Abstract
Residues comprising the guanine nucleotide-binding sites of the α subunits of heterotrimeric (large) G-proteins (Gα subunits), as well as the Ras-related (small) G-proteins, are highly conserved. This is especially the case for the phosphate-binding loop (P-loop) where both Gα subunits and Ras-related G-proteins have a conserved serine or threonine residue. Substitutions for this residue in Ras and related (small) G-proteins yield nucleotide-depleted, dominant-negative mutants. Here we have examined the consequences of changing the conserved serine residue in the P-loop to asparagine, within a chimeric Gα subunit (designated αT*) that is mainly comprised of the α subunit of the retinal G-protein transducin and a limited region from the α subunit of Gi1. The αT*(S43N) mutant exhibits a significantly higher rate of intrinsic GDP-GTP exchange compared with wild-type αT*, with light-activated rhodopsin (R*) causing only a moderate increase in the kinetics of nucleotide exchange on αT*(S43N). The αT*(S43N) mutant, when bound to either GDP or GTP, was able to significantly slow the rate of R*-catalyzed GDP-GTP exchange on wild-type αT*. Thus, GTP-bound αT*(S43N), as well as the GDP-bound mutant, is capable of forming a stable complex with R*. αT*(S43N) activated the cGMP phosphodiesterase (PDE) with a dose-response similar to wild-type αT*. Activation of the PDE by αT*(S43N) was unaffected if either R* or β1γ1 alone was present, whereas it was inhibited when R* and the β1γ1 subunit were added together. Overall, our studies suggest that the S43N substitution on αT* stabilizes an intermediate on the G-protein activation pathway consisting of an activated G-protein-coupled receptor, a GTP-bound Gα subunit, and the β1γ1 complex.
Keywords: G Protein-coupled Receptors (GPCR), G Proteins, Phototransduction, Rhodopsin, Signal Transduction, Gα, Gβγ, Transducin
Introduction
G protein-coupled receptors (GPCR)2 are one of the largest families of membrane proteins and are involved in various physiological functions. In the past several years, significant advances have been made in the determination of structures at an atomic level of GPCRs (1, 2), their cognate G-proteins, and their downstream targets (3). In addition, structures have been solved for complexes of G-proteins with their downstream targets as well as their regulators (e.g. the regulators of G-protein signaling (RGS) proteins) (3). However, one of the central unresolved questions in this field involves the mechanism utilized by a GPCR to catalyze the release of GDP from its cognate G-protein (4, 5).
The x-ray crystal structures of various Gα subunits have shown that they are composed of two distinct domains: one that highly resembles the GTPase domain of the small G-protein Ras, and a second domain which is mainly α-helical in content and thus referred to as the helical domain. The guanine nucleotide is nestled between these two domains (4, 5). The binding of GTP to αT induces structural changes within 3 regions of the GTPase domain, designated as Switches 1, 2, and 3.
The vertebrate visual system in rod cells has provided an excellent model system for understanding how GPCRs activate heterotrimeric G-proteins and subsequently their downstream targets (6, 7). In the visual transduction pathway, the absorption of a photon leads to the isomerization of the covalently-bound chromophore, 11-cis-retinal, to its all-trans configuration. This converts rhodopsin to its functionally active conformation referred to as metarhodopsin II (R*). The heterotrimeric G-protein transducin is made up of a GDP-bound α subunit (αT-GDP), as well as the β1 and γ1 subunits, which are noncovalently complexed to each other and can be dissociated only by denaturation. The R* species binds to heterotrimeric transducin (αT-GDP/β1γ1) with an extremely high affinity. This results in a weakening of the affinity of αT for GDP such that it dissociates from the G-protein. GTP then binds to αT yielding a species (αT-GTP) that has a reduced affinity for both R* and β1γ1. The structural changes in the switch regions of αT-GTP allow it to bind and activate the downstream effector enzyme, the cGMP phosphodiesterase (PDE). The PDE is made up of two catalytic subunits (αPDE and βPDE) and two smaller regulatory subunits (γPDE) and catalyzes the rapid hydrolysis of cGMP to GMP. The αT-GTP species binds to γPDE and relieves its inhibition of the catalytic activity of the αPDE and βPDE subunits. The activation of PDE is terminated by the hydrolysis of GTP by αT, which is catalyzed by RGS9. The conversion of cGMP to GMP leads to a closure of cGMP-gated channels on the rod cell membrane. This hyperpolarization results in an inhibition of neurotransmitter release, which represents the signal that is conveyed to the optic nerve.
Our laboratory has been interested in identifying and biochemically characterizing αT mutants that might help to shed light on the mechanism by which GDP is released from a Gα subunit, thus leading to G-protein activation (8–12). These efforts have resulted in the identification of various Gα mutants with novel biochemical properties. Owing to the difficulties in overexpressing native αT in Escherichia coli, we have used a chimera of αT and αi1, referred to as αT*, as a starting Gα-backbone for these studies (13).
G-proteins belonging to both the monomeric (small) and heterotrimeric (large) G-protein families have a conserved serine or threonine residue in the P-loop. Mutation of this residue in the small G-protein Ras results in the stabilization of its nucleotide-free form relative to both its GDP- and GTP-bound forms (14). This yields a Ras protein that has an enhanced affinity for Ras-guanine nucleotide exchange factors (Ras-GEFs) and thereby has been used as a dominant-negative inhibitor of signaling by endogenous Ras in cells. Mutations of this residue have also been performed in some Gα subunits. In the case of Gαo or Gαi2, such changes resulted in their having an enhanced affinity for the Gβγ subunit complex (15, 16). A similar mutation in GαS gave rise to an enhanced affinity for the β-adrenergic receptor (17).
In this study, we have changed the conserved serine residue in the P-loop of αT* to an asparagine residue (S43N). This mutant, unlike wild-type αT*, is able to undergo GDP-GTP exchange at a significant rate even in the absence of R*. Similar to the phenotype shown by the GαS mutant toward the β-adrenergic receptor, the αT*(S43N) mutant, when in the GDP-bound state, slows R*-stimulated GDP-GTP exchange upon wild-type αT*. In addition, we show that the GTPγS-bound form of αT*(S43N) is capable of activating the PDE and of slowing R*-stimulated GDP-GTP exchange upon wild-type αT*. Moreover, using the activation of PDE as a probe, we have been able to show that GTPγS-bound αT*(S43N) stabilizes what may represent an intermediate complex that forms along the G-protein activation pathway and consists of both R* and the β1γ1 subunit complex, together with the αT subunit.
During the course of our studies, Artemyev and co-workers (18) reported the characterization of the same mutation within the chimeric αT* subunit. However, they reported that αT*(S43N) was not capable of undergoing an exchange of GTP for GDP. We believe that this was likely due to a step in their purification protocol that was carried out at room temperature that would have resulted in the loss of the bound nucleotide. We have indeed observed that prolonged incubation of the αT*(S43N) mutant at room temperature renders it unable to bind GTPγS, and while it is still capable of binding to R* and preventing it from activating wild-type αT*, it no longer exhibits many of the features that we describe below.
EXPERIMENTAL PROCEDURES
Materials
Frozen dark-adapted bovine retina were obtained from Lawson (Lincoln, NE). All other chemicals were from Sigma.
Purification of Visual Transduction Proteins
Rod outer segment (ROS) membranes were isolated as described (19). Holo-transducin and PDE were obtained from ROS membranes essentially as described (20). The PDE was further purified by gel filtration chromatography on a HiLoad Superdex 200 HR26/60 column (GE Healthcare) equilibrated with HMAG buffer (20 mm Na-HEPES, pH 7.5, 5 mm MgCl2, 1 mm Na-azide, and 10% glycerol). Urea-washed disc membranes were prepared as described and served as the source of R* (21).
Purification of the β1γ1 Complex
Holo-transducin was applied to two 5-ml Hitrap Blue-Sepharose columns (GE Healthcare), connected in tandem, that had been pre-equilibrated in G1G0A buffer (10 mm Na-HEPES, pH 7.5, 6 mm MgCl2, 1 mm DTT, and 10% glycerol). The columns were first washed with 250 ml of G1G0A buffer. This was followed by a 250 ml of low salt wash (G1G0A buffer + 100 mm KCl) to elute the β1γ1 complex. The αT subunit was then eluted with 250 ml of high salt buffer (G1G0A buffer + 500 mm KCl). The β1γ1 subunit complex was further purified by ion exchange chromatography using a 5-ml Hitrap Q-Sepharose column by applying a NaCl gradient (0–500 mm, 150 ml) in HMAG buffer (20 mm Na-HEPES, pH 7.5, 5 mm MgCl2, 1 mm Na-azide, and 10% glycerol). The β1γ1 complex eluted at a salt concentration of ∼100 mm and was then concentrated, flash-frozen, and stored at −80 °C.
Expression and Purification of αT*
We have used a construct designated pHis6Chi8, which was obtained from Dr. Heidi Hamm (Vanderbilt University). This chimera has the corresponding region from αi1 inserted between residues 215 and 295 of αT. In addition, residues 244 and 247 were changed back to the original amino acids in αT, yielding what we refer to as αT*. The recombinant αT* subunits, both wild-type and S43N, were expressed in BL21 (DE3) supercompetent cells and purified as described (11, 13). The proteins were further purified by ion exchange chromatography as outlined for the β1γ1 complex. The proteins were concentrated, aliquoted, snap-frozen, and stored at −80 °C. The final yield of chimeric αT* was typically ∼1–2 mg of pure protein/liter of bacterial culture. Nucleotide occupancy of the purified recombinant αT* and αT*(S43N) was determined using HPLC (11).
Determination of Active Concentrations of αT* Subunits
The amount of active, functional αT* was determined by assaying [35S]GTPγS binding activity. Rhodopsin was light activated (R*) by incubation in ambient light for 5 min on ice. The αT* subunits (500 nm) were incubated in the presence of 50 nm R*, 500 nm β1γ1, and 50 μm [35S]GTPγS, in HMDM buffer (20 mm HEPES, pH 7.5, 5 mm MgCl2, and 0.01% (w/v) dodecylmaltoside) at a total volume of 100 μl, for 1–2 h at room temperature. Subsequently, 40-μl aliquots were applied, in duplicate, to prewet nitrocellulose filters (Schleicher & Schuell, pore size, 0.45 μm) on a suction manifold. The filters were washed twice with HM buffer (20 mm HEPES, pH 7.5, 5 mm MgCl2), added to scintillation liquid (30% LSC Scintisafe Mixture), and counted in a scintillation counter (LS6500 multipurpose scintillation counter). All protein concentrations of αT* were determined by this procedure.
Time Course of GTPγS Binding
R* and β1γ1 were mixed with [35S]GTPγS to give a final GTPγS concentration of 50 μm in HMDM buffer. The GDP-GTP exchange reaction was initiated by the addition of αT*, with the final volume of the reaction mixture being 300 μl. At different times, 20-μl aliquots were added to prewetted nitrocellulose filters and processed as described in the previous section.
Fluorescence Measurements
Fluorescence measurements were carried out using a Varian eclipse spectrofluorimeter. The binding of aluminum fluoride (AlF4−) was measured by monitoring the intrinsic tryptophan fluorescence of αT* (excitation: 280 nm; emission: 340 nm). αT* (300 nm) was mixed with 1 ml of HMDM buffer at room temperature and the tryptophan fluorescence emission was monitored continuously for 1 min. Subsequently, AlF4− (final concentrations: 5 mm NaF and 100 μm AlCl3) was added while monitoring the tryptophan fluorescence emission in real-time.
GTPγS binding to αT* was monitored by premixing R* (prepared as described above) and β1γ1 in HMDM buffer containing GTPγS (50 μm) and monitoring tryptophan fluorescence (excitation: 300 nm; emission: 345 nm) in real-time. Subsequently, αT* (300 nm) was added. All kinetic traces were corrected for the fluorescence from R* and β1γ1.
The inhibition of GDP-GTP exchange upon αT*(wild-type) by the GDP-bound form of αT*(S43N) was measured by monitoring the kinetics of GTPγS binding using tryptophan fluorescence. This was carried out with 300 nm αT*(wild-type), R* (5 nm), β1γ1 (300 nm), 50 μm GTPγS, and varying concentrations of αT*(S43N).
The GTPγS-bound form of αT*(S43N) was prepared by incubating the protein (5 μm) with 100 μm GTPγS in HMDM buffer at room temperature for 2 h. The protein was subsequently placed on ice. αT*(S43N)-GTPγS was added at varying concentrations to a cuvette containing a mixture of R* (5 nm), β1γ1 (300 nm), and GTPγS (50 μm). Tryptophan fluorescence was monitored for 1 min and αT*(wild-type) was added, and the kinetics of GTPγS binding were monitored. All kinetic data were fitted to a single exponential Equation 1,
where F = fluorescence signal at any time t, F0 = fluorescence signal at time t = 0, F∞ = fluorescence signal at t = ∞, kobs = observed rate constant.
Labeling the β1γ1 Subunit Complex
The β1γ1 complex (10 μm) was incubated in a buffer containing 20 mm Na-HEPES (pH 7.5) and 5 mm MgCl2 with a 0.5 mm solution of 5-((((2-iodoacetyl)amino)ethyl)amino)naphthalene-1-sulfonic acid (IAEDANS), dissolved in dimethyl formamide, at room temperature in the dark for 2 h. The reaction was quenched by the addition of β-mercaptoethanol at 11.2 mm. The labeled β1γ1 was separated from free probe and exchanged into HMAG buffer on a PD-10 desalting column (Amersham Biosciences). The extent of incorporation of the IAEDANS moiety was calculated using an extinction coefficient of 5600 m−1 cm−1 at 336 nm. The stoichiometry of labeling was determined by correcting the absorbance at 280 nm for IAEDANS absorption and measuring the concentration of β1γ1 using the calculated extinction coefficient of 53,600 m−1 cm−1. The stoichiometry of labeling was determined to be 1.2 ± 0.1 mol per mol β1γ1.
Fluorescence Anisotropy Measurements
Anisotropy measurements of IAEDANS-labeled β1γ1 complexes were carried out on a Cary eclipse spectrofluorimeter in the L-format. All reported anisotropy values have been corrected by determination of G-factors using horizontally polarized light. The excitation and emission wavelengths were set at 336 nm (bandwidth = 10 nm) and 490 nm (bandwidth = 20 nm), respectively. An integration time of 10 s was used for all measurements.
Measurements of cGMP PDE Activity
The analysis of cGMP hydrolysis by the retinal PDE was carried out as described (22). Briefly, a pH microelectrode was used to measure the decrease in pH resulting from the production of a proton for each molecule of cGMP hydrolyzed by PDE. All assays were carried out at 22 °C in a final volume of 300 μl containing 5 mm HEPES (pH 7.4), 100 mm NaCl, and 2 mm MgCl2 (AB buffer). The dose response for the activation of PDE by αT* was determined by mixing R* (118 nm), β1γ1 (370 nm), 100 μm GTPγS, and αT* in AB buffer. This mixture was incubated at room temperature for 1–2 h. PDE (70 nm) was added and incubated at room temperature for 5 min. The reaction was initiated by the addition of 2 mm cGMP. At the end of each assay period, the buffering capacity (in mV/nmol) was determined by adding 500 nmol of sodium hydroxide. The rate of hydrolysis of cGMP (nmol/s) was determined from the ratio of the initial slope of the pH record (mV/nmol) and the buffering capacity of the assay buffer (mV/nmol).
GTPγS-bound αT*(S43N) was prepared by incubating the αT* subunit (5 μm) with GTPγS (100 μm) in AB buffer for 2 h at room temperature. To determine the effects of R* and the β1γ1 subunit complex on PDE activation, R* and/or β1γ1 were mixed with GTPγS-bound αT*(S43N), diluted in AB buffer containing 100 μm GTPγS to a final concentration of 250 nm, and incubated at room temperature for 5 min. PDE and cGMP were added to this mixture and the PDE activity measured as described above.
RESULTS
The Purified Recombinant αT*(S43N) Mutant Is Isolated with Bound GDP
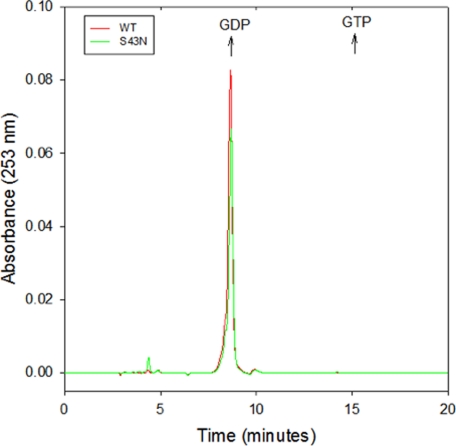
Both the Gα subunits of large G-proteins and the Ras-related small G-proteins bind to GDP with a very high affinity. This explains the requirement for an activated GPCR or a GEF to catalyze the dissociation of GDP from Gα subunits and Ras-related G-proteins, respectively, in order for GDP-GTP exchange to occur. In the case of the small G-proteins, the highly conserved serine/threonine residues in the P-loop help to coordinate Mg2+, which is essential for the high affinity binding of GDP, such that substitutions at this site yield nucleotide-depleted, dominant-negative mutants (14). Magnesium is not necessary for the high affinity binding of GDP to the Gα subunits of large G-proteins, possibly because of their helical domain, which is lacking in the small G-proteins (23). However, the x-ray crystal structure of the GDP-bound form of αT shows that the main-chain nitrogen of the serine residue at position 43 makes a hydrogen bond with one of the non-bridging oxygens on the β-phosphate of GDP, thereby suggesting a potentially important role in high-affinity binding (24). In addition, there have been suggestions that substitutions for the conserved P-loop serine/threonine residue in Gα subunits yield dominant-negative inhibitors, raising the question of whether these mutants are also nucleotide-depleted (16–18, 25–27). Thus, we performed HPLC analysis to determine whether the αT*(S43N) mutant, following its purification, still contained bound guanine nucleotide. We found that as in the case for the wild-type αT* subunit, the αT*(S43N) mutant was purified with stoichiometric amounts of bound GDP (Fig. 1), i.e. with stoichiometries of 1 ± 0.1 mol GDP per mol αT* and 0.8 ± 0.1 mol GDP per mol αT*(S43N).
FIGURE 1.
HPLC analysis of the nucleotide occupancy of wild-type αT* and αT* (S43N). Wild-type αT* (red) and αT*(S43N) (green) at concentrations of 100 μm were incubated in the presence of 7.5% acetonitrile for 5 min at room temperature and centrifuged at 16,000 × g for 10 min at room temperature. An aliquot of the supernatant (20 μl) was injected into a C18 reverse phase HPLC column equilibrated with 100 mm KH2PO4, pH 6.5, 10 mm tetrabutylammonium bromide, 0.2% NaN3, and 7.5% acetonitrile, and then isocratic elution was performed. The absorbance at 253 nm was monitored. The elution times of GDP and GTP standards are indicated by arrows.
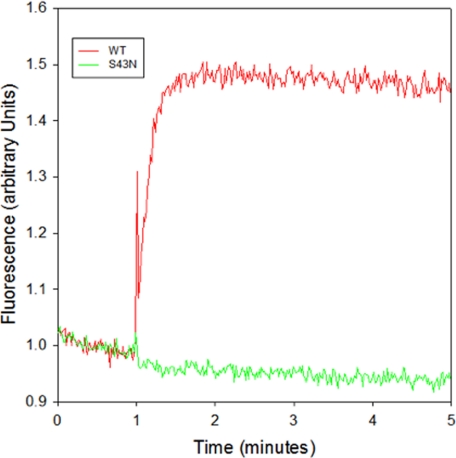
The x-ray crystal structure for the aluminum fluoride (AlF4−)/GDP-bound form of αT shows that AlF4− occupies the γ-phosphate-binding site of GTP (28). This structure has been postulated to resemble the transition state for GTP hydrolysis. When AlF4− binds to the GDP-bound form of αT, there is an increase in the intrinsic tryptophan fluorescence due to a conformational change in the Switch 2 region, one of three regions of αT that change conformation upon binding GTP (29). As expected, a robust increase in tryptophan fluorescence was observed upon addition of AlF4− to the wild-type αT* subunit (Fig. 2). However, the addition of AlF4− to the αT*(S43N) mutant did not cause a detectable change in tryptophan fluorescence. This was not entirely unexpected since the presence of Mg2+ is required for the AlF4−-induced enhancement in tryptophan fluorescence. The x-ray crystal structures of both the GTPγS- and AlF4−-bound forms of αT show that the side-chain hydroxyl oxygen of Ser-43 is one of the coordination sites for the bound divalent cation (Mg2+ in the GTPγS-bound αT structure and Ca2+ in the AlF4−-bound αT structure) (28, 30). Thus, the αT*(S43N) mutant would have a reduced affinity for Mg2+ and, consequently, might not be able to undergo the AlF4−-induced conformational change that alters the position of Switch 2 and enhances its intrinsic tryptophan fluorescence.
FIGURE 2.
AlF4− response of wild-type αT* and αT* (S43N). Wild-type αT* (red) and αT*(S43N) (green) were incubated in HMDM buffer. At the indicated time, 20 μl of a premixed aliquot containing 250 mm NaF and 150 μm AlCl3 to generate AlF4− was added and the enhancement in tryptophan fluorescence (excitation: 280 nm; emission: 340 nm) was monitored.
R* Has Only Minor Effects on GDP-GTP Exchange within the αT*(S43N) Mutant
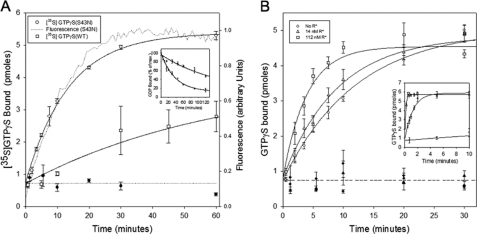
Wild-type αT* undergoes very slow guanine nucleotide exchange when assayed in the absence of R* and β1γ1, as monitored by either the enhancement in the intrinsic tryptophan fluorescence that accompanies the exchange of GDP for GTPγS (kobs = 0.01 min−1), or by using a filter binding assay to measure the exchange of GDP for [35S]GTPγS (0.02 min−1) (Fig. 3A, open squares). This is similar to the spontaneous nucleotide exchange rate reported earlier (31). In contrast, we found that the αT*(S43N) mutant undergoes nucleotide exchange with a rate constant that is about 4–8-fold faster than that for the wild-type αT* subunit (kobs = 0.09 min−1) as monitored using intrinsic tryptophan fluorescence (Fig. 3A, dotted line). A similar rate constant was obtained when using a filter binding assay to measure the exchange of GDP for [35S]GTPγS (kobs = 0.08 min−1). A filter binding assay was used to measure the rate of dissociation of GDP using wild-type αT* or αT*(S43N) that had been pre-loaded with [3H]GDP. This experiment showed that the rate of release of GDP from αT*(S43N) was 4-fold faster than that of wild-type αT* (Fig. 3A, inset). Thus, changing the serine to asparagine at position 43 within the αT* subunit apparently causes a change in the conformation of the P-loop such that there is an increase in the rate of GDP dissociation, which has been suggested to be the rate-limiting step for GDP-GTP exchange (32).
FIGURE 3.
The R*-independent and R*-dependent GDP-GTP exchange activity of αT* (S43N). A, 50 μm GTPγS or [35S]GTPγS was added to 300 nm αT*(S43N) or wild-type αT*. In the case of GTPγS (dotted: αT*(S43N)), the tryptophan fluorescence emission was monitored in real-time with the excitation wavelength set at 300 nm and the emission wavelength at 345 nm. When assaying [35S]GTPγS binding to either αT*(S43N) (○) or wild-type αT* (□), aliquots (20 μl) of the reaction mixture were removed at the indicated times and added directly to pre-wetted nitrocellulose filters on a suction manifold to quench the reaction. The filters were subsequently washed twice with HM buffer and added to 3 ml of scintillation fluid and counted on a scintillation counter. The y-axis shows the number of pmol of GTPγS in 20 μl of the filtered reaction mix. The radioactivity data is shown as mean ± S.E. of three independent experiments. A combined single exponential fit (solid line) of all 3 data sets for αT*(S43N) yielded a rate constant of 0.076 ± 0.005 min−1 (± S.E.). A single exponential fit of the fluorescence data for αT*(S43N) yielded a rate constant of 0.085 ± 0.003 min−1 (mean ± S.E.; n = 21). Data from experiments carried out in the absence of αT*(S43N) or wild-type αT* are shown as closed circles. Inset, αT*(S43N) or wild-type αT* (5 μm) was incubated in the presence of 100 μm [3H]GDP for either 2 or 3 h, respectively. The mixtures were diluted 10-fold into HM buffer containing 1 mm GTPγS and incubated at room temperature. At different times, aliquots (20 μl) of the reaction mixture were removed and treated as described above. The y-axis shows the percentage of [3H]GDP remaining bound to the αT* subunits, relative to the amount bound after 15 s of incubation. Wild-type αT*: □. αT*(S43N) : ○. B, 50 μm [35S]GTPγS was added to 300 nm αT*(S43N) in the presence of 400 nm β1γ1 and the indicated concentrations of R* (○: no R*; △: 14 nm R*; □: 112 nm R*). The samples were filtered and counted as described in panel A. The y-axis shows the number of pmol of GTPγS in 20 μl of the reaction mix. The radioactivity data are shown as mean ± S.E. of 3 independent experiments. Data from experiments carried out in the absence of αT*(S43N) are shown as corresponding closed symbols. Inset, 50 μm [35S]GTPγS was added to 300 nm wild-type αT* in the presence of 400 nm β1γ1 and the indicated concentrations of R* (○: no R*; △: 14 nm R*; □: 112 nm R*). The samples were filtered and counted as described in panel A.
When R* was added together with the β1γ1 subunit complex to wild-type αT*, there was the expected marked increase in the rate of GDP-GTP exchange. For example, the addition of 12 nm R*, along with 300 nm β1γ1, to 300 nm wild-type αT* increased the rate constant for guanine nucleotide exchange by at least 500-fold (Fig. 3B, inset, open triangles). This rate enhancement due to light-activated rhodopsin is similar to what has been reported earlier (10). However, the addition of even relatively high levels of R* to the αT*(S43N) mutant had only minimal effects on the rate of nucleotide exchange (Fig. 3B). Specifically, when the αT*(S43N) mutant was assayed in the presence of 112 nm R* and 400 nm β1γ1, the rate constant for GDP-GTP exchange was increased by only ∼3-fold. The rate of GDP-GTP exchange upon wild-type αT* was too fast to be measured under these conditions (Fig. 3B, inset, open squares). These findings suggested either that the αT*(S43N) mutant is impaired in its ability to interact with R* or that the release of R* from the activated, GTPγS-bound mutant is slow relative to its rate of release from wild-type αT*, thus compromising the ability of R* to act catalytically.
The β1γ1 Subunit Complex Has No Effect on the GDP-GTP Exchange Activity of the αT* (S43N) Mutant
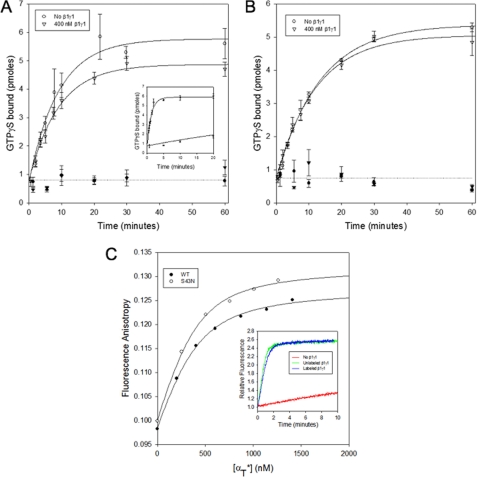
The β1γ1 subunit complex has been shown to increase the affinity of GPCRs like R* for their Gα-signaling partners, as well as to help GPCRs to further increase the rate of G-protein activation (33–37). The stimulatory actions of the β1γ1 subunit complex were clearly evident when assaying nucleotide exchange on the wild-type αT* subunit, as the rate constant for R*-stimulated GDP-GTP exchange increased from 0.02 min−1 in the absence of β1γ1 to 0.78 min−1 in the presence of 400 nm β1γ1 (Fig. 4A, inset). This rate enhancement, due to the β1γ1 complex, is similar to that reported in earlier studies (10). However, this was not the case when assaying the αT*(S43N) mutant, as the rate of nucleotide exchange upon the addition of 400 nm β1γ1 to αT*(S43N) in the presence of 14 nm R* was 0.11 min−1 (Fig. 4A), similar to the rate constant obtained in the absence of β1γ1 (0.11 min−1). Similarly, the β1γ1 subunit complex had no effect on the GDP-GTP exchange activity of the S43N mutant in the absence of R* (Fig. 4B). These findings raised the possibility that the αT*(S43N) mutant might not be able to effectively interact with the β1γ1 subunit complex. We then used a fluorescence anisotropy assay that allows for the direct monitoring of the binding of Gα subunits to the β1γ1 subunit complex labeled with IAEDANS, and found that the αT*(S43N) mutant bound to β1γ1 with an affinity that was similar to that for the binding of wild-type αT* to β1γ1 (Fig. 4C). This indicates that the β1γ1 subunit complex is able to bind to the αT*(S43N) mutant but it is unable to help accelerate the nucleotide exchange reaction in the presence of R*.
FIGURE 4.
Effects of β1γ1 on the GDP-GTP exchange activity of αT* (S43N). A, 50 μm [35S]GTPγS was added to 300 nm αT*(S43N) and 14 nm R* in the absence (○) or presence of 400 nm β1γ1 (▿). The samples were filtered and counted as described in the legend for Fig. 3A. The radioactivity data is shown as the mean ± S.E. of three independent experiments. The lines through the data show a simultaneous single exponential fit of all 3 data sets. Data from experiments carried out in the absence of αT*(S43N) are shown as corresponding closed symbols. Inset, 50 μm [35S]GTPγS was added to 300 nm wild-type αT* and 14 nm R* in the absence or presence of 400 nm β1γ1. The samples were filtered and counted as described in the legend for Fig. 3A. B, 50 μm [35S]GTPγS was added to 300 nm αT*(S43N) in the absence (○) or presence of 400 nm β1γ1 (▿). The samples were filtered and counted as described in the legend for Fig. 3A. The y-axis shows the number of pmol of GTPγS in 20 μl of the reaction mix. The radioactivity data is shown as the mean ± S.E. of three independent experiments. The lines through the data show a simultaneous single exponential fit of all 3 data sets. Data from experiments carried out in the absence of αT*(S43N) are shown as corresponding closed symbols. C, aliquots of wild-type αT* (○) and αT*(S43N) (●) were added successively to the β1γ1 complex (400 nm) labeled with IAEDANS in HMDM buffer and the fluorescence anisotropy was monitored (excitation = 336 nm, emission = 490 nm). The data were fit to a bimolecular binding model. Inset, 50 μm GTPγS was added to 300 nm wild-type αT* and 10 nm R* in the absence (red line) or the presence of either 60 nm unlabeled β1γ1 complex (green), or the β1γ1 complex labeled with IAEDANS (blue line) in HMDM buffer, and the tryptophan fluorescence emission was monitored in real-time with the excitation wavelength set at 300 nm and the emission wavelength at 345 nm.
As expected, the rate of R*-stimulated GDP-GTP exchange on wild-type αT* was very slow in the absence of any added β1γ1. However, the rate of GDP-GTP exchange was enhanced in the presence of 60 nm β1γ1. Importantly, a similar rate of GDP-GTP exchange was observed when the IAEDANS-labeled β1γ1 was used instead of unlabeled β1γ1 (Fig. 4C, inset), demonstrating that the IAEDANS-labeled β1γ1 was functionally equivalent to its unlabeled counterpart.
αT* (S43N) Slows the Rate of R*-dependent GDP-GTP Exchange on Wild-type αT*
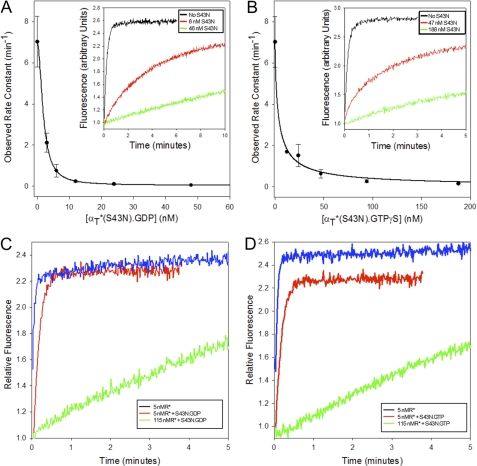
It was first shown for the small G-protein Ras, that changing the serine residue at position 17, corresponding to Ser-43 in αT*, to asparagine (S17N) yielded a potent dominant-negative inhibitor of Ras signaling by sequestering upstream GEFs (38, 39). Likewise, the αT*(S43N) mutant, even at relatively low concentrations in comparison to wild-type αT*, was able to inhibit R*-stimulated GDP-GTP exchange on the wild-type αT* subunit (Fig. 5A, inset). Specifically, the addition of ∼20 nm αT*(S43N) slowed the rate of R*-stimulated nucleotide exchange on wild-type αT* (300 nm) by ∼70-fold (Fig. 5A), indicating that the GDP-bound form of αT*(S43N) was able to bind to R* with high affinity and prevent it from binding and activating the wild-type αT* subunit.
FIGURE 5.
Effects of αT* (S43N) on the R*-dependent GDP/GTP exchange activity of wild-type αT*. A, 50 μm GTPγS was added to 6 nm R*, 300 nm wild-type αT*, 300 nm β1γ1, and varying concentrations of αT*(S43N) bound to GDP. The tryptophan fluorescence emission of the sample was monitored in real time with the excitation wavelength set at 300 nm and the emission wavelength set at 345 nm. Rate constants obtained from single exponential fits of the data are plotted as a function of the concentration of added αT*(S43N). Inset: intrinsic tryptophan fluorescence is plotted as a function of time at different concentrations of αT*(S43N) (black: none, red: 6 nm, green: 46 nm). B, 50 μm GTPγS was added to 6 nm R*, 300 nm wild-type αT*, 300 nm β1γ1, and varying concentrations of αT*(S43N) bound to GTPγS. The tryptophan fluorescence emission of the sample was monitored in real time with the excitation wavelength set at 300 nm and the emission wavelength set at 345 nm. Rate constants obtained from single exponential fits of the data are plotted as a function of the concentration of added αT*(S43N). Inset: intrinsic tryptophan fluorescence is plotted as a function of time at different concentrations of αT*(S43N) (black: none, red: 47 nm, green: 188 nm). C, 50 μm GTPγS was added to a mixture containing either 5 nm (red or green line) or 115 nm R* (blue line), 300 nm wild-type αT*, 300 nm β1γ1, in the presence (green or blue line) or absence (red line) of 30 nm αT*(S43N) bound to GDP. The tryptophan fluorescence emission of the sample was monitored in real time with the excitation wavelength set at 300 nm and the emission wavelength set at 345 nm. D, 50 μm GTPγS was added to a mixture containing either 5 nm (red or green line) or 115 nm R* (blue line), 300 nm wild-type αT*, 300 nm β1γ1, in the presence (green or blue line) or absence (red line) of 100 nm αT*(S43N) bound to GTPγS. The tryptophan fluorescence emission of the sample was monitored in real time with the excitation wavelength set at 300 nm and the emission wavelength set at 345 nm.
Interestingly, we found that the GTPγS-bound form of the αT*(S43N) mutant was also capable of forming a stable complex with R*. As shown in Fig. 5B, increasing concentrations of the GTPγS-bound form of this αT* mutant were able to reduce the rate of R*-stimulated GDP-GTP exchange on wild-type αT*. For example, the addition of ∼50 nm GTPγS-bound αT*(S43N) slowed the rate of GDP-GTP exchange on wild-type αT* by ∼10-fold (Fig. 5B, inset).
Addition of light-activated rhodopsin at 115 nm was able to relieve the inhibition of nucleotide exchange on wild-type αT* caused by the addition of either GDP-bound (Fig. 5C) or GTPγS-bound (Fig. 5D) αT*(S43N). This suggests the idea that the αT*(S43N) mutant is acting by forming a stable complex with R* and blocking its interaction with wild-type αT*.
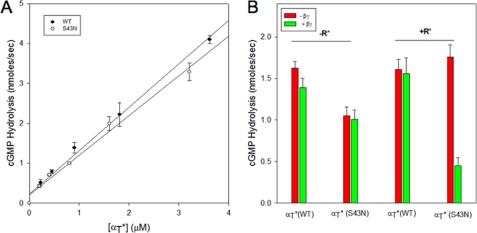
R* and the β1γ1 Subunit Complex Together Inhibit PDE Activation by αT* (S43N)
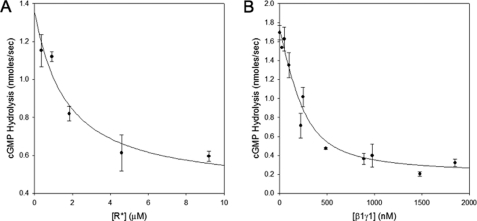
Given the indications that the GTPγS-bound αT*(S43N) mutant can form a stable complex with R*, we were interested in seeing whether the αT*(S43N)-GTPγS species was also capable of engaging and regulating the cyclic GMP PDE. We found that like GTPγS-bound wild-type αT*, the GTPγS-bound αT*(S43N) mutant was able to bind to the PDE and stimulate its activity in a dose-dependent manner (Fig. 6A). The activation of the PDE by wild-type αT* was unaffected when R* and the β1γ1 subunit complex were added separately or together. This is because αT*-GTPγS has a very low affinity for R* and β1γ1. Similarly, the ability of αT*(S43N)-GTPγS to activate PDE was unaffected when R* and the β1γ1 complex were added individually to αT*(S43N). However, surprisingly, the addition of R* and the β1γ1 subunit complex together to αT*(S43N) inhibited its ability to activate the PDE (Fig. 6B). The inhibition of the αT*(S43N)-stimulated PDE activity occurred in a dose-dependent manner with respect to either R* (Fig. 7A) or the β1γ1 subunit complex (Fig. 7B). This suggests that R*, the activated form of αT*(S43N), and the β1γ1 subunit complex are able to form a ternary complex that prevents αT*(S43N) from stimulating PDE activity.
FIGURE 6.
PDE activation by αT* (S43N). A, wild-type αT* (●) or αT*(S43N) (○) at various concentrations was preincubated with 100 μm GTPγS in the presence of 118 nm R* and 370 nm β1γ1 for 1 h in a buffer containing 5 mm HEPES, pH 7.4 (AB buffer), at room temperature. PDE (70 nm) was added to this mixture and the pH of the sample was monitored. Subsequently, cGMP (2 mm) was added to initiate the reaction and the change in pH was monitored over time. At the end of the assay period, the buffering capacity (mV/nmol) was determined by the addition of 600 nmol of NaOH to the reaction mixture. The rate of hydrolysis of cGMP (nmol/s) was determined from the ratio of the slope of the pH record (mV/s) and the buffering capacity of the assay buffer (mV/nmol) and plotted on the Y-axis. The data are shown as the mean ± S.E. from three independent experiments. B, GTPγS-bound αT*(S43N) or wild-type αT* was prepared by incubating either αT* subunit with 100 μm GTPγS at room temperature for 2 h and then placed on ice. The incubation mixture contained 1 μm R* and 1 μm β1γ1 in the case of wild-type αT*. Subsequently, the GTPγS-bound αT* subunits were diluted into AB buffer to a final concentration of 250 nm in the presence or absence of R* (4.8 μm) and β1γ1 (400 nm) and incubated at room temperature for 5 min. PDE was added to a final concentration of 160 nm, and its activation was measured as described for panel A. The data are presented as a bar graph with the y-axis showing the PDE activity in nmol/s. The red bars show data in the absence of β1γ1, and the green bars show data in the presence of β1γ1. The presence or absence of R* and the identity of the αT* subunits are shown on the plots.
FIGURE 7.
Effects of R* and β1γ1 on PDE activation by αT* (S43N). αT*(S43N) bound to GTPγS was prepared as described in Fig. 6B. This was diluted in AB buffer to a final concentration of 250 nm. Subsequently, the β1γ1 subunit complex (500 nm, panel A) or R* (4.8 μm, panel B) was added to the sample in the presence of varying concentrations of R* (panel A) or β1γ1 (panel B). PDE was added to a final concentration of 160 nm, and its activation was measured as described for Fig. 6B.
DISCUSSION
The visual phototransduction pathway has been proven to be a valuable system for understanding how a heptahelical GPCR can lead to the activation of its cognate G-protein and to the stimulation of an intracellular effector activity (40). However, despite an extensive number of biochemical and structural studies, the exact sequence of events that occurs during this process is not clear. According to the traditional model for G-protein activation, a GPCR that is activated by an external stimulus like light or hormones binds to a heterotrimeric G-protein. This binding triggers the release of GDP from the Gα subunit and its replacement by GTP. The binding of GTP has been shown to cause structural changes in the Gα subunit (24). These structural changes have been proposed to cause the dissociation of the Gα subunit from both the βγ subunit complex and the activated GPCR. The GTP-bound Gα subunit can then bind to and regulate its downstream targets.
Our strategy for gaining a better understanding of the mechanism of G-protein activation has been to identify and characterize Gα mutants that might provide insight into different stages of the activation process (10–12). In particular, we have generated mutations within a chimeric αT subunit, referred to as αT*, that contains an insertion between residues 215 and 295 of the corresponding region from Gαi1. Although the insertion comprises 79 residues, there are only 21 amino acid substitutions, of which 12 are conservative. This insertion includes the substitution of residues in the conformationally sensitive Switch 3 region of αT. Despite these alterations, αT* is similar to retinal αT in undergoing GDP-GTP exchange in a R*-dependent manner and activating the cGMP phosphodiesterase.
In this study, we have changed the conserved serine residue within the region referred to as the P-loop to an asparagine residue. In the x-ray crystal structures of αT, in complex with either GDP or GTPγS, the main-chain nitrogen of Ser43 forms a hydrogen bond with the non-bridging oxygen of the β-phosphate of the nucleotide (24, 30). In addition, the side-chain hydroxyl oxygen of Ser-43 forms hydrogen bonds with the carboxyl oxygen of Asp-196, located in the Switch 2 region, in both the GDP- and GTPγS-bound forms of αT, and with the side-chain hydroxyl of Thr-177 in the GTPγS-bound structure of αT.
One of the first observations we made regarding the αT*(S43N) mutant was its ability to undergo spontaneous GDP-GTP exchange, in the absence of R*. The rate-limiting step of the GDP-GTP exchange reaction is the release of GDP from the αT subunit (32). Thus, the S43N mutation apparently causes a change in the conformation of the P-loop to enhance the rate of GDP release. Still despite its weakened affinity, a substantial fraction of the purified αT*(S43N) mutant contains bound GDP as indicated by reverse-phase HPLC.
Surprisingly, R* was much less effective at stimulating GDP-GTP exchange on αT*(S43N) compared with the wild-type αT* subunit. The main binding site for R* on the αT subunit is located at the C terminus, which is a considerable distance from the P-loop (4). This suggested that the impaired ability of R* to catalyze GDP-GTP exchange on αT*(S43N) might not be due to R* having a reduced affinity for the GDP-bound form of αT*(S43N). Instead, the αT*(S43N) mutant may be impaired in its ability to undergo the necessary conformational change following GTP binding that enables a rapid release of R*, thereby making R* a less efficient catalyst of the nucleotide exchange reaction.
Mutation of the homologous serine residue in the monomeric G-protein Ras to an asparagine (S17N) yields a protein whose nucleotide-free form has an extremely high affinity for its GEFs (14, 38). Introduction of this mutant into cells inhibits the activation of wild-type Ras, such that it behaves as a dominant-negative inhibitor (38). Similar to the S17N mutant of Ras, the αT*(S43N) mutant inhibits the activation of wild-type αT*. Because GTPγS is able to bind to αT*(S43N) in the absence of R*, we were able to prepare GTPγS-bound αT*(S43N) and examine its effects on the GDP-GTP exchange activity of wild-type αT*. We found that the GTPγS-bound form of αT*(S43N) was also able to inhibit R*-dependent GDP-GTP exchange on wild-type αT*, in a dose-dependent manner. These findings suggest that upon the binding of GTPγS, the αT*(S43N) mutant was still capable of associating with the activated receptor.
GTPγS-bound wild-type αT*, that was prepared in the presence of low concentrations of R* and β1γ1, activated the cGMP PDE very effectively. The presence of high concentrations of R* and β1γ1, either separately or together, had no effect on the ability of GTPγS-bound αT* to activate the PDE. This is consistent with the observation that upon binding GTPγS, wild-type αT* dissociates from R* and β1γ1 (41). The GTPγS-bound form of αT*(S43N) is able to activate the cGMP PDE with a dose-response that is similar to that for wild-type αT*. Neither the addition of R* alone, nor β1γ1 alone, had any effect on the activation of the PDE by GTPγS-bound αT*(S43N). However, the combined addition of R* and β1γ1 strongly inhibited the activation of the PDE by αT*(S43N). This provides evidence for the existence of a ternary complex consisting of R*, GTPγS-bound αT*(S43N), and β1γ1. The existence of such a ternary complex was initially postulated based on the enhancement of the cholera toxin-catalyzed ADP-ribosylation of retinal αT that was induced in the presence of R*, the β1γ1 subunit complex, and the non-hydrolyzable GTP analog Gpp(NH)p (42, 43). The ability of a ternary complex to form consisting of an activated receptor, an activated Gα subunit, and a Gβγ subunit complex carries interesting implications for the mechanisms that describe the reaction pathway for the activation of heterotrimeric G-proteins, as it raises important questions concerning whether Gβγ has a role in influencing the binding of GTP and the ensuing GTP-induced activating conformational change within Gα, as well as when the activated GTP-bound Gα subunit dissociates from Gβγ and the receptor, as this has been been traditionally assumed to be necessary for Gα to engage and regulate its effector activity.
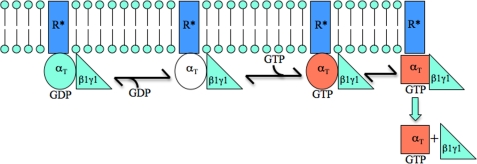
Based on these findings, we propose the following model (Fig. 8). The exchange of GTP for GDP within a Gα subunit complexed to β1γ1 and R* leads to the formation of a ternary complex. We postulate that the αT*(S43N) mutant stabilizes the ternary complex, such that it persists even following GDP-GTP exchange on αT*(S43N). It has been shown that the binding of Mg2+ is essential for the enhancement in intrinsic trytophan fluorescence that accompanies the conformational change in Switch 2 that occurs upon the activation of retinal αT (44). In the case of the αT*(S43N) mutant, we observe changes in its intrinsic tryptophan fluorescence upon binding GTPγS, but these changes are substantially smaller than those for wild-type αT*. The Switch 2 region is one of two regions on the αT subunit, with the other being the N-terminal helix, that is involved in binding to the β1γ1 complex (45). This subunit complex has been shown to increase the affinity of αT for R* (34). Thus, in order for GTP-bound αT to dissociate from R*, the affinity of β1γ1 for αT has to be reduced and this might be an outcome of a Mg2+-induced conformational change that occurs in Switch 2. The inability of the GTP-bound αT*(S43N) mutant to undergo this Mg2+-dependent conformational change would then stabilize a ternary complex consisting of R*, αT*(S43N)-GTPγS, and β1γ1. However, the free GTPγS-bound αT*(S43N) species (i.e. when not associated with either R* or β1γ1) is able to activate the cGMP PDE as effectively as wild-type αT*. This indicates that upon GDP-GTP exchange, αT*(S43N) is able to couple to its biological effector (i.e. the PDE), provided that neither R* or β1γ1 is bound to this mutant so as to interfere with effector binding.
FIGURE 8.
The αT* (S43N) mutant makes it possible to trap a GPCR-Gα-GTP-Gβγ complex. The activation of a GPCR (e.g. R*) promotes its interaction with a heterotrimeric G-protein (αT-GDP-β1γ1). This results in GDP-GTP exchange on αT, leading to a conformational change (i.e. depicted by the change from a red circle to a red square) that enables αT-GTP to dissociate from β1γ1 and R*. Apparently, the binding of Mg2+ by αT is necessary for this conformational change. Thus, the GTP-bound αT*(S43N) mutant, that is incapable of binding Mg2+ and undergoing such a conformational change, is able to form a stable complex with R* and β1γ1.
In summary, we have characterized an S43N mutant of a Gα subunit and shown that it can stabilize a ternary complex that also includes a GPCR (R*) and a Gβγ complex (β1γ1). Thus, the αT*(S43N) acts as a dominant negative inhibitor of wild-type αT by sequestering R* and β1γ1, even when it is bound to GTP. Perhaps more importantly, this αT* mutant makes it possible to trap a previously uncharacterized intermediate along the G-protein activation pathway (i.e. consisting of the receptor, the GTP-bound Gα subunit, and the Gβγ complex).
Acknowledgments
We thank Cindy Westmiller for excellent secretarial assistance and Jon Erickson for comments on the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant GM047458.
- GPCR
- G protein-coupled receptor
- PDE
- cGMP-phosphodiesterase
- cGMP
- cyclic guanosine monophosphate
- GTPγS
- guanosine-5′-O-(3-thiotriphosphate)
- ROS
- rod outer segment
- HPLC
- high pressure liquid chromatography
- P-loop
- phosphate-binding loop
- AlF4−
- aluminum fluoride
- Gpp(NH)p
- guanosine 5′-(β,γ-imido)triphosphate.
REFERENCES
- 1. Kobilka B., Schertler G. F. (2008) Trends Pharmacol. Sci. 29, 79–83 [DOI] [PubMed] [Google Scholar]
- 2. Gether U., Kobilka B. K. (1998) J. Biol. Chem. 273, 17979–17982 [DOI] [PubMed] [Google Scholar]
- 3. Sprang S. R., Chen Z., Du X. (2007) Adv. Protein Chem. 74, 1–65 [DOI] [PubMed] [Google Scholar]
- 4. Oldham W. M., Hamm H. E. (2008) Nat. Rev. Mol. Cell Biol. 9, 60–71 [DOI] [PubMed] [Google Scholar]
- 5. Oldham W. M., Hamm H. E. (2007) Adv. Protein Chem. 74, 67–93 [DOI] [PubMed] [Google Scholar]
- 6. Burns M. E., Arshavsky V. Y. (2005) Neuron 48, 387–401 [DOI] [PubMed] [Google Scholar]
- 7. Chen C. K. (2005) Rev. Physiol. Biochem. Pharmacol. 154, 101–121 [DOI] [PubMed] [Google Scholar]
- 8. Mittal R., Erickson J. W., Cerione R. A. (1996) Science 271, 1413–1416 [DOI] [PubMed] [Google Scholar]
- 9. Li Q., Cerione R. A. (1997) J. Biol. Chem. 272, 21673–21676 [DOI] [PubMed] [Google Scholar]
- 10. Majumdar S., Ramachandran S., Cerione R. A. (2004) J. Biol. Chem. 279, 40137–40145 [DOI] [PubMed] [Google Scholar]
- 11. Majumdar S., Ramachandran S., Cerione R. A. (2006) J. Biol. Chem. 281, 9219–9226 [DOI] [PubMed] [Google Scholar]
- 12. Pereira R., Cerione R. A. (2005) J. Biol. Chem. 280, 35696–35703 [DOI] [PubMed] [Google Scholar]
- 13. Skiba N. P., Thomas T. O., Hamm H. E. (2000) Methods Enzymol. 315, 502–524 [DOI] [PubMed] [Google Scholar]
- 14. John J., Rensland H., Schlichting I., Vetter I., Borasio G. D., Goody R. S., Wittinghofer A. (1993) J. Biol. Chem. 268, 923–929 [PubMed] [Google Scholar]
- 15. Slepak V. Z., Quick M. W., Aragay A. M., Davidson N., Lester H. A., Simon M. I. (1993) J. Biol. Chem. 268, 21889–21894 [PubMed] [Google Scholar]
- 16. Slepak V. Z., Katz A., Simon M. I. (1995) J. Biol. Chem. 270, 4037–4041 [DOI] [PubMed] [Google Scholar]
- 17. Cleator J. H., Mehta N. D., Kurtz D. T., Hildebrandt J. D. (1999) FEBS Lett. 443, 205–208 [DOI] [PubMed] [Google Scholar]
- 18. Natochin M., Barren B., Artemyev N. O. (2006) Biochemistry 45, 6488–6494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fawzi A. B., Northup J. K. (1990) Biochemistry 29, 3804–3812 [DOI] [PubMed] [Google Scholar]
- 20. Kroll S., Phillips W. J., Cerione R. A. (1989) J. Biol. Chem. 264, 4490–4497 [PubMed] [Google Scholar]
- 21. Min K. C., Gravina S. A., Sakmar T. P. (2000) Protein Expr. Purif. 20, 514–526 [DOI] [PubMed] [Google Scholar]
- 22. Liebman P. A., Evanczuk A. T. (1982) Methods Enzymol. 81, 532–542 [DOI] [PubMed] [Google Scholar]
- 23. Higashijima T., Ferguson K. M., Sternweis P. C., Smigel M. D., Gilman A. G. (1987) J. Biol. Chem. 262, 762–766 [PubMed] [Google Scholar]
- 24. Lambright D. G., Noel J. P., Hamm H. E., Sigler P. B. (1994) Nature 369, 621–628 [DOI] [PubMed] [Google Scholar]
- 25. Cleator J. H., Ravenell R., Kurtz D. T., Hildebrandt J. D. (2004) J. Biol. Chem. 279, 36601–36607 [DOI] [PubMed] [Google Scholar]
- 26. Berlot C. H. (2002) J. Biol. Chem. 277, 21080–21085 [DOI] [PubMed] [Google Scholar]
- 27. Iiri T., Bell S. M., Baranski T. J., Fujita T., Bourne H. R. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 499–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sondek J., Lambright D. G., Noel J. P., Hamm H. E., Sigler P. B. (1994) Nature 372, 276–279 [DOI] [PubMed] [Google Scholar]
- 29. Phillips W. J., Cerione R. A. (1988) J. Biol. Chem. 263, 15498–15505 [PubMed] [Google Scholar]
- 30. Noel J. P., Hamm H. E., Sigler P. B. (1993) Nature 366, 654–663 [DOI] [PubMed] [Google Scholar]
- 31. Natochin M., Granovsky A. E., Muradov K. G., Artemyev N. O. (1999) J. Biol. Chem. 274, 7865–7869 [DOI] [PubMed] [Google Scholar]
- 32. Ferguson K. M., Higashijima T., Smigel M. D., Gilman A. G. (1986) J. Biol. Chem. 261, 7393–7399 [PubMed] [Google Scholar]
- 33. Phillips W. J., Cerione R. A. (1992) J. Biol. Chem. 267, 17032–17039 [PubMed] [Google Scholar]
- 34. Phillips W. J., Wong S. C., Cerione R. A. (1992) J. Biol. Chem. 267, 17040–17046 [PubMed] [Google Scholar]
- 35. Fawzi A. B., Fay D. S., Murphy E. A., Tamir H., Erdos J. J., Northup J. K. (1991) J. Biol. Chem. 266, 12194–12200 [PubMed] [Google Scholar]
- 36. Wildman D. E., Tamir H., Leberer E., Northup J. K., Dennis M. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 794–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jian X., Clark W. A., Kowalak J., Markey S. P., Simonds W. F., Northup J. K. (2001) J. Biol. Chem. 276, 48518–48525 [DOI] [PubMed] [Google Scholar]
- 38. Feig L. A., Cooper G. M. (1988) Mol. Cell. Biol. 8, 3235–3243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Feig L. A. (1999) Nat. Cell Biol. 1, E25–E27 [DOI] [PubMed] [Google Scholar]
- 40. Arshavsky V. Y., Lamb T. D., Pugh E. N., Jr. (2002) Annu. Rev. Physiol. 64, 153–187 [DOI] [PubMed] [Google Scholar]
- 41. Fung B. K. (1983) J. Biol. Chem. 258, 10495–10502 [PubMed] [Google Scholar]
- 42. Abood M. E., Hurley J. B., Pappone M. C., Bourne H. R., Stryer L. (1982) J. Biol. Chem. 257, 10540–10543 [PubMed] [Google Scholar]
- 43. Navon S. E., Fung B. K. (1984) J. Biol. Chem. 259, 6686–6693 [PubMed] [Google Scholar]
- 44. Zelent B., Veklich Y., Murray J., Parkes J. H., Gibson S., Liebman P. A. (2001) Biochemistry 40, 9647–9656 [DOI] [PubMed] [Google Scholar]
- 45. Lambright D. G., Sondek J., Bohm A., Skiba N. P., Hamm H. E., Sigler P. B. (1996) Nature 379, 311–319 [DOI] [PubMed] [Google Scholar]