Abstract
Neuronal calcium sensor (NCS) proteins transduce Ca2+ signals and are highly conserved from yeast to humans. We determined NMR structures of the NCS-1 homolog from fission yeast (Ncs1), which activates a phosphatidylinositol 4-kinase. Ncs1 contains an α-NH2-linked myristoyl group on a long N-terminal arm and four EF-hand motifs, three of which bind Ca2+, assembled into a compact structure. In Ca2+-free Ncs1, the N-terminal arm positions the fatty acyl chain inside a cavity near the C terminus. The C14 end of the myristate is surrounded by residues in the protein core, whereas its amide-linked (C1) end is flanked by residues at the protein surface. In Ca2+-bound Ncs1, the myristoyl group is extruded (Ca2+-myristoyl switch), exposing a prominent patch of hydrophobic residues that specifically contact phosphatidylinositol 4-kinase. The location of the buried myristate and structure of Ca2+-free Ncs1 are quite different from those in other NCS proteins. Thus, a unique remodeling of each NCS protein by its myristoyl group, and Ca2+-dependent unmasking of different residues, may explain how each family member recognizes distinct target proteins.
Keywords: Calcium, Calcium-binding Proteins, Calmodulin, Lipid Synthesis, Membrane Trafficking, Neurological Diseases, NMR, Phosphatidylinositol Kinase, Signal Transduction
Introduction
Neuronal calcium sensor (NCS)2 proteins (1–3) are a conserved subclass of the calmodulin (CaM) superfamily that regulate signal transduction in the brain and retina. All members of the NCS family includes ∼200 residues, are N-myristoylated on their α-NH2 group, and possess four EF-hand motifs, but the first contains a diagnostic CPXG sequence that disables its ability to bind Ca2+ (4, 5). Recoverin, the most intensively studied NCS protein, is a Ca2+ sensor in rod and cone cells, where it controls desensitization of rhodopsin (6–9). The guanylate cyclase-activating proteins, GCAP1 (10) and GCAP2 (11), are NCS proteins also found in rods and cones, which regulate the recovery phase of visual excitation and are genetically linked to retinal diseases (12, 13). Brain NCS family members include neurocalcin (14), hippocalcin (15), visinin, and visinin-like proteins (16), KChIPs (17), and NCS-1 (also called frequenin) (18). Brain NCS proteins have diverse functions. Neurocalcins and visinin-like proteins regulate guanylate cyclase and nicotinamide acetylcholine receptors implicated in synaptic plasticity (16). KChIPs (17), hippocalcin (19), and NCS-1 (20) bind to various ion channels and thus control neuronal excitability.
Remarkably, even the genomes of yeasts (Saccharomyces cerevisiae and Schizosaccharomyces pombe) encode a protein that is more than 60% identical to mammalian NCS-1 (Fig. 1A). The budding yeast (S. cerevisiae) homolog (Frq1) is essential for cell growth (21) and activates a PtdIns 4-kinase (Pik1) (22, 23). The fission yeast (S. pombe) homolog (Ncs1) regulates sporulation (24) and confers Ca2+ tolerance (25). Sporulation defects in Ncs1 knock-out fission yeast are rescued by overexpressing S. cerevisiae Frq1 or Pik1, suggesting that Ncs1 activates the homologous S. pombe PtdIns 4-kinase. Indeed, the Frq1-binding site in Pik1 (26) is conserved in its fission yeast ortholog (also called Pik1) (Fig. 1B) (27).
FIGURE 1.
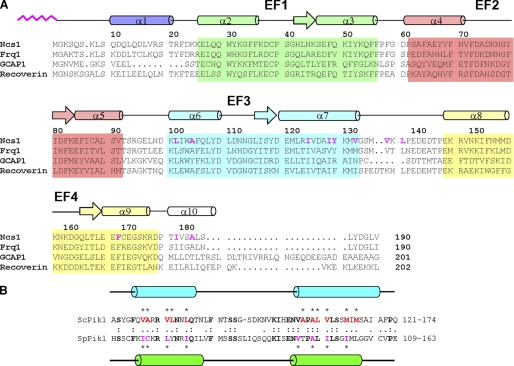
A, amino acid sequence alignment of S. pombe Ncs1 with other NCS proteins (sequence numbering is for S. pombe Ncs1). Secondary structure elements (helices and strands), EF-hand motifs (EF1, green; EF2, red; EF3, cyan; and EF4, yellow), and residues that interact with the myristoyl group (highlighted magenta) are mapped onto the amino acid sequence of Ncs1. Swiss Protein Data base accession numbers are Q09711 (S. pombe Ncs1), Q06389 (S. cerevisiae Frq1), P21457 (bovine recoverin), and P43080 (human GCAP1). Secondary structural elements indicated schematically were derived from the analysis of NMR data (3JHNHα, chemical shift index (54), and sequential NOE patterns). B, amino acid sequence alignment (one-letter code) of the N-terminal Frq1-binding region of S. cerevisiae Pik1 (ScPik1) and the N-terminal Ncs1-binding region of S. pombe Pik1 (ScPik1). Identities are indicated in boldface with a colon; conservative substitutions are indicated with a period. Residues in ScPik1 implicated in Frq1 binding are indicated in boldface red with an asterisk (26); residues in SpPik1 implicated in Ncs1 binding are indicated in boldface purple with an asterisk (this study). Residues that are α-helical in the ScPik1-Frq1 complex (125–135 and 156–169) (26) are depicted as blue cylinders; residues that are α-helical in the SpPik1-Ncs1 complex (114–127 and 143–156) (this study) are depicted as green cylinders.
The high degree of sequence identity among NCS proteins suggests that their three-dimensional structures should be quite similar. Indeed, the overall structures of the Ca2+-bound state of the unmyristoylated forms of several NCS family members look rather similar, as determined by x-ray crystallography (28–32) or nuclear magnetic resonance (NMR) spectroscopy (33–35). The four EF-hands are packed in a tandem array, in contrast to the dumbbell-shaped arrangement seen in CaM (36) and troponin C (37). However, much less is known about the structure of the Ca2+-free and/or myristoylated forms of NCS proteins. The fact that NCS proteins, like recoverin, GCAP1, and NCS-1, all recognize different physiological target proteins suggests that they must have some distinguishing structural characteristic that may be conferred by the interaction of the N-myristoyl moiety with the rest of the protein. Consistent with this notion, the structures of myristoylated recoverin (PDB code 1iku) and myristoylated GCAP1 (PDB code 2r2i) are quite different from each other. Moreover, although there is only one NCS protein (recoverin) whose structure has been determined for its myristoylated form in both the absence (38) and presence of Ca2+ (39), the structure of Ca2+-free recoverin is quite different from the structure of Ca2+-bound recoverin. In its Ca2+-free state, the N-terminal myristoyl group of recoverin is sequestered inside a deep hydrophobic cavity in the N-domain. In its Ca2+-bound state, the N-myristoyl group is extruded, permitting the now solvent-exposed fatty acyl chain to interact with membranes (40, 41), allowing recoverin (42) and other NCS proteins (30, 43, 44) to bind to target membranes when the Ca2+ level is high. The Ca2+-induced conformational change that exposes the myristoyl group has been dubbed a Ca2+-myristoyl switch.
Frq1 in budding yeast both activates the PtdIns 4-kinase Pik1 (21, 23) and its N-terminal myristoyl group enhances its membrane binding. When bound to the enzyme, Frq1 occupies residues 121–174 of Pik1, which forms a U-shaped structure that lies upstream of the catalytic domain (residues 792–1066) (26). Mammalian NCS-1 can interact with yeast Pik1 (45) and reportedly regulates PtdIns 4-kinase activity in animal cells (46, 47). Ca2+-dependent activation of PtdIns 4-kinase by NCS-1 may be especially important in neurons because modulation of phosphoinositide synthesis by intracellular Ca2+ controls synaptic vesicle exocytosis (48) and is involved in synaptic plasticity (49). However, previously, we were unable to obtain structural information for myristoylated Frq1.
Here, we report the NMR structures of Ca2+-free myristoylated Ncs1 in solution and the Ca2+-loaded form of the same protein bound to its target site (residues 111–159) in fission yeast Pik1, hereafter referred to as Pik1(111–159). Strikingly, the location of the myristoyl-binding site in Ca2+-free Ncs1 and the resulting structure of Ncs1 are quite different from that of either recoverin (38) or GCAP1 (50). Our data support the conclusion that myristoylation shapes each NCS protein into a distinct structure. Moreover, we find that Ncs1 undergoes large Ca2+-induced conformational changes that lead to extrusion of the myristoyl group, causing hydrophobic residues in the C-domain that sequester the fatty acyl chain in Ca2+-free Ncs1 to become solvent-exposed in Ca2+-bound Ncs1 and thus available to interact with Pik1(111–159). In vivo, this Ca2+-myristoyl switch presumably promotes membrane localization of Ncs1 and its association with Pik1. Furthermore, based on the structure of Pik1-bound Ncs1, we propose a mechanism for simultaneous Ca2+-induced membrane localization and activation of the enzyme. Finally, given the profound structural differences between the Ca2+-free states of myristoylated Ncs1 (this study), myristoylated recoverin (38), and myristoylated GCAP1 (50), we propose the general idea that N-terminal myristoylation is critical for shaping each NCS family member into a unique structure, which upon Ca2+-induced extrusion of the myristoyl group exposes a unique set of previously masked residues, thereby accounting for the target specificity of each NCS protein.
EXPERIMENTAL PROCEDURES
Protein Expression and Purification
Expression and purification of recombinant myristoylated S. pombe Ncs1 (and S. cerevisiae Frq1) was described previously (24). Briefly, Ncs1 and Frq1 (without any affinity tags) were expressed using pET11 vector harboring the ncs1 (or FRQ1) coding sequence and was co-expressed with yeast N-myristoyl-CoA transferase using pBB131 vector harboring the N-myristoyl-CoA transferase coding sequence in Escherichia coli strain BL21(DE3). Ncs1 was labeled with 15N or 15N/13C isotopes by growing cells at 37 °C in M9 minimal media supplemented with 15N-labeled ammonium chloride and 13C-labeled glucose as described previously (26). Unlabeled or 13C14-labeled myristic acid was added to the culture 20 min prior to induction that is needed for N-terminal myristoylation. Cells were harvested, lysed, and spun down to collect supernatant. Protein was then purified from the supernatant using hydrophobic interaction (butyl-Sepharose), anion exchange (DEAE-Sepharose), and size exclusion (Superdex 200) columns. The final protein purity is ∼95% judged by SDS-PAGE, and more than 90% of the protein was myristoylated determined by mass spectrometry analysis.
A functional polypeptide fragment of S. pombe Pik1 (residues 111–159, named Pik1(111–159)) uniformly labeled with nitrogen-15 and/or carbon-13 and tagged with a C-terminal His6 tract was expressed in E. coli strain BL21(DE3)-RIL (Stratagene) carrying the pET23d vector (Novagen) harboring the PIK1(111–159) coding sequence grown in M9 medium containing [15N]NH4Cl and [13C]glucose (26). Labeled Pik1(111–159) was isolated from the insoluble fraction of bacterial cell lysates dissolved in 8 m urea buffer and purified using Ni2+-chelate affinity chromatography on a nitrilotriacetate resin (Qiagen), according to the manufacturer's instructions. Peak fractions were then dialyzed extensively against 4 liters of 25 mm sodium acetate (pH 5.0) to remove urea. After dialysis, the Pik1(111–159) polypeptide remained soluble at pH 5.0 and was concentrated about 10-fold to a final concentration of 1 mm used in NMR experiments.
The Ncs1-Pik1(111–159) complex used in NMR experiments was prepared by mixing a relatively dilute stock solution of Ca2+-bound Ncs1 (0.05 mm Ncs1 in 2 mm CaCl2 and 5 mm dithiothreitol at pH 7.0) to 1 eq of Pik1(111–159) (0.05 mm Pik1 in 5 mm sodium acetate, 2 mm CaCl2, and 5 mm dithiothreitol at pH 5.0). The dilute preparation was then concentrated 20-fold to generate a final sample used in NMR studies (0.3 ml of 1 mm Ncs1-Pik1(111–159) complex).
Lipid Kinase Assay
PtdIns-4-OH kinase activity of S. pombe Pik1 was assayed using a procedure described previously (27) with minor modifications. The reaction product, [32P]PtdIns 4-phosphate, was visualized by autoradiography of TLC plates. The band intensity on the TLC plates was measured and quantified using a PhosphorImager (GE Healthcare). The activity of Pik1 in cell-free extracts prepared from cells completely lacking Ncs1 was studied. For this purpose, a fission yeast strain that lacked Ncs1 expression (h+ his3-D1 ade6-M216 leu1-32 ura4-D18 ncs1Δ::his3 (24)) with overexpression of Pik1 (using plasmid pREP1-PIK1 (a kind gift from S. Hemmingsen) was grown at 30 °C to midexponential phase in minimal medium lacking His and Ura to ensure maintenance of the plasmid, and extracts were prepared by glass bead breakage and clarified by brief centrifugation at 500 × g to remove beads, unbroken cells, and large debris. Samples were assayed under conditions specific for Pik1 activity (51).
NMR Spectroscopy
NMR samples of Ca2+-free myristoylated or unmyristoylated Ncs1 (∼0.7 mm) or Frq1 (0.5 mm) were prepared in 90:10% H2O/D2O or 100% D2O with 5 mm Tris-d11 (pH 7.4) buffer, 4 mm DTT-d11, and 0.3 mm EDTA-d12. NMR samples of Ca2+-bound Ncs1 bound to Pik1(111–159) consisted of 15N-labeled or 13C/15N-labeled Ncs1 bound to 1 eq of unlabeled Pik1(111–159) (1.0 mm) in 0.3 ml of a 95% H2O, 5% {2H}H2O solution containing 5 mm sodium acetate and 2 mm CaCl2 (pH 5.0). Reverse labeled samples (i.e. 15N- or 13C/15N-labeled Pik1(111–174) bound to 1 eq of unlabeled Ncs1) were also prepared for some of the NMR experiments. All NMR experiments were performed at 37 °C on Bruker Avance 600 or 800 spectrometers with an Ultrashield Bruker magnet equipped with a four-channel interface, triple resonance probe, and cryo-probe with z axis pulsed field gradients. 15N-1H HSQC spectra were recorded on samples of 15N-labeled Ncs1 in the presence or absence of unlabeled Pik1(111–159) in 95% H2O, 5% 2H2O. The number of complex points and acquisition times were as follows: 256, 180 ms (15N (F1)) and 512, 64 ms (1H (F2)). The 13C(F1)-edited and 13C(F3)-filtered NOESY-HSQC spectra (see Figs. 4A and 5A) were recorded on a sample of unlabeled Ca2+-free Ncs1 protein attached to a 13C-labeled myristoyl group (Fig. 4A) or unlabeled Ncs1 bound to 13C-labeled Pik1(111–159) (Fig. 5A) as well as 13C-labeled Ncs1 bound to unlabeled Pik1(111–159) (data not shown). Intermolecular NOESY experiments were performed as described previously (52). Stereospecific assignments of chiral methyl groups of valine and leucine were obtained by analyzing 1H-13C HSQC experiments performed on a sample that contained 10% 13C labeling in either Ncs1 or Pik1(111–159). All triple resonance experiments were performed, processed, and analyzed as described previously (53) on a sample of 13C/15N-labeled Ncs1 in the presence or absence of unlabeled Pik1(111–159) (in 95% H2O, 5% 2H2O) with the following number of complex points and acquisition times: HNCO {15N (F1) 32, 23.7 ms; 13CO (F2) 64, 42.7 ms; 1H (F3) 512, 64 ms}; HNCACB {15N (F1) 32, 23.7 ms; 13C (F2) 48, 6.3 ms; 1H (F3) 512, 64 ms}; CBCACONNH {15N (F1) 32, 23.7 ms; 13C (F2) 48, 6.3 ms; 1H (F3) 512, 64 ms}; CBCACOCAHA {13C (F1) 52, 6.8 ms, 13CO (F2) 64, 42 ms, 1H (F3) 384, 64 ms}; and HBHACONNH {15N (F1) 32, 23.7 ms, 1Hab (F2) 64 21 ms, 1H (F3) 512, 64 ms}.
FIGURE 4.
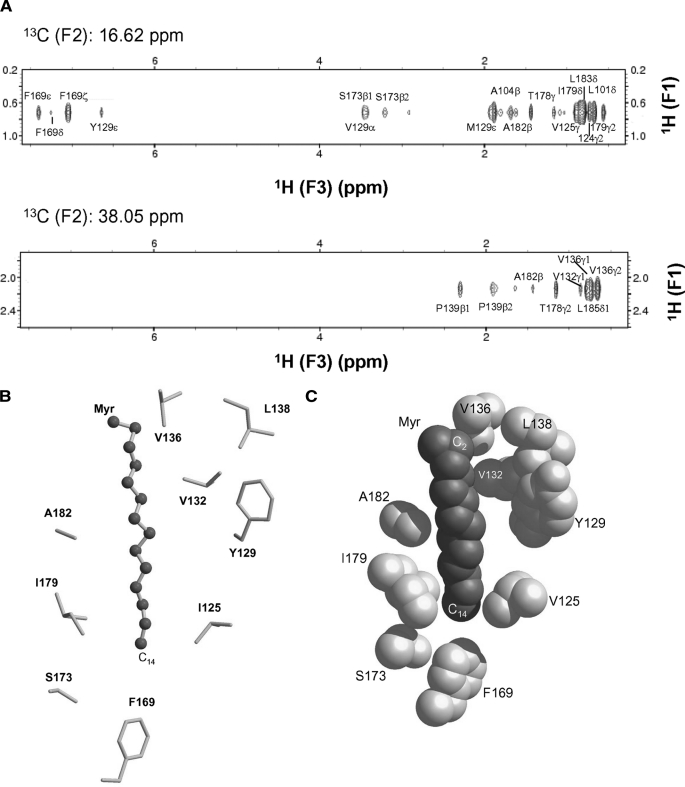
Myristoyl-binding site environment in Ncs1. A, selected slices of 13C(F1)-edited and 13C(F3)-filtered NOESY-HSQC spectra that selectively probe resonances of Ncs1 less than 5 Å away from C14-methyl (top panel) and C2 carbonyl group (bottom panel) of myristate. B, ball-and-stick model of myristoyl group and C-terminal hydrophobic side chain atoms located less than 5 Å away. C, space-filling model of myristate and hydrophobic side chain atoms with same view as in B.
FIGURE 5.
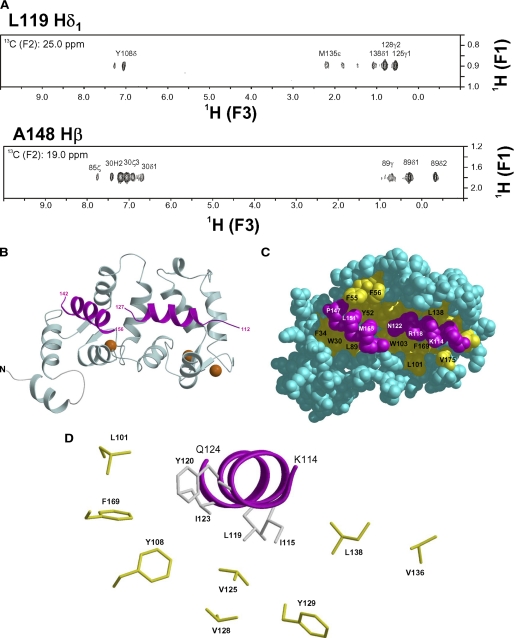
NMR-derived structure of Ca2+-bound Ncs1 bound to Pik(111–159). A, selected slices of 13C(F1)-edited/13C(F3)-filtered NOESY-heteronuclear multiple quantum coherence spectra of 13C-labeled Pik1(111–159) bound to unlabeled Ncs1. B, ribbon diagram of main chain structure of Ca2+-bound Ncs1 bound to Pik(111–159). EF-hands are colored as in Fig. 2. Pik(111–159) is highlighted magenta. C, space-filling representation of Ca2+-bound Ncs1 with same view as in B. D, close-up view of Pik1(111–159) (magenta) with hydrophobic side chains in Ncs1.
The triple resonance and NOESY spectra measured above were analyzed to determine secondary and tertiary structure in Ca2+-free myristoylated Ncs1 and Ca2+-bound unmyristoylated Ncs1-Pik1(111–159) complex. The chemical shift index (see Ref. 54) for detailed description), 3JNHα coupling constants, and NOE connectivity patterns for each residue were analyzed and provided a measure of the overall secondary structure. Small 3JNHα coupling constants (<5 Hz), strong NOE connectivities (NN(i,i + 1) and αN(i,i + 3)), and positive chemical shift index are characteristic of residues in an α-helix. Conversely, large 3JNHα coupling constants (>8 Hz), strong αN(i,i + 1), and weak NN(i,i + 1) NOE connectivities and negative chemical shift index are characteristic of residues in a β-strand. The results of the secondary structure analysis of Ncs1 and Pik1(111–159) are summarized schematically in Fig. 1.
Structure Calculation
Three-dimensional 15N-NOESY-HSQC and 13C-NOESY-HSQC and two-dimensional homonuclear NOESY spectra of Ca2+-free myristoylated Ncs1 and Ca2+-bound Ncs1 (bound to Pik1(111–159)) were analyzed to obtain 1553 and 1225 NOE distance restraints used in the structure calculations, respectively. In addition to the NOE-derived distance constraints, the following additional constraints were included in the structure calculation: 18 distance constraints involving Ca2+ bound to loop residues 1, 3, 5, 7, and 12 in each EF-hand motif (EF-2, EF-3, and EF-4); 124 distance constraints for 80 hydrogen bonds; and 200 dihedral angle constraints (φ and ψ) derived from TALOS (55). Fifty independent structures were calculated by XPLOR-NIH software (56) implemented with YASAP protocol (57), and the 15 structures of lowest energy were selected and overlaid with r.m.s.d. of 0.69 Å (Ca2+-free) and 0.9 Å (Ca2+-bound). Structures of Ca2+-free unmyristoylated Ncs1 were also calculated in a similar fashion. Figures of NMR structures in this paper were prepared with PyMOL or VMD (University of Illinois at Urbana-Champaign).
RESULTS
Structure of Ca2+-free Myristoylated Ncs1
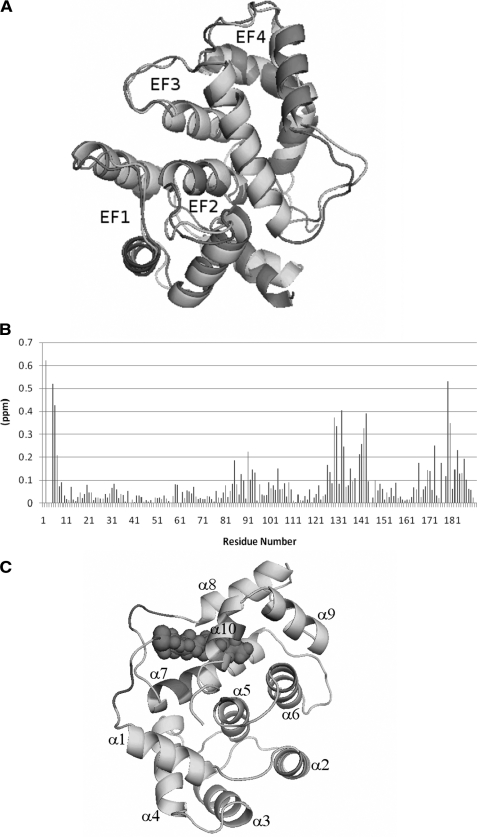
15N-1H HSQC NMR spectra of Ca2+-free Ncs1 (both myristoylated and unmyristoylated forms) exhibited the expected number of highly dispersed peaks with uniform intensities, indicating that Ca2+-free Ncs1 adopts a stable three-dimensional fold. Analysis of 15N relaxation parameters (T1 and T2) indicates an average rotational correlation time of 9.65 ± 0.5 ns, suggesting that Ca2+-free Ncs1 is monomeric in solution under NMR conditions. The spectral similarity for both myristoylated and unmyristoylated forms of Ca2+-free Ncs1 suggests their overall protein structures are similar, and thus we were able to determine the NMR structures of both myristoylated and unmyristoylated forms of Ca2+-free Ncs1. The sequence-specific NMR assignments of Ca2+-free myristoylated Ncs1 were analyzed and described previously (BMRB 16446) (58). The assigned resonances in the HSQC spectrum represent main chain and side chain amide groups that serve as fingerprints of the overall conformation. Three-dimensional protein structures derived from the NMR assignments were calculated on the basis of NOE data, chemical shift analysis, and 3JNHα spin-spin coupling constants (see “Experimental Procedures”). The final NMR-derived structures of Ca2+-free myristoylated Ncs1 are illustrated in Fig. 2, A and B (atomic coordinates have been deposited in the RCSB Protein Data bank, code 2l2e). Table 1 summarizes the structural statistics calculated for the 15 lowest energy conformers.
FIGURE 2.
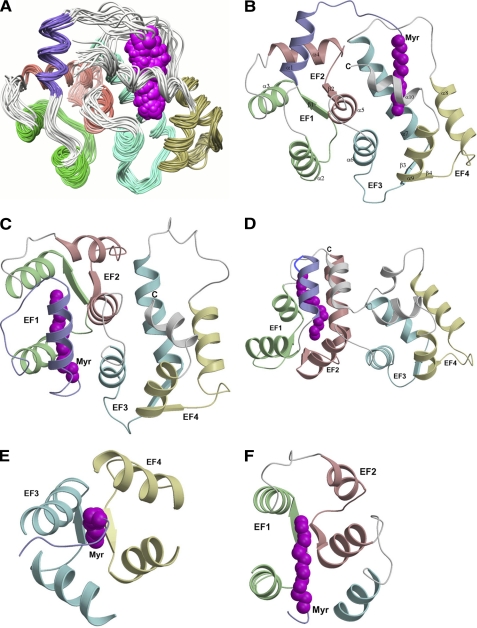
NMR-derived structures of Ca2+-free Ncs1 and comparison with recoverin (Protein Data Bank code 1iku) and GCAP1 (Protein Data Bank code 2rlr). A, superimposed ensemble of NMR structures for Ca2+-free, myristoylated Ncs1. Overall r.m.s.d. is 0.69 Å for main chain atoms (see Table 1). EF-hands and myristoyl group (magenta) are colored as defined in Fig. 1. Ribbon diagrams of Ca2+-free myristoylated Ncs1 (B), recoverin (Protein Data Bank code 1iku) (C), and GCAP1 (Protein Data Bank code 2r2i) (D) are shown. Close-up views of the myristate binding pocket in Ncs1 (E) and recoverin (F) are shown.
TABLE 1.
Structural statistics for Ca2+-free Ncs1
| Myristoylated | Unmyristoylated | |
|---|---|---|
| NMR restraints | ||
| Short range NOE (i to i + j, j = 1–4) | 1010 | 506 |
| Long range NOE (i to i + j, j >4) | 446 | 268 |
| Hydrogen bonds | 124 | 124 |
| Dihedral angle restraints | 200 | 200 |
| Protein-myristate | 18 | |
| r.m.s.d. to the mean coordinates | ||
| Backbone of structured regionsa | 0.69 ± 0.05 Å | 0.85 ± 0.09 Å |
| Heavy atoms of structured regions | 1.17 ± 0.08 Å | 1.34 ± 0.1 Å |
| r.m.s.d. from idealized geometry | ||
| Bond lengths | 0.0064 ± 0.0001 Å | 0.0059 ± 0.0001 Å |
| Bond angles | 2.00 ± 0.0014° | 2.00 ± 0.0014° |
| Impropers | 0.9 ± 0.005° | 0.9 ± 0.005° |
| Ramachandran statistics of 15 structures | ||
| Most favored regions | 85% | 82% |
| Additional allowed regions | 12% | 15% |
| Generously allowed regions | 2% | 3% |
| Disallowed regions | 1% | 0% |
a Pairwise r.m.s.d. was calculated among 15 refined structures: residues in regions of regular secondary structure (10–20, 25–37, 42–55, 62–72, 79–89, 98–108, 115–130, 146–156, 163–174, 178–183).
Aside from the last two residues at the C terminus, the entire polypeptide chain of Ca2+-free myristoylated Ncs1 could be determined from the NMR data. Ncs1 contains a total of 10 α-helices and 4 β-strands: α1 (residues 10–20), α2 (residues 25–35), α3 (residues 46–56), α4 (residues 61–71), α5 (residues 84–91), α6 (100–109), α7 (residues 120–133), α8 (residues 146–157), α9 (residues 167–176), and α10 (residues 178–184) and β1 (residues 42–44), β2 (residues 79–81), β3 (residues 115–117), and β4 (residues 163–165) (Fig. 1A). The covalently attached myristoyl group at Gly2 is connected to a long extended structure (residues Gly2–Arg21) called an N-terminal arm (highlighted purple in Fig. 2B) that reaches more than 15 Å to insert the fatty acyl chain inside a protein cavity near the C terminus. Ncs1 contains two domains comprising four EF-hands (Fig. 2, A and B) as follows: EF1 (green, residues 26–55) and EF2 (red, residues 62–91) are linked and form the N-domain; likewise, EF3 (cyan, residues 101–130) and EF4 (yellow, residues 146–175) form the C-domain. The interface between the two domains is established by interactions between EF2 (Asp84, Cys87, Ala88, and Ser90 in helix α5) and EF3 (Lys100, Trp103, Ala104, and Leu107 in helix α6; and Arg123, Ala127, Ile128, and Met131 in helix α7) that connect the two domains. The domain interface in Ncs1 is similar to that in Ca2+-free myristoylated recoverin (Fig. 2C) but very different from that of myristoylated GCAP1 (Fig. 2D) (50). Each EF-hand in Ncs1 consists of a helix-turn-helix structure similar to the closed structure of Ca2+-free EF-hands seen in previous structures of Ca2+-free recoverin (38) and apo-CaM (59). The interhelical angles for the EF-hands of Ncs1 are 146.9° (EF1), 121.7° (EF2), 119.3° (EF3), and 107.8° (EF4). The four EF-hands of Ncs1 are grouped in a compact globular topology (Fig. 2, A and B) only somewhat similar to that found in Ca2+-free recoverin (Fig. 2C) and very different from the tandem EF-hand arrangement seen in GCAP1 (Fig. 2D). The C-terminal helix (α10) of Ncs1 is displaced somewhat from the protein core to make room for the N-terminal myristoyl group that inserts inside a cavity formed between EF3 and EF4 (Fig. 2B). The fatty acyl chain in the cavity is nearly parallel to the helices of EF3 and EF4 that form walls that surround the myristoyl moiety (Fig. 2E). This arrangement is in stark contrast to recoverin where the myristate is wedged perpendicularly between the helices of EF1 and EF2 (Fig. 2F). Indeed, the overall main chain structure of Ca2+-free myristoylated Ncs1 (Fig. 2B) is different from the myristoylated forms of recoverin (Fig. 2C) and GCAP1 (Fig. 2D). When comparing the main chain atoms of Ca2+-free Ncs1 with those of recoverin and GCAP1, the root mean square deviations are 2.3 and 3.4 Å, respectively.
Structure of Ca2+-free Unmyristoylated Ncs1
The NMR chemical shift assignments and structure for Ca2+-free myristoylated Ncs1 are very similar to those of Ca2+-free unmyristoylated Ncs1 (see overlaid structures in Fig. 3A). The root mean square deviation between the two structures is 1.18 Å. The main structural differences are detected in the N-terminal arm (residues Gly2–Arg21) and C-terminal helix, both of which are somewhat destabilized and shortened by the absence of the myristoyl group (e.g. helix α1, residues 10–18, and helix α10, residues 178–186). The 15N chemical shift differences between myristoylated and unmyristoylated Ncs1 are plotted as a function of residue number in Fig. 3B. The largest chemical shift differences are observed for the residues that contact the myristoyl group (Fig. 3C). The overall main chain structures for myristoylated and unmyristoylated Ncs1 are nearly identical in the EF-hand regions (r.m.s.d. = 1.04 Å). Thus, the myristoyl group does not alter the main chain structure of the EF-hands in Ca2+-free Ncs1, but instead the myristoyl chain penetrates inside the protein by displacing hydrophobic side chains. Indeed, the Ca2+-free myristoylated Ncs1 has a slightly higher melting temperature compared with unmyristoylated Ncs1 (measured by differential scanning calorimetry, data not shown), consistent with stabilization of the hydrophobic core by the sequestered myristoyl group.
FIGURE 3.
A, superposition of Ca2+-free myristoylated Ncs1 (light gray) onto Ca2+-free unmyristoylated Ncs1 (dark gray). Overall, the two structures are superimposed with a main chain r.m.s.d. of 1.18 Å. B, chemical shift difference (amide 15N) between myristoylated and unmyristoylated Ncs1 plotted versus residue number showing structural differences at the N and C termini and loop region between EF3 and EF4, where the myristate interacts. C, ribbon diagram of Ca2+-free Ncs1 highlighting the residues in dark gray that contact the myristoyl group and show the largest 15N chemical shift differences from B.
Myristoyl-binding Site in Ncs1
The structural environment around the covalently attached myristoyl group in Ncs1 was determined by analyzing NMR experiments (three-dimensional (13C/F1) edited and (13C/F3) filtered NOESY-HSQC) performed on unlabeled Ca2+-free Ncs1 samples that contained a 13C-labeled myristoyl group (Fig. 4A). These NMR spectra selectively probed atoms in Ncs1 that lie within 5 Å of the 13C-labeled fatty acyl chain. We analyzed nuclear Overhauser effect (NOE) dipolar interactions between the C14 methyl of the myristoyl group (13C14, F2 = 16.62 ppm) and the protein (Fig. 4A, upper panel), and between the C2 methylene of the myristoyl chain (13C2, F2 = 38.05 ppm) and the protein (Fig. 4A, lower panel). The spectra probing the C14 methyl group (Fig. 4A, upper panel) exhibit many off-diagonal peaks, which could be assigned to residues with aromatic ring protons (Tyr129 and Phe169) and to protons in aliphatic side chains (Leu101, Ile124, Val125, Met121, Ile179, and Leu183). Thus, the C14 methyl group is surrounded by hydrophobic side chains from residues in EF3 and EF4 and the C-terminal helix. The spectra probing the C2 position of the myristoyl moiety (Fig. 4A, lower panel) exhibit off-diagonal peaks assigned to residues in the loop between EF3 and EF4 (Val132, Val136, and Pro139) and the C-terminal helix (Thr178, Ala182, and Leu185). On the basis of these NMR data, the N-terminal myristoyl group in Ncs1 resides inside a protein cavity located in the C-terminal domain; in marked contrast, the myristoyl group in recoverin (38) and in GCAP1 (50) is housed in an N-terminal cavity in both proteins. The myristoyl group attached to Ncs1 adopts an extended conformation (Fig. 4B) that is about 75% buried inside the protein (Fig. 4C). The C14 methyl group of the myristate makes close contacts with hydrophobic side chains from Val125, Phe169, and Ile179 located inside the hydrophobic core (Fig. 4, B and C). Thus, the C14 methyl end of the myristoyl group protrudes deep inside the protein. The middle of the fatty acyl chain makes hydrophobic contacts with side chains of Tyr129 and Ala182. The carbonyl end of the myristate contacts the side chains of Val132, Val136, and Leu138 on the protein surface. The hydrophobic environment around the myristoyl group in Ncs1 consists of mainly aliphatic side chain groups (Leu101, Val125, Val132, Val136, Ile179, and Ala182) that is quite different from the nearly all-aromatic environment around the myristoyl moiety in recoverin (Trp31, Tyr32, Phe49, Tyr53, Phe56, Phe57, and Tyr86) (38, 60).
Ca2+-induced Activation of Fission Yeast PtdIns 4-Kinase by Ncs1
In budding yeast, Frq1 activates a PtdIns 4-kinase isoform called Pik1 (21). Both Frq1 and Pik1 are highly conserved in fission yeast, and therefore we set out to verify whether S. pombe Pik1 is similarly activated by Ncs1. The effects of Ca2+ and Ncs1 on the catalytic activity of PtdIns 4-kinase in fission yeast were monitored using an in vitro enzyme assay described previously (see supplemental Fig. 1) (27). Although S. pombe Pik1 displayed detectable basal activity in the absence of exogenously added Ncs1 and Ca2+, the enzyme was stimulated close to 5-fold in the presence of a saturating concentration of Ca2+ and myristoylated Ncs1 (supplemental Fig. 1). Thus, Ca2+-bound Ncs1 activates S. pombe Pik1 similar to Frq1 activation of S. cerevisiae Pik1 (21). In the absence of added Ca2+, addition of myristoylated Ncs1 had a negligible stimulatory effect (supplemental Fig. 1). The most likely explanation for this observation is that sequestration of the myristoyl group in Ca2+-free Ncs1 (Fig. 2) blocks the residues in Ncs1 that are necessary to contact its binding site on Pik1. In agreement with this view, unmyristoylated Ncs1 yielded modest, but detectable, stimulation of the lipid kinase even in the absence of added Ca2+ (supplemental Fig. 1). Furthermore, as we demonstrate below, Ncs1 undergoes a Ca2+-myristoyl switch in which Ca2+-induced extrusion of the myristoyl group exposes critical residues involved in interacting with the PtdIns 4-kinase (see under “Discussion”).
Structure of Ca2+-bound Ncs1 Bound to PtdIns 4-Kinase
Previous studies showed that budding yeast Frq1 interacts with a localized region of Pik1 (residues 121–174, called Pik1(121–174) (22, 26). Fission yeast Pik1 contains a very similar sequence at a very similar location (Fig. 1B). We therefore tested whether a peptide fragment of this region of S. pombe Pik1 (residues 111–159), hereafter Pik1(111–159), might interact with Ncs1. Isothermal titration calorimetry data (supplemental Fig. 2) show that S. pombe Pik1(111–159) does indeed bind to Ca2+-bound Ncs1 (Kd = 0.5 ± 0.3 μm and ΔH = 2.4 kcal/mol), very similar to the binding of Frq1 to Pik1(121–174) in budding yeast (22). The high affinity and endothermic reaction of Ca2+-bound Ncs1 with Pik(111–159) indicates that Ca2+-bound Ncs1 must bind to the PtdIns 4-kinase in a largely entropy-driven manner, suggesting primarily a hydrophobic interaction, as was described previously for S. cerevisiae Pik1-Frq1 association (22).
Next, we set out to determine the NMR structure of Ca2+-bound Ncs1 bound to S. pombe Pik(111–159), as was done previously for Frq1 bound to Pik1(121–174) (26). The NMR spectra and assignments of Ca2+-bound Ncs1-Pik1(111–159) complex are shown in supplemental Fig. 5. More than 85% of the backbone assignments for Ncs1 and Pik1(111–159) were obtained as described under “Experimental Procedures.” The unassigned residues were located in unstructured regions as follows: loop residues 134–139 for Pik1(111–159) and the last eight residues at the C terminus for Ncs1. The NMR assignments in supplemental Fig. 5 then served as the basis for analyzing both intramolecular and intermolecular NOESY spectra as described for the Frq1-Pik1(121–174) complex (26). This analysis of the NOESY spectra provides distance constraints for determining the overall protein fold and probing contacts from key residues at the protein interface (Fig. 5A). The NMR-derived structure of Ca2+-bound Ncs1-Pik1(111–159) complex is shown in Fig. 5, B–D, and structural statistics are given in Table 2.
TABLE 2.
Structural statistics for the Ca2+-bound Ncs1-Pik1 complex
| Ncs1 | Pik1(111–159) | |
|---|---|---|
| NMR restraints | ||
| Short range NOE (i to i + j, j = 1–3) | 715 | 112 |
| Long range NOE (i to i + j, j > 3) | 250 | 62 |
| Hydrogen bonds | 124 | 38 |
| Calcium bound at EF2, EF3, and EF4 | 18 | |
| Dihedral angle restraints | 200 | 54 |
| Intermolecular distance restraints | 66 | |
| r.m.s.d. to the mean coordinates | ||
| Backbone of structured regionsa | 0.9 ± 0.07 Å | 0.9 ± 0.09 Å |
| Heavy atoms of structured regions | 1.4 ± 0.08 Å | 1.4 ± 0.1 Å |
| r.m.s.d. from idealized geometry | ||
| Bond lengths | 0.007 ± 0.0001 Å | 0.009 ± 0.0001 Å |
| Bond angles | 2.07 ± 0.04° | 2.09 ± 0.04° |
| Impropers | 0.9 ± 0.05° | 0.95 ± 0.05° |
| Ramachandran statistics of 15 structures | ||
| Most favored regions | 76% | 80% |
| Additional allowed regions | 17% | 15% |
| Generously allowed regions | 6% | 4% |
| Disallowed regions | 1% | 1% |
a Residues in regions of regular secondary structure: Ncs1, residues 9–16, 24–37, 42–55, 62–72, 79–92, 99–108, 115–129, 145–156, 163–175; Pik1, residues 113–125, 144–156.
The four EF-hands in Ncs1 are arranged in a tandem array and, overall, form a globular structure with a concave solvent-exposed groove lined by two separate hydrophobic patches (highlighted yellow in Fig. 5C). These two hydrophobic surfaces represent bipartite binding sites on Ncs1 that interact with Pik1(111–159). The structure of Pik1(111–159) in the complex adopts a conformation that contains two α-helices (residues 114–127 and 143–156) connected by a disordered loop. The N-terminal helix contains hydrophobic residues (Ile115, Cys116, Leu119, and Ile123) that contact C-terminal residues of Ncs1 (Leu101, Trp103, Val125, Val128, Leu138, Ile152, Leu155, and Phe169) (see Fig. 5D). Interestingly, these same hydrophobic residues in Ca2+-free Ncs1 make close contacts with the myristoyl group. Therefore, Ca2+-induced extrusion of the myristoyl group causes exposure of hydrophobic residues in Ncs1 that forms part of the Pik1-binding site. This hydrophobic interaction was further confirmed using site-specific mutagenesis in which the L119A mutant of Pik1(111–159) binds to Ncs1 with ∼3-fold lower affinity (supplemental Fig. 2). The opposite face of the Pik1 N-terminal helix contains polar and positively charged residues (Lys119, Arg120, Asn123, and Arg124) that point outward toward the solvent. The C-terminal helix of Pik1(111–159) contains many hydrophobic residues (Val145, Ala148, Ile150, and Ile154) that contact the exposed N-terminal hydrophobic groove of Ncs1 (Trp30, Phe34, Phe48, Ile51, Tyr52, Phe55, Phe85, and Leu89). The two helices of Pik1(111–159) do not interact with one another or with the unstructured connecting loop and are highly stabilized by interactions with Ncs1.
Ca2+-induced Conformational Changes in Ncs1
Comparing the structures of Ca2+-free (Fig. 2B) and Ca2+-bound (Fig. 5B) Ncs1 reveals large Ca2+-induced protein conformational changes (see supplemental Movie 1) analogous to the Ca2+-myristoyl switch in recoverin (39, 60). The structures of Ca2+-free and Ca2+-bound Ncs1 have an overall r.m.s.d. of 7 Å when comparing all heavy atoms. The topology of Ca2+-free Ncs1 in the EF-hand regions is somewhat similar to that of Ca2+-free recoverin (r.m.s.d. = 1.8 Å) except that the N-terminal myristoyl group is buried in a cavity formed by EF3 and EF4 (Fig. 2E) rather than the cavity in recoverin formed by EF1 and EF2 (Fig. 2F). Ca2+-binding at EF2, EF3, and EF4 in Ncs1 causes the familiar closed-to-open transition in the EF-hands that promotes a 45° swiveling about Gly95 in the domain linker and results in a repacking of the domain interface (supplemental Fig. 3). The swiveling of the two domains then pulls the N-terminal myristoyl group out of the protein cavity at the C terminus, resulting in Ca2+-induced exposure of the myristoyl group and a concomitant exposure of hydrophobic residues (Leu101, Val125, Val128, Leu138, Ile152, Leu155, and Phe169) that contact the myristoyl group in the Ca2+-free protein. Ca2+-induced conformational changes in the N-domain occur simultaneously and result in the exposure of many additional hydrophobic residues (Trp30, Phe34, Phe48, Ile51, Tyr52, Phe55, Phe85, and Leu89), forming an exposed hydrophobic crevice (Fig. 5C), similar to those seen in all other Ca2+-bound NCS proteins examined to date (28–32). In essence, Ca2+ binding to Ncs1 leads to domain swiveling that causes extrusion of the myristoyl group. As a result of conformational changes in both domains, two separate hydrophobic patches are formed on the surface of Ca2+-bound Ncs1 that can now accommodate the hydrophobic faces of the two amphipathic helices from Pik1 (Fig. 5, B–D).
DISCUSSION
In this study, we determined NMR structures for Ca2+-free myristoylated Ncs1 and Ca2+-bound Ncs1 complexed to a fragment (residues 111–159) of a fission yeast PtdIns 4-kinase. Ca2+-free Ncs1 adopts a novel structure where the N-terminal myristoyl group is sequestered inside a protein cavity located near the C terminus (Figs. 2B and 4C). The structural location and environment around the myristoyl group in Ncs1 is very different from that in either recoverin (38) or GCAP1 (Fig. 2) (50). We suggest that each NCS protein adopts a distinct structure because its N-terminal myristoyl group associates with patches of hydrophobic residues that are unique to that protein; thus, upon Ca2+-evoked extrusion of the myristoyl group, a distinctive ensemble of hydrophobic residues is unmasked, exposing surface residues that allow each class of NCS protein to associate specifically with a particular physiological target. This scenario explains how a Ca2+ signal can cause each NCS family member to engage a different physiological target, despite the high degree of sequence similarity among NCS family members (1).
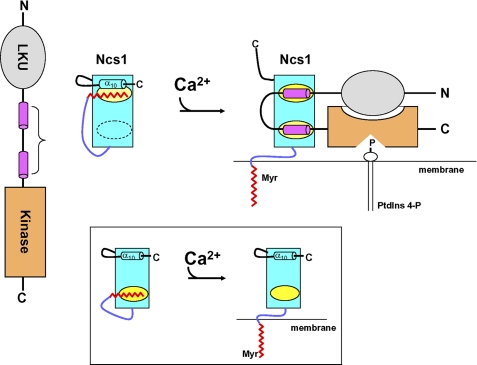
As we have documented here, Ca2+ binding to Ncs1 causes very large protein structural changes that causes extrusion of the myristoyl group, quite analogous to the Ca2+-myristoyl switch described previously for recoverin (39). The Ca2+-induced exposure of the myristoyl group for Ncs1 explains why myristoylated (but not unmyristoylated) Ncs1 binds to S. pombe cell membranes only at high Ca2+ levels (24). The Ca2+-induced extrusion of the myristoyl moiety also exposes two hydrophobic patches on a concave surface of the Ca2+-bound protein that provide sites for making important contacts with PtdIns 4-kinase (Fig. 5). Therefore, the Ca2+-myristoyl switch promotes both the capacity of Ncs1 to bind and activate the lipid kinase and controls the delivery of the Ncs1-Pik1 complex to the membrane where the substrate for this enzyme resides (Fig. 6).
FIGURE 6.
Model of Ca2+-induced activation or PtdIns 4-kinase by Ncs1. Left panel shows schematic diagrams of Pik1 and Ncs1 at low Ca2+. LKU motif (residues 23–98) is in gray; Ncs1-binding region (111–159) is in magenta, and catalytic (kinase) domain (residues 642–916) is in orange. In Ca2+-free Ncs1, an N-terminal arm (purple) places the myristoyl group (red) in a hydrophobic cavity (yellow) flanked by a C-terminal helix (α10). Right panel shows Ca2+-induced conformational changes in Ncs1 that cause exposure of myristate and two hydrophobic patches (yellow), followed by structural rearrangement in Pik1 induced by its binding to Ca2+-bound Ncs1. Ncs1 binding to Pik1 imposes a U-turn in the main chain of Pik1 that is necessary to allow the LKU domain (gray) to interact with the catalytic domain (orange). In addition, N-myristoylation of Ncs1 (red) helps deliver the complex to membranes where it can bind to substrate (PtdIns). Inset shows schematic diagram of a somewhat different Ca2+-myristoyl switch mechanism for recoverin.
Previous studies have shown that various frequenins (mammalian NCS-1 (30) and yeast Frq1 (61)) appear localized to membranes even at low Ca2+ levels, suggesting that NCS-1 and Frq1 may not possess a functional myristoyl switch (62). Indeed, NMR structural studies on Frq1 suggested the Ca2+-free myristoylated Frq1 protein is in a partially unfolded molten-globule state, and the myristoyl group remains solvent-exposed regardless of Ca2+ level (63). These observations of a constitutively exposed myristoyl group are in stark contrast with the Ca2+-induced extrusion of the myristoyl group in recoverin (39) and Ncs1 (this study). Mutagenesis studies have suggested that particular residues in NCS-1 (62) might be responsible for preventing a Ca2+-myristoyl switch. However, these residues are somewhat conserved in both S. pombe Ncs1 and mammalian NCS-1 and do NOT prevent the Ca2+-myristoyl switch in this case. The very high sequence identity (≥60%) among NCS-1, Frq1, and Ncs1, would imply that the three-dimensional structures of Ncs1, NCS-1, and Frq1 must all be very similar. We considered the possibility that the persistent exposure of the myristoyl group in Frq1 and NCS-1 observed in prior work might be an artifact caused by protein misfolding due to the tags (His6 or GFP) attached to the C terminus in those previous studies because our structure of Ncs1 shows that the myristoyl group makes important contacts with residues close to the C terminus (Ile179, Leu183, and Leu185). Indeed, NMR spectra of Ca2+-free myristoylated Frq1 prepared without a C-terminal His6 tag exhibit methyl resonances below 0 ppm (due to aromatic ring currents in the hydrophobic core), which are similar to those observed for Ncs1 and characteristic of a folded protein (supplemental Fig. 4). By stark contrast, NMR spectra of Ca2+-free myristoylated Frq1 that contains a C-terminal His6 tag completely lack any resonances below 0 and above 9 ppm, indicative of a molten-globule (unfolded) state. Based on these findings and our structure of untagged myristoylated Ncs1 in the absence and presence of Ca2+, we feel that it is highly likely that all frequenins (from mammalian NCS-1 to yeast Frq1) will have structures very similar to that of Ncs1 (Figs. 2 and 5) and will undergo a Ca2+-myristoyl switch that is critical for its function. Thus, it seems clear from our findings that some aspects of studies of the subcellular localization and cellular roles of NCS family members might have been compromised by the use of tagged derivatives that may have caused significant structural perturbations.
Nonconserved residues of NCS proteins at the C terminus (α10) and immediately following EF3 (Fig. 1A) interact closely with the N-terminal myristoyl group in Ncs1 and thus help stabilize the novel structure of Ca2+-free Ncs1 (Fig. 2B). The corresponding residues in recoverin and GCAP1 do not contact the myristoyl group. Instead, both recoverin and GCAP1 have nonconserved residues near the N terminus (called an N-terminal arm highlighted purple in Fig. 2) that make specific contacts with the myristoyl moiety. GCAP1 also contains an extra helix at the C terminus that contacts the N-terminal arm and myristoyl group (Fig. 2D). Thus, nonconserved residues at the N and C termini and loop between EF3 and EF4 all play a role in creating a unique environment around the myristoyl group. In Ncs1, the long N-terminal arm and particular hydrophobic residues in the C-terminal helix are crucial for placing the C14 fatty acyl chain in a cavity between EF3 and EF4 (Fig. 2E). By contrast, the much shorter N-terminal arm in both recoverin and GCAP1 prevents the myristoyl group from reaching the C-terminal cavity and instead places the fatty acyl chain between EF1 and EF2 (Fig. 2F). Likewise, nonconserved residues at the N and C termini and/or loop between EF3 and EF4 may play a role in forming unique myristoyl binding environments in other NCS proteins, such as visinin-like proteins, neurocalcins, and hippocalcins that may explain their capacity to associate with functionally diverse targets once they have undergone a Ca2+-induced conformational change.
Aside from NCS family members, N-terminal myristoylation confers important structural effects in many other classes of proteins. The structures of myristoylated forms of ARF (64), Bcr-Abl (65), c-Abl (66), and the HIV-1 matrix protein (67) all reveal intimate contacts between the protein and the fatty acyl chain that help mold these proteins into biologically active structures. Interaction of the myristoyl group with oncogenic Bcr-Abl protein is critical for activating tyrosine kinase activity, and drugs (e.g. imatinib) important for treating human leukemias prevent access to the active conformation by occluding the myristoyl binding pocket (65). In the ARF protein, the myristoyl moiety interacts with important switch residues that control GTPase activity involved in regulating vesicular trafficking. Finally, interactions of the myristoyl group with other parts of the HIV-1 matrix protein control its oligomerization, an important step in how this virus targets a host cell. These highly diverse structural interactions demonstrate that N-terminal myristoylation is an important tool for shaping protein structures into distinct and physiologically active conformations.
The structures of Ca2+-free Ncs1 and Ncs1-Pik1 complex (Figs. 2 and 5) suggest how a Ca2+-myristoyl switch might promote activation of PtdIns 4-kinase (Fig. 6). Under resting basal conditions, cytosolic Ca2+ levels are presumably maintained below 100 nm and Ncs1 exists in its Ca2+-free state with a sequestered myristoyl group buried in the C-domain that covers part of its binding site for PtdIns 4-kinase (highlighted yellow in Fig. 6) and prevents binding of Ncs1 to Pik1. The fatty acyl chain has the same molecular dimensions (length and width) as the N-terminal helix of Pik1(111–159), which explains why the myristoyl group and Pik1 helix can effectively compete for the same binding site in Ncs1. A rise in cytosolic Ca2+ will cause Ca2+-induced conformational changes in Ncs1, resulting in extrusion of the N-terminal myristoyl group (see supplemental Movie 1). Ca2+-induced extrusion of the myristoyl group exposes a hydrophobic crevice in the C-terminal domain of Ncs1, and concomitantly, Ca2+-induced structural changes in its N-domain result in formation of a second exposed hydrophobic crevice, also seen in all other Ca2+-bound NCS proteins examined to date (26, 28–31). These two separate hydrophobic sites on the surface of Ca2+-bound Ncs1 are different from Ca2+-bound recoverin that contains only one exposed hydrophobic patch (Fig. 6, inset) that interacts with a single target helix in rhodopsin kinase (68). The two exposed hydrophobic sites on Ncs1 bind to the hydrophobic faces of the two antiparallel amphipathic α-helices in Pik1(111–159) (colored magenta in Figs. 5B and 6), akin to the mechanism proposed previously for S. cerevisiae Frq1 and Pik1(121–174) (26). The Ca2+-induced binding of Ncs1 to PtdIns 4-kinase may promote a long range structural interaction between the LKU and catalytic domains, which are conserved in both S. cerevisiae and S. pombe Pik1 (26), leading to acquisition of the conformation optimal for lipid kinase activity. Simultaneously, Ncs1 binding to PtdIns 4-kinase will also promote membrane localization of the lipid kinase, because Ca2+-bound Ncs1 contains an extruded myristoyl group that serves as a membrane anchor. Thus, Ncs1 controls both delivery of PtdIns 4-kinase to the membrane where its substrates are located and formation of the optimally active state of the enzyme. We propose that a corresponding Ca2+-induced membrane localization and activation of PtdIns 4-kinase-β by NCS-1 may take place in neurons and may serve to couple phosphoinositide cascades with calcium signaling pathways, which is thought to be important in synaptic plasticity (49).
Acknowledgments
We are grateful to Dr. Mitsuhiko Ikura for critical comments; Dr. Jerry Dallas for help with NMR experiments; Dr. Sean Hemmingsen for providing the pREP1-Pik1 plasmid; and Frank Delaglio for writing computer software for NMR data processing and analysis.
This work was supported, in whole or in part, by National Institutes of Health Grant EY012347 (to J. B. A.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–5 and Movie S1.
The atomic coordinates and structure factors (code 2l2e) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
- NCS
- neuronal calcium sensor
- HSQC
- heteronuclear single quantum coherence
- r.m.s.d.
- root mean squared deviation
- PtdIns 4-kinase
- phosphatidylinositol 4-kinase.
REFERENCES
- 1. Burgoyne R. D., O'Callaghan D. W., Hasdemir B., Haynes L. P., Tepikin A. V. (2004) Trends Neurosci. 27, 203–209 [DOI] [PubMed] [Google Scholar]
- 2. Burgoyne R. D., Weiss J. L. (2001) Biochem. J. 353, 1–12 [PMC free article] [PubMed] [Google Scholar]
- 3. Braunewell K. H., Gundelfinger E. D. (1999) Cell Tissue Res. 295, 1–12 [DOI] [PubMed] [Google Scholar]
- 4. Ikura M., Ames J. B. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 1159–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ames J. B., Tanaka T., Stryer L., Ikura M. (1996) Curr. Opin. Struct. Biol. 6, 432–438 [DOI] [PubMed] [Google Scholar]
- 6. Dizhoor A. M., Ray S., Kumar S., Niemi G., Spencer M., Brolley D., Walsh K. A., Philipov P. P., Hurley J. B., Stryer L. (1991) Science 251, 915–918 [DOI] [PubMed] [Google Scholar]
- 7. Chen C. K., Inglese J., Lefkowitz R. J., Hurley J. B. (1995) J. Biol. Chem. 270, 18060–18066 [DOI] [PubMed] [Google Scholar]
- 8. Kawamura S. (1993) Nature 362, 855–857 [DOI] [PubMed] [Google Scholar]
- 9. Erickson M. A., Lagnado L., Zozulya S., Neubert T. A., Stryer L., Baylor D. A. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 6474–6479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Palczewski K., Subbaraya I., Gorczyca W. A., Helekar B. S., Ruiz C. C., Ohguro H., Huang J., Zhao X., Crabb J. W., Johnson R. S. (1994) Neuron 13, 395–404 [DOI] [PubMed] [Google Scholar]
- 11. Dizhoor A. M., Olshevskaya E. V., Henzel W. J., Wong S. C., Stults J. T., Ankoudinova I., Hurley J. B. (1995) J. Biol. Chem. 270, 25200–25206 [DOI] [PubMed] [Google Scholar]
- 12. Payne A. M., Downes S. M., Bessant D. A., Taylor R., Holder G. E., Warren M. J., Bird A. C., Bhattacharya S. S. (1998) Hum. Mol. Genet. 7, 273–277 [DOI] [PubMed] [Google Scholar]
- 13. Sokal I., Li N., Surgucheva I., Warren M. J., Payne A. M., Bhattacharya S. S., Baehr W., Palczewski K. (1998) Mol. Cell 2, 129–133 [DOI] [PubMed] [Google Scholar]
- 14. Hidaka H., Okazaki K. (1993) Neurosci. Res. 16, 73–77 [DOI] [PubMed] [Google Scholar]
- 15. Kobayashi M., Takamatsu K., Saitoh S., Noguchi T. (1993) J. Biol. Chem. 268, 18898–18904 [PubMed] [Google Scholar]
- 16. Braunewell K. H., Klein-Szanto A. J., Szanto A. J. (2009) Cell Tissue Res. 335, 301–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. An W. F., Bowlby M. R., Betty M., Cao J., Ling H. P., Mendoza G., Hinson J. W., Mattsson K. I., Strassle B. W., Trimmer J. S., Rhodes K. J. (2000) Nature 403, 553–556 [DOI] [PubMed] [Google Scholar]
- 18. Pongs O., Lindemeier J., Zhu X. R., Theil T., Engelkamp D., Krah-Jentgens I., Lambrecht H. G., Koch K. W., Schwemer J., Rivosecchi R. (1993) Neuron 11, 15–28 [DOI] [PubMed] [Google Scholar]
- 19. Tzingounis A. V., Kobayashi M., Takamatsu K., Nicoll R. A. (2007) Neuron 53, 487–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tsujimoto T., Jeromin A., Saitoh N., Roder J. C., Takahashi T. (2002) Science 295, 2276–2279 [DOI] [PubMed] [Google Scholar]
- 21. Hendricks K. B., Wang B. Q., Schnieders E. A., Thorner J. (1999) Nat. Cell Biol. 1, 234–241 [DOI] [PubMed] [Google Scholar]
- 22. Huttner I. G., Strahl T., Osawa M., King D. S., Ames J. B., Thorner J. (2003) J. Biol. Chem. 278, 4862–4874 [DOI] [PubMed] [Google Scholar]
- 23. Strahl T., Hama H., DeWald D. B., Thorner J. (2005) J. Cell Biol. 171, 967–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hamasaki-Katagiri N., Molchanova T., Takeda K., Ames J. B. (2004) J. Biol. Chem. 279, 12744–12754 [DOI] [PubMed] [Google Scholar]
- 25. Hamasaki-Katagiri N., Ames J. B. (2010) J. Biol. Chem. 285, 4405–4414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Strahl T., Huttner I. G., Lusin J. D., Osawa M., King D., Thorner J., Ames J. B. (2007) J. Biol. Chem. 282, 30949–30959 [DOI] [PubMed] [Google Scholar]
- 27. Park J. S., Steinbach S. K., Desautels M., Hemmingsen S. M. (2009) PLoS One 4, e6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Flaherty K. M., Zozulya S., Stryer L., McKay D. B. (1993) Cell 75, 709–716 [DOI] [PubMed] [Google Scholar]
- 29. Vijay-Kumar S., Kumar V. D. (1999) Nat. Struct. Biol. 6, 80–88 [DOI] [PubMed] [Google Scholar]
- 30. Bourne Y., Dannenberg J., Pollmann V., Marchot P., Pongs O. (2001) J. Biol. Chem. 276, 11949–11955 [DOI] [PubMed] [Google Scholar]
- 31. Zhou W., Qian Y., Kunjilwar K., Pfaffinger P. J., Choe S. (2004) Neuron 41, 573–586 [DOI] [PubMed] [Google Scholar]
- 32. Stephen R., Palczewski K., Sousa M. C. (2006) J. Mol. Biol. 359, 266–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ames J. B., Dizhoor A. M., Ikura M., Palczewski K., Stryer L. (1999) J. Biol. Chem. 274, 19329–19337 [DOI] [PubMed] [Google Scholar]
- 34. Ames J. B., Hendricks K. B., Strahl T., Huttner I. G., Hamasaki N., Thorner J. (2000) Biochemistry 39, 12149–12161 [DOI] [PubMed] [Google Scholar]
- 35. Lusin J. D., Vanarotti M., Li C., Valiveti A., Ames J. B. (2008) Biochemistry 47, 2252–2264 [DOI] [PubMed] [Google Scholar]
- 36. Babu Y. S., Bugg C. E., Cook W. J. (1988) J. Mol. Biol. 204, 191–204 [DOI] [PubMed] [Google Scholar]
- 37. Herzberg O., James M. N. (1988) J. Mol. Biol. 203, 761–779 [DOI] [PubMed] [Google Scholar]
- 38. Tanaka T., Ames J. B., Harvey T. S., Stryer L., Ikura M. (1995) Nature 376, 444–447 [DOI] [PubMed] [Google Scholar]
- 39. Ames J. B., Ishima R., Tanaka T., Gordon J. I., Stryer L., Ikura M. (1997) Nature 389, 198–202 [DOI] [PubMed] [Google Scholar]
- 40. Valentine K. G., Mesleh M. F., Opella S. J., Ikura M., Ames J. B. (2003) Biochemistry 42, 6333–6340 [DOI] [PubMed] [Google Scholar]
- 41. Valentine K. G., Peterson R. W., Saad J. S., Summers M. F., Xu X., Ames J. B., Wand A. J. (2010) Structure 18, 9–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zozulya S., Stryer L. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 11569–11573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhao C., Anand R., Braunewell K. H. (2009) Cell. Mol. Neurobiol. 29, 273–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ladant D. (1995) J. Biol. Chem. 270, 3179–3185 [PubMed] [Google Scholar]
- 45. Strahl T., Grafelmann B., Dannenberg J., Thorner J., Pongs O. (2003) J. Biol. Chem. 278, 49589–49599 [DOI] [PubMed] [Google Scholar]
- 46. Zhao X., Várnai P., Tuymetova G., Balla A., Tóth Z. E., Oker-Blom C., Roder J., Jeromin A., Balla T. (2001) J. Biol. Chem. 276, 40183–40189 [DOI] [PubMed] [Google Scholar]
- 47. Gromada J., Bark C., Smidt K., Efanov A. M., Janson J., Mandic S. A., Webb D. L., Zhang W., Meister B., Jeromin A., Berggren P. O. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 10303–10308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zheng Q., Bobich J. A., Vidugiriene J., McFadden S. C., Thomas F., Roder J., Jeromin A. (2005) J. Neurochem. 92, 442–451 [DOI] [PubMed] [Google Scholar]
- 49. Nahorski S. R., Young K. W., John Challiss R. A., Nash M. S. (2003) Trends Neurosci. 26, 444–452 [DOI] [PubMed] [Google Scholar]
- 50. Stephen R., Bereta G., Golczak M., Palczewski K., Sousa M. C. (2007) Structure 15, 1392–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Flanagan C. A., Thorner J. (1992) J. Biol. Chem. 267, 24117–24125 [PubMed] [Google Scholar]
- 52. Lee W., Revington M. J., Arrowsmith C., Kay L. E. (1994) FEBS Lett. 350, 87–90 [DOI] [PubMed] [Google Scholar]
- 53. Clore G. M., Gronenborn A. M. (1997) Nat. Struct. Biol. 4, 849–853 [PubMed] [Google Scholar]
- 54. Wishart D. S., Sykes B. D., Richards F. M. (1992) Biochemistry 31, 1647–1651 [DOI] [PubMed] [Google Scholar]
- 55. Cornilescu G., Delaglio F., Bax A. (1999) J. Biomol. NMR 13, 289–302 [DOI] [PubMed] [Google Scholar]
- 56. Schwieters C. D., Kuszewski J. J., Tjandra N., Clore G. M. (2003) J. Magn. Reson. 160, 65–73 [DOI] [PubMed] [Google Scholar]
- 57. Badger J., Kumar R. A., Yip P., Szalma S. (1999) Proteins 35, 25–33 [PubMed] [Google Scholar]
- 58. Lim S., Ames J. B. (2009) Biomol. NMR Assign. 3, 269–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang M., Tanaka T., Ikura M. (1995) Nat. Struct. Biol. 2, 758–767 [DOI] [PubMed] [Google Scholar]
- 60. Ames J. B., Hamasaki N., Molchanova T. (2002) Biochemistry 41, 5776–5787 [DOI] [PubMed] [Google Scholar]
- 61. Hendricks K. B. (1999) The FRQ1 Gene Product Is a Positive Regulator of Phosphatidylinositol 4-Kinase in the Yeast Saccharomyces cerevisiae. Ph.D. thesis, University of California, Berkeley [Google Scholar]
- 62. O'Callaghan D. W., Burgoyne R. D. (2004) J. Biol. Chem. 279, 14347–14354 [DOI] [PubMed] [Google Scholar]
- 63. Ames J. B., Ikura M., Stryer L. (2000) Methods Enzymol. 316, 121–132 [DOI] [PubMed] [Google Scholar]
- 64. Goldberg J. (1998) Cell 95, 237–248 [DOI] [PubMed] [Google Scholar]
- 65. Zhang J., Adrián F. J., Jahnke W., Cowan-Jacob S. W., Li A. G., Iacob R. E., Sim T., Powers J., Dierks C., Sun F., Guo G. R., Ding Q., Okram B., Choi Y., Wojciechowski A., Deng X., Liu G., Fendrich G., Strauss A., Vajpai N., Grzesiek S., Tuntland T., Liu Y., Bursulaya B., Azam M., Manley P. W., Engen J. R., Daley G. Q., Warmuth M., Gray N. S. (2010) Nature 463, 501–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hantschel O., Nagar B., Guettler S., Kretzschmar J., Dorey K., Kuriyan J., Superti-Furga G. (2003) Cell 112, 845–857 [DOI] [PubMed] [Google Scholar]
- 67. Tang C., Loeliger E., Luncsford P., Kinde I., Beckett D., Summers M. F. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ames J. B., Levay K., Wingard J. N., Lusin J. D., Slepak V. Z. (2006) J. Biol. Chem. 281, 37237–37245 [DOI] [PubMed] [Google Scholar]