Abstract
Cellular oxygen consumption is a determinant of intracellular oxygen levels. Because of the high demand of mitochondrial respiration during insulin secretion, pancreatic β-cells consume large amounts of oxygen in a short time period. We examined the effect of insulin secretion on cellular oxygen tension in vitro. We confirmed that Western blotting of pimonidazole adduct was more sensitive than immunostaining for detection of cellular hypoxia in vitro and in vivo. The islets of the diabetic mice but not those of normal mice were hypoxic, especially when a high dose of glucose was loaded. In MIN6 cells, a pancreatic β-cell line, pimonidazole adduct formation and stabilization of hypoxia-inducible factor-1α (HIF-1α) were detected under mildly hypoxic conditions. Inhibition of respiration rescued the cells from becoming hypoxic. Glucose stimulation decreased cellular oxygen levels in parallel with increased insulin secretion and mitochondrial respiration. The cellular hypoxia by glucose stimulation was also observed in the isolated islets from mice. The MIN6 cells overexpressing HIF-1α were resistant to becoming hypoxic after glucose stimulation. Thus, glucose-stimulated β-cells can become hypoxic by oxygen consumption, especially when the oxygen supply is impaired.
Keywords: Diabetes, Glucose, Hypoxia, Insulin Secretion, Respiration
Introduction
Hypoxia is a common challenge for living organisms that depend on oxygen (1). Although each organism has evolved to adapt to this challenge, hypoxia is closely related to various pathological conditions (2). Cellular oxygen tension is determined by the balance between supply and demand of oxygen. In mammals, oxygen is delivered by the circulatory system and is consumed by the cells, especially by oxidative phosphorylation in mitochondria. Although hypoxia due to poor oxygen supply by factors such as impaired perfusion has been intensively studied, hypoxia as a consequence of an imbalance between oxygen demand and supply has not been well recognized.
Cellular oxygen consumption is one of the determinants of intracellular oxygen levels (3, 4). Hagen et al. (3) reported that inhibitors of mitochondrial respiration, such as nitric oxide, prevent the stabilization of hypoxia-inducible factor (HIF)2 during hypoxia. HIF-1α is a transcription factor that regulates gene expression under hypoxic conditions (5). HIF-1α protein is constitutively synthesized but degraded under normoxic conditions by the ubiquitin/proteasome system, whereas it is stabilized under hypoxic conditions because of the requirement for molecular oxygen in the degradation machinery. Once stabilized, HIF-1α binds to HIF-1β to form heterodimers and acts as a transcription factor. Thus, expression levels of HIF-1α are sensitive to intracellular oxygen levels. Inhibition of mitochondrial oxygen consumption increases intracellular oxygen levels, and consequently HIF degradation is maintained even under extracellular hypoxic conditions (3). In addition, Doege et al. (4) showed that at an intermediate oxygen concentration (3% O2), the formation of pimonidazole adduct can be detected, although pimonidazole is supposed to form adducts with cellular macromolecules only under severely hypoxic conditions (6). The presence of pimonidazole adducts was diminished by inhibition of mitochondrial respiration (4).
Under conditions of high oxygen demand, cells can become hypoxic due to high oxygen consumption (7–9). For example, neurosecretory cells require high mitochondrial activity, mostly due to the requirement of ATP to re-establish the resting membrane potential and to maintain intracellular Ca2+ equilibrium by ion pumps (7). In working skeletal muscle cells (8), in which increased cellular ATP demand is facilitated by mitochondrial biogenesis, elevated oxygen demand stimulates the expression of a cohort of hypoxia-inducible genes (9).
The insulin secretion from pancreatic β-cells is critical for the homeostasis of systemic glucose metabolism. How glucose triggers insulin release from pancreatic β-cells has been intensively studied (10–12). Glucose is metabolized in the cytoplasm via the glycolytic pathway to pyruvate, which is rapidly degraded in the mitochondrion to produce ATP by oxidative phosphorylation. A rise in the cytoplasmic ATP/ADP ratio triggers the closure of the ATP-sensitive potassium (KATP) channels, which leads to depolarization of the plasma membrane and the opening of voltage-sensitive Ca2+ channels. Consequently, Ca2+ influx into a cell triggers Ca2+-dependent exocytosis, resulting in insulin release from β-cells. Thus, β-cells require large amounts of oxygen to produce ATP for the insulin secretion process. Insulin secretion and mitochondrial functions are tightly linked because insulin secretion is impaired in patients with mitochondrial DNA mutations as well as in cells in which mitochondrial DNA is artificially removed (13).
We undertook a series of studies to examine the effect of insulin secretion on cellular oxygen tension. Considering the formation of pimonidazole adducts and also the elevated expression levels of HIF-1α proteins, β-cells can be hypoxic after high glucose loads due to high oxygen consumption under mildly hypoxic or at physiological oxygen tension. Response to the cellular hypoxia might play a role in β-cell function.
EXPERIMENTAL PROCEDURES
Reagents
Antimycin A, rotenone, carbonyl cyanide 3-chlorophenylhydrazone (CCCP), and bovine serum albumin (BSA) were purchased from Sigma-Aldrich.
Cells and Cell Culture
The mouse insulinoma cell line, MIN6, was a gift from Dr. J. Miyazaki (Osaka University) (14). MIN6 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) containing 450 mg/dl glucose, 10% fetal bovine serum, penicillin, and streptomycin, 50 μm β-mercaptoethanol at 37 °C under 5% CO2, 95% air conditions. A human pancreatic cancer cell line, PANC-1, was obtained from ATCC. PANC-1 cells were cultured in DMEM, containing 450 mg/dl glucose, 10% fetal bovine serum, penicillin, and streptomycin. Hypoxic culture was performed in a Multigas incubator (ASTEC, Fukuoka, Japan) or an Invivo2400 hypoxia workstation (Ruskinn, Leeds, UK). Anoxia (0% O2) was achieved by the AnaeroPack system (Mitsubishi Gas Chemical, Tokyo, Japan).
Measurement of Oxygen Consumption
MIN6 cells were trypsinized to prepare single cells and preincubated with Krebs-Ringer-bicarbonate HEPES (KRBH) buffer containing 4 mg/dl glucose for 1 h. The cells (5 × 106 cells) and the medium were added to a small closed chamber and incubated at 37 °C for 30 min. Oxygen tension was measured using a Clark-type oxygen electrode system (model 203, Instech Laboratories, Plymouth Meeting, PA). After injection of 400 mg/dl glucose, MIN6 cells were incubated for 30 min. The oxygen tension was constantly recorded before and after glucose stimulation. Oxygen consumption was calculated as previously described (15).
Measurement of Insulin Concentration
Insulin secretion into the culture medium from MIN6 cells was measured by Mesacup Insulin ELISA (MBL, Nagoya, Japan) according to the manufacturer's protocol. For details, see the supplemental material.
Immunocytochemistry
Immunocytochemistry was performed as described previously (16). The details are described in the supplemental material. The fluorescein isothiocyanate (FITC)-labeled Hypoxyprobe-1 monoclonal antibody (mAb1) (NPI, Belmont, MA) was used.
Flow Cytometric Analysis
The cells were treated with pimonidazole in the same manner as in the immunocytochemistry assays. The cells were dispersed with trypsin/EDTA for 15 min at 37 °C. Cells were washed with phosphate-buffered saline (PBS) once, fixed with 2% formalin overnight, and washed in PBS twice. The cells were permeabilized with 0.5% Triton, 0.5% BSA in PBS for 10 min at room temperature. The cells were then incubated with the Hypoxyprobe-1 mAb1 (1:1000 in 0.5% BSA in PBS) in the dark for 30 min, washed once, passed through a 40-μm filter, and subjected to flow cytometric analysis. Stained cells were analyzed using a FACSCaliber apparatus (BD Biosciences) with FlowJo software (Tomy Digital Biology, Tokyo, Japan). The data were reported as percentage of maximum, which is the number of cells in each bin divided by the number of cells in the bin that contains the largest number of cells.
Western Blotting Analysis
Western blotting analysis was done as described previously (17) (see the supplemental material). The primary antibody against HIF-1α (NB100-105, NB100-479) was purchased from Novus Biologicals (Littleton, CO), HIF-1α (clone 54) from BD, β-actin (A5060) from Sigma, HIF-1β (clone 29) from BD Biosciences, and pimonidazole (Hypoxyprobe-1 Mab1) from NPI (Belmont, MA).
Plasmids
The construction of pMX-HIF-1α CA5 is described in the supplemental material. Retroviral transfection was carried out as described previously (18). After selection with puromycin for 1 week, the cells were subjected to further experiments.
Animal Studies
The animal studies were approved by the animal care committee of Osaka Medical Center for Cancer and Cardiovascular Diseases. C57BL6/J mice and leptin-deficient (ob/ob) male mice were purchased from Charles River Laboratories Japan Inc. (Yokohama, Japan). KK-Ay female mice were from Japan CREA (Tokyo, Japan). Mice were maintained on a 12-h light/12-h dark cycle with free access to water and normal chow and housed in specific pathogen-free barrier facilities. To detect tissue hypoxia, pimonidazole (60 mg/kg body weight) was intraperitoneally injected to the mice fed ad libitum. At the same time, glucose (2 g/kg body weight) or saline was intraperitoneally injected into both control and diabetic mice. After 2 h, the islets were isolated as described below, lysed, and subjected to further analysis. Pimonidazole adduct formation was evaluated by Western blotting. The image of the Western blotting film was captured by an image scanner, GT-9300UF (EPSON, Tokyo, Japan), and the intensity of the bands from 50 to 150 kDa was measured using Photoshop (Adobe Systems Inc., San Jose, CA). The pimonidazole/HIF-1β ratio was calculated to standardize the protein loading. The lysate of the MIN6 cells cultured at 15% oxygen tension for 3 h was included in each experiment and used as an internal control for standardization.
Pancreatic Islet Isolation Studies
Pancreatic islets of Langerhans were isolated by collagenase digestion as described previously (19). The isolated islets were cultured overnight in RPMI1640 (Invitrogen) medium containing 200 mg/dl glucose, 10% fetal bovine serum, 10 mm HEPES, 1 mm sodium pyruvate, penicillin, and streptomycin, at 37 °C under 5% CO2, 95% air. All islets were preincubated in KRBH buffer containing 40 mg/dl glucose for 30 min and divided into two groups. One group was maintained in KRBH buffer containing 40 mg/dl glucose and pimonidazole (10 μm) for 2 h, and the other was stimulated in KRBH buffer containing 400 mg/dl glucose and pimonidazole for 2 h. For immunocytochemistry, islets were embedded in collagen gel using Cell Matrix Type 1A (Nitta Gelatin, Osaka, Japan) and fixed with formalin for paraffin sections. The isolated islets were divided into three groups by their diameters: small (<50 μm), intermediate (50–100 μm), and large (>100 μm). The pimonidazole staining patterns were divided into three groups: positive staining, where islets were stained overall by pimonidazole; central staining, where the central region, occupying less than 50% of total area, was stained by pimonidazole; and negative staining, where no pimonidazole staining was observed. These samples were also subjected to Western blotting.
Statistical Analysis
Statistical analysis was performed with GraphPad Prism 4 (GraphPad Software, La Jolla, CA). The statistical significance of the results was tested with the unpaired t test. A value of p < 0.05 was considered to be statistically significant.
RESULTS
Pimonidazole Adduct Was Detected under Mildly Hypoxic Conditions in MIN6 Cells
Because it is difficult to measure accurate oxygen tension inside a living cell with current technology, we estimated oxygen tension within a cell by pimonidazole staining, which has been widely used to detect cellular and tissue hypoxia (4, 20). Although pimonidazole forms adducts with intracellular biomolecules only in severe hypoxia, it can also detect the decrease of cellular oxygen tension when the cells were cultured under moderately hypoxic conditions (4).
Because pancreatic islet and β-cell lines are known to consume substantial amounts of oxygen when the cells secrete insulin in response to glucose stimulation (21–23), the cultured β-cell line would also show severe cellular hypoxia under moderately hypoxic culture conditions upon the secretion of insulin.
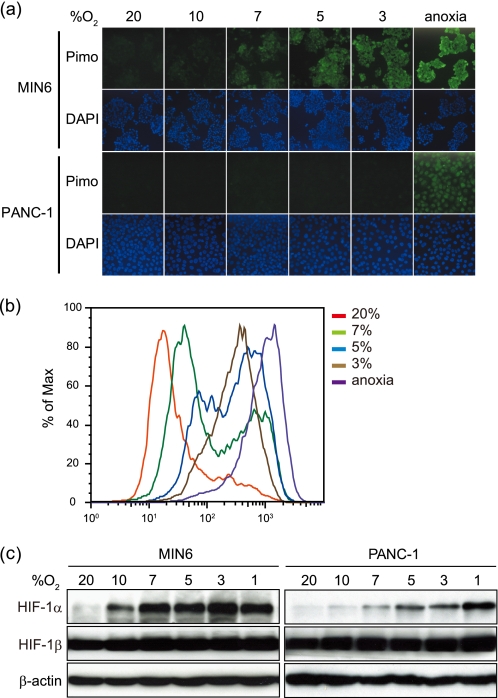
We examined the pimonidazole staining pattern in MIN6 cells in comparison with non-endocrine cancer cells, PANC-1, by immunocytochemistry (Fig. 1a). We detected pimonidazole staining in PANC-1 cells only under anoxic conditions but not at 3% oxygen tension. In striking contrast, MIN6 cells showed positive pimonidazole staining even at 7% oxygen tension. Intensity of the staining was higher in MIN6 cells than in PANC-1 cells at each oxygen tension examined below 7%. Furthermore, pimonidazole staining was examined by flow cytometry (Fig. 1b). Consistent with the observations by immunocytochemistry, the fluorescence was inversely correlated with the oxygen tension. The shift of the peak was observed at 7% oxygen tension in MIN6 cells.
FIGURE 1.
Pimonidazole binding under mildly hypoxic conditions was greater in MIN6 cells than in PANC-1 cells. a, pimonidazole immunocytochemistry of the cells. MIN6 cells and PANC-1 cells were incubated at various oxygen tensions as indicated in the presence of pimonidazole (10 μm) for 3 h. Green, pimonidazole; blue, DAPI. b, the MIN6 cells in a were subjected to flow cytometric analysis of pimonidazole staining. c, MIN6 cells and PANC-1 cells were incubated at various oxygen tensions as indicated for 6 h. The cell lysates were subjected to Western blotting for HIF-1α and HIF-1β. Blotting of β-actin is shown as a control.
We next examined the protein levels of HIF-1α (Fig. 1c). In several cell lines, HIF-1α levels were stabilized at oxygen tensions below 5% (24, 25). Indeed, in PANC-1 cells, HIF-1α was detected at oxygen tensions below 5% but only weakly in cells exposed to 7% oxygen tension (Fig. 1c). In striking contrast, in MIN6 cells, HIF-1α was detected even at 10% oxygen tension, whereas no change was observed in HIF-1β levels (Fig. 1c). The increased mRNA levels of HIF-1α target genes, including BNIP3 (BCL2/adenovirus E1B 19-kDa interacting protein 3), PGK1 (phosphoglycerate kinase 1), PDK1 (pyruvate dehydrogenase kinase isozyme 1), and LDH-A (lactate dehydrogenase A), that occurred even at 7% oxygen tension, indicate that HIF-1α was transcriptionally active under conditions of mild hypoxia (supplemental Fig. 1a). HIF-1α is known to be stabilized by factors other than hypoxia, such as the presence of reactive oxygen species (26, 27). To assess the role of reactive oxygen species in HIF-1α protein levels in MIN6 cells under mildly hypoxic conditions, the cells were treated with antioxidants, such as N-acetyl-l-cysteine or Trolox (supplemental Fig. 1b). Because HIF-1α levels did not change after treatment with these antioxidants, contributions of reactive oxygen species, if any, were subtle. These results also confirmed that MIN6 cells become hypoxic more easily than PANC-1 cells.
Western Blotting Was Useful for Detecting Pimonidazole Adduct in Vitro and in Vivo
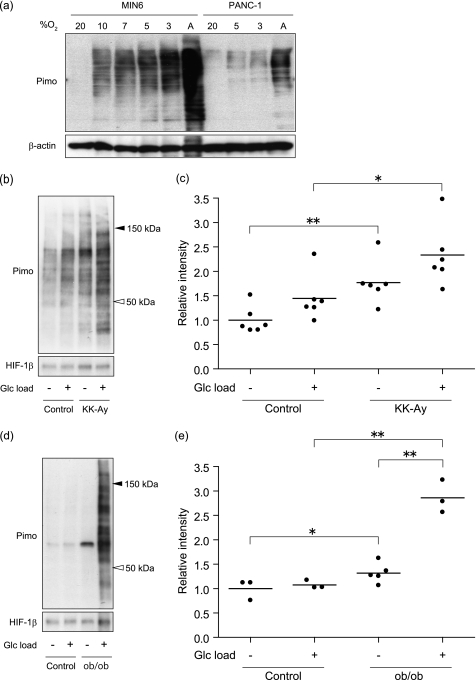
Next, pimonidazole adduct formation was assessed by Western blotting. When lysates of MIN6 cells in Fig. 1a were subjected to Western blotting, multiple bands were detected, consistent with the nature of pimonidazole, which nonspecifically forms adduct with molecules in the cells (Fig. 2a). As observed in Fig. 1, the intensity of Western blotting was inversely correlated with the oxygen tension. The intensity of the bands was higher in MIN6 cells than in PANC-1 cells at each oxygen tension examined. It should be noted that Western blotting was more sensitive than immunocytochemistry because the bands were detectable at higher oxygen tension. Because the islets in diabetes mice were suggested to be hypoxic (28), we applied Western blotting to the islets in vivo to detect pimonidazole adduct formation (Fig. 2, b–e). The pimonidazole adduct in the islets was remarkably increased in two different model mice of diabetes, KK-Ay (Fig. 2b) and ob/ob (Fig. 2d), compared with respective C57BL/6 control mice matched in age and sex. Quantification analysis revealed that the increase of adduct formation was statistically significant in KK-Ay (Fig. 2c) and ob/ob (Fig. 2e), whether glucose was loaded or not. Immunohistochemical analysis failed to detect pimonidazole adduct not only in pancreatic islet cells but also in other types of cells, including CD31+ blood vessels (supplemental Fig. 2). Blood glucose concentrations of diabetic mice were higher than in control mice (supplemental Fig. 3a). Because high levels of blood glucose per se might affect the pimonidazole adduct formation in the islets, non-diabetic control mice were injected with glucose at 4 g/kg. Although blood glucose levels at 30 min after the glucose load increased more than 600 mg/dl, we did not observe the difference in pimonidazole adduct formation between saline- and glucose-loaded mice (supplemental Fig. 3, b and c), suggesting that pimonidazole adduct formation in islets is not accelerated solely by blood glucose levels.
FIGURE 2.
Western blotting was useful for detecting pimonidazole adduct in vitro and in vivo. a, cell lysates from the cells in Fig. 1a were subjected to Western blotting, and pimonidazole-adducted proteins were detected in MIN6 cells and in PANC-1 cells. Blotting of β-actin is shown as a control. A, anoxia. b and d, Western blotting of pimonidazole adducts (Pimo) in the pancreatic islets of KK-Ay mice, 13 weeks of age (b), and ob/ob mice, 12 weeks of age (d), compared with age-matched C57BL/6 mice (Control) injected with saline (Glc load −) or 2 g/kg glucose (Glc load +), respectively. Blotting of HIF-1β is shown as a loading control. Representative results are shown. c and e, relative intensity of pimonidazole in KK-Ay mice (n = 6) (c) and ob/ob mice (n = 3–5) (e), compared with control mice under the same conditions as in b and d. Each value from a pool of the islets of a mouse is plotted as a dot, and the mean of values is shown as a bar (*, p < 0.05; **, p < 0.01).
As Mitochondrial Respiration Increased, MIN6 Cells Became Hypoxic
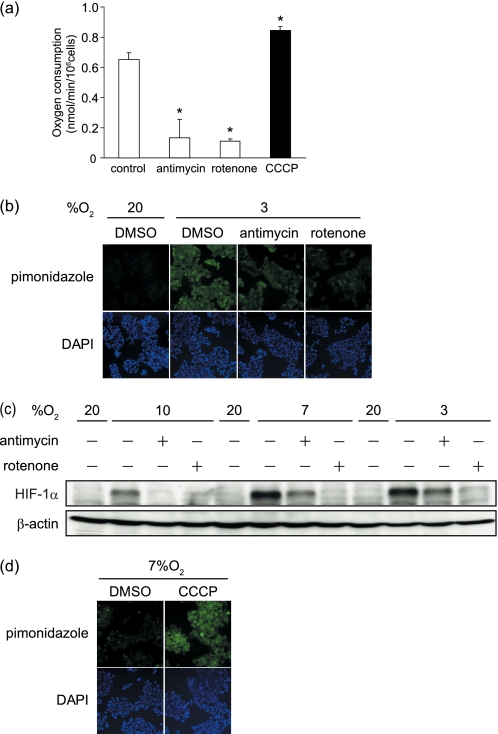
To examine the contributions of mitochondrial respiration on the generation of cellular hypoxia, we applied inhibitors of the respiratory chain, such as rotenone (an inhibitor of complex I) and antimycin A (an inhibitor of complex III). Decreased oxygen consumption was confirmed in the cells treated with either of the inhibitors (Fig. 3a). The pimonidazole adduct in MIN6 cells at 3% oxygen tension was remarkably decreased by the inhibitor treatments (Fig. 3b and supplemental Fig. 4a). In addition, HIF-1α protein levels were strongly suppressed with rotenone and partially suppressed with antimycin A (Fig. 3c). The different potencies of the inhibitors in suppressing HIF-1α expression were consistent with previously reports (25). On the other hand, the uncouplers, such as carbonyl cyanide 4-phenylhydrazone and CCCP, have been reported to stimulate oxygen consumption through facilitating the proton influx into the mitochondrial matrix, bypassing ATP synthetase (29), which was confirmed in MIN6 cells (Fig. 3a). At 7% oxygen tension, where immunocytological staining of the pimonidazole adduct was barely detectable, the staining became detectable after treatment with CCCP (Fig. 3d). By flow cytometric analysis, a stronger signal was observed in CCCP-treated cells than in the non-treated cells under 7% oxygen (supplemental Fig. 4b). These results indicate that cellular hypoxia of MIN6 cells can be generated by intracellular oxygen consumption by mitochondrial respiration.
FIGURE 3.
Mitochondrial oxygen consumption contributed to cellular hypoxia in MIN6 cells. a, the effect of respiratory inhibitors and an uncoupler on oxygen consumption of MIN6 cells. The cells were incubated with the indicated reagents (antimycin A (10 nm), rotenone (100 nm), and CCCP (5 μm)) for 30 min. Oxygen consumption of the cells were measured, and the means ± S.D. of values from each group are shown (*, p < 0.001). The experiments were repeated at least three times. b, immunocytochemistry of pimonidazole staining (green) of MIN6 cells treated with antimycin A, rotenone, and DMSO as a control for 3 h at 20 or 3% oxygen tension. The cell nuclei were stained with DAPI (blue). c, the effect of respiratory inhibitors on HIF-1α protein levels. Western blotting of HIF-1α in the MIN6 cells cultured under the indicated conditions for 6 h is shown. d, immunocytochemistry of pimonidazole staining (green) of MIN6 cells treated with CCCP or with DMSO as a control for 3 h at 7% oxygen tension. The cell nuclei were stained with DAPI (blue).
MIN6 Cells Became More Hypoxic by Glucose Stimulation
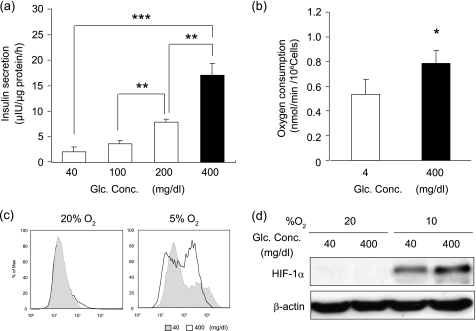
MIN6 cells consume oxygen when the cells secrete insulin in response to glucose stimulation (21). Mitochondrial oxidative phosphorylation is stimulated to fulfill the demand of ATP that is necessary for β-cell function (10). When MIN6 cells were exposed to elevated levels of glucose (400 mg/dl), insulin secretion increased 7-fold over the values obtained with 40 mg/dl glucose (Fig. 4a). We examined the change in oxygen consumption as a function of the glucose concentration of the medium. The oxygen consumption was higher when cells were cultured with higher concentrations of glucose (Fig. 4b). Furthermore, pimonidazole adduct formation was stronger at high glucose concentration than low concentration at 5% oxygen tension (Fig. 4c). Higher glucose concentrations yielded higher intensity of the HIF-1α band at 10% oxygen tension (Fig. 4d). Taken together, our data indicate that glucose-stimulated insulin secretion drove MIN6 cells into hypoxia.
FIGURE 4.
Cellular hypoxia of MIN6 cells was dependent on glucose concentration in vitro. a, glucose-dependent insulin secretion. MIN6 cells were stimulated by incubation in various glucose concentrations as indicated for 1 h, and insulin secretion was calculated as the difference between the insulin concentrations of the medium before and after stimulation. b, glucose-dependent oxygen consumption. MIN6 cells were cultured with 4 mg/dl for 30 min, and oxygen consumption was measured. Then, 30 min after adding glucose to 400 mg/dl, oxygen consumption was measured. In a and b, the means ± S.D. (error bars) of values from each group are shown (*, p < 0.05; **, p < 0.01; ***, p < 0.001). The experiments were repeated at least three times. c, glucose-dependent pimonidazole adduct formation in vitro. Shown is flow cytometric analysis of pimonidazole-stained MIN6 cells incubated in 40 or 400 mg/dl glucose as indicated at 20 or 5% oxygen tension for 3 h. d, glucose-dependent expression levels of HIF-1α protein. Western blotting of HIF-1α in MIN6 cells cultured under the indicated conditions for 6 h is shown.
Mouse Isolated Islets Became More Hypoxic by Glucose Stimulation
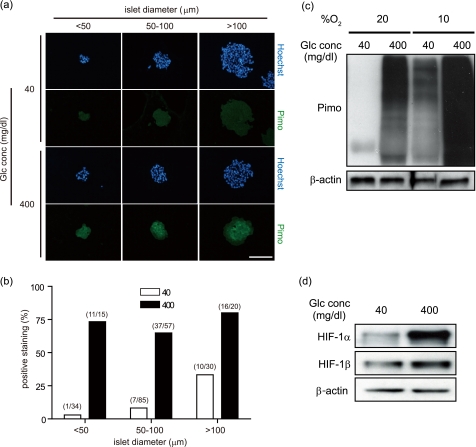
Because the behavior of MIN6 cells is known not to perfectly mimic primary β-cell physiology (30), we examined the islets isolated from mice and cultured in vitro. First, the islets were subjected to immunohistochemistry for detection of pimonidazole. In the islets cultured under normoxic conditions, more pimonidazole-positive islets were observed in samples stimulated by high glucose than in those cultured in low glucose medium, independent of the size of the islets (Fig. 5, a and b). Pimonidazole staining was rather homogenous in the high glucose-stimulated islets compared with that in the relatively large islets in which pimonidazole staining was observed only in the core region, central staining (Fig. 5a and supplemental Fig. 5, a and b). Furthermore, Western blotting revealed that pimonidazole adduct was observed in the isolated islets stimulated by high glucose under normoxic conditions (Fig. 5c). When the islets were cultured under moderately hypoxic conditions, the pimonidazole adduct was observed even in the islets in low glucose medium, and more adduct was evident in the islets stimulated by high glucose levels. The expression levels of HIF-1α protein under the same conditions as in Fig. 5c were dramatically increased by treatment with high glucose, whereas HIF-1β expression was slightly increased (Fig. 5d). Thus, isolated islets became hypoxic when they were stimulated by high glucose.
FIGURE 5.
Glucose-dependent cellular hypoxia in the cultured isolated islets in vitro. a, immunohistochemistry of pimonidazole (Pimo) (green) and Hoechst 33342 (Hoechst) (blue) of isolated islets incubated with 40 or 400 mg/dl glucose at 20% oxygen tension for 1 h. Isolated islets were divided into three groups (<50, 50–100, and >100 μm) by their diameters. Representative images are shown. Scale bar, 100 μm. b, percentage of pimonidazole-positive ratio in the same conditions as in a. The islets incubated with 40 or 400 mg/dl glucose were represented as an open bar or closed bar, respectively. The number of the islets was indicated as (positive staining/total islets) above each bar. c, isolated islets incubated with 40 or 400 mg/dl glucose at 20 or 10% oxygen tension for 2 h were subjected to Western blotting for detection of pimonidazole adducts. Blotting of β-actin is shown as a loading control. d, expression levels of HIF-1α protein. Western blotting of HIF-1α and HIF-1β in the isolated islets cultured as in c is shown. Blotting of β-actin is shown as a loading control.
HIF-1α Suppressed Insulin Secretion and Cellular Hypoxia Concurrently
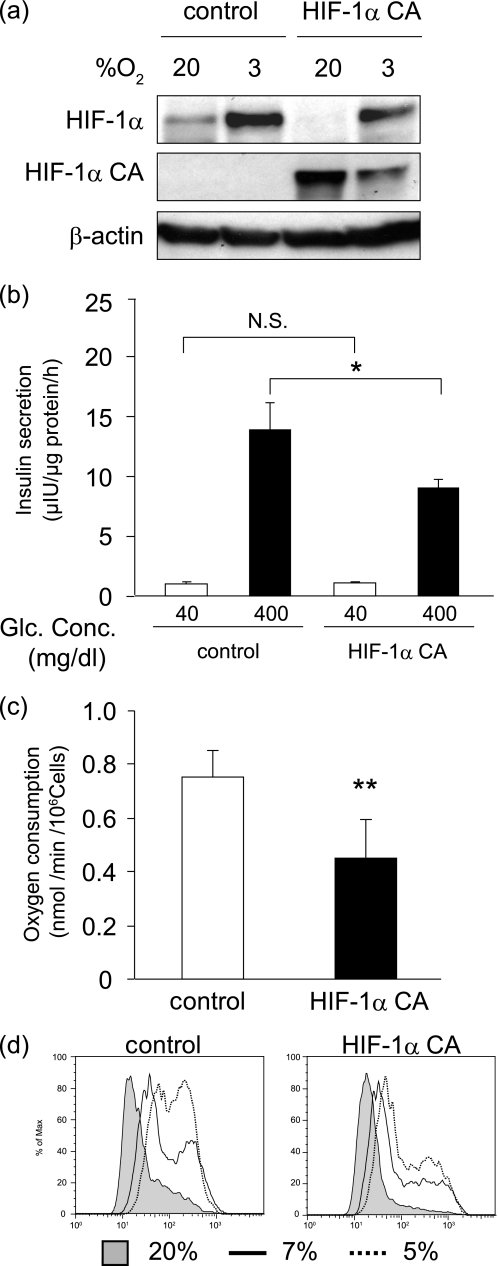
Our observations that HIF-1α was induced in mild hypoxia in MIN6 cells prompted us to investigate the functional role of HIF-1α in the cellular hypoxia of MIN6 cells. We introduced the constitutively active form of HIF-1α, HIF-1α CA, into the MIN6 cells. HIF-1α CA proteins were confirmed to be expressed in HIF-1α CA cells even under normoxic conditions (Fig. 6a). In HIF-1α CA cells, the expression of GLUT-1, one of the HIF-1α target genes, was up-regulated (supplemental Fig. 6). Insulin secretion after glucose stimulation was impaired in HIF-1α CA cells (Fig. 6b), an outcome consistent with previous reports (28, 31, 32). Oxygen consumption was decreased in HIF-1α CA cells (Fig. 6c), and pimonidazole adduct formation under mildly hypoxic conditions was reduced in HIF-1α CA cells compared with control cells (Fig. 6d), findings that are consistent with the previous report using mouse embryonic fibroblasts (33). Thus, high levels of HIF-1α suppress insulin secretion and oxygen consumption, avoiding a decrease of the cellular oxygen levels in the insulin-secreting cells.
FIGURE 6.
Cellular hypoxia is attenuated in HIF-1α-overexpressing MIN6 cells. a, generation of MIN6 cells carrying the constitutive active form of HIF-1α (HIF-1α CA). Control MIN6 cells and HIF-1α CA MIN6 cells were incubated at the indicated oxygen tension for 6 h, and the expression of endogenous as well as exogenous HIF-1α protein was assessed. Blotting of β-actin is shown as a loading control. b, impairment of insulin secretion in HIF-1α CA MIN6 cells. MIN6 cells were stimulated in a 40 and 400 mg/dl glucose concentration for 1 h, and insulin secretion was estimated as in Fig. 4a. c, decrease of oxygen consumption in HIF-1α CA MIN6 cells. The MIN6 cells were cultured in a 400 mg/dl glucose concentration, and oxygen consumption was measured. In b and c, the means ± S.D. (error bars) of values from each group are shown (*, p > 0.05; **, p < 0.01). N.S., not significant. The experiments were repeated at least three times. d, cellular hypoxia is attenuated in HIF-1α CA5 MIN6 cells. Flow cytometric analysis of pimonidazole-stained cells at the indicated oxygen tensions is shown.
DISCUSSION
Pimonidazole is a derivative of 2-nitroimidazole, which forms adducts with intracellular macromolecules only at low oxygen tension (6). Nitroreductases within the cells transfer electrons to the parent compound and generate the nitro radical anion. Oxygen reoxidizes the nitro radical anion, thereby blocking further reduction of the parent compound. In the absence of oxygen, the nitro radical anion is further reduced and binds to -SH-containing molecules. The drawback of pimonidazole as a hypoxic marker is that it detects only severe hypoxia. Adduct formation of misonidazole, another 2-nitroimidazole derivative, steeply increases below partial oxygen pressure, 10 mm Hg (34).
On the other hand, the advantage of the pimonidazole detection is that it forms irreversible adducts under severely hypoxic conditions, so that the adduct formation serves as a record of the lowest oxygen tension to which cells have been exposed. The metabolic change following insulin secretion is transient and happens over a short time. The nature of irreversible adduct formation of pimonidazole is suitable to detect transient hypoxic events.
Formation of pimonidazole adduct is accelerated in reduced status. Thus, high levels of NADPH or glutathione might affect the adduct formation. Because glucose stimulation reportedly results in an increase of the NADPH/NADP+ ratio under normoxic conditions in islet β-cells (35), pimonidazole adduct formation is promoted not only by low oxygen tension but also possibly by an increase of the NADPH/NADP+ ratio. Arteel et al. (36) reported that under hypoxic conditions, pimonidazole adduct formation mainly depends on oxygen tension rather than redox status. In addition, we did not observe an increase of pimonidazole adduct formation in normoxic MIN6 cells by glucose stimulation (Fig. 4c). Therefore, change of redox status by glucose stimulation might not be enough to increase pimonidazole adduct formation to the levels of our detection system. Thus, pimonidazole adduct formation is likely to reflect low oxygen tension in our experimental settings.
We demonstrated here that the pancreatic islet cells in two types of mouse diabetes model were hypoxic using Western blotting analysis. We failed to detect the signal by immunohistochemistry, indicating that Western blotting is more sensitive than immunostaining in vivo. It was previously suggested that the islet cells may be exposed to hypoxia in the progression of diabetes (28). Indeed, the hypoxia-related genes, including HIF-1α, VEGF-A, PAI-1, and LDH-A, are up-regulated in prediabetic ZDF, Zucker Diabetic Fatty, rat islets (37). The mechanism underlying islet cell hypoxia in the diabetes mice is not clear. Impairment of microcirculation and consequent decrease of blood supply might be a cause of islet hypoxia in these mice. Alternatively, as shown in vitro in this study, increased oxygen consumption by glucose stimulation is another possible cause. Further studies are needed to elucidate the mechanism.
There are two possibilities through which intracellular oxygen tension is decreased by acute oxygen consumption. One possibility is that intracellular hypoxia occurs secondary to pericellular hypoxia. Alternatively, intracellular hypoxia can occur despite the existence of adequate extracellular oxygen tension. Oxygen does not freely pass through the cellular membrane because oxygen diffusion is limited by the lipid bilayer (38). A water channel, aquaporin, is reportedly a gate for oxygen to enter into a cell (39). Therefore, cellular hypoxia can occur in those cells that consume large amounts of oxygen in a short period of time. These two possibilities are not mutually exclusive and can occur at the same time. It should be noted that the physiological oxygen tension of the normal tissue is between 24 and 66 mm Hg (3.3–9.2%) (6), which is often termed “moderately hypoxic” when the cells are cultured in vitro.
The islets of non-diabetic mice in vivo did not accumulate the pimonidazole adduct even after a challenge of high glucose (Fig. 2 and supplemental Fig. 3). In contrast, the isolated islets easily accumulated pimonidazole adduct when stimulated by high glucose (Fig. 5). Some protective mechanisms must exist to circumvent crisis in vivo, which might be impaired in the diabetic mice. The increase of blood flow by increased blood glucose levels can be a protective mechanism in vivo (40). High oxygen consumption due to the aerobic glucose metabolism of the isolated islets (41) might make the cellular hypoxia evident in the absence of the compensation mechanism.
The mice with the Hif-1α or Hif-1β deletion in β-cells showed impairment of glucose tolerance, not by the decrease of islet mass but by impaired insulin secretion of β-cells (42, 43). Lack of a hypoxia response mechanism might damage the islet cells over an extended time period. In MIN6 cells, we observed that basal as well as glucose-stimulated insulin secretion was intact under normoxic conditions when Hif-1α gene expression was suppressed,3 an observation that supports the previous reports that the mice with Hif-1α deletion in β-cells do not show any defect in glucose homeostasis (28, 31).
Our data demonstrate that high glucose is a trigger of cellular hypoxia of pancreatic β-cells in vitro. In developing type 2 diabetes, blood glucose levels are elevated because of the peripheral glucose intolerance. In such a condition, the demand of the insulin secretion increases, and then the consequent increase of oxygen consumption might contribute to the β-cell hypoxia. Induction of HIF-1α by hypoxia might reduce insulin secretion, therefore suppressing the metabolic demand. Consequently, decreased oxygen consumption would offset severe cellular hypoxia in β-cells. Further investigation will be required to clarify whether islet hypoxia plays a role in deterioration of β-cell function in diabetes.
The findings here might also be applicable to islet transplantation. Preparing the intact islet from donors is one of the most important factors for the success of islet transplantation. During the process of islet isolation from donors and engraftment to recipients, hypoxia is a major cause of islet cell damage (44). Transiently reducing insulin secretion and consequent oxygen consumption might be beneficial for oxygenating the core hypoxic region of islets.
Acknowledgments
We thank Junichi Miyazaki for generously providing MIN6 cells and Toshiko Yasuda for secretarial support.
This work was supported in part by a grant-in-aid for scientific research from the Japan Society for the Promotion of Science.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–6.
Y. Sato and M. Inoue, unpublished data.
- HIF
- hypoxia-inducible factor
- CCCP
- carbonyl cyanide 3-chlorophenylhydrazone
- KRBH
- Krebs-Ringer-bicarbonate HEPES
- CA
- constitutively active.
REFERENCES
- 1. Hochachka P. W., Somero G. N. (2002) Biochemical Adaptation, Oxford University Press, New York [Google Scholar]
- 2. Semenza G. L. (2000) Genes Dev. 14, 1983–1991 [PubMed] [Google Scholar]
- 3. Hagen T., Taylor C. T., Lam F., Moncada S. (2003) Science 302, 1975–1978 [DOI] [PubMed] [Google Scholar]
- 4. Doege K., Heine S., Jensen I., Jelkmann W., Metzen E. (2005) Blood 106, 2311–2317 [DOI] [PubMed] [Google Scholar]
- 5. Semenza G. L. (2007) Science 318, 62–64 [DOI] [PubMed] [Google Scholar]
- 6. Höckel M., Vaupel P. (2001) J. Natl. Cancer. Inst. 93, 266–276 [DOI] [PubMed] [Google Scholar]
- 7. Zhdanov A. V., Ward M. W., Prehn J. H., Papkovsky D. B. (2008) J. Biol. Chem. 283, 5650–5661 [DOI] [PubMed] [Google Scholar]
- 8. Aragonés J., Schneider M., Van Geyte K., Fraisl P., Dresselaers T., Mazzone M., Dirkx R., Zacchigna S., Lemieux H., Jeoung N. H., Lambrechts D., Bishop T., Lafuste P., Diez-Juan A., Harten S. K., Van Noten P., De Bock K., Willam C., Tjwa M., Grosfeld A., Navet R., Moons L., Vandendriessche T., Deroose C., Wijeyekoon B., Nuyts J., Jordan B., Silasi-Mansat R., Lupu F., Dewerchin M., Pugh C., Salmon P., Mortelmans L., Gallez B., Gorus F., Buyse J., Sluse F., Harris R. A., Gnaiger E., Hespel P., Van Hecke P., Schuit F., Van Veldhoven P., Ratcliffe P., Baes M., Maxwell P., Carmeliet P. (2008) Nat. Genet. 40, 170–180 [DOI] [PubMed] [Google Scholar]
- 9. O'Hagan K. A., Cocchiglia S., Zhdanov A. V., Tambawala M. M., Cummins E. P., Monfared M., Agbor T. A., Garvey J. F., Papkovsky D. B., Taylor C. T., Allan B. B. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 2188–2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wiederkehr A., Wollheim C. B. (2006) Endocrinology 147, 2643–2649 [DOI] [PubMed] [Google Scholar]
- 11. Jensen M. V., Joseph J. W., Ronnebaum S. M., Burgess S. C., Sherry A. D., Newgard C. B. (2008) Am. J. Physiol. Endocrinol Metab. 295, E1287–E1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eliasson L., Abdulkader F., Braun M., Galvanovskis J., Hoppa M. B., Rorsman P. (2008) J. Physiol. 586, 3313–3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maechler P., Wollheim C. B. (2001) Nature 414, 807–812 [DOI] [PubMed] [Google Scholar]
- 14. Miyazaki J., Araki K., Yamato E., Ikegami H., Asano T., Shibasaki Y., Oka Y., Yamamura K. (1990) Endocrinology 127, 126–132 [DOI] [PubMed] [Google Scholar]
- 15. Guppy M., Kong S. E., Niu X., Busfield S., Klinken S. P. (1997) J. Cell. Physiol. 170, 1–7 [DOI] [PubMed] [Google Scholar]
- 16. Mukai M., Kusama T., Hamanaka Y., Koga T., Endo H., Tatsuta M., Inoue M. (2005) Cancer Res. 65, 9121–9125 [DOI] [PubMed] [Google Scholar]
- 17. Okuyama H., Krishnamachary B., Zhou Y. F., Nagasawa H., Bosch-Marce M., Semenza G. L. (2006) J. Biol. Chem. 281, 15554–15563 [DOI] [PubMed] [Google Scholar]
- 18. Endo H., Murata K., Mukai M., Ishikawa O., Inoue M. (2007) Cancer Res. 67, 8095–8103 [DOI] [PubMed] [Google Scholar]
- 19. Fukui K., Yang Q., Cao Y., Takahashi N., Hatakeyama H., Wang H., Wada J., Zhang Y., Marselli L., Nammo T., Yoneda K., Onishi M., Higashiyama S., Matsuzawa Y., Gonzalez F. J., Weir G. C., Kasai H., Shimomura I., Miyagawa J., Wollheim C. B., Yamagata K. (2005) Cell. Metab. 2, 373–384 [DOI] [PubMed] [Google Scholar]
- 20. He F., Deng X., Wen B., Liu Y., Sun X., Xing L., Minami A., Huang Y., Chen Q., Zanzonico P. B., Ling C. C., Li G. C. (2008) Cancer Res. 68, 8597–8606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Soejima A., Inoue K., Takai D., Kaneko M., Ishihara H., Oka Y., Hayashi J. I. (1996) J. Biol. Chem. 271, 26194–26199 [DOI] [PubMed] [Google Scholar]
- 22. Wang W., Upshaw L., Strong D. M., Robertson R. P., Reems J. (2005) J. Endocrinol. 185, 445–455 [DOI] [PubMed] [Google Scholar]
- 23. Gilbert M., Jung S. R., Reed B. J., Sweet I. R. (2008) J. Biol. Chem. 283, 24334–24342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bracken C. P., Fedele A. O., Linke S., Balrak W., Lisy K., Whitelaw M. L., Peet D. J. (2006) J. Biol. Chem. 281, 22575–22585 [DOI] [PubMed] [Google Scholar]
- 25. Brown S. T., Nurse C. A. (2008) Am. J. Physiol. 294, C1305–C1312 [DOI] [PubMed] [Google Scholar]
- 26. Brunelle J. K., Bell E. L., Quesada N. M., Vercauteren K., Tiranti V., Zeviani M., Scarpulla R. C., Chandel N. S. (2005) Cell. Metab. 1, 409–414 [DOI] [PubMed] [Google Scholar]
- 27. Guzy R. D., Hoyos B., Robin E., Chen H., Liu L., Mansfield K. D., Simon M. C., Hammerling U., Schumacker P. T. (2005) Cell. Metab. 1, 401–408 [DOI] [PubMed] [Google Scholar]
- 28. Zehetner J., Danzer C., Collins S., Eckhardt K., Gerber P. A., Ballschmieter P., Galvanovskis J., Shimomura K., Ashcroft F. M., Thorens B., Rorsman P., Krek W. (2008) Genes Dev. 22, 3135–3146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Daunt M., Dale O., Smith P. A. (2006) Endocrinology 147, 1527–1535 [DOI] [PubMed] [Google Scholar]
- 30. Poitout V., Olson L. K., Robertson R. P. (1996) Diabetes Metab. 22, 7–14 [PubMed] [Google Scholar]
- 31. Cantley J., Selman C., Shukla D., Abramov A. Y., Forstreuter F., Esteban M. A., Claret M., Lingard S. J., Clements M., Harten S. K., Asare-Anane H., Batterham R. L., Herrera P. L., Persaud S. J., Duchen M. R., Maxwell P. H., Withers D. J. (2009) J. Clin. Invest. 119, 125–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Puri S., Cano D. A., Hebrok M. (2009) Diabetes 58, 433–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Papandreou I., Cairns R. A., Fontana L., Lim A. L., Denko N. C. (2006) Cell. Metab. 3, 187–197 [DOI] [PubMed] [Google Scholar]
- 34. Gross M. W., Karbach U., Groebe K., Franko A. J., Mueller-Klieser W. (1995) Int. J. Cancer 61, 567–573 [DOI] [PubMed] [Google Scholar]
- 35. Ivarsson R., Quintens R., Dejonghe S., Tsukamoto K., in 't Veld P., Renström E., Schuit F. C. (2005) Diabetes 54, 2132–2142 [DOI] [PubMed] [Google Scholar]
- 36. Arteel G. E., Thurman R. G., Raleigh J. A. (1998) Eur. J. Biochem. 253, 743–750 [DOI] [PubMed] [Google Scholar]
- 37. Li X., Zhang L., Meshinchi S., Dias-Leme C., Raffin D., Johnson J. D., Treutelaar M. K., Burant C. F. (2006) Diabetes 55, 2965–2973 [DOI] [PubMed] [Google Scholar]
- 38. Ivanov I. I., Fedorov G. E., Gus'kova R. A., Ivanov K. I., Rubin A. B. (2004) Biochem. Biophys. Res. Commun. 322, 746–750 [DOI] [PubMed] [Google Scholar]
- 39. Echevarría M., Muñoz-Cabello A. M., Sánchez-Silva R., Toledo-Aral J. J., López-Barneo J. (2007) J. Biol. Chem. 282, 30207–30215 [DOI] [PubMed] [Google Scholar]
- 40. Jansson L., Hellerström C. (1986) Am. J. Physiol. 251, E644–E647 [DOI] [PubMed] [Google Scholar]
- 41. Schuit F., De Vos A., Farfari S., Moens K., Pipeleers D., Brun T., Prentki M. (1997) J. Biol. Chem. 272, 18572–18579 [DOI] [PubMed] [Google Scholar]
- 42. Cheng K., Ho K., Stokes R., Scott C., Lau S. M., Hawthorne W. J., O'Connell P. J., Loudovaris T., Kay T. W., Kulkarni R. N., Okada T., Wang X. L., Yim S. H., Shah Y., Grey S. T., Biankin A. V., Kench J. G., Laybutt D. R., Gonzalez F. J., Kahn C. R., Gunton J. E. (2010) J. Clin. Invest. 120, 2171–2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gunton J. E., Kulkarni R. N., Yim S., Okada T., Hawthorne W. J., Tseng Y. H., Roberson R. S., Ricordi C., O'Connell P. J., Gonzalez F. J., Kahn C. R. (2005) Cell 122, 337–349 [DOI] [PubMed] [Google Scholar]
- 44. Miao G., Ostrowski R. P., Mace J., Hough J., Hopper A., Peverini R., Chinnock R., Zhang J., Hathout E. (2006) Am. J. Transplant. 6, 2636–2643 [DOI] [PubMed] [Google Scholar]