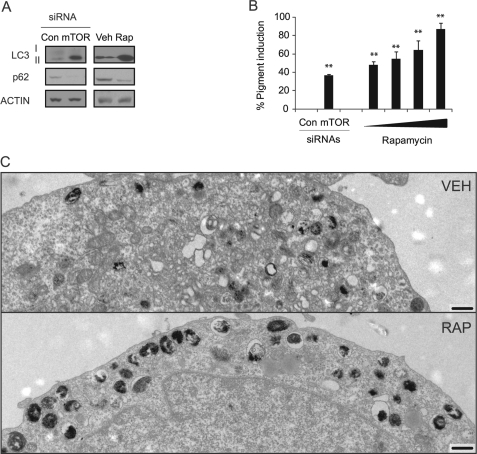
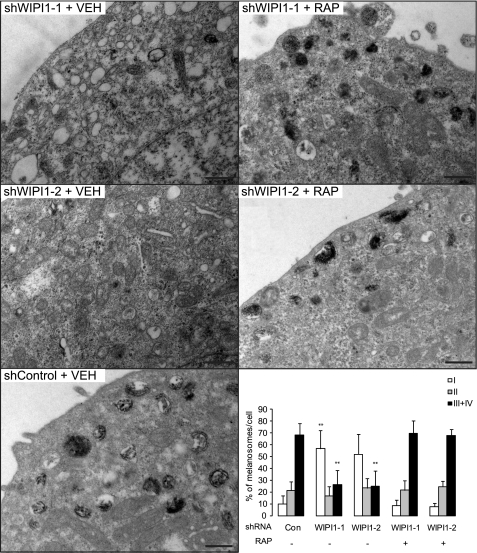
To further verify that WIPI1 regulates melanosome maturation by repressing MTOR signaling, we examined the consequences of pharmacologic MTOR suppression on melanosome maturation. As low doses of rapamycin suppressed melanocyte growth, we used MNT-1 cells for these studies. Rapamycin or MTOR siRNA treatment increased LC3-II accumulation but inhibited p62 accumulation (Fig. 7A), consistent with other studies that demonstrate that rapamycin increases both the number of autophagosomes and autophagosome turnover (26). MTOR inhibition also increased overall levels of pigment production (Fig. 7B), consistent with a role for MTOR signaling in repressing melanogenesis. Electron microscopy analysis revealed that rapamycin-treated cells accumulated mature melanosomes (stage III-IV) (Fig. 7C) but did not accumulate conventional autophagosomes (Fig. 7C) or stage I-II melanosomes, consistent with the lack of p62 accumulation (Fig. 7A). Rapamycin treatment also induced the accumulation of mature melanosomes in WIPI1-depleted cells (Fig. 8), suggesting that WIPI1 controls both stage I-II melanosome maturation and MITF target gene transcription by modulating MTOR signaling.
FIGURE 7.
TORC1 inhibition stimulates melanosome maturation and pigment production. A, MNT-1 cells transfected with MTOR siRNA or treated with rapamycin (100 nm for 5 days) were lysed and subjected to immunoblot analysis using LC3B, p62-SQSTM1, and actin antibodies. B, MNT-1 cells were either transfected with control (Con) or MTOR siRNA or treated with 0, 0.1, 1, 10, or 100 nm rapamycin for 5 days. Melanin accumulation was measured using a spectrophotometry-based melanin quantitation assay. Data shown are mean ± S.D. (n = 5). C, MNT-1 cells were treated with either vehicle (Veh) or 100 nm rapamycin (Rap) for 5 days, fixed, and imaged by TEM. Scale bar, 2 μm. Each experiment was performed three times in triplicate.
FIGURE 8.
TORC1 inhibition rescues melanosome formation defect caused by WIPI1 depletion. MNT-1 melanoma cells expressing WIPI1 or control shRNA were incubated in the presence of vehicle (VEH) or 100 nm rapamycin (RAP) for 5 days, fixed, and imaged by TEM as indicated in the top left of each panel. Scale bar, 0.5 μm. Stage I–IV melanosomes were quantified (right lower panel). Data shown are mean ± S.D. (n = 10). **, p < 0.01 using a Student's t test versus sh control (Con) + vehicle.
TORC1 Signaling Has a Distinct Function in Controlling Melanogenesis
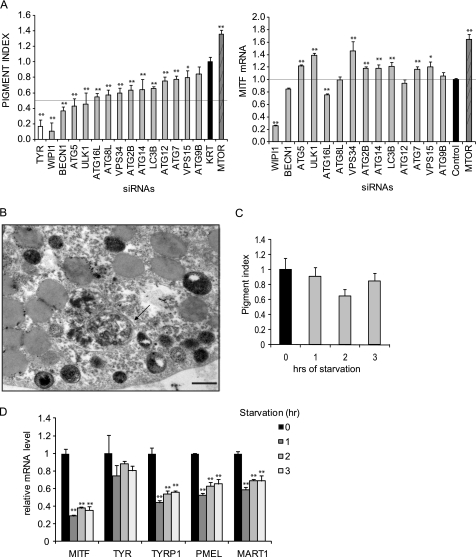
Rapamycin-treated MNT-1 cells accumulated mature melanosomes but not autophagosomes, suggesting that TORC1 may regulate melanosome biogenesis independently of its role in autophagy. To investigate this further, we first examined whether other autophagy proteins regulate melanogenic enzyme transcription in a fashion similar to that observed for WIPI1. Although depletion of many autophagy regulators marginally controlled melanin accumulation as measured by our spectrophotometric assay (Fig. 9A), only depletion of WIPI1, BECN1, or ATG5 inhibited melanin accumulation greater than 2-fold. Moreover, only depletion of WIPI1, MTOR, and BECN1 had any impacts on MITF mRNA accumulation, suggesting that WIPI1 controls melanogenesis via a mechanism distinct from other autophagy genes. To determine whether autophagy induction was sufficient to induce melanogenesis, we examined whether starvation was sufficient to induce melanin accumulation. Although starvation was sufficient to induce autophagosome formation (Fig. 9B), it did not stimulate melanin accumulation (Fig. 9C) and resulted in the decreased accumulation of MITF mRNA (Fig. 9D). Taken together, these results suggest that TORC1 signaling modulates melanosome biogenesis via a mechanism that is distinct from conventional autophagy.
FIGURE 9.
Starvation-induced autophagy does not stimulate melanogenesis. A, left, MNT-1 melanoma cells were transfected with the indicated siRNA pooled from three different individual siRNAs targeting the same gene. Seven days after transfection, relative melanin accumulation was measured spectrophotometrically as described. Data shown are mean ± S.D. (n = 5) from a representative experiment repeated three times. *, p < 0.05; **, p < 0.01 using a Student's t test versus si Keratin-7 (KRT) control. Right, MNT-1 cells were transfected with the indicated siRNA pools. 72 h after transfection, cell lysates were subjected to RT-qPCR to detect the relative MITF mRNA level. Data shown are mean ± S.D. (n = 3) *, p < 0.05; **, p < 0.01 using a Student's t test versus si control. Each experiment was performed three times in triplicate. B, MNT-1 cells were starved for 3 h as described, fixed, and subjected to TEM. The arrow indicates an autophagosome. Scale bar, 0.5 μm. C, MNT-1 melanoma cells were starved for 1, 2, or 3 h as described. Relative pigment accumulation was measured using a spectrophotometric assay as described. Data shown are mean ± S.D. (n = 5). Each experiment was performed three times. A Student's t test was performed, but there was no significant difference between the non-starved cells and the cells starved for 1–3 h. D, MNT-1 melanoma cells were starved for 1, 2, or 3 h as described. Cell lysates were subjected to RT-qPCR to detect relative MITF mRNA levels. *, p < 0.05; **, p < 0.01 using a Student's t test versus non-starved cells. Data shown are mean ± S.D. (n = 3) from an experiment repeated three times in triplicate.
DISCUSSION
WIPI1 Controls Transcription of Melanogenic Enzymes
WIPI1 depletion inhibited the transcription of MITF and MITF-M target genes. Our loss-of-function studies indicated that WIPI1 normally represses TORC1, leading to TORC2 activation, β-Catenin accumulation, and increased MITF transcription (Fig. 10). These findings are consistent with other studies demonstrating that rapamycin treatment stimulates melanogenic enzyme transcription (35, 36) and that PI3K inhibitors stimulates melanogenesis (49, 50). Our results are also consistent with previous studies that show that GSK3β inhibition leads to increased melanogenesis via a mechanism that involves increased β-Catenin accumulation (45). β-Catenin regulates the expression of MITF in both the hair follicle and retinal pigment epithelium (44). MITF-luciferase reporter constructs that contain mutations in the LEF1 binding site have a 10-fold decrease in promoter activity (51), indicating that β-Catenin/LEF1 is dominant among other transcription factors that regulate the MITF promoter. Although our studies suggest that WIPI1 regulates β-Catenin via TORC2, recently published studies have determined that autophagy can also directly regulate Wnt signaling (52). Future studies will determine the mechanism by which WIPI1 regulates β-Catenin in pigmented cells.
Interruption of vesicle trafficking pathways in other contexts can also inhibit gene transcription. Disruption of the ESCRT pathway, a pathway that regulates multivesicular body biogenesis, can regulate gene transcription (53). The ESCRT protein TSG101 is a transcriptional activator of steroid hormone receptors and their co-activators (54). ESCRT-III subunits can also function to suppress transcription (55). Our results demonstrated that autophagy signals, like signals generated from other vesicle trafficking pathways, could regulate enzyme transcription through a mechanism involving β-Catenin.
WIPI1 Regulates Transcription of Melanogenic Enzymes and Melanosome Maturation
Our results revealed that WIPI1 controls both melanosome maturation and the transcription of melanogenic enzymes via a mechanism involving TORC1 and β-Catenin (Fig. 3). As WIPI1-depleted cells accumulated p70S6K and phospho-MTOR, the normal role of WIPI1 is to inhibit TORC1 signaling. WIPI1-depleted cells accumulated a large number of stage I melanosome-like vesicles (Fig. 5, B and C) and LC3-II and p62 (Fig. 6B), consistent with a role for TORC1 activation in repressing melanosome maturation. Inhibition of TORC1 signaling by rapamycin induced melanosome maturation even in the context of WIPI1 depletion, suggesting that WIPI1 controls melanogenesis by modulating TORC1. WIPI1-depleted cells also accumulated structures that resemble endosomes with lumenal fibrillar material (Fig. 5, A–C). Immunofluorescence analysis did not reveal the accumulation of the early endosome marker EAA1 or the late endosomal marker LAMP2 in these cells (data not shown), making the origin of these vesicles unclear. Nevertheless, our results do indicate that WIPI1 regulates the biogenesis of melanosomes, organelles that are endosomally derived (34).
Our results are consistent with a model where WIPI1 inhibits TORC1 signaling (Fig. 10). Inhibition of TORC1 signaling leads to the activation of TORC2 signaling, leading to increased transcription of melanogenic enzymes via a mechanism involving β-Catenin. The accumulated melanogenic enzymes are then incorporated into the developing melanosome. In the absence of WIPI1, TORC1 is active, and TORC2 is repressed, resulting in the inhibition of melanogenic enzyme transcription (Fig. 10). As the melanosome components are not transcribed, they cannot be incorporated into the melanosome. This results in the accumulation of stage I melanosome vesicles.
Other published studies are consistent with our observations that modulation of autophagy signaling leads to the arrest of melanosome maturation. The protein complex composed of FIG4-VAC14-FAB1 is a protein kinase-phosphatase complex that mediates the conversion between phosphatidylinositol 3-phosphate and phosphatidylinositol 3,5-bisphosphate in yeast and mammalian cells (56, 57). The yeast WIPI1 homologue ATG18 is known to associate with FAB1 (58) and regulate its activity in membrane recruitment (59). Mice that have mutations in two other genes that regulate endosome/autophagosome fusion processes (FIG4 and VAC14) exhibit vacuolar accumulation in neural tissue and dramatically decreased melanin and mature melanosome accumulation in the hair follicle (60, 61). It is currently unclear whether these mice possess defects in melanogenic enzyme transcription, which eventually lead to decreased melanosome accumulation.
Autophagy Regulators Control Maturation of Stage I Melanosome
WIPI1-deficient melanocytic cells accumulated small (0.4-μm) single membrane vesicles with intracellular vesicles within them as well as fibrin material that resemble stage I melanosomes (Fig. 5). These cells also accumulated the autophagy markers p62 and LC3-II (Fig. 6A). Immunofluorescence analysis revealed that the early melanosome marker PMEL17 partially colocalized with the autophagy markers LC3 and p62, whereas the late melanosome marker TYRP1 only partially colocalized with LC3 (Fig. 6C), indicating that LC3 is a marker for both early and late melanosomes. The yeast WIPI1 homologue ATG18 functions to retrieve LC3 from the phagophore. In melanocytic cells, depletion of WIPI1 resulted in the accumulation of stage I melanosome vesicles that contain LC3-II and also p62, suggesting that WIPI1 also regulates membrane retrieval from the melanosome, an LC3-containing vesicle. Consistent with these findings, pharmacologic activation of autophagy resulted in decreased accumulation of p62 (a protein present in early melanosomes), increased accumulation of LC3-II (a marker that is present in early and late melanosomes), and increased accumulation of stage III-IV melanosomes. Interestingly, our studies did not detect LC3-I high base-line levels in melanocytic cells. Others report variability in LC3-I and LC3-II levels from cell type to cell type (46). As the cells examined in our studies produce melanosomes, the abundance of LC3-II in these cells could simply be explained by the accumulation of melanosomes in these cells that also contain LC3-II.
Other data support a role for autophagy regulators in both endosome-based trafficking pathways and melanosome biogenesis. Distinct BECN1 subcomplexes regulate the formation of multivesicular bodies, late endosome vesicles (62–64). BECN1-haploinsufficient mice possess pigmentary defects, consistent with a role for autophagy regulators in the formation of the stage I melanosome in vivo (21). Depletion of ESCRT-I from melanocytic cells induces autophagosome accumulation, implicating a role for autophagy in the trafficking of the melanogenic enzyme TYRP1 (65). Deletion of VAC14, another protein that regulates the maturation of late endosomes, also impacts pigment accumulation in the mouse hair follicle (66). Neural tissue from VAC14 knock-out mice accumulates intracellular vacuoles that are stained with both late endosome and autophagy markers (61), suggesting that these cells also possess defects in melanosome biogenesis.
Autophagy Signaling Plays a Distinct Role in Melanosome Biogenesis
The studies presented here lead to the novel conclusion that WIPI1 regulates the maturation of stage I melanosomes and also represses MTOR, leading to the increased transcription of melanogenic enzymes that are required for the formation of the mature melanosome. Although WIPI1 depletion leads to the decreased transcription of several components of stage III-IV melanosomes, it had minimal impact on the expression of MART1. These results are consistent with previously published ChIP studies demonstrating that MITF exhibited more robust binding to the PMEL17 promoter compared with the MART1 promoter (67).
More importantly, our results help answer a central question raised from our RNAi screen (21): does autophagy control melanogenesis, or do genes that regulate autophagy also play a distinct role in regulating melanogenesis? Immunofluorescence colocalization studies revealed that melanosome markers and autophagy markers did not exhibit complete colocalization (Fig. 6C), giving the first hints that the melanosome may be a subset of cellular organelles that contain autophagy markers. Although depletion of many autophagy regulators had some impact on melanin accumulation (Fig. 9A), only depletion of WIPI1, BECN1, and ATG5 inhibited melanin accumulation at least 2-fold (Fig. 9A), consistent with a role for only a subset of autophagy genes in melanogenesis. Moreover, only WIPI1 depletion inhibited both the accumulation of melanin and the accumulation of MITF mRNA, consistent with a specific role for WIPI1 in modulating MTOR. Consistent with a specific role for MTOR signaling in melanogenesis, rapamycin treatment induced melanosome accumulation but not autophagosome accumulation in MNT-1 cells (Fig. 7C). In contrast, starvation, a known inducer of autophagosome formation, was sufficient to induce autophagosome induction (Fig. 9C) but not melanin accumulation (Fig. 9D) or MITF mRNA accumulation (Fig. 9E). Taken together, these results are consistent with a role for distinct components of the autophagy signaling machinery in melanogenesis and help explain previously published transgenic mouse studies that revealed that although BECN1-haploinsufficient mice possess pigmentary defects (21) other autophagy-deficient mice do not (68).
Although our studies revealed that WIPI1 represses MTOR signaling, which activates melanogenesis, it is currently unclear how WIPI1 regulates MTOR signaling. Immunoprecipitation studies from our laboratory (data not shown) as well as published studies have revealed that WIPI1 does not interact with MTOR directly in melanocytic cells or other mammalian cell lines (30). Although rapamycin treatment consistently stimulated MITF and TYR transcription, depletion of different components of the TORC1 complex had differential effects on the accumulation of MITF and TYR mRNA. These results could be secondary to the differential impacts of each TORC1 component on MTOR catalytic activity or the differential efficacies of the siRNAs used in our experiments.
Recent studies have defined a connection between endosomal trafficking and MTOR signaling. In human cells, TORC1 associates with the late endosomes (69). Disruption of endosomal maturation leads to an inhibition of TORC1 (69), consistent with a model in which the accumulation of early endosomes/stage I melanosomes should inhibit TORC1 and lead to increased transcription of melanogenic enzymes. Additionally, it was reported that in TORC1-active cells p70S6K regulates the activity of GSK3β (70), further supporting the connection of TORC1 downstream signaling and β-Catenin accumulation. Finally, a recent study determined that components of the Wnt pathway and autophagy machinery colocalize (52), documenting another connection between autophagy signaling and MITF transcription. Future studies are required to determine which MTOR complex is modulated by WIPI1 and delineate how MTOR modulation leads to stabilization of β-Catenin.
In summary, our results define a novel role for autophagy signaling in coordinating the transcription of vesicle cargo with the maturation of the early melanosome. This regulation is distinct from the regulation of autophagosome formation as rapamycin-treated melanocytic cells accumulate melanosomes but not autophagosomes, whereas starved cells accumulate autophagosomes but not melanin.
Acknowledgments
We thank Dr. Tatiana Krasieva (Laser Microbeam and Medical Program, Beckman Laser Institute, University of California, Irvine) for assistance in acquiring confocal images. We thank Charles E. Ribak and Zhi-yin Shan (Electronic Microscope Core Facility, University of California, Irvine) for assistance with TEM imaging. We thank Shigeki Shibahara for kindly providing us MITF-M and TYR core promoter-luciferase reporter constructs. We thank Dr. Marian Waterman for kindly providing us superTOPflash reporter, β-Catenin, and LEF1 overexpression constructs. We thank Michael A. White and Bogi Andersen for assistance with preparing the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants 1RO3AR057150 and 1K08AR056001 (to A. K. G.) and by Biomedical Technology Resource Grant P41-RR01192 (for the microscopy studies at the University of California, Irvine. This work was also supported by a Dermatology Foundation research grant.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- MITF-M
- melanocyte-specific microphthalmia-associated transcription factor
- WIPI1
- WD repeat domain, phosphoinositide-interacting 1
- MTOR
- mammalian target of rapamycin
- TORC
- target of rapamycin complex
- GSK3β
- glycogen synthase kinase 3β
- MITF
- microphthalmia transcription factor
- BIO
- 2′Z,3′E)-6-bromoindirubin-3′-oxime
- qPCR
- quantitative PCR
- TEM
- transmission electron microscopy
- ChIP
- chromatin immunoprecipitation
- TYR
- tyrosinase
- ESCRT
- endosomal sorting complex required for transport
- TYRP1
- tyrosinase-related protein 1
- GBL
- G-protein β-subunit-like.
REFERENCES
- 1. Slominski A., Tobin D. J., Shibahara S., Wortsman J. (2004) Physiol. Rev. 84, 1155–1228 [DOI] [PubMed] [Google Scholar]
- 2. Hu D. N., Simon J. D., Sarna T. (2008) Photochem. Photobiol. 84, 639–644 [DOI] [PubMed] [Google Scholar]
- 3. Tachibana M. (1999) Pigment Cell Res. 12, 344–354 [DOI] [PubMed] [Google Scholar]
- 4. Nicolaus B. J. (2005) Med. Hypotheses 65, 791–796 [DOI] [PubMed] [Google Scholar]
- 5. Double K. L. (2006) J. Neural. Transm. 113, 751–756 [DOI] [PubMed] [Google Scholar]
- 6. El-Mofty M. A., Esmat S. M., Abdel-Halim M. R. (2007) Dermatol. Clin. 25, 401–417, x [DOI] [PubMed] [Google Scholar]
- 7. Passeron T., Mantoux F., Ortonne J. P. (2005) Clin. Dermatol. 23, 56–67 [DOI] [PubMed] [Google Scholar]
- 8. Salopek T. G., Jimbow K. (1996) J. Investig. Dermatol. Symp. Proc. 1, 195–202 [PubMed] [Google Scholar]
- 9. Raposo G., Marks M. S. (2007) Nat. Rev. Mol. Cell Biol. 8, 786–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Theos A. C., Truschel S. T., Raposo G., Marks M. S. (2005) Pigment Cell Res. 18, 322–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hoashi T., Watabe H., Muller J., Yamaguchi Y., Vieira W. D., Hearing V. J. (2005) J. Biol. Chem. 280, 14006–14016 [DOI] [PubMed] [Google Scholar]
- 12. Hurbain I., Geerts W. J., Boudier T., Marco S., Verkleij A. J., Marks M. S., Raposo G. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 19726–19731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gautam R., Novak E. K., Tan J., Wakamatsu K., Ito S., Swank R. T. (2006) Traffic 7, 779–792 [DOI] [PubMed] [Google Scholar]
- 14. Hearing V. J. (2005) J. Dermatol. Sci. 37, 3–14 [DOI] [PubMed] [Google Scholar]
- 15. Wasmeier C., Hume A. N., Bolasco G., Seabra M. C. (2008) J. Cell Sci. 121, 3995–3999 [DOI] [PubMed] [Google Scholar]
- 16. Yamaguchi Y., Hearing V. J. (2009) Biofactors 35, 193–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cheli Y., Ohanna M., Ballotti R., Bertolotto C. (2010) Pigment Cell Melanoma Res. 23, 27–40 [DOI] [PubMed] [Google Scholar]
- 18. Steingrímsson E., Copeland N. G., Jenkins N. A. (2004) Annu. Rev. Genet. 38, 365–411 [DOI] [PubMed] [Google Scholar]
- 19. Costin G. E., Hearing V. J. (2007) FASEB J. 21, 976–994 [DOI] [PubMed] [Google Scholar]
- 20. Sturm R. A. (2009) Hum. Mol. Genet. 18, R9–17 [DOI] [PubMed] [Google Scholar]
- 21. Ganesan A. K., Ho H., Bodemann B., Petersen S., Aruri J., Koshy S., Richardson Z., Le L. Q., Krasieva T., Roth M. G., Farmer P., White M. A. (2008) PLoS Genet. 4, e1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jeffries T. R., Dove S. K., Michell R. H., Parker P. J. (2004) Mol. Biol. Cell 15, 2652–2663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Proikas-Cezanne T., Ruckerbauer S., Stierhof Y. D., Berg C., Nordheim A. (2007) FEBS Lett. 581, 3396–3404 [DOI] [PubMed] [Google Scholar]
- 24. Proikas-Cezanne T., Pfisterer S. G. (2009) Methods Enzymol. 452, 247–260 [DOI] [PubMed] [Google Scholar]
- 25. Reggiori F., Tucker K. A., Stromhaug P. E., Klionsky D. J. (2004) Dev. Cell 6, 79–90 [DOI] [PubMed] [Google Scholar]
- 26. Levine B., Kroemer G. (2008) Cell 132, 27–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dove S. K., Piper R. C., McEwen R. K., Yu J. W., King M. C., Hughes D. C., Thuring J., Holmes A. B., Cooke F. T., Michell R. H., Parker P. J., Lemmon M. A. (2004) EMBO J. 23, 1922–1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guan J., Stromhaug P. E., George M. D., Habibzadegah-Tari P., Bevan A., Dunn W. A., Jr., Klionsky D. J. (2001) Mol. Biol. Cell 12, 3821–3838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barth H., Meiling-Wesse K., Epple U. D., Thumm M. (2001) FEBS Lett. 508, 23–28 [DOI] [PubMed] [Google Scholar]
- 30. Behrends C., Sowa M. E., Gygi S. P., Harper J. W. (2010) Nature 466, 68–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Takeda K., Yasumoto K., Takada R., Takada S., Watanabe K., Udono T., Saito H., Takahashi K., Shibahara S. (2000) J. Biol. Chem. 275, 14013–14016 [DOI] [PubMed] [Google Scholar]
- 32. Shibata K., Muraosa Y., Tomita Y., Tagami H., Shibahara S. (1992) J. Biol. Chem. 267, 20584–20588 [PubMed] [Google Scholar]
- 33. Rabinovich A., Jin V. X., Rabinovich R., Xu X., Farnham P. J. (2008) Genome Res. 18, 1763–1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Raposo G., Tenza D., Murphy D. M., Berson J. F., Marks M. S. (2001) J. Cell Biol. 152, 809–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ohguchi K., Banno Y., Nakagawa Y., Akao Y., Nozawa Y. (2005) J. Cell. Physiol. 205, 444–451 [DOI] [PubMed] [Google Scholar]
- 36. Buscà R., Bertolotto C., Ortonne J. P., Ballotti R. (1996) J. Biol. Chem. 271, 31824–31830 [DOI] [PubMed] [Google Scholar]
- 37. Chiang G. G., Abraham R. T. (2005) J. Biol. Chem. 280, 25485–25490 [DOI] [PubMed] [Google Scholar]
- 38. Navé B. T., Ouwens M., Withers D. J., Alessi D. R., Shepherd P. R. (1999) Biochem. J. 344, 427–431 [PMC free article] [PubMed] [Google Scholar]
- 39. Dibble C. C., Asara J. M., Manning B. D. (2009) Mol. Cell. Biol. 29, 5657–5670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sarbassov D. D., Guertin D. A., Ali S. M., Sabatini D. M. (2005) Science 307, 1098–1101 [DOI] [PubMed] [Google Scholar]
- 41. Hajduch E., Litherland G. J., Hundal H. S. (2001) FEBS Lett. 492, 199–203 [DOI] [PubMed] [Google Scholar]
- 42. Cross D. A., Alessi D. R., Cohen P., Andjelkovich M., Hemmings B. A. (1995) Nature 378, 785–789 [DOI] [PubMed] [Google Scholar]
- 43. Verheyen E. M., Gottardi C. J. (2010) Dev. Dyn. 239, 34–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Larue L., Kumasaka M., Goding C. R. (2003) Pigment Cell Res. 16, 312–317 [DOI] [PubMed] [Google Scholar]
- 45. Bellei B., Flori E., Izzo E., Maresca V., Picardo M. (2008) Cell. Signal. 20, 1750–1761 [DOI] [PubMed] [Google Scholar]
- 46. Klionsky D. J., Abeliovich H., Agostinis P., Agrawal D. K., Aliev G., Askew D. S., Baba M., Baehrecke E. H., Bahr B. A., Ballabio A., Bamber B. A., Bassham D. C., Bergamini E., Bi X., Biard-Piechaczyk M., Blum J. S., Bredesen D. E., Brodsky J. L., Brumell J. H., Brunk U. T., Bursch W., Camougrand N., Cebollero E., Cecconi F., Chen Y., Chin L. S., Choi A., Chu C. T., Chung J., Clarke P. G., Clark R. S., Clarke S. G., Clavé C., Cleveland J. L., Codogno P., Colombo M. I., Coto-Montes A., Cregg J. M., Cuervo A. M., Debnath J., Demarchi F., Dennis P. B., Dennis P. A., Deretic V., Devenish R. J., Di Sano F., Dice J. F., Difiglia M., Dinesh-Kumar S., Distelhorst C. W., Djavaheri-Mergny M., Dorsey F. C., Dröge W., Dron M., Dunn W. A., Jr., Duszenko M, Eissa N. T., Elazar Z., Esclatine A., Eskelinen E. L., Fésüs L., Finley K. D., Fuentes J. M., Fueyo J., Fujisaki K., Galliot B., Gao F. B., Gewirtz D. A., Gibson S. B., Gohla A., Goldberg A. L., Gonzalez R., González-Estévez C., Gorski S., Gottlieb R. A., Häussinger D., He Y. W., Heidenreich K., Hill J. A., Høyer-Hansen M., Hu X., Huang W. P., Iwasaki A., Jäättelä M., Jackson W. T., Jiang X., Jin S., Johansen T., Jung J. U., Kadowaki M., Kang C., Kelekar A., Kessel D. H., Kiel J. A., Kim H. P., Kimchi A., Kinsella T. J., Kiselyov K., Kitamoto K., Knecht E., Komatsu M., Kominami E., Kondo S., Kovács A. L., Kroemer G., Kuan C. Y., Kumar R., Kundu M., Landry J., Laporte M., Le W., Lei H. Y., Lenardo M. J., Levine B., Lieberman A., Lim K. L., Lin F. C., Liou W., Liu L. F., Lopez-Berestein G., López-Otín C., Lu B., Macleod K. F., Malorni W., Martinet W., Matsuoka K., Mautner J., Meijer A. J., Meléndez A., Michels P., Miotto G., Mistiaen W. P., Mizushima N., Mograbi B., Monastyrska I., Moore M. N., Moreira P. I., Moriyasu Y., Motyl T., Münz C., Murphy L. O., Naqvi N. I., Neufeld T. P., Nishino I., Nixon R. A., Noda T., Nürnberg B., Ogawa M., Oleinick N. L., Olsen L. J., Ozpolat B., Paglin S., Palmer G. E., Papassideri I., Parkes M., Perlmutter D. H., Perry G., Piacentini M., Pinkas-Kramarski R., Prescott M., Proikas-Cezanne T., Raben N., Rami A., Reggiori F., Rohrer B., Rubinsztein D. C., Ryan K. M., Sadoshima J., Sakagami H., Sakai Y., Sandri M., Sasakawa C., Sass M., Schneider C., Seglen P. O., Seleverstov O., Settleman J., Shacka J. J., Shapiro I. M., Sibirny A., Silva-Zacarin E. C., Simon H. U., Simone C., Simonsen A., Smith M. A., Spanel-Borowski K., Srinivas V., Steeves M., Stenmark H., Stromhaug P. E., Subauste C. S., Sugimoto S., Sulzer D., Suzuki T., Swanson M. S, Tabas I., Takeshita F., Talbot N. J., Tallóczy Z., Tanaka K., Tanaka K., Tanida I., Taylor G. S., Taylor J. P., Terman A., Tettamanti G., Thompson C. B., Thumm M., Tolkovsky A. M., Tooze S. A., Truant R., Tumanovska L. V., Uchiyama Y., Ueno T., Uzcátegui N. L., van der Klei I., Vaquero E. C., Vellai T., Vogel M. W., Wang H. G., Webster P., Wiley J. W., Xi Z., Xiao G., Yahalom J., Yang J. M., Yap G., Yin X. M., Yoshimori T., Yu L., Yue Z,., Yuzaki M., Zabirnyk O., Zheng X., Zhu X., Deter R. L. (2008) Autophagy 4, 151–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mills I. G., Jones A. T., Clague M. J. (1999) Mol. Membr. Biol. 16, 73–79 [DOI] [PubMed] [Google Scholar]
- 48. Eskelinen E. L. (2006) Mol. Aspects Med. 27, 495–502 [DOI] [PubMed] [Google Scholar]
- 49. Oka M., Nagai H., Ando H., Fukunaga M., Matsumura M., Araki K., Ogawa W., Miki T., Sakaue M., Tsukamoto K., Konishi H., Kikkawa U., Ichihashi M. (2000) J. Invest. Dermatol. 115, 699–703 [DOI] [PubMed] [Google Scholar]
- 50. Kim J. H., Baek S. H., Kim D. H., Choi T. Y., Yoon T. J., Hwang J. S., Kim M. R., Kwon H. J., Lee C. H. (2008) J. Invest. Dermatol. 128, 1227–1235 [DOI] [PubMed] [Google Scholar]
- 51. Saito H., Yasumoto K., Takeda K., Takahashi K., Fukuzaki A., Orikasa S., Shibahara S. (2002) J. Biol. Chem. 277, 28787–28794 [DOI] [PubMed] [Google Scholar]
- 52. Gao C., Cao W., Bao L., Zuo W., Xie G., Cai T., Fu W., Zhang J., Wu W., Zhang X., Chen Y. G. (2010) Nat. Cell Biol. 12, 781–790 [DOI] [PubMed] [Google Scholar]
- 53. Roxrud I., Stenmark H., Malerød L. (2010) Biol. Cell 102, 293–318 [DOI] [PubMed] [Google Scholar]
- 54. Burgdorf S., Leister P., Scheidtmann K. H. (2004) J. Biol. Chem. 279, 17524–17534 [DOI] [PubMed] [Google Scholar]
- 55. Weiss P., Huppert S., Kölling R. (2009) Biochem. J. 424, 89–97 [DOI] [PubMed] [Google Scholar]
- 56. Michell R. H., Heath V. L., Lemmon M. A., Dove S. K. (2006) Trends Biochem. Sci. 31, 52–63 [DOI] [PubMed] [Google Scholar]
- 57. Sbrissa D., Ikonomov O. C., Fu Z., Ijuin T., Gruenberg J., Takenawa T., Shisheva A. (2007) J. Biol. Chem. 282, 23878–23891 [DOI] [PubMed] [Google Scholar]
- 58. Georgakopoulos T., Koutroubas G., Vakonakis I., Tzermia M., Prokova V., Voutsina A., Alexandraki D. (2001) Yeast 18, 1155–1171 [DOI] [PubMed] [Google Scholar]
- 59. Efe J. A., Botelho R. J., Emr S. D. (2007) Mol. Biol. Cell 18, 4232–4244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chow C. Y., Zhang Y., Dowling J. J., Jin N., Adamska M., Shiga K., Szigeti K., Shy M. E., Li J., Zhang X., Lupski J. R., Weisman L. S., Meisler M. H. (2007) Nature 448, 68–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ferguson C. J., Lenk G. M., Meisler M. H. (2010) Autophagy 6, 170–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Matsunaga K., Noda T., Yoshimori T. (2009) Autophagy 5, 876–877 [DOI] [PubMed] [Google Scholar]
- 63. Matsunaga K., Saitoh T., Tabata K., Omori H., Satoh T., Kurotori N., Maejima I., Shirahama-Noda K., Ichimura T., Isobe T., Akira S., Noda T., Yoshimori T. (2009) Nat. Cell Biol. 11, 385–396 [DOI] [PubMed] [Google Scholar]
- 64. Zhong Y., Wang Q. J., Li X., Yan Y., Backer J. M., Chait B. T., Heintz N., Yue Z. (2009) Nat. Cell Biol. 11, 468–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Truschel S. T., Simoes S., Setty S. R., Harper D. C., Tenza D., Thomas P. C., Herman K. E., Sackett S. D., Cowan D. C., Theos A. C., Raposo G., Marks M. S. (2009) Traffic 10, 1318–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jin N., Chow C. Y., Liu L., Zolov S. N., Bronson R., Davisson M., Petersen J. L., Zhang Y., Park S., Duex J. E., Goldowitz D., Meisler M. H., Weisman L. S. (2008) EMBO J. 27, 3221–3234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Du J., Miller A. J., Widlund H. R., Horstmann M. A., Ramaswamy S., Fisher D. E. (2003) Am. J. Pathol. 163, 333–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Komatsu M., Waguri S., Ueno T., Iwata J., Murata S., Tanida I., Ezaki J., Mizushima N., Ohsumi Y., Uchiyama Y., Kominami E., Tanaka K., Chiba T. (2005) J. Cell Biol. 169, 425–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Flinn R. J., Yan Y., Goswami S., Parker P. J., Backer J. M. (2010) Mol. Biol. Cell 21, 833–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhang H. H., Lipovsky A. I., Dibble C. C., Sahin M., Manning B. D. (2006) Mol. Cell 24, 185–197 [DOI] [PMC free article] [PubMed] [Google Scholar]