Abstract
L-type calcium currents conducted by CaV1.2 channels initiate excitation-contraction coupling in cardiac and vascular smooth muscle. In the heart, the distal portion of the C terminus (DCT) is proteolytically processed in vivo and serves as a noncovalently associated autoinhibitor of CaV1.2 channel activity. This autoinhibitory complex, with A-kinase anchoring protein-15 (AKAP15) bound to the DCT, is hypothesized to serve as the substrate for β-adrenergic regulation in the fight-or-flight response. Mice expressing CaV1.2 channels with the distal C terminus deleted (DCT−/−) develop cardiac hypertrophy and die prematurely after E15. Cardiac hypertrophy and survival rate were improved by drug treatments that reduce peripheral vascular resistance and hypertension, consistent with the hypothesis that CaV1.2 hyperactivity in vascular smooth muscle causes hypertension, hypertrophy, and premature death. However, in contrast to expectation, L-type Ca2+ currents in cardiac myocytes from DCT−/− mice were dramatically reduced due to decreased cell-surface expression of CaV1.2 protein, and the voltage dependence of activation and the kinetics of inactivation were altered. CaV1.2 channels in DCT−/− myocytes fail to respond to activation of adenylyl cyclase by forskolin, and the localized expression of AKAP15 is reduced. Therefore, we conclude that the DCT of CaV1.2 channels is required in vivo for normal vascular regulation, cell-surface expression of CaV1.2 channels in cardiac myocytes, and β-adrenergic stimulation of L-type Ca2+ currents in the heart.
Keywords: Calcium Channels, Heart, Membrane Proteins, Protein Kinase A (PKA), Smooth Muscle
Introduction
The CaV1.2 channel conducts L-type calcium (Ca2+) current in cardiomyocytes, where Ca2+ enters through the channel and initiates excitation-contraction coupling via Ca2+-induced Ca2+ release (1). Normal expression of CaV1.2 channels is required for cardiac contractile function and for survival beyond embryonic day 14 (2). Lack of the CaV1.2 channel also abolishes the development of myogenic tone and disrupts hormonal regulation of blood pressure (3). In contrast, deletion of CaV1.3, which also conducts L-type Ca2+ currents, causes sinoatrial nodal dysfunction and cardiac arrhythmias but does not impair contractility or cause premature death (4). Overall, these gene deletion studies illustrate that L-type Ca2+ currents are essential for normal cardiovascular function and for normal development.
CaV1 channels are multisubunit complexes composed of a pore-forming α1 subunit and auxiliary β, α2δ, and in some cases γ subunits (5–7). They are a primary target for regulation by numerous hormones, protein kinases, and phosphoprotein phosphatases (5–7). In the “fight-or-flight” response, increased force of contraction is achieved largely through regulation of CaV1.2 channels in the heart by the sympathetic nervous system through activation of β-adrenergic receptors, adenylyl cyclase, and cyclic AMP-dependent protein kinase (PKA) and resulting phosphorylation of CaV1.2 channels (1, 5, 6, 8). β-Adrenergic regulation of CaV1.2 channels requires A-kinase anchoring protein 15 (AKAP15),2 which anchors the kinase to the distal C terminus of CaV1.2 via a modified leucine zipper (LZ) motif (9–11).
The C terminus of CaV1 channels undergoes proteolytic processing in vivo in skeletal and cardiac muscle (12–15). In cardiac muscle, the α1 subunit of CaV1.2 channels is present in two size forms of ∼240 and 210 kDa, which differ by truncation of the distal C terminus (DCT) (15). This truncation leads to enhanced activity of CaV1.2 channels expressed in Xenopus oocytes and mammalian cell lines (16, 17). Single channel conductance, modulation by β and α2δ subunits, and sensitivity to Ca2+ channel agonists such as Bay K8644 remain unchanged (16, 17). The proteolytically cleaved DCT binds to the truncated channel and acts as potent autoinhibitor (18). Mutations of key charged residues at the interface between distal and proximal C-terminal domains of CaV1.2 relieves autoinhibition (18). Moreover, recent studies indicate that regulation of CaV1.2 channels can be reconstituted in transfected nonmuscle cells expressing a signaling complex of truncated CaV1.2, DCT, and AKAP15 (19).
To determine the regulatory roles of the DCT in vivo, we created a mouse line expressing only the truncated CaV1.2 channel (CaV1.2ΔDCT) by introducing a premature stop codon at the site of proteolytic cleavage. Mice expressing only truncated CaV1.2 channels developed severe cardiac hypertrophy and died perinatally. Drugs that reduce peripheral vascular resistance improved survival, suggesting that premature death results from failure of vascular regulation. Cell-surface expression of CaV1.2 channels was dramatically reduced in myocytes from embryonic hearts but not in coronary vascular smooth muscle cells. Deletion of the DCT abolished regulation by PKA and reduced localized expression of AKAP15. Overall, our results reveal that the DCT of CaV1.2 channels is required for normal cardiovascular regulation, expression of CaV1.2 channels in cardiac myocytes, and regulation by β-adrenergic stimulation in vivo.
EXPERIMENTAL PROCEDURES
The present study conformed to the guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health and protocols approved by the Institutional Animal Care and Use Committee of the University of Washington. Animals were maintained under a standard 12:12-h light-dark cycle regime and had ad libitum access to standard chow and water except for specified drug treatments.
Generation of CaV1.2 ΔDCT Mice
A mouse line carrying a premature stop codon that results in the expression of the truncated channel was generated by Ingenious Targeting Laboratory (Stony Brook, NY). Approximately 11.5 kb of the Cacna1c gene containing exons 44 through 49 were isolated from a 129SvJ mouse genomic library. A targeting vector was constructed containing 1.1 kb of intronic sequence that extended from a site 0.3 kb upstream of exon 44 as its short arm followed by a 1.9-kb neomycin positive-selection cassette flanked by loxP sites and a 11.2-kb long arm that began at a site 0.3 kb upstream of exon 44 and extended to the NsiI site downstream of exon 49. To truncate the C terminus of CaV1.2 channels after G1796, a three-way stop codon cassette (5′-ctgagtaagta) was ligated into the NcoI site in exon 44 of the targeting construct. An NcoI-linearized targeting construct was transfected into 129Sv/Ev embryonic stem cells by electroporation. Clones were selected and expanded in the presence of G418. PCR analysis was used to identify clones that had undergone homologous recombination. The correctly targeted embryonic stem cell clones were microinjected into C57BL/6J blastocysts (The Jackson Laboratory, Bar Harbor, ME). Germ line transmission of the mutated Cacna1c allele was confirmed for one of three chimeric mice, and the resulting progeny were backcrossed into C57Bl/6J for more than 10 generations to generate heterozygous (Het) and homozygous CaV1.2ΔDCT (KO) animals used in this study.
Genotyping
Wild-type (WT) and mutant animals were identified by PCR analysis of tail-tip DNA using primers FH258 (CCCACTGCACATCAACAAGAC) and FH254 (GTCCTGTGTGGAAGACTCAAGGAG). The amplified product was further digested by NcoI, which gives a single band at 702 bp for the WT allele and at 908 bp for the mutated allele due to elimination of the NcoI restriction site in exon 44.
Drug Treatments of DCT−/− Mice
Heterozygotes (DCT+/−, Het) were used to generate DCT−/− (KO) mice that only express CaV1.2ΔDCT. Pregnant Het female mice were sacrificed at gestational day 16–18. Embryos were removed by Cesarean section, and a tail sample was used for PCR analysis for genotyping. Due to the embryonic lethality of KO mice, a series of drugs were administered during the last week of gestation to prolong survival. Isradipine (1 or 3 mg/kg) and cyclosporin A (50 mg/kg) were administered via daily subcutaneous injections to pregnant female mice starting at E13. Tadalafil (0.5 μg/ml) and captopril (200 mg/liter) were given via drinking water from E13 to E18. Hearts were dissected and blotted dry for weight measurement to evaluate hypertrophy.
Patency of Ductus Arteriosus
Embryos at E18 were dissected after Cesarean section. The thoracic cavity was opened, and the atria were removed for better visualization of the ductus arteriosus. Ink was injected into the right ventricle and visualized under a dissection microscope.
Isolation and Culture of Embryonic Cardiomyocytes
Embryonic cardiomyocytes were isolated similarly to Davies et al. (20). Atria were separated from the heart. The ventricles were cut into small pieces with a razor blade and transferred to ADS buffer containing 5 mm NaCl, 20 mm HEPES, 1 mm NaH2PO4, 5.5 mm glucose, 5 mm KCl, 0.8 mm MgSO4 (pH 7.35). After a short centrifugation, the buffer was replaced with ADS containing 0.5 mg/ml Collagenase II (Worthington Biochemical). The tissue blocks were digested for two 20-min cycles at 37 °C on a rotating plate followed by gentle trituration. After a quick centrifugation, the supernatant was transferred to a tissue culture dish or plated on laminin-treated glass coverslips. Cells were cultured in 37 °C in DMEM with 10% fetal bovine serum and used within 48 h after isolation for electrophysiological measurements.
Histology and Immunocytochemistry
Embryos were fixed in 4% paraformaldehyde and cryoprotected by sinking in 30% (w/v) sucrose in 0.1 m sodium phosphate at 4 °C for 72 h. Tissues were cut into 80-μm coronal sections and mounted on subbed slides for hematoxylin and eosin staining and immunocytochemistry. As described previously (21), tissues slices and myocytes were permeabilized by 0.025% Triton X-100 after fixation and probed with corresponding antibodies. Mouse monoclonal antibody (CaV1.2m, clone L57/46) against the proximal C terminus of CaV1.2 channels was purchased from UC Davis/NIH NeuroMab Facility. Polyclonal anti-CaV1.2 (anti-CNC1) and anti-CaV1.3 (anti-CND1) were generated against amino acid sequences in the intracellular loop betweens domains II and III of CaV1.2 and CaV1.3 channels (22) and characterized previously (9, 23). Monoclonal antibody against RyR2 was purchased from Millipore Corp. The DCT was immunostained using anti-CH1 that specifically recognizes the DCT of CaV1.2 (15, 24). A specific anti-peptide antibody against AKAP15 was prepared as previously described (25).
Electrophysiology
The whole-cell configuration of the patch clamp technique was used to record Ca2+ currents from embryonic ventricular myocytes isolated at E16-E18. Currents were recorded with an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA). Data acquisition and command potentials were controlled by Pulse software (Heka Electronics), and data were stored for later analysis with IGOR (Wavemetrics, Lake Oswego, OR). Residual leak and capacitive transients were subtracted using a P/4 protocol. The extracellular bath solution contained 140 mm tetraethylammonium chloride, 2 mm MgCl2, 20 mm BaCl2, 10 mm Hepes, and 10 mm glucose (pH 7.4). The intracellular solution contained 100 mm CsCl, 20 mm tetraethylammonium chloride, 1 mm MgCl2, 5 mm MgATP, 10 mm Hepes, and 10 mm EGTA (pH 7.3). Control and forskolin-containing medium was rapidly perfused from a gravity-fed system. Cells were held at −40 mV to inactivate T-type Ca2+ channels and Na+ channels, and 100-ms test pulses were applied from −40 to +40 mV in 10-mV increments. Peak Ba2+ current amplitude was plotted versus voltage to generate current-voltage (I-V) relationships. To measure the voltage dependence of activation, tail currents were recorded after repolarization to −50 mV after test pulses from the holding potential to potentials from −50 to +150 mV in 10-mV increments. To measure inactivation kinetics, Ba2+ current was recorded after a series of 1-s depolarizations from the holding potential of −40 mV to potentials from 0 to +30 mV in 10-mV increments. The results were normalized to peak Ba2+ current amplitude recorded at +10 mV. For analysis of inactivation kinetics, individual data traces were smoothed using a binomial algorithm with an interval of 20 points. Gating-charge movement was measured by applying a series of test pulses at 10-s intervals from a holding potential of −40 mV to potentials between +60 to +80 mV in 2-mV increments and integrating the gating charge movement at the reversal potential for the ionic currents (18). Patch pipettes (2–4 megaohms) were pulled from micropipette glass and fire-polished before use. Recordings were discarded if the series resistance was >7 megaohms before compensation. All experiments were performed at room temperature.
Quantification of Immunocytochemical Images
For analysis of fluorescence intensity of CaV1.2 channels and AKAP15, whole regions of interest were taken from the images, and mean pixel intensities were determined.
RT-PCR
Total RNA was extracted from whole hearts of WT, Het, and KO embryos. CaV1.2 mRNA expression was analyzed by RT-PCR using fluorescent SYBR Green technology on a StepOnePlus system (Applied Biosystems). Standard curves were constructed from cDNA standards, and the results were normalized by expression as a molar ratio to acidic ribosomal protein.
Statistics and Significance
All of the data are reported as the means ± S.E. One-way ANOVA with a Bonferroni posttest (GraphPad Prism 4.0) was used to compare the averages from multiple groups.
RESULTS
Genotype
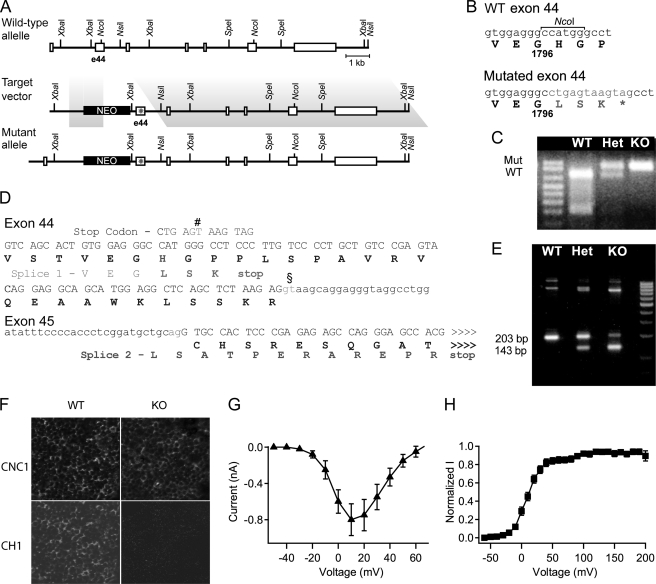
A mouse line expressing the truncated CaV1.2 channel was generated by introducing a premature stop codon at G1796 (Fig. 1A). Genotype was determined by loss of a NcoI restriction site (Fig. 1, B–D). Germ line transmission was confirmed using RT-PCR with different primer pairs (Fig. 1E). When probed with an anti-CaV1.2 antibody that recognizes an epitope in the intracellular loop connecting domains II and III, sections from hearts of E16 WT and KO mice had easily detectable levels of CaV1.2 channels (Fig. 1F). In contrast, no signal was detected using an antibody against the truncated distal C terminus in DCT−/− heart, confirming successful deletion of the DCT. Two unexpected splice variants with short extensions of 3 and 12 residues beyond G1796 were identified (Fig. 1D). mRNA encoding the truncated CaV1.2 channel with the 12-amino acid extension was most abundant. However, this 12-residue extension has no effect on expression and function of the truncated CaV1.2 channel in transfected tsA-201 embryonic kidney cells (Fig. 1, G and H).
FIGURE 1.
Generation of CaV1.2ΔDCT mice. A, a diagram of the targeting strategy used to generate CaV1.2ΔDCT mice by homologous recombination to truncate the CaV1.2 channel. Homologous recombination of the targeting construct with the Cacna1c gene resulted in a mutated allele in which a three-way stop codon cassette was inserted. The targeting vector consists of a short arm of 1.1 kb followed by neomycin (NEO) cassette flanked by loxP sites and an 11.2-kb long-arm that began at a site 0.3 kb upstream of exon 44 bound by a NsiI site downstream of exon 49. A three-way stop codon cassette (5′-ctgagtaagtag) was ligated into a blunted NcoI site in exon 44 of the targeting construct to engineer a premature stop. Homologous recombination leads to incorporation of Neo selection cassette and premature stop codon into genomic DNA of the Cacna1c gene. B, partial amplicons obtained by the primer pair used for genotyping were sequenced and aligned with the cDNA and amino acid sequence of CaV1.2. Lowercase letters, sequence of exon 44 for wild-type and mutant; second line, amino acid sequence of wild-type and mutant protein. *, premature stop codon downstream of the three-way stop codon cassette. C, PCR amplification products from genomic DNAs of WT, Het (DCT+/−), and KO (DCT−/−) embryos were subjected to NcoI digestion. An NcoI site is present in the WT but not the mutant construct. A 702-bp product is obtained for the WT allele, whereas the uncut mutant allele is 908 bp. D, DNA sequence analyses of the RT-PCR products from mRNAs encoding the C terminus of CaV1.2ΔDCT. The expected ∼210-bp RT-PCR product encodes the expected C-terminal sequence ending at Gly-1796 with an extension of Leu-Ser-Lys-stop (bold). However, there is also an unexpected alternative RNA splicing variant due to a splice donor site (§) in Exon 44 and a splice acceptor site in Exon 45. Splicing via the alternative splice donor site (#) produces a +1 nucleotide shift of the Exon 45 reading frame, and a 12-amino acid-residue peptide extension (LSATPERAREPR) after Gly-1796 in the truncated C terminus of CaV1.2 channels. E, RT-PCR of brain mRNA from WT and mutant CaV1.2ΔDCT mice is shown. Multiple sets of oligonucleotides were used to amplify mRNA splice products spanning the exons 44 and 45 splice junction. The alternative splice variant gives rise to a ∼143-bp RT-PCR product instead of the predicted ∼210 bp for mutant allele. The ratio of RT-PCR products indicates that the 143-bp splice product accounts for the majority of the mRNA. F, coronal heart sections from WT and KO mice at E16 were analyzed by immunocytochemistry. Top, anti-CNC1 recognizes the intracellular loop between domains I and II of CaV1.2 channels. Bottom, anti-CH1 recognizing the distal C-terminal tail of CaV1.2 (CaV1.2DCT). Scale bar = 25 μm. G, I-V relationship. H, voltage dependence of activation. These parameters were unchanged relative to unextended CaV1.2 ΔDCT channels (not shown) expressed in tsA-201 cells.
Premature Death of DCT−/− Mice
Heterozygous CaV1.2ΔDCT mice containing one truncated allele show no distinguishable phenotypic differences from WT littermates, whereas no homozygous mice were found at weaning. Further analysis revealed that DCT−/− mice died shortly after birth, usually within a few hours. No survival was observed after 24 h. To determine the timeline of death of DCT−/− mice, we examined the genotype distribution during embryonic development from E13 to E18. Live DCT−/− embryos were found at a lower than expected Mendelian ratio (Fig. 2A, p < 0.01). Death of DCT−/− embryos occurred as early as E15 and throughout the later gestational stage until birth. These results indicate that regulation of the CaV1.2 channel by its DCT is required for normal embryonic development in vivo.
FIGURE 2.
Phenotypes of CaV1.2ΔDCT mice. A, CaV1.2ΔDCT KO mice have reduced survival rates during embryonic development from E13 to E19 as indicated by the sub-Mendelian numbers of KO animals. Deaths occur as early as E15, but some embryos survive until hours after birth. Right panel, examples of KO embryos show marked abdominal blood pooling. B, cardiac hypertrophy of KO mice. Top, examples of WT and KO hearts. Bottom, coronal sections of WT (left) and KO (right) hematoxylin and eosin-stained hearts. Scale bar = 1 mm. C, ratio of heart weight to body weight is shown. D, a histogram of distribution of cell capacitances for cells isolated from animals of each genotype (colors correspond to genotype as in panel C). E, cells isolated from WT hearts (upper panel) and KO hearts (lower panel) stained with wheat germ agglutinin labeled with Alexa 555 as a cell membrane marker (red) (anti-WGA), anti-RyR2 (green), or anti-CaV1.2. KO cells were hypertrophied with blebbing and extension of membrane at the end of the cell (arrows in lower two panels) compared with WT Cells (upper panel). Scale bar = 10 μm.
Cardiac Hypertrophy and Heart Failure in DCT−/− Mice
Significant abdominal blood pooling was observed in all dead DCT−/− embryos and pups collected after birth (Fig. 2A), a hallmark feature of congestive heart failure. No other difference in gross structure or morphology was observed between WT and DCT−/− embryos. DCT−/−embryos had an ∼31% increase in heart weight to body weight ratio compared with WT (Fig. 2C, 5.5 ± 0.2 versus 4.2 ± 0.1 mg/g, p < 0.001), indicating severe cardiac hypertrophy. Unlike the left ventricular hypertrophy observed in humans and animal models of heart failure, the hypertrophy in DCT−/− embryos occurs preferentially in the right ventricle as shown in Fig. 2B. Enlarged hearts were observed in all dead DCT−/− embryos and pups. Further histological analysis with hematoxylin and eosin staining demonstrated significant concentric hypertrophy in the right ventricular chamber (Fig. 2B), which would limit the diastolic volume and thereby impair contractile function and pumping capacity. Therefore, the right ventricular hypertrophy we have observed may cause impaired pumping capacity and pooling of blood in internal organs such as the gastrointestinal track and liver, contributing to heart failure and death of DCT−/− mice.
Membrane capacitance is proportional to cell surface area, providing a quantitative estimate of cell size. Dissociated myocytes from DCT−/− mice have a shift in distribution of cell-surface area, with fewer myocytes having single-cell capacitances of 20–25 picofarads and more myocytes having capacitances of 30–35 picofarads (Fig. 2D). Hypertrophied myocytes often appeared larger in cell culture and typically expressed lower levels of CaV1.2 channels in cytoplasm and plasma membrane in immunocytochemical images (Fig. 2E). Overall, these results indicate that truncation of CaV1.2 channels leads to hypertrophy of individual cardiac myocytes, which increases the size and impairs the function of the right ventricle.
Rescue of DCT−/− Embryos with Vasodilator Treatment
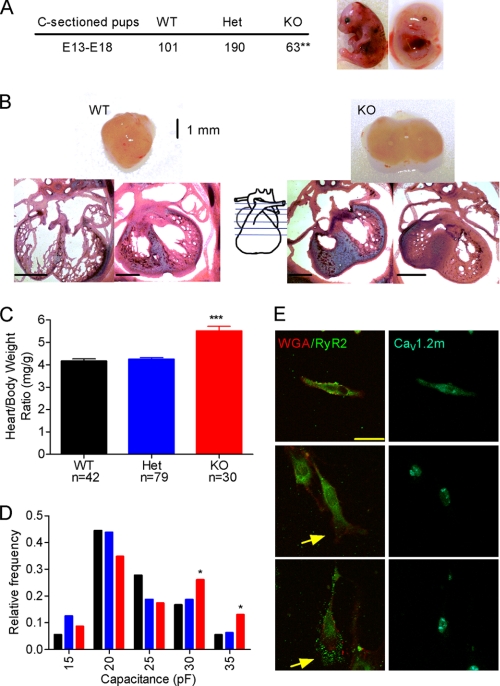
The preferential hypertrophy of right ventricle prompted us to investigate whether the ductus arteriosus remains patent in DCT−/− embryos. The ductus arteriosus is a specialized vascular structure for fetal circulation that connects the pulmonary trunk to the descending aorta and allows shunting of 95% of the blood away from the high resistance pulmonary vasculature. Because of this shunt, the right ventricle plays a much more important role in pumping blood to the peripheral circulation compared with adult heart. Premature closure of the ductus arteriosus results in cardiac hypertrophy and right-sided heart failure (26). We examined live DCT−/− embryos, all of which show enlarged right ventricles but no closure of the ductus arteriosus (Fig. 3A). These results rule out anatomical closure of the ductus arteriosus as the cause of hypertrophy in DCT−/− mice, but it remains possible that increased contracture of the ductus arteriosus could contribute to right ventricular hypertrophy in DCT−/− mice.
FIGURE 3.
Role of ductus arteriosus and peripheral vascular resistance in producing hypertrophy. A, images of the ink-filled ductus arteriosus (arrows) in hearts from animals of each genotype. No gross abnormalities were detected in the vascular structure of KO mice. B and C, cardiac hypertrophy (B) and embryonic survival (C) were evaluated after the indicated drug treatments from E13 to E18. For each drug or drug combination, at least five litters of animals were tested. Significant differences in hypertrophy and survival are indicated by asterisks. Isra, isradipine; CsA, cyclosporin; Tad, tadalafil.
Based on our in vitro data (18), we expected that truncation of the DCT would lead to up-regulation of CaV1.2 channel activity in cardiac and vascular smooth muscle cells, and this increased activity could lead to increased peripheral vascular resistance, hypertension, and heart failure. Human hypertension is treated with dihydropyridine calcium channel blockers, which preferentially inhibit CaV1.2 channels in vascular smooth muscle cells in vivo (27, 28). The calcium channel antagonist isradipine (1 and 3 mg/kg) reduced the heart to body weight ratio significantly and increased survival of DCT−/− embryos assayed at E18 (Fig. 3, B and C). This effect was dose-dependent, but no further improvement was observed at 10 mg/kg. This result suggests the possibility that overactive Ca2+ channels in vascular smooth muscle are responsible for hypertrophy and premature death in DCT−/− mice.
Angiotensin-converting enzyme inhibitors are the most widely used treatment for human hypertension. We found that captopril plus isradipine did not substantially improve heart weight or survival compared with isradipine alone (Fig. 3, B and C). The specific cyclic nucleotide phosphodiesterase-5 (PDE5) inhibitor tadalafil is an effective treatment for pulmonary hypertension in humans, and it has also been shown to inhibit PDE5 in ductus arteriosus and cause dilation of the shunt in the fetus (29). Tadalafil was approximately as effective as isradipine in reducing heart weight and premature death when given alone, but the combined effects of tadalafil and isradipine were not greater than isradipine alone (Fig. 3, B and C).
These results suggested that increased vascular resistance due to overactive Ca2+ channels contributes significantly to the development of cardiac hypertrophy and thereby to premature death of DCT−/− animals during embryonic development. To test whether the cardiac hypertrophy per se results in the death of DCT−/− embryos, we used the calcineurin inhibitor cyclosporin A, which counteracts the effects of alterations in Ca2+ signaling and effectively protects the heart from cardiac hypertrophy (30–33). Treatment with cyclosporin A almost completely prevented hypertrophy in DCT−/− embryos (Fig. 3B). Surprisingly, however, the survival ratio of DCT−/− embryos did not change (Fig. 3C). These results suggest that cardiac hypertrophy per se is not the primary cause of premature death of DCT−/− embryos and point to increased contracture of vascular smooth muscle in resistance vessels and resulting hypertension as a primary cardiovascular defect that causes premature death in DCT−/− mice.
Even though treatment with isradipine, captopril, and tadalafil improved the survival of DCT−/− embryos to E18, none of them increased the number of pups surviving to weaning. The combination of captopril and isradipine was the most effective treatment, supporting survival to postnatal day 4 for a small fraction of DCT−/− pups. All other treatments failed to improve postnatal survival beyond 24 h.
Reduced L-type Ca2+ Current and CaV1.2 Expression in DCT−/− Hearts
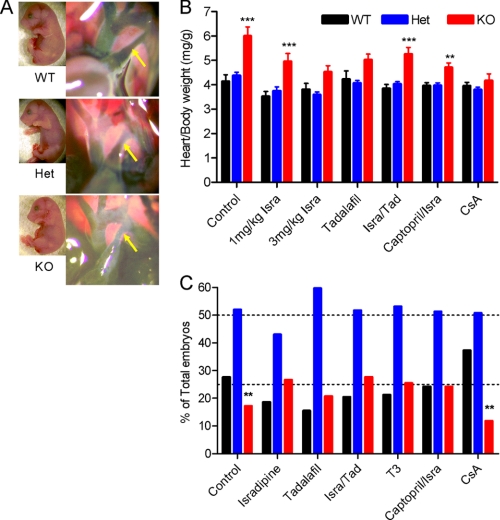
Using the whole-cell voltage clamp technique, we examined L-type Ca2+ current in acutely dissociated embryonic cardiomyocytes. Current amplitude in WT myocytes is very small, even with 3.6 mm extracellular Ca. Therefore, 20 mm Ba2+ was used as the charge carrier to achieve a better signal-to-noise ratio. L-type Ba2+ current amplitude (Fig. 4, A and B) is dramatically reduced in cells of DCT−/− mice compared with those of WT and Het. At a holding potential of −40 mV, which inactivates Na+ current and T-type Ca2+ current, 10 μm isradipine completely blocked Ba2+ current in both WT and DCT−/− cells, indicating that the dihydropyridine-sensitive L-type Ca2+ current is selectively recorded under these conditions. The reduced L-type current in DCT−/− myocytes is surprising because truncated CaV1.2 channels expressed in non-muscle cells in vitro exhibit enhanced activity (17, 18).
FIGURE 4.
Function and modulation of Ba2+ currents through L-type channels. A, Ba2+ current from myocytes isolated from WT (left) and KO (right) animals in control and in the presence of 10 μm isradipine. B, mean I-V relationships for cells of each phenotype derived from peak currents during depolarizations to the indicated potentials. C, activation curves for L-type Ba2+ currents derived from tail currents recorded at −50 mV after depolarizations to the indicated potentials and normalized to the largest tail current in each series of test pulses. D, mean currents recorded from cells of each genotype during 1-s depolarizations from −40 to +10 mV. E, mean gating charge measured at the reversal potential for cells of each genotype. pC, pico coulomb.
Measurements of gating charge movement provide an estimate of the number of functional voltage-gated ion channels on the cell surface whose voltage sensors can be activated by depolarization (34). Most of the rapid gating charge movement in single dissociated cardiac myocytes upon depolarization from a holding potential of −40 mV reflects activation of CaV1.2 channels (35, 36). The gating charge movement measured at the reversal potential was significantly lower in DCT−/− cells compared with WT (Fig. 4E). This suggests that the surface expression of truncated CaV1.2 channels is dramatically reduced, which explains the lower peak current amplitude in these cells.
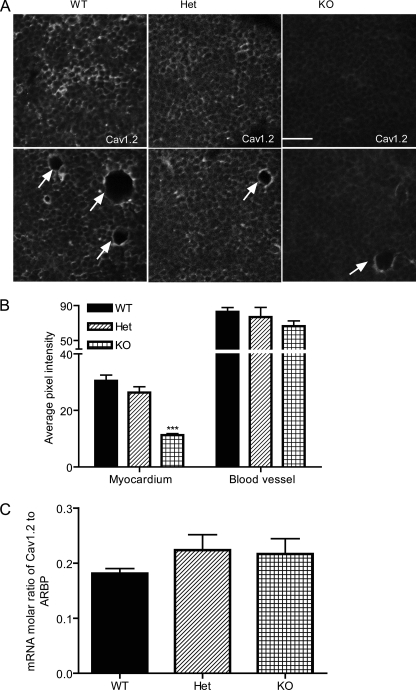
We used immunocytochemistry with specific antibodies to further assess the expression of CaV1.2 channels in DCT−/− myocytes and vascular smooth muscle cells. In both acutely dissociated cardiomyocytes (not shown) and transverse sections of heart from DCT−/− mice, specific immunostaining of CaV1.2 channel was greatly reduced (Fig. 5). On the other hand, the expression of other proteins that are involved in Ca2+ signaling in cardiomyocytes such as ryanodine receptor and CaV1.3 channels remained unchanged (data not shown). These results confirm that expression of CaV1.2 channels in cardiac myocytes is impaired by deletion of the DCT. In contrast, specific immunostaining of CaV1.2 channel in blood vessels (indicated by arrows) remained unchanged (Fig. 5, A, lower panels, and B). The level of mRNA encoding CaV1.2 channels in the heart remains the same for all three genotypes (Fig. 5C), suggesting that deletion of the DCT impairs the protein assembly and/or lifetime at the plasma membrane of cardiomyocytes rather than gene expression. The reduced expression of CaV1.2 channels in cardiac myocytes, but not in vascular smooth muscle, may create an imbalance in the cardiovascular system in which cardiac output is compromised by reduced CaV1.2 channel expression in cardiac myocytes at the same time as peripheral vascular resistance is increased by deletion of the DCT and increased activity of the normal number of CaV1.2 channels in vascular smooth muscle.
FIGURE 5.
Reduced expression of CaV1.2 channels in KO hearts but not blood vessels. A, representative ventricular tissue slices from WT, Het, and KO E18 hearts stained with antibody recognizing the proximal C terminus of CaV1.2. Representative blood vessels (arrows) are shown in the lower panel. B, mean pixel intensity for CaV1.2 staining of WT, Het, and KO in myocytes versus blood vessels. C, mRNA levels of CaV1.2 expressed as a molar/molar ratio to acidic ribosomal protein (ARBP). Scale bar = 25 μm.
Activation and Inactivation of CaV1.2ΔDCT
In addition to the large reduction in peak L-type Ba2+ current, we also observed a substantial negative shift in voltage dependence of activation of CaV1.2ΔDCT channels (Fig. 4C). However, the precise extent of this negative shift should be viewed with caution considering the small current amplitude in DCT−/− cells. In addition to the negative shift in the voltage dependence of activation, voltage-dependent inactivation of the L-type Ba2+ current conducted by CaV1.2ΔDCT channels was significantly slowed (Fig. 4D). Interestingly, voltage-dependent inactivation was also slowed in Het cells, where the current amplitude and voltage dependence of channel activation remained indistinguishable from WT, suggesting that the rate of inactivation may be preferentially affected by the loss of DCT. This result is consistent with our recent studies showing that the DCT is required for the normal enhancement of voltage-dependent inactivation of CaV1.2 channels by physiological levels of intracellular Mg2+ (37).
Regulation of CaV1.2ΔDCT Channels by the β-Adrenergic/PKA Pathway
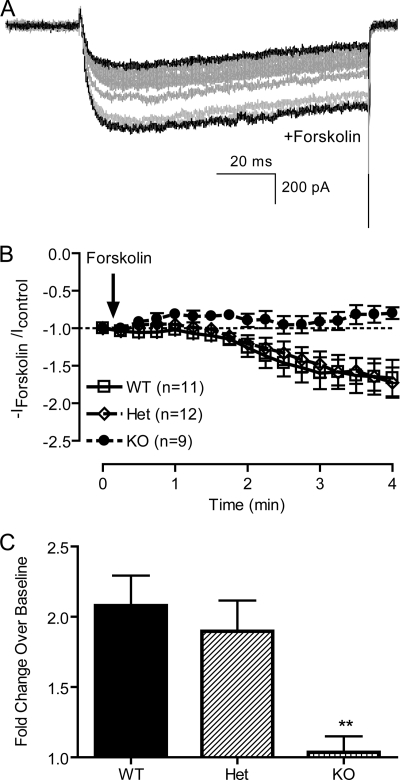
Upon β-adrenergic activation of cardiac myocytes, PKA phosphorylates α1 and β subunits of the CaV1.2 channel (15, 23, 38, 39), which increases peak Ca2+ currents, increases the maximal probability of channel opening and negatively shifts the voltage dependence of channel activation (6, 8, 40). The β-adrenergic receptor agonist isoproterenol increases Ca2+ current of embryonic cardiomyocytes isolated at E16-E18 by ∼2-fold and shifts the voltage dependence of activation to more negative membrane potential (data not shown), indicating that the fetal cardiomyocyte is a valid system to examine the regulation of β-adrenergic signaling pathway. However, this response is heavily dependent on the maturation of cells because robust coupling of β-adrenergic receptors to G proteins is not fully established until birth (41). Therefore, we chose to activate adenylyl cyclase directly, downstream of β-adrenergic receptors and G proteins, using forskolin. Extracellular perfusion of 10 μm forskolin increased the current amplitude in both Het and WT cells by ∼2-fold (Fig. 6). In contrast, no enhancement of Ba2+ current was observed in DCT−/− cells. This result suggests that the DCT is a critical regulatory element that is required for regulation by the β-adrenergic/PKA pathway in vivo. It is also consistent with other studies showing that the full-length α1C subunit but not the truncated form is phosphorylated in response to elevated cAMP in vitro (15, 42–45). Our results provide the first demonstration in vivo that the truncated CaV1.2 channel alone, without the proteolytically processed DCT, is not sufficient for up-regulation by β-adrenergic stimulation.
FIGURE 6.
Forskolin regulation of Ba2+ current in cardiac myocytes. A, Ba2+ current elicited by depolarization from −40 to +10 mV was measured during extracellular perfusion with control solution or solution containing 10 μm forskolin. Traces recorded every 15 s from a representative cell are shown. B, peak current amplitude was derived from each depolarization and normalized to base-line current amplitude. -Fold change over base line was plotted. C, maximal response to forskolin in each cell was normalized to base-line Ba2+ current, and the means of -fold change for cells of each genotype are shown.
Association of AKAP15 with CaV1.2ΔDCT Channels
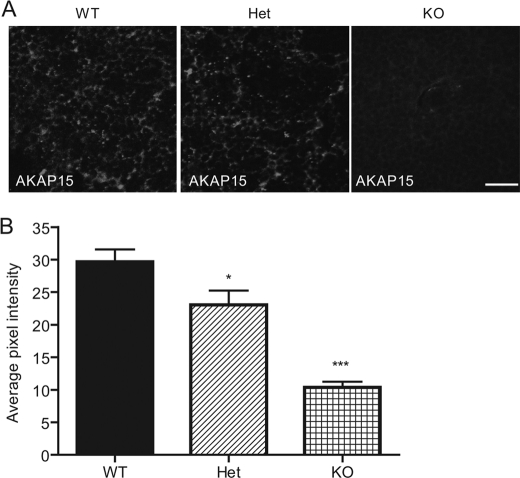
AKAP15 has been demonstrated to anchor PKA to CaV1.2 channels in heart through interaction with the AKAP binding domain located in the DCT (9). This interaction is required for effective regulation by PKA even though the AKAP binding domain is located beyond the point of proteolytic truncation of the DCT (9). To assess the importance of the DCT in localization of AKAP15 in vivo, we measured its expression and localization by immunocytochemistry with specific antibodies. We found that the specific staining of AKAP15 is reduced in cardiac cells dissociated from DCT−/− animals (data not shown). A similar reduction in immunostaining was also observed in transverse sections from heart tissue (Fig. 7). These results indicate that the DCT of CaV1.2 channels is required for normal expression and localization of AKAP15 in cardiac myocytes.
FIGURE 7.
Expression of AKAP15 in WT, Het, and KO hearts. A, representative ventricular tissue sections from WT, Het, and KO E18 hearts stained with antibody recognizing AKAP15. B, mean pixel intensity of AKAP15 in ventricular slices. Scale bar = 25 μm.
DISCUSSION
Functional Roles of the DCT of CaV1.2 Channels in Vivo
Our studies of mice with deletion of the DCT of CaV1.2 channels have shown that this regulatory domain is required for life and has at least three essential functions. First, we find that deletion of the DCT induces cardiac hypertrophy and failure, possibly as a result of impairment of regulation of the peripheral vasculature. These results demonstrate an essential role for the DCT in cardiovascular regulation in vivo. Second, against expectations from in vitro expression studies, we find that deletion of the DCT reduced the function and cell-surface localization of CaV1.2 channels, demonstrating a requirement of the DCT for normal functional channel expression in cardiac myocytes. Third, our results show that CaV1.2ΔDCT channels cannot be regulated by the β-adrenergic/PKA signaling pathway and cannot support normal expression and localization of AKAP15. Each of these functional effects of the DCT is considered in more detail below.
Deletion of the DCT of CaV1.2 Channels Causes Right Ventricular Hypertrophy and Heart Failure
Increased peripheral vascular resistance leads to hypertension and heart failure (46). Hypertension in humans is effectively treated with the calcium channel blocker isradipine and the angiotensin-converting enzyme inhibitor captopril (27, 28, 47). In addition, pulmonary hypertension in humans is effectively treated with tadalafil (48). Our experiments show that deletion of the DCT of CaV1.2 channels in mice causes dramatic right ventricular hypertrophy and heart failure that are improved, but not cured, by treatment with isradipine, captopril, tadalafil, and combinations of those drugs. These results demonstrate a requirement for the DCT of CaV1.2 channels in normal regulation of the cardiovascular system in vivo.
CaV1.2 knock-out mice die before embryonic day 14, and their death was proposed to be caused by lack of functional CaV1.2 channels in the heart (2). This hypothesis was confirmed by the generation of viable conditional knock-out animals with CaV1.2 channels inactivated in vascular smooth muscle cells (3), in insulin-secreting cells in the pancreas (49), or in the hippocampus and neocortex of the brain (50). These studies show that loss of function of CaV1.2 channels in vascular smooth muscle is not lethal, whereas loss of function in cardiac myocytes leads to premature death before E14. In light of these previous results, it is most likely that the failure of cardiovascular function in CaV1.2ΔDCT mice is caused by the combination of gain-of-function of the normal number of CaV1.2 channels in vascular smooth muscle cells with loss of expression in cardiac myocytes. Together, these two effects would create an imbalance between increased peripheral vascular resistance and decreased ventricular contractility, eventually leading to heart failure and premature death.
Unfortunately, the small size of E16–18 embryos precludes detailed studies of the function of the cardiovascular system to determine the source of failure of cardiovascular regulation precisely. However, CaV1.2 channels have been suggested to act as Ca2+ sensors for depolarization-induced Ca2+ release in vascular smooth muscle (51), thereby playing an important role in maintaining myogenic tone and peripheral resistance. CaV1.2 channels also mediate blood pressure regulation by various hormones, such as angiotensin II (3). Therefore, it is expected that up-regulation of CaV1.2 channel activity in vascular smooth muscle cells would lead to increased vascular resistance and pressure overload to the heart. Our results show that CaV1.2 channels are expressed at normal levels in the coronary vasculature, in contrast to the loss of expression in cardiac myocytes, and it is expected that these channels in the vasculature would be hyperactive because of loss of the autoinhibitory DCT. Future studies of regulation of contractility and Ca2+ channel function in dissociated vascular smooth muscle cells from CaV1.2ΔDCT mice may provide further information on the source of impaired cardiovascular regulation.
Differential Effects of DCT Truncation of Cardiac CaV1.2 Channels in Vitro and in Vivo
Using in vitro heterologous expression systems, several groups have shown that removal of as many as 478 amino acids from distal C terminus of CaV1.2 increases current density, gating charge movement, and channel opening without affecting cell surface expression (16–18). Although the proteinase responsible for the proteolytic processing of CaV1.2 channels in vivo remains unknown, introduction of trypsin or carboxypeptidase A into cardiomyocytes results in an enhancement in L-type Ca2+ currents (52, 53). Therefore, we expected that cardiac myocytes expressing only truncated CaV1.2 channels would have enhanced channel activity. In contrast to these expectations, however, the L-type Ca2+ current measured in dissociated cardiomyocytes is dramatically reduced as a consequence of reduced cell-surface expression of the truncated channels. The drastic down-regulation of CaV1.2 channels in cardiac myocytes observed in our model is not characteristic of heart failure in general. Although the alteration in CaV1.2 channel expression in hypertrophied and failing hearts remains controversial and inconclusive, no drastic up- or down-regulation was observed in most studies (54–57). Therefore, down-regulation of CaV1.2 channel expression is more likely to be a direct consequence of deletion of the DCT rather than a secondary consequence of heart failure. Deletion of the DCT would impair in vivo proteolytic processing at the point of truncation, which may be required for normal functional expression and cell-surface localization in cardiac myocytes. It is also possible that deletion of the DCT may result in failure of regulation in CaV1.2 gene expression, as the DCT has been shown to act as a transcriptional regulator (58) and to autoregulate CaV1.2 promoter activity in vivo in cardiac myocytes (59). However, we found no effect of deletion of the DCT on the level of mRNA encoding CaV1.2 channels in the heart. Therefore, it is most likely that the loss of expression of CaV1.2 channels in DCT−/− mice is caused by failure of proteolytic processing and assembly of the channel complex at the cell surface or by enhanced internalization from the cell surface and degradation.
The DCT of CaV1.2 Channels Is Required for β-Adrenergic/PKA Regulation in Cardiomyocytes in Vivo
Stimulation of cardiac L-type Ca2+ current by epinephrine was first described in 1966 and hypothesized to be mediated by phosphorylation by PKA (8, 60–62). The increase in Ca2+ currents observed after activation of PKA is due to an increase in the open-state probability of the channel resulting from a shift in gating mode (5, 42). Regulation of CaV1.2 channel requires PKA anchored to the DCT by AKAP15 or another AKAP (9, 10, 18, 19, 43), and the DCT is required for regulation of CaV1.2 channels in virally infected cardiac myocytes (63). The full-length α1C subunit is phosphorylated both in vitro and in vivo by PKA in response to elevated cAMP, whereas the truncated CaV1.2 channel is not (15, 42–45). Multiple potential PKA phosphorylation sites in α1C subunit have been identified (64). Phosphorylation of serine 1928 in the DCT is observed upon β-adrenergic stimulation and PKA activation in transfected cells and in cardiac myocytes; however, the functional impact of phosphorylation of serine 1928 in vivo remains uncertain (15, 21, 45, 63, 65, 66). Recent results from reconstitution of the regulation of CaV1.2 channels expressed in nonmuscle cells show that PKA phosphorylation of sites at the interface between the DCT and the proximal C terminus is responsible for regulation (19).
Although peak L-type Ba2+ currents are much reduced in DCT−/− myocytes, these cells provide a unique experimental opportunity to examine the role of the DCT in regulation by the β-adrenergic/PKA pathway in vivo. Our results demonstrate for the first time in native cardiomyocytes that truncation of DCT abolishes the regulation of CaV1.2 channels by the β-adrenergic/PKA pathway. Deletion of the DCT also disrupts the expression and localization of AKAP15, which may contribute to impairment of PKA regulation. The requirement of the DCT for β-adrenergic stimulation of L-type Ca2+ current is consistent with the hypothesis that β-adrenergic receptor/PKA signaling pathway acts through phosphorylation of sites at the interface between the proximal and distal C terminus, thereby relieving autoinhibition exerted by the cleaved, noncovalently associated DCT (18, 19).
In mammalian myocardium, CaV1.2 channels consist of three forms: full-length, truncated, and truncated channel with noncovalently associated DCT. Only ∼20% is full-length channel (15). This proportion of channel forms provides a quantitative fit to the L-type Ca2+ current in cardiac myocytes before and after treatment with isoproterenol if primarily the truncated channels with noncovalently bound DCT are up-regulated by PKA phosphorylation (18). Moreover, in recent studies, we have found that reconstitution of PKA regulation in nonmuscle cells requires formation of an autoinhibitory complex of truncated CaV1.2 channels with the noncovalently associated DCT and AKAP15 (19). These previous results are consistent with our current findings and support the conclusion that the DCT is required for β-adrenergic regulation of cardiac L-type Ca2+ current. DCT−/− mice provide a unique model for study of the molecular mechanism of Ca2+ channel regulation in the heart.
Acknowledgments
The monoclonal antibody against CaV1.2 channels was developed by and obtained from the UC Davis/NIH NeuroMab Facility, supported by National Institutes of Health Grant U24NS050606 and maintained by the Department of Neurobiology, Physiology, and Behavior, College of Biological Sciences, University of California, Davis, CA 95616.
This work was supported, in whole or in part, by National Institutes of Health Training Grants T32 GM07750 (to M. R. M.) and T32 NS07332 (to J. P. C.) and Research Grants P01 HL44948 and R01 HL85372 (to W. A. C.). This work was also supported by American Heart Association Postdoctoral Research Fellowship 10POST2630160 (to Y. F.).
- AKAP15
- A-kinase anchoring protein 15
- Het
- heterozygous
- DCT
- distal C terminus.
REFERENCES
- 1. Bers D. M. (2000) Circ. Res. 87, 275–281 [DOI] [PubMed] [Google Scholar]
- 2. Seisenberger C., Specht V., Welling A., Platzer J., Pfeifer A., Kühbandner S., Striessnig J., Klugbauer N., Feil R., Hofmann F. (2000) J. Biol. Chem. 275, 39193–39199 [DOI] [PubMed] [Google Scholar]
- 3. Moosmang S., Schulla V., Welling A., Feil R., Feil S., Wegener J. W., Hofmann F., Klugbauer N. (2003) EMBO J. 22, 6027–6034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Platzer J., Engel J., Schrott-Fischer A., Stephan K., Bova S., Chen H., Zheng H., Striessnig J. (2000) Cell 102, 89–97 [DOI] [PubMed] [Google Scholar]
- 5. Striessnig J. (1999) Cell. Physiol. Biochem. 9, 242–269 [DOI] [PubMed] [Google Scholar]
- 6. Catterall W. A. (2000) Annu. Rev. Cell Dev. Biol. 16, 521–555 [DOI] [PubMed] [Google Scholar]
- 7. Ertel E. A., Campbell K. P., Harpold M. M., Hofmann F., Mori Y., Perez-Reyes E., Schwartz A., Snutch T. P., Tanabe T., Birnbaumer L., Tsien R. W., Catterall W. A. (2000) Neuron 25, 533–535 [DOI] [PubMed] [Google Scholar]
- 8. Reuter H. (1979) Annu. Rev. Physiol. 41, 413–424 [DOI] [PubMed] [Google Scholar]
- 9. Hulme J. T., Lin T. W., Westenbroek R. E., Scheuer T., Catterall W. A. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 13093–13098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gray P. C., Scott J. D., Catterall W. A. (1998) Curr. Opin. Neurobiol. 8, 330–334 [DOI] [PubMed] [Google Scholar]
- 11. Hulme J. T., Ahn M., Hauschka S. D., Scheuer T., Catterall W. A. (2002) J. Biol. Chem. 277, 4079–4087 [DOI] [PubMed] [Google Scholar]
- 12. Hulme J. T., Konoki K., Lin T. W., Gritsenko M. A., Camp D. G., 2nd, Bigelow D. J., Catterall W. A. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 5274–5279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. De Jongh K. S., Warner C., Colvin A. A., Catterall W. A. (1991) Proc. Natl. Acad. Sci. U.S.A. 88, 10778–10782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De Jongh K. S., Merrick D. K., Catterall W. A. (1989) Proc. Natl. Acad. Sci. U.S.A. 86, 8585–8589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. De Jongh K. S., Murphy B. J., Colvin A. A., Hell J. W., Takahashi M., Catterall W. A. (1996) Biochemistry 35, 10392–10402 [DOI] [PubMed] [Google Scholar]
- 16. Wei X., Neely A., Lacerda A. E., Olcese R., Stefani E., Perez-Reyes E., Birnbaumer L. (1994) J. Biol. Chem. 269, 1635–1640 [PubMed] [Google Scholar]
- 17. Gao T., Cuadra A. E., Ma H., Bunemann M., Gerhardstein B. L., Cheng T., Ten Eick R., Hosey M. M. (2001) J. Biol. Chem. 276, 21089–21097 [DOI] [PubMed] [Google Scholar]
- 18. Hulme J. T., Yarov-Yarovoy V., Lin T. W., Scheuer T., Catterall W. A. (2006) J. Physiol. 576, 87–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fuller M. D., Emrick M. A., Sadilek M., Scheuer T., Catterall W. A. (2010) Sci. Signal. 3, ra70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Davies M. P., An R. H., Doevendans P., Kubalak S., Chien K. R., Kass R. S. (1996) Circ. Res. 78, 15–25 [DOI] [PubMed] [Google Scholar]
- 21. Hulme J. T., Westenbroek R. E., Scheuer T., Catterall W. A. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 16574–16579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Snutch T. P., Tomlinson W. J., Leonard J. P., Gilbert M. M. (1991) Neuron 7, 45–57 [DOI] [PubMed] [Google Scholar]
- 23. Hell J. W., Yokoyama C. T., Wong S. T., Warner C., Snutch T. P., Catterall W. A. (1993) J. Biol. Chem. 268, 19451–19457 [PubMed] [Google Scholar]
- 24. Westenbroek R. E., Hell J. W., Warner C., Dubel S. J., Snutch T. P., Catterall W. A. (1992) Neuron 9, 1099–1115 [DOI] [PubMed] [Google Scholar]
- 25. Gray P. C., Johnson B. D., Westenbroek R. E., Hays L. G., Yates J. R., 3rd, Scheuer T., Catterall W. A., Murphy B. J. (1998) Neuron 20, 1017–1026 [DOI] [PubMed] [Google Scholar]
- 26. Toyoshima K., Momma K., Imamura S., Nakanishi T. (2006) Biol. Neonate 89, 251–256 [DOI] [PubMed] [Google Scholar]
- 27. Brogden R. N., Sorkin E. M. (1995) Drugs 49, 618–649 [DOI] [PubMed] [Google Scholar]
- 28. Schachter M. (1991) J. Clin. Pharm. Ther. 16, 79–91 [DOI] [PubMed] [Google Scholar]
- 29. Momma K., Toyoshima K., Imamura S., Nakanishi T. (2005) Pediatr. Res. 58, 42–45 [DOI] [PubMed] [Google Scholar]
- 30. Luo Z., Shyu K. G., Gualberto A., Walsh K. (1998) Nat. Med. 4, 1092–1093 [DOI] [PubMed] [Google Scholar]
- 31. Sussman M. A., Lim H. W., Gude N., Taigen T., Olson E. N., Robbins J., Colbert M. C., Gualberto A., Wieczorek D. F., Molkentin J. D. (1998) Science 281, 1690–1693 [DOI] [PubMed] [Google Scholar]
- 32. Meguro T., Hong C., Asai K., Takagi G., McKinsey T. A., Olson E. N., Vatner S. F. (1999) Circ. Res. 84, 735–740 [DOI] [PubMed] [Google Scholar]
- 33. Molkentin J. D., Lu J. R., Antos C. L., Markham B., Richardson J., Robbins J., Grant S. R., Olson E. N. (1998) Cell 93, 215–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bezanilla F. (2000) Physiol. Rev. 80, 555–592 [DOI] [PubMed] [Google Scholar]
- 35. Hadley R. W., Lederer W. J. (1991) J. Gen. Physiol. 98, 265–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hadley R. W., Lederer W. J. (1992) Am. J. Physiol. 262, H472–H477 [DOI] [PubMed] [Google Scholar]
- 37. Brunet S., Scheuer T., Catterall W. A. (2009) J. Gen. Physiol. 134, 81–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hulme J. T., Scheuer T., Catterall W. A. (2004) J. Mol. Cell. Cardiol. 37, 625–631 [DOI] [PubMed] [Google Scholar]
- 39. Bünemann M., Gerhardstein B. L., Gao T., Hosey M. M. (1999) J. Biol. Chem. 274, 33851–33854 [DOI] [PubMed] [Google Scholar]
- 40. Tsien R. W., Bean B. P., Hess P., Lansman J. B., Nilius B., Nowycky M. C. (1986) J. Mol. Cell Cardiol. 18, 691–710 [DOI] [PubMed] [Google Scholar]
- 41. An R. H., Davies M. P., Doevendans P. A., Kubalak S. W., Bangalore R., Chien K. R., Kass R. S. (1996) Circ. Res. 78, 371–378 [DOI] [PubMed] [Google Scholar]
- 42. Kamp T. J., Hell J. W. (2000) Circ. Res. 87, 1095–1102 [DOI] [PubMed] [Google Scholar]
- 43. Gao T., Puri T. S., Gerhardstein B. L., Chien A. J., Green R. D., Hosey M. M. (1997) J. Biol. Chem. 272, 19401–19407 [DOI] [PubMed] [Google Scholar]
- 44. Steinberg S. F. (2004) J. Mol. Cell. Cardiol. 37, 407–415 [DOI] [PubMed] [Google Scholar]
- 45. Gao T., Yatani A., Dell'Acqua M. L., Sako H., Green S. A., Dascal N., Scott J. D., Hosey M. M. (1997) Neuron 19, 185–196 [DOI] [PubMed] [Google Scholar]
- 46. Deedwania P. C. (1997) Am. J. Hypertens. 10, 280S–288S [DOI] [PubMed] [Google Scholar]
- 47. Hansson L., Lindholm L. H., Niskanen L., Lanke J., Hedner T., Niklason A., Luomanmäki K., Dahlöf B., de Faire U., Mörlin C., Karlberg B. E., Wester P. O., Björck J. E. (1999) Lancet 353, 611–616 [DOI] [PubMed] [Google Scholar]
- 48. Anderson J. R., Nawarskas J. J. (2010) Cardiol. Rev. 18, 148–162 [DOI] [PubMed] [Google Scholar]
- 49. Schulla V., Renström E., Feil R., Feil S., Franklin I., Gjinovci A., Jing X. J., Laux D., Lundquist I., Magnuson M. A., Obermüller S., Olofsson C. S., Salehi A., Wendt A., Klugbauer N., Wollheim C. B., Rorsman P., Hofmann F. (2003) EMBO J. 22, 3844–3854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Moosmang S., Haider N., Klugbauer N., Adelsberger H., Langwieser N., Müller J., Stiess M., Marais E., Schulla V., Lacinova L., Goebbels S., Nave K. A., Storm D. R., Hofmann F., Kleppisch T. (2005) J. Neurosci. 25, 9883–9892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fernández-Tenorio M., González-Rodríguez P., Porras C., Castellano A., Moosmang S., Hofmann F., Ureña J., López-Barneo J. (2010) Circ. Res. 106, 1285–1289 [DOI] [PubMed] [Google Scholar]
- 52. Hescheler J., Trautwein W. (1988) J. Physiol. 404, 259–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mikala G., Bodi I., Klockner U., Varadi M., Varadi G., Koch S. E., Schwartz A. (2003) Mol. Cell. Biochem. 250, 81–89 [DOI] [PubMed] [Google Scholar]
- 54. Piacentino V., 3rd, Weber C. R., Chen X., Weisser-Thomas J., Margulies K. B., Bers D. M., Houser S. R. (2003) Circ. Res. 92, 651–658 [DOI] [PubMed] [Google Scholar]
- 55. Beuckelmann D. J., Erdmann E. (1992) Basic Res. Cardiol. 87, 235–243 [DOI] [PubMed] [Google Scholar]
- 56. Gómez A. M., Valdivia H. H., Cheng H., Lederer M. R., Santana L. F., Cannell M. B., McCune S. A., Altschuld R. A., Lederer W. J. (1997) Science 276, 800–806 [DOI] [PubMed] [Google Scholar]
- 57. Chen X., Piacentino V., 3rd, Furukawa S., Goldman B., Margulies K. B., Houser S. R. (2002) Circ. Res. 91, 517–524 [DOI] [PubMed] [Google Scholar]
- 58. Gomez-Ospina N., Tsuruta F., Barreto-Chang O., Hu L., Dolmetsch R. (2006) Cell 127, 591–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schroder E., Byse M., Satin J. (2009) Circ. Res. 104, 1373–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sperelakis N., Schneider J. A. (1976) Am. J. Cardiol. 37, 1079–1085 [DOI] [PubMed] [Google Scholar]
- 61. Reuter H., Scholz H. (1977) J. Physiol. 264, 17–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tsien R. W., Giles W., Greengard P. (1972) Nat. New Biol. 240, 181–183 [DOI] [PubMed] [Google Scholar]
- 63. Ganesan A. N., Maack C., Johns D. C., Sidor A., O'Rourke B. (2006) Circ. Res. 98, e11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Norman R. I., Leach R. N. (1994) Biochem. Soc. Trans. 22, 492–496 [DOI] [PubMed] [Google Scholar]
- 65. Mitterdorfer J., Froschmayr M., Grabner M., Moebius F. F., Glossmann H., Striessnig J. (1996) Biochemistry 35, 9400–9406 [DOI] [PubMed] [Google Scholar]
- 66. Lemke T., Welling A., Christel C. J., Blaich A., Bernhard D., Lenhardt P., Hofmann F., Moosmang S. (2008) J. Biol. Chem. 283, 34738–34744 [DOI] [PMC free article] [PubMed] [Google Scholar]