Abstract
The recognition that malfunction of the microtubule (MT) associated protein tau is likely to play a defining role in the onset and/or progression of a number of neurodegenerative diseases, including Alzheimer's disease, has resulted in the initiation of drug discovery programs that target this protein. Tau is an endogenous MT-stabilizing agent that is highly expressed in the axons of neurons. The MT-stabilizing function of tau is essential for the axonal transport of proteins, neurotransmitters and other cellular constituents. Under pathological conditions, tau misfolding and aggregation results in axonal transport deficits that appear to have deleterious consequences for the affected neurons, leading to synapse dysfunction and, ultimately, neuronal loss. This review focuses on both progress and unresolved issues surrounding the development of novel therapeutics for the treatment of neurodegenerative tauopathies, which are based on (A) MT-stabilizing agents to compensate for the loss of normal tau function, and (B) small molecule inhibitors of tau aggregation.
INTRODUCTION
The modulation of protein-protein interactions, which are intimately involved in the vast majority of biological processes, holds considerable promise as a strategy for the development of new therapies. Thus far, several successful examples of this approach have appeared, particularly in the area of therapeutic antibodies, where a number of biologics are now part of the medical armamentarium for the treatment of various diseases while many others are presently undergoing clinical development [1]. In comparison, the discovery of small molecule modulators of protein-protein interactions has proven far more challenging, partly due to the fact that in the majority of cases there are no known natural small ligands that can be employed as starting points for drug-design [2]. Furthermore, since the interactions between macromolecules frequently take place over relatively large, mostly flat and ill defined surfaces (i.e., 750 to 1,500 Å2), the identification of specific regions that may serve as binding pockets for small ligands is often difficult, even when protein-protein interfaces can be studied in detail by means of X-ray crystallography [3, 4]. Nonetheless, despite these significant challenges, some notable instances provide clear evidence that modulation of protein-protein interactions by means of small molecules is indeed possible. Such examples include the immunosuppressants cyclosporine A and FK506 [5, 6], the microtubule (MT)-stabilizing (e.g., taxanes) and destabilizing (e.g., vinca alkaloids) natural products [7], and several drug-like RGD-mimetics [3]. Furthermore, a growing number of protein-protein interactions are considered to be potentially druggable targets [3, 4, 8]. In this review, we highlight both progress and challenges associated with the use of MT-stabilizing agents and small molecule inhibitors of tau aggregation as potential treatments of Alzheimer's disease (AD) and related neurodegenerative diseases, collectively known as tauopathies. In the first part of the review, we summarize our current understanding of tau-mediated neurodegeneration as well as the rationale for therapeutic intervention based on MT-stabilizing agents and inhibitors of tau aggregation. Next, we review different classes of MT-stabilizing agents with a particular emphasis on their potential to be developed as novel treatments for central nervous system (CNS) diseases like AD and related tauopathies. Finally, we present the “state-of-the-art” in the area of small molecule inhibitors of tau aggregation.
Tauopathies and tau-mediated neurodegeneration
A number of neurodegenerative tauopathies [9], which include AD, Pick's disease and frontotemporal dementia (FTD) with Parkinsonism linked to chromosome 17 (FTDP-17), are characterized by the presence of insoluble proteinaceous deposits comprised of hyperphosphorylated tau proteins, referred to as neurofibrillary tangles (NFTs), and dystrophic processes (axons, dendrites) or neuropil threads. In addition to their diagnostic significance, such lesions are believed to play a significant role in the onset and/or progression of these diseases. Under physiological conditions, the primary function of tau, which is highly expressed in the axons of neurons, is to stabilize the MTs. The MT-stabilizing function of tau is essential for the axonal transport of protein, trophic factors and other cellular constituents. Although the exact mechanism(s) underlying tau-mediated neurodegeneration has not been fully elucidated, under pathological conditions, tau malfunctions can lead to synaptic dysfunction and neuronal loss at least in part by causing axonal transport deficits [10, 11].
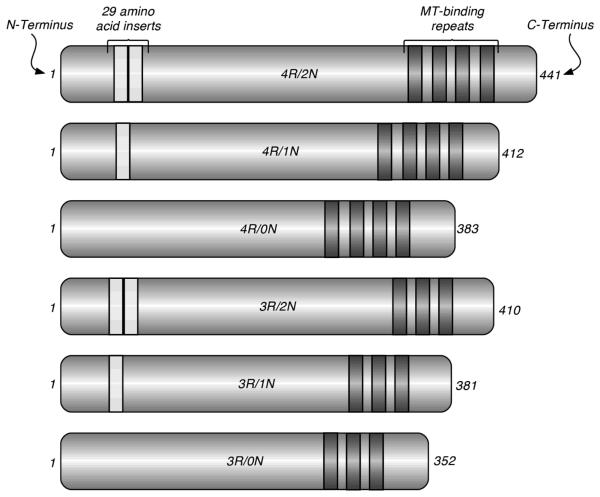
In the adult human brain there are six tau isoforms (Figure 1). Although these isoforms are functionally similar in that all are capable of binding to and stabilizing MTs, there are qualitative and quantitative differences, particularly between the three repeat (3R) and the four repeat (4R) tau species, with 4R-tau binding MTs with greater avidity than 3R-tau [12]. Furthermore, there is evidence to suggest that while 4R-tau is a potent inhibitor of MT-shortening, 3R-tau isoforms have a minimal effect on this parameter of MT-dynamics [13]. Thus, the concerted action of the 3R and 4R isoforms is likely to be essential in maintaining MT-dynamics within physiological ranges. Indeed, in the healthy adult brain the 4R/3R tau ratio is ~1, while deviations from this ratio are found in the brains of patients with FTD due to taupathologies, known as the tau variants of frontotemporal lobar degeneration (FTLD) or FTLD-Tau [14, 15]. The interaction of tau with the MT, schematically illustrated in Figure 2, has been a subject of intense study [16].
Figure 1.
The six tau isoforms are generated from the same tau (MAPT) gene by alternative splicing; they differ by the presence of either 3 or 4 MT-binding amino acid repeats in the MT-binding domains, as well as by the presence of 0, 1, or 2 29-amino acid-long inserts in the N-terminal region.
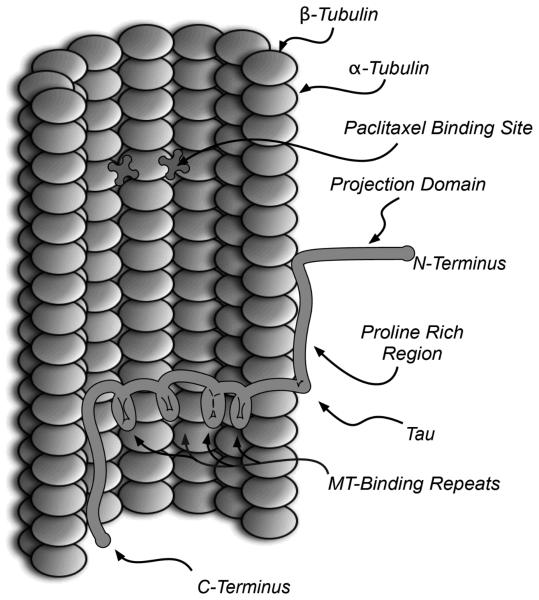
Figure 2.
Schematic representation (adapted from [16]) showing a section of a MT bound to 4R tau and the paclitaxel binding sites.
MT-stabilization is promoted by the interaction of the 3 or 4 MT-binding amino acid repeats present in the MT-binding domains of tau with highly conserved binding pockets (taxoid binding site) found in the β-tubulin subunits of adjacent protofilaments (see Figure 2). Furthermore, electrostatic interactions between the positively-charged proline rich region of tau with the negatively charged surface of the MTs further stabilize the tau-MT complex, while the negatively charged N-terminal region (i.e., the projection domain) extends away from the surface of the MT due to electrostatic repulsion [16].
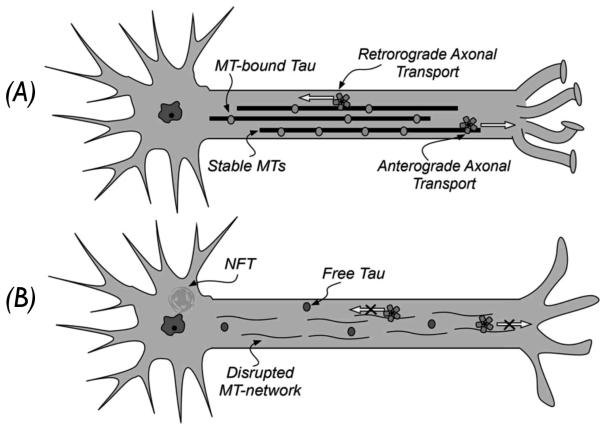
As schematically illustrated in Figure 3, the dynamics of the MT-network can greatly influence the efficiency of the axonal transport, which is responsible for the movement of signaling molecules, trophic factors and other essential cellular constituents along the axons. As such, the action that tau exerts on the MTs is key to maintaining an appropriate dynamic of the MT-network. Under physiological conditions, there is a constant dynamic equilibrium between free tau in the cytosol and MT-bound tau, with the vast majority of the 6 tau isoforms in neurons (~99%) being associated with MTs [17, 18]. Such equilibrium is believed to be post-translationally regulated, mainly by serine/threonine-directed phosphorylation of tau as higher phosphorylation states of the protein are known to reduce the binding affinity of tau for the MTs [19]. Thus, the opposing actions of tau-kinases and phosphatases are thought to play an important role in regulating the dynamic equilibrium between unbound and MT-bound tau. Under pathological conditions, this equilibrium is perturbed, resulting in an aberrant disengagement of tau from the MTs with a concomitant increase in the cytosolic tau concentration. When bound to the MTs, tau is believed not to be prone to fibrillization; however, when free in the cytosol, this natively unfolded protein can misfold thereby initiating the aggregation cascade that culminates in the formation of tau fibrils that deposit in NFTs and neuropil threads. The misfolding of tau is likely to be a stochastic phenomenon [20], which becomes more probable at higher cytosolic tau concentrations.
Figure 3.
Schematic representation of axonal transport in normal (A) and diseased neurons (B).
The pathological consequences of tau aggregation have been conceptualized as resulting from a combination of a loss of normal tau function with possible gain(s) of pathological functions by various forms of aggregated tau [10]. Thus, while the loss of the MT-stabilizing function of tau would lead to axonal transport deficits via disturbances in the normal dynamics of the MT-network, toxic functions could be also ascribed to tau aggregates. Although NFTs and neuropil threads comprise the diagnostic signatures of neurodegenerative tauopathies, there is increasing evidence to suggest that smaller soluble oligomeric tau species may be toxic to neurons [21]. The exact mechanism(s) of toxicity caused by the various forms of aggregated tau remains an area of intense investigation. It is plausible, however, that tau aggregation would impart toxicity at least in part via an amplification of the aforementioned loss of function by facilitating further sequestration of normal tau. Moreover large insoluble aggregates such as NFTs or neuropil threads, could also pose a direct physical disruption to cellular functions, including the occlusion of axons and dendrites, which could block axonal transport.
Thus, based on our current understanding of tau-mediated neurodegeneration, a number of potential targets have been identified that could be exploited for therapeutic intervention [22]. Among these, the modulation of MT-dynamics in diseased neurons appears particularly tractable given the evidence from several recent proof-of-concept studies in tau transgenic mouse models of AD-like tau pathology [23, 24] (vide infra). Furthermore, there is increasing evidence that tau misfolding and aggregation may also be effectively targeted by small-molecules.
MT-stabilizing compounds for the treatment of neurodegenerative tauopathies
Historically, MT-stabilizing agents have been employed as antineoplastic agents. Nonetheless, a number of studies suggested that these agents hold considerable promise as potential treatments for other serious conditions including rheumatoid arthritis [25], psoriasis [26] and neurodegenerative diseases [27-29]. In the context of AD and related tauopathies, the rationale for therapeutic intervention with MT-stabilizing agents is to compensate for the loss of MT-stabilizing tau function, as described above [30]. Importantly, the therapeutic potential of MT-stabilizing agents to treat neurodegenerative tauopathies was validated by in vivo efficacy studies conducted in 2005 by the Lee and Trojanowski laboratory [23]. In these studies, T44 tau transgenic (Tg) mice were treated weekly for 8 weeks via intraperitoneal injection with low (10 mg/m2), medium (25 mg/m2), and high (40 mg/m2) doses of paclitaxel in a micelle vehicle (PaxceedTM, Angiotech). The outcome of these studies demonstrated that paclitaxel treatment can compensate for the loss of MT-stabilizing function of tau and, as a result, prevent axonal transport deficits in diseased neurons with consequent improvement in the neurodegenerative phenotype. It should be noted, however, that to see improvements in axonal transport in the Tg animal model employed in these studies, paclitaxel was not required to cross the blood-brain barrier (BBB) since affected CNS motor neurons took up paclitaxel via endocytosis through axon terminals at the neuromuscular junction, where there is no BBB. Thus, since paclitaxel is known to have limited CNS exposure [31], this compound is not suitable for further development, and appropriate drug candidates for further preclinical investigations of efficacy and safety will have to be brain-penetrant. To this end, selected compounds discussed below hold considerable potential.
In addition to brain penetration, one further challenge in the development of MT-stabilizing drugs for AD is the potential for toxic side effects. Although the toxicity of MT-stabilizing agents in cancer therapy has been well documented [32-35], the doses required to promote MT-stabilization in non-dividing (i.e., postmitotic) neurons could well be considerably lower than those needed to trigger apoptosis in rapidly dividing cells. Evidence to support this possibility derives from the same proof-of-concept studies by Trojanowski, Lee and co-workers, wherein both low and medium dose regimes of paclitaxel (10 to 25 mg/m2) required for amelioration of motor impairments in tau Tg mice were well tolerated. Moreover, in these studies higher doses, similar to those used as cancer chemotherapeutic agents, proved less effective.
Taxanes
The discovery that paclitaxel, a diterpenoid natural product isolated from the Pacific yew tree Taxus brevifolia, is capable of stabilizing the interactions between α and β-tubulin heterodimers and thereby favoring the polymerization of microtubules (MTs), is one of the most prominent examples of a small-molecule modulator of protein-protein interactions. Paclitaxel interacts with the polymer form of tubulin at a binding site localized to the lumen of the MT in the β-tubulin subunit [36]. Notably, the amino acid repeats of the MT-binding domain of tau were found to bind to a site on β-tubulin that overlaps with the paclitaxel binding site [37]. Although the exact mechanism of paclitaxel-promoted MT-polymerization is not fully understood, paclitaxel binding to β-tubulin is believed to induce conformational changes that favors the interaction with the neighboring α-tubulin, thereby stabilizing the MT structure [38]. Interestingly, although paclitaxel is a potent inducer of MT-polymerization, when present in a ~1:1 stoichiometry with β-tubulin, a marked suppression of MT-dynamics without significant changes in overall MT-polymerization is achievable at the much reduced ratio of one paclitaxel molecule per several hundred β-tubulin molecules [39].
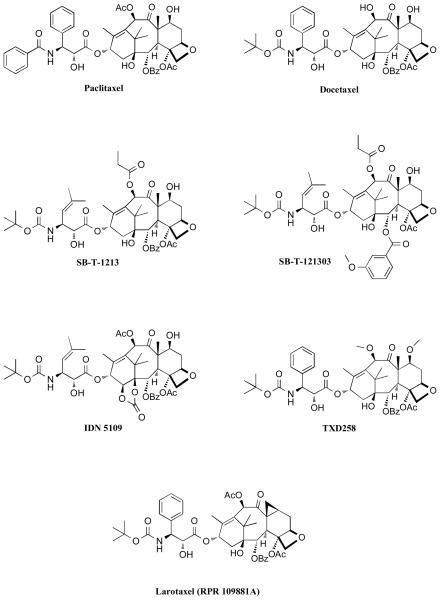
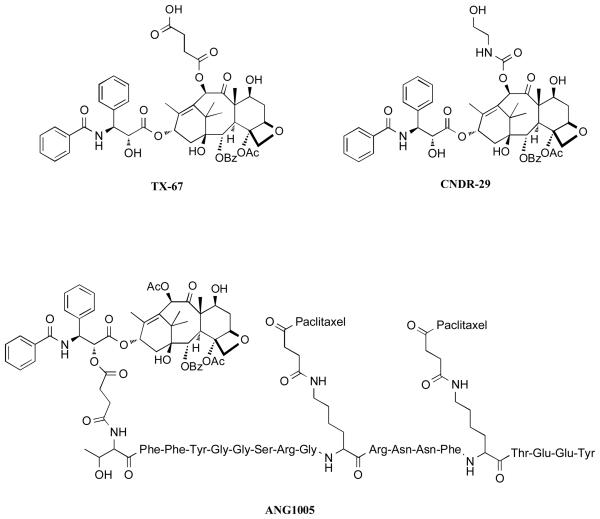
Paclitaxel (Taxol™) and the closely related semi-synthetic derivative, docetaxel (Taxotere™), have been widely employed as chemotherapeutic agents to treat solid tumors [39]. However, the use of these drugs for the treatment of CNS diseases has been largely precluded by limited brain uptake. Additional notable shortcomings of both drugs include limited water solubility, lack of oral bioavailability, and toxicities at high doses. The limited oral bioavailability and brain penetration of paclitaxel is believed to result, at least in part, from P-glycoprotein (Pgp) mediated efflux of the drug. This conclusion is based on the observation that paclitaxel brain levels are significantly higher in Pgp-knockout mice compared to wild-type animals [40]; and that co-administration of paclitaxel with Pgp-inhibitors can lead to an increase in both oral bioavailability as well as brain penetration of the drug [41-44]. Pgp is a member of the ATP-binding cassette (ABC) family of active transporters, which is highly expressed in the BBB, in addition to the gut, liver and kidneys [45]. As a result, compounds that are substrates for this active transporter often exhibit limited brain penetration and oral bioavailability. Furthermore, Pgp-mediated efflux is known to be an important contributing factor in the development of multi-drug resistance (MDR). Thus, over the past several years, there has been considerable interest in the discovery and development of taxanes that could overcome the action of Pgp. Structure-activity relationship (SAR) studies by Ojima and co-workers demonstrated that modifications in the taxane side chain as well as in the C-2 and/or C-10 position could lead to potent antimitotic compounds, as typified by compound SB-T-1213 and SB-T-121303 (Figure 4) whose anti-proliferative activity is retained in Pgp-overexpressing cancer cell-lines that are resistant to paclitaxel [46-48]. Subsequent studies revealed that SB-T-1213 and other related “second generation” taxoids effectively overcome Pgp-mediated efflux by virtue of Pgp-inhibition [49, 50]. Among these compounds, IDN5109, which exhibited approximately a 15-fold higher brain to plasma ratio (B/P), compared to paclitaxel [51], was reported to be a potent and broad-spectrum modulator of ABC-transporters [52]. Other taxanes exhibiting similar abilities to overcome Pgp and cross the BBB, include the 7-O-methyl-10-O-methyldocetaxel, TXD258, as well as Larotaxel (RPR-109881A), both developed by Sanofi-Aventis [53-55]. In the case of Larotaxel, however, published reports do not clarify whether this compound is devoid of Pgp-interactions or acts as a modulator of the active transporter. Indeed, such distinctions may be important, particularly in the context of neurodegenerative diseases as Pgp represents an important defense mechanism to limit CNS exposure to xenobiotics. Disruption of Pgp-function may thus potentially lead to serious CNS toxicities. Indeed, recent studies have shown that Pgp-deficiency at the BBB in an AD mouse model resulted in a marked increase in Aβ deposition [56]. This observation suggests that taxane derivatives lacking Pgp-interactions may be potentially safer, particularly in the treatment of AD and related disorders compared to those that inhibit Pgp. To this end, several C-10 acylated paclitaxel derivatives such as TX-67 [57] and CNDR-29 [58] (Figure 5), have been found to be devoid of Pgp-interactions (i.e., not substrates or inhibitors of the active transporter) in bi-directional permeability studies. Furthermore, ex-vivo experiments with these paclitaxel analogues indicated the potential for higher brain uptake compared to paclitaxel [57, 59].
Figure 4.
Selected taxanes
Figure 5.
Examples of C-10 acylated paclitaxel derivatives that are devoid of Pgp-interactions (TX-67 and CNDR-29); BBB-permeable paclitaxel prodrug (ANG1005).
An alternative approach for the delivery of taxanes into the CNS without disruption of Pgp-function involves the use of targeted delivery systems that take advantage of receptor-mediated uptake. An example of one such strategy comes from the prodrug ANG1005 [60], in which three paclitaxel molecules are linked to different residues of a 19 amino acid peptide, named Angiopep-2. ANG1005 was found to cross the BBB via transcytosis upon binding to the low-density lipoprotein receptor-related protein [61].
Epothilones
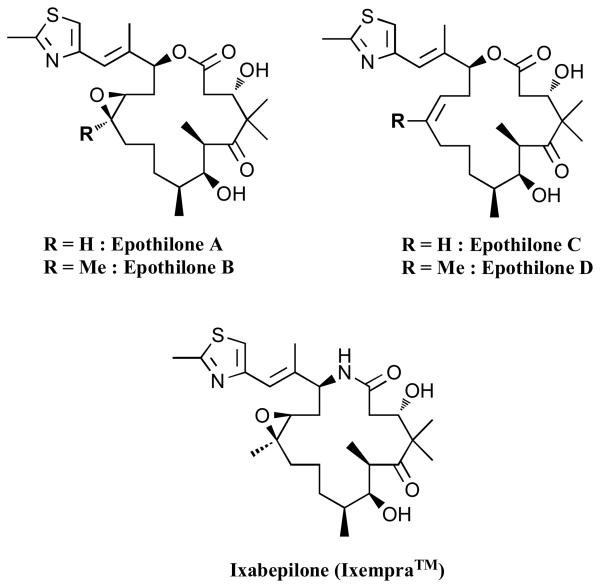
Originally described as fungicidal macrolide natural products [62], epothilones A and B (Figure 6) were subsequently found to promote MT-polymerization in vitro at submicromolar concentrations via a mechanism similar, although not identical to that of paclitaxel [63-66]. Over the past several years, considerable effort has been directed towards the synthesis and the biological evaluation of the epothilones [67-69]. Currently, Ixabepilone (Ixempra™, Bristol-Myers Squibb) is the only epothilone approved for the treatment of cancer [70], however, several other congeners are in advanced stages of development. Although the epothilones have been primarily studied as anti-cancer agents, different reports suggest the possible therapeutic benefits of these MT-stabilizing compounds for the treatment of CNS diseases [29, 30, 71].
Figure 6.
Selected epothilones.
The epothilones are of lower molecular weight than taxanes, which may suggest a higher potential for these compounds to cross the BBB via passive diffusion. Furthermore, the activity of a number of epothilones, including epothilone B, the desoxyepothilones (epothilones C and D) and related congeners, were found to be only minimally affected by over-expression of Pgp in cell culture models [72]. Pharmacokinetic (PK) studies in the patent literature showed that certain epothilones could reach significant concentrations in the brain [73]. In addition, recent PK studies with epothilone B revealed that this compound could readily distribute from plasma into the CNS of rodents, where it was retained for prolonged periods of time [74]. To investigate further the ability of epothilones to permeate the BBB and to stabilize MTs within the CNS, we recently completed a series of studies involving epothilone D and a number of related congeners [75]. As part of these studies, we monitored both the drug concentrations in the brain and plasma, as well as the elevation in acetylated tubulin in the brain of mice, a biomarker of MT stabilization [76, 77], which was used as pharmacodynamic (PD) readout. These studies demonstrated that epothilone D and several related compounds are capable of reaching considerable brain concentrations (i.e., low μM) after an intraperitoneal (i.p.) administration of 3 mg/Kg of the compound [75]. Furthermore, similarly to what was reported for epothilone B [74], we noted that the brain half-life of epothilone D is considerably longer than the plasma half-life. Our studies also showed a significant elevation in acetylated tubulin in the brain of mice 7 days after i.p. administration of 1 mg/kg of epothilone D. Our PK/PD data are in general agreement with a recent report from Bristol-Myers Squibb (BMS), that has appeared in the patent literature [24]. In addition, the BMS report revealed that treatment of Tg4510 tau Tg mice [78] with low weekly doses of epothilone D (i.e., 1 mg/Kg, i.p. injection, once a week for 2 months, corresponding to a ~100 fold lower dose employed in oncology) resulted significant improvement of cognitive functions relative to vehicle-treated animals. Notably, Tg animals treated with a higher dose of epothilone D (i.e., 10 mg/Kg) showed less improvement compared with the low-dose treatment group, suggesting that optimal therapeutic effects may be achieved without significant toxicities. Collectively, these important observations suggest that epothilone D has distinctive characteristics in terms of both efficacy and PK properties that could make this compound appropriate for the treatment of CNS diseases. It is possible that other members of the same class of compounds may have similar attributes.
Other MT-stabilizing agents natural products
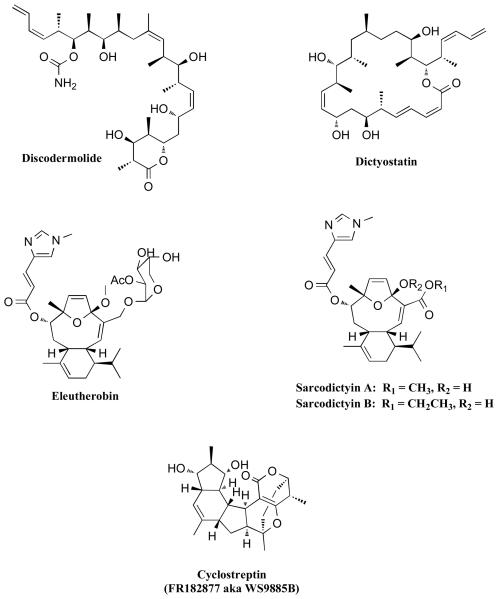
In addition to taxanes and epothilones, several other natural product classes (Figures 7 and 8) have been reported to stabilize the MTs in a manner similar to paclitaxel. Among these, discodermolide, dictyostatin, eleutherobin, and sarcodictyins (Figure 7) have all been found to bind to the taxoid binding site on β-tubulin, as revealed by competition assays [79-81]. Interestingly, despite the considerable differences in structure, these MT-stabilizing agents may all share a common pharmacophore [82]. Nonetheless, a number of significant functional differences have been discovered. For example, discodermolide is the only compound among the taxoid-site MT-stabilizing agents that acts synergistically with paclitaxel in both in vitro and in vivo experiments [83-85]. This observation is somewhat surprising and suggests that discodermolide may have alternative binding sites or multiple mechanisms to promote MT-stabilization [85]. Furthermore, unlike eleutherobin and sarcodictyins [86] and similar to many of the epothilones, discodermolide and dictyostatin are active against cancer cell-lines that are resistant to paclitaxel because of Pgp-overexpression [79, 87]. In addition, significant differences have been found in the way these drugs interact with the MTs. For example, cyclostreptin (FR182877) [88] is the only example that was found to covalently modify tubulin [89, 90]. Moreover, comparative studies involving discodermolide, epothilones and paclitaxel revealed significant difference in the kinetics of polymerization and morphology of the MTs when treated with these compounds [91, 92]. For example, the initial rate of MT polymerization is dramatically faster in the presence of discodermolide than with paclitaxel or the epothilones. However, the lengths of the MTs are considerably longer in the presence of paclitaxel. The potential implications of such differences in the overall ability of these MT-stabilizing drugs to restore axonal transport in diseased neurons have not been studied.
Figure 7.
Other natural product MT-stabilizing agents that bind to the taxoid site on β-tubulin.
Figure 8.
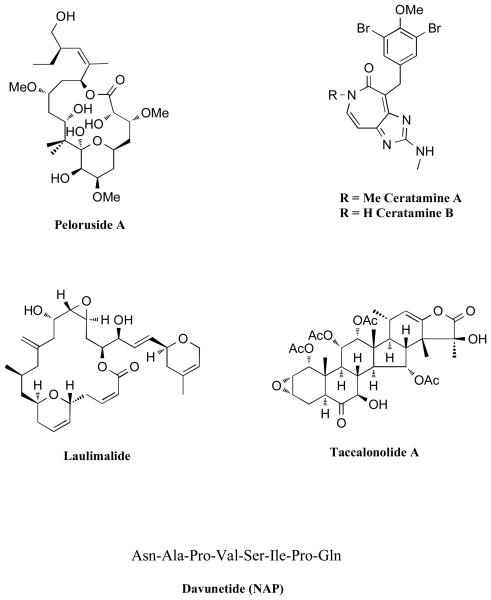
MT-stabilizing agents that are likely to act by a different mechanism than paclitaxel and other taxoid site drugs.
Compounds that can promote MT-stabilization in a manner similar to paclitaxel, but that have been found to bind to a site distinct from the taxoid site on β-tubulin include peloruside [93, 94], laulimalide [95, 96] and ceratamine A and B [97]. Interestingly, while peloruside A and laulimalide can act synergistically with other taxoid site compounds, they are not synergistic with each other, suggesting that these two compounds bind to the same site [98]. An additional group of naturally occurring compounds, the taccalonolides [99], can also effectively stabilize MTs. However, these compounds are unable to stabilize MTs in a cell-free tubulin-assembly assay, indicating a different mechanism of action for this class of compounds [100]. Finally, in addition to the MT-stabilizing natural products discussed above, Gozes and co-workers have shown that davunetide (Asn-Ala-Pro-Val-Ser-Ile-Pro-Gln; also known as “NAP”), an octapeptide derived from the activity dependent neuroprotective protein (ADNP), is capable of promoting MT-stabilization [101]. The observation that paclitaxel substantially reduces, albeit not completely, NAP binding to tubulin, suggests that there may be a partial overlapping in the binding sites of these two compounds [101]. Davunetide has been described as neuroprotective against a wide range of insults, including β-amyloid peptide, N-methyl-D-aspartate, electrical blockade, H2O2, dopamine, nutrient starvation and zinc overload [102]. Furthermore, davunetide was found to reduce tau-hyperphosphorylation both in vitro [101] as well as in ADNP+/− mice [103]. Importantly, PK studies on davunetide showed that significant amounts of the intact peptide can be detected in the brain of rats after i.v. administration of 30 mg/Kg of the compound [104]. NAP is being developed by Allon Therapeutics Inc. (Vancouver, Canada) and is currently in clinical trials for AD and soon will enter clinical trials for progressive supranuclear palsy, an FTLD-Tau disorder with dementia and parkinsonism.
Small molecule inhibitors of tau aggregation
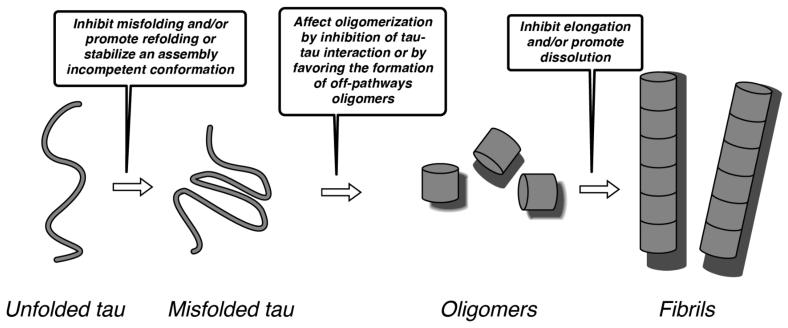
The rationale for therapeutic intervention based on small molecule inhibitors of tau aggregation relies on the notion that prevention or reduction in tau fibrillization would ameliorate the neuropathological consequences arising from the loss of tau function, as well as from the gain of toxic function(s) by tau multimers. The fibrillization of tau is a complex, multistep phenomenon (highlighted in Figure 9), which begins with tau misfolding and progresses to the formation of intermediate diffusible oligomeric structures [21, 105]. As previously noted, there is increasing evidence to suggest that these oligomeric species are important contributors to neuropathology [21] although their exact mechanism of toxicity has not as yet been elucidated. The transition from oligomers to full fibrils involves both elongation, as well as conformational changes to reveal the characteristic pleated β-sheet motif found in amyloid fibrils. Finally, these fibrils can further assemble in vivo to generate relatively large aggregates such as NFTs.
Figure 9.
Schematic representation of key steps involved in the tau fibrillization process; specific possible steps that might be targeted by potential inhibitors are highlighted in the boxes (see Brunden et al. [21], and Xu et al. [105] for details).
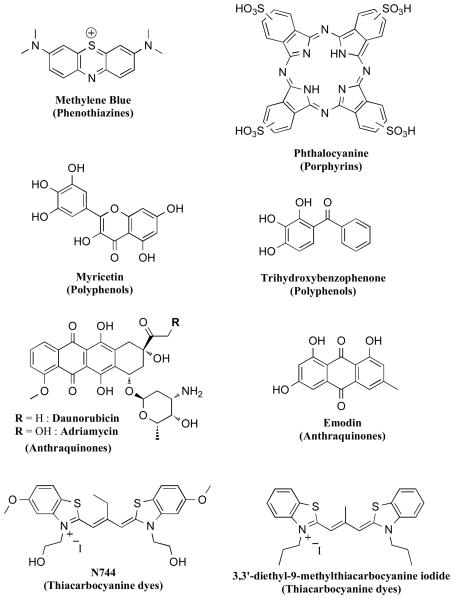
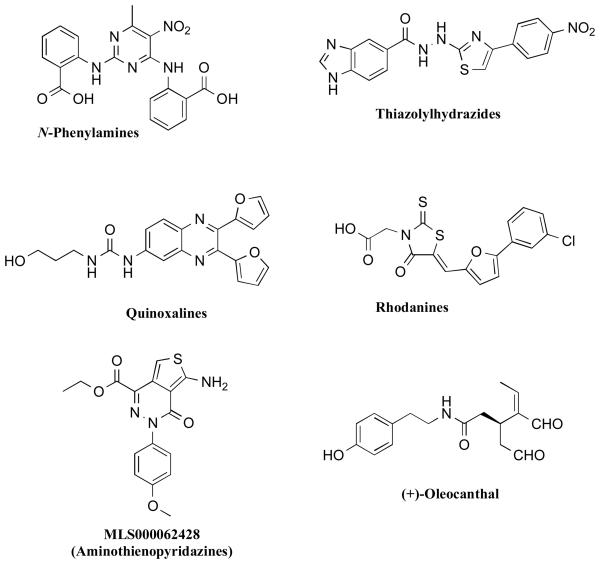
Efforts directed towards the discovery of inhibitors of tau aggregation initially faced significant challenges as tau fibrillization would only proceed in vitro under non-physiologically high tau concentrations and impractically long reaction times. This situation improved, however, with the discovery that anionic co-factors such as heparin [106] or arachidonic acid [107] induce faster fibrillization kinetics with lower concentrations of tau and truncated fragments thereof. This observation ultimately resulted in the development of several assays, some of which were amenable to high throughput screening (HTS) [108-110]. In the majority of cases, the in vitro assays relied on β-sheet specific dyes, such as thioflavine-T (ThT) or thioflavine-S (ThS), to monitor the fibrillization reaction, as these reagents are known to be highly effective indicators of amyloid fibrils that are rich in pleated β-sheet structures [111]. Collectively, these screening efforts resulted in the identification of a number of compound classes (Figure 10) that reduce the formation of tau fibrils in vitro [112, 113]. Representative classes include phenothiazines [108, 114], anthraquinones [109, 114], polyphenols [114], thiacarbocyanine dyes [115], N-phenylamines [116], thiazolyl-hydrazides [117], rhodanines [118], quinoxalines [108], and aminothienopyridazines [110]. In addition, a natural product isolated from extra-virgin olive oil, (+)-oleocanthal [119, 120], was also found to inhibit tau fibrillization [121]. In the majority of these examples, the exact mechanism of action is not known. However, the activities of a number of inhibitors have been extensively characterized in a variety of in vitro models. These efforts revealed several significant differences in the inhibitory properties of different compound classes. For example, Taniguchi et al. reported that while phthalocyanine (Figure 10), a member of the porphyrin class of inhibitors, was both able to prevent the assembly of tau as well as to dissolve pre-formed fibrils, methylene blue (phenothiazine class) and trihydroxybenzophenone (polyphenol class) did not exhibit the same ability to dissolve fibrils [114]. Further differences among these inhibitors were evident by comparing the compound-mediated effects on soluble and insoluble tau fractions, which with some compounds, such as methylene blue, revealed the formation of high molecular weight tau species by SDS-PAGE [114]. Interestingly, in addition to porphyrins, other classes of inhibitors, namely anthraquinones, rhodanines, phenylthiazolyl-hydrazides, and N-phenylamines, were reported to inhibit the fibrillization reaction and to dissolve pre-formed tau fibrils both in cell-free as well as cell-based assays [109, 116-118, 122]. However, the effect of these inhibitors on non-fibrillar tau aggregates has not as yet been reported. If such compounds are acting predominantly via a disruption of the transition between oligomers to full fibrils, this mechanism of inhibition could potentially lead to a reduction of the fibrils with a concomitant build-up of oligomeric tau species.
Figure 10.
Representative structures of different classes of small molecule inhibitors of tau aggregation.
Finally, while the majority of tau aggregation inhibitors reported to date appear to target predominantly the elongation phase of the fibrillization reaction, oleocanthal, a naturally occurring compound found in cold-pressed extra-virgin olive oil, selectively modifies early stage events in the fibrillization process. Indeed, this compound was found to interact with monomeric tau and prevent tau misfolding by covalently modifying the protein via the formation of Schiff bases with the ε-NH2 of lysine residues [121]. SAR studies demonstrated that both aldehyde moieties are required for inhibition of tau fibrillization. Interestingly, despite the evidence of covalent modifications of tau, oleocanthal, did not seem to impact the normal MT-stabilizing function of tau [121].
A different class of inhibitors, the thiacarbocyanine dyes (e.g., N744, Figure 10) are also believed to inhibit tau fibrillization via disruption of the transition from tau oligomers to full fibrils [123]. Indeed, in vitro studies have revealed that N744 can interfere with tau filament extension with no disruption of the initial nucleation phase [123]. Further studies with this compound and related analogues revealed that the thiacarbocyanine dyes can function both as inhibitors or promoters of tau fibrillization depending on the compound concentration. Thus, while sub-stoichiometric concentrations of N744 produced significant reduction of tau filament formation (i.e., IC50 ~300 nM at 4 μM tau40), compound concentrations > 10 μM resulted in an enhanced tau aggregation [124]. This surprising observation was initially attributed to the different aggregation states of the thiacarbocyanine dye present at different concentrations [124]. Subsequent studies, however, indicate the absence of a clear correlation between the inhibitory activity of these compounds and their aggregation state [125]. Also noteworthy, at concentration ranges that are optimal for inhibition of tau aggregation, the thiacarbocyanine dyes did not appear to interfere with tau-MT binding, while higher concentrations that promote tau assembly have been found to reduce the levels of MT-bound tau [126].
In addition to the compound classes mentioned above, we have recently reported the discovery of a new series of inhibitors, the aminothienopyridazines, which exhibit anti-fibrillization activities with IC50s near the stoichiometric equivalence with tau [110]. Interestingly, although not as yet clear whether these inhibitors act by disrupting the initial oligomerization or the elongation phase, or both, size-exclusion chromatography of the soluble fraction obtained after centrifugation of the compound-treated fibrillizing mixture demonstrated that MLS000062428 can maintain the majority of total tau as free monomer, although an appreciable amount of soluble tau oligomers was also detected [110]. It is not yet known whether the oligomers detected in the soluble fractions are the same transient species that form en route to full fibrils or whether these aggregates may be off-pathway species that are unable to elongate.
To date, only one tau fibrillization inhibitor, the phenothiazine methylene blue, has entered in vivo evaluation. Methylene blue, which was originally synthesized in 1876 by Caro, has been extensively employed as a diagnostic and therapeutic agent for a wide range of conditions [127]. The wide spectrum of activities of this compound are thought to be largely due to the ability of methylene blue to enter redox cycles in biological systems [127]. Notably, PK studies have shown that methylene blue can reach relatively high brain concentrations in rats after i.v. and oral administration of the compound [128]. The tau anti-fibrillization properties of methylene blue and a number of related phenothiazines were first discovered by Wishick in 1996 [129]. Methylene blue (also known as Rember™, developed by TauRx Therapeutics), was recently evaluated in a randomized Phase 2 study involving 321 patients with mild or moderate AD. The results from these studies were presented at the 2008 International Conference on Alzheimer's Disease (ICAD) in Chicago, IL and indicated a significant reduction in cognitive decline after 50 weeks of continuous treatment. These promising results have not as yet been published in the peer-reviewed literature but if confirmed, will provide firm support to the notion that tau aggregation inhibitors can be therapeutically useful. However, in light of the broad range of biological activities and targets known to be affected by methylene blue, it is not yet clear whether the positive outcome of the clinical studies may be ascribable to a direct inhibition of tau aggregation. Thus, further in vivo efficacy studies involving less promiscuous compounds may be required to evaluate fully the therapeutic potential of tau-aggregation inhibitors. Towards this end, viable candidate compounds will have to exhibit acceptable combinations of efficacy, selectivity, and PK properties. With respect to in vitro efficacy, the thiacarbocyanines are the only class that showed significant inhibition of tau fibrillization when present in sub-stoichiometric concentrations relative to tau; all other classes appear to be most effective when compound concentrations approach an ~1:1 molar ratio with tau. Although the critical free tau concentration needed to trigger the fibrillization reaction in diseased neurons is not known, the total physiological intraneuronal tau concentration (i.e., MT-bound and MT-unbound tau) is believed to be in the low μM range [17, 18]. As previously noted, tau is in a constant dynamic equilibrium between being bound to MTs and free in the cytosol, with the vast majority of the protein (~99%) bound to MTs under physiological conditions. The fraction of total tau that is unbound from the MTs under pathological conditions is likely to increase, reaching conceivably the high nM range. This reasoning suggests that candidate tau fibrillization inhibitors will need to achieve comparable brain concentrations in order to be potentially effective. To date, with the sole exception of phenothiazines (i.e., methylene blue) there are no other reports of brain penetrant tau aggregation inhibitors. However, as part of our ongoing investigations into the properties of the aminothienopyridazines, we have identified a number of analogues that reach significant brain concentrations in mice, after either i.v. or oral administration of the compound [130]. These findings indicate that the aminothienopyridazines may be viable candidate compounds for in vivo evaluation of efficacy.
Concluding remarks
Despite significant challenges, a number of drug discovery efforts have successfully targeted specific protein-protein interactions and, among these programs, some hold considerable potential for new therapies. In the area of neurodegenerative diseases, in recent years, there has been a growing interest in the development of strategies that could target tau dysfunction and the neuropathological consequences of tau aggregation. This has resulted in a number of significant advances. For example, the discovery that the MT-stabilizing compound epothilone D can readily cross the BBB and distribute in the brain parenchyma paves the way for further in vivo evaluation of efficacy and safety of this and related molecules. Furthermore, in the area of small-molecule inhibitors of tau aggregation, a number of recent HTS campaigns have led to the discovery of several classes of compounds, some of which exhibit clear potential to be developed into candidate compounds for in vivo proof-of-concept studies. In addition to further screenings and hit/lead optimization efforts, future studies are likely to focus on elucidating the mode of action of tau aggregation inhibitors.
List of Abbreviations
- MT
microtubule
- AD
Alzheimer's disease
- CNS
central nervous system
- FTD
frontotemporal dementia
- FTDP-17
frontotemporal dementia with Parkinsonism linked to chromosome 17
- NFT
neurofibrillary tangle
- FTLD
frontotemporal lobar degeneration
- BBB
blood-brain barrier
- Pgp
P-glycoprotein
- ABC
ATP-binding cassette
- MDR
multi-drug resistance
- SAR
structure-activity relationship
- B/P
brain to plasma ratio
- PK
pharmacokinetic
- PD
pharmacodynamic
- BMS
Bristol-Myers Squibb
- ADNP
activity dependent neuroprotective protein
- HTS
high throughput screening
- ThT
thioflavine-T
- ThS
thioflavine-S
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- ICAD
International Conference on Alzheimer's Disease
References
- 1.Stockwin LH, Holmes S. Antibodies as therapeutic agents: vive la renaissance! Expert Opin. Biol. Ther. 2003;3:1133–1152. doi: 10.1517/14712598.3.7.1133. [DOI] [PubMed] [Google Scholar]
- 2.Toogood PL. Inhibition of protein-protein association by small molecules: approaches and progress. J. Med. Chem. 2002;45:1543–1558. doi: 10.1021/jm010468s. [DOI] [PubMed] [Google Scholar]
- 3.Fry D. Protein-protein interactions as targets for small molecule drug discovery. Biopolymers. 2006;84:535–552. doi: 10.1002/bip.20608. [DOI] [PubMed] [Google Scholar]
- 4.Arkin MR, Wells JA. Small-molecule inhibitors of protein-protein interactions: progressing towards the dream. Nat. Rev. Drug. Discov. 2004;3:301–317. doi: 10.1038/nrd1343. [DOI] [PubMed] [Google Scholar]
- 5.Liu J, Albers MW, Wandless TJ, Luan S, Alberg DG, Belshaw PJ, Cohen P, MacKintosh C, Klee CB, Schreiber SL. Inhibition of T cell signaling by immunophilin-ligand complexes correlates with loss of calcineurin phosphatase activity. Biochemistry. 1992;31:3896–3901. doi: 10.1021/bi00131a002. [DOI] [PubMed] [Google Scholar]
- 6.Liu J, Farmer JD, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 7.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 8.Berg T. Modulation of protein-protein interactions with small organic molecules. Angew. Chem. Int. Ed. Engl. 2003;42:2462–2481. doi: 10.1002/anie.200200558. [DOI] [PubMed] [Google Scholar]
- 9.Lee VM-Y, Goedert M, Trojanowski JQ. Neurodegenerative Tauopathies. Ann. Rev. Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- 10.Ballatore C, Lee VM-Y, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nat. Rev. Neurosci. 2007;8:663–672. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- 11.Roy S, Zhang B, Lee VM-Y, Trojanowski JQ. Axonal transport defects: a common theme in neurodegenerative diseases. Acta Neuropathol. (Berl.) 2005;109:5–13. doi: 10.1007/s00401-004-0952-x. [DOI] [PubMed] [Google Scholar]
- 12.Panda D, Samuel JC, Massie M, Feinstein SC, Wilson L. Differential regulation of microtubule dynamics by three-and four-repeat tau: implications for the onset of neurodegenerative disease. Proc. Natl. Acad. Sci. U. S. A. 2003;100:9548–9553. doi: 10.1073/pnas.1633508100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bunker JM, Wilson L, Jordan MA, Feinstein SC. Modulation of Microtubule Dynamics by Tau in Living Cells: Implications for Development and Neurodegeneration. Mol. Biol. Cell. 2004;15:2720–2728. doi: 10.1091/mbc.E04-01-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mackenzie I, Neumann M, Bigio E, Cairns N, Alafuzoff I, Kril J, Kovacs G, Ghetti B, Halliday G, Holm I. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol (Berl) 2010;119:1–4. doi: 10.1007/s00401-009-0612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong M, Zhukareva V, Vogelsberg-Ragaglia V, Wszolek Z, Reed L, Miller BI, Geschwind DH, Bird TD, McKeel D, Goate A, Morris JC, Wilhelmsen KC, Schellenberg GD, Trojanowski JQ, Lee VMY. Mutation-Specific Functional Impairments in Distinct Tau Isoforms of Hereditary FTDP-17. Science. 1998;282:1914–1917. doi: 10.1126/science.282.5395.1914. [DOI] [PubMed] [Google Scholar]
- 16.Amos LA. Microtubule structure and its stabilisation. Org. Biomol. Chem. 2004;2:2153–2160. doi: 10.1039/b403634d. [DOI] [PubMed] [Google Scholar]
- 17.Congdon EE, Kim S, Bonchak J, Songrug T, Matzavinos A, Kuret J. Nucleation-dependent tau filament formation: the importance of dimerization and an estimation of elementary rate constants. J. Biol. Chem. 2008;283:13806–13816. doi: 10.1074/jbc.M800247200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drubin DG, Feinstein SC, Shooter EM, Kirschner MW. Nerve growth factor-induced neurite outgrowth in PC12 cells involves the coordinate induction of microtubule assembly and assembly-promoting factors. J. Cell Biol. 1985;101:1799–1807. doi: 10.1083/jcb.101.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazanetz MP, Fischer PM. Untangling tau hyperphosphorylation in drug design for neurodegenerative diseases. Nat. Rev. Drug. Discov. 2007;6:464–479. doi: 10.1038/nrd2111. [DOI] [PubMed] [Google Scholar]
- 20.Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat. Med. 2004;(10 Suppl):S10–7. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- 21.Brunden KR, Trojanowski JQ, Lee VM-Y. Evidence that non-fibrillar tau causes pathology linked to neurodegeneration and behavioral impairments. J. Alzheimers Dis. 2008;14:393–399. doi: 10.3233/jad-2008-14406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brunden KR, Trojanowski JQ, Lee VM-Y. Advances in tau-focused drug discovery for Alzheimer's disease and related tauopathies. Nat. Rev. Drug. Discov. 2009;8:783–793. doi: 10.1038/nrd2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang B, Maiti A, Shively S, Lakhani F, McDonald-Jones G, Bruce J, Lee EB, Xie SX, Joyce S, Li C, Toleikis PM, Lee VM-Y, Trojanowski JQ. Microtubule-binding drugs offset tau sequestration by stabilizing microtubules and reversing fast axonal transport deficits in a tauopathy model. Proc. Natl. Acad. Sci. U. S. A. 2005;102:227–231. doi: 10.1073/pnas.0406361102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albright CF, Barten DM, Lee FY. Use of epothilone D in treating tau-associated diseases including Alzheimer's disease. 2009 US2009/0270465A1. [Google Scholar]
- 25.Akira Kurose WYMYTS. Effects of paclitaxel on cultured synovial cells from patients with rheumatoid arthritis. Cytometry. 2001;44:349–354. doi: 10.1002/1097-0320(20010801)44:4<349::aid-cyto1126>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 26.Ehrlich A, Booher S, Becerra Y, Borris DL, Figg WD, Turner ML, Blauvelt A. Micellar paclitaxel improves severe psoriasis in a prospective phase II pilot study. J. Am. Acad. Dermatol. 2004;50:533–540. doi: 10.1016/j.jaad.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 27.Moscarello MA, Mak B, Nguyen TA, Wood DD, Mastronardi F, Ludwin SK. Paclitaxel (Taxol) attenuates clinical disease in a spontaneously demyelinating transgenic mouse and induces remyelination. Mult. Scler. 2002;8:130–138. doi: 10.1191/1352458502ms776oa. [DOI] [PubMed] [Google Scholar]
- 28.Lee VM-Y, Daughenbaugh R, Trojanowski JQ. Microtubule stabilizing drugs for the treatment of Alzheimer's disease. Neurobiol. Aging. 1994;15(Suppl 2):S87–89. doi: 10.1016/0197-4580(94)90179-1. [DOI] [PubMed] [Google Scholar]
- 29.Andrieux A, Salin P, Schweitzer A, Begou M, Pachoud B, Brun P, Gory-Faure S, Kujala P, Suaud-Chagny MF, Hofle G, Job D. Microtubule Stabilizer Ameliorates Synaptic Function and Behavior in a Mouse Model for Schizophrenia. Biol. Psychiatry. 2006;60:1224–1230. doi: 10.1016/j.biopsych.2006.03.048. [DOI] [PubMed] [Google Scholar]
- 30.Trojanowski JQ, Smith AB, Huryn D, Lee VM-Y. Microtubule-stabilizing drugs for therapy of Alzheimer's disease and other neurodegenerative disorders with axonal transport impairments. Expert Opin. Pharmacother. 2005;6:683–686. doi: 10.1517/14656566.6.5.683. [DOI] [PubMed] [Google Scholar]
- 31.Eiseman JL, Eddington ND, Leslie J, MacAuley C, Sentz DL, Zuhowski M, Kujawa JM, Young D, Egorin MJ. Plasma pharmacokinetics and tissue distribution of paclitaxel in CD2F1 mice. Cancer Chemother. Pharmacol. 1994;34:465–471. doi: 10.1007/BF00685656. [DOI] [PubMed] [Google Scholar]
- 32.Markman M. Managing taxane toxicities. Support. Care Cancer. 2003;11:144–7. doi: 10.1007/s00520-002-0405-9. [DOI] [PubMed] [Google Scholar]
- 33.Von Hoff DD. The taxoids: same roots, different drugs. Semin. Oncol. 1997;24:S13-3–S13-10. [PubMed] [Google Scholar]
- 34.Marupudi NI, Han JE, Li KW, Renard VM, Tyler BM, Brem H. Paclitaxel: a review of adverse toxicities and novel delivery strategies. Expert Opin. Drug Saf. 2007;6:609–621. doi: 10.1517/14740338.6.5.609. [DOI] [PubMed] [Google Scholar]
- 35.Lee J, Swain SM. Peripheral Neuropathy Induced by Microtubule-Stabilizing Agents. J. Clin. Oncol. 2006;24:1633–1642. doi: 10.1200/JCO.2005.04.0543. [DOI] [PubMed] [Google Scholar]
- 36.Nogales E, Wolf SG, Khan IA, Luduena RF, Downing KH. Structure of tubulin at 6.5 A and location of the taxol-binding site. Nature. 1995;375:424–427. doi: 10.1038/375424a0. [DOI] [PubMed] [Google Scholar]
- 37.Kar S, Fan J, Smith MJ, Goedert M, Amos LA. Repeat motifs of tau bind to the insides of microtubules in the absence of taxol. Embo J. 2003;22:70–77. doi: 10.1093/emboj/cdg001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amos LA, Lowe J. How Taxol stabilises microtubule structure. Chem. Biol. 1999;6:R65–69. doi: 10.1016/s1074-5521(99)89002-4. [DOI] [PubMed] [Google Scholar]
- 39.Derry WB, Wilson L, Jordan MA. Substoichiometric binding of taxol suppresses microtubule dynamics. Biochemistry. 1995;34:2203–2211. doi: 10.1021/bi00007a014. [DOI] [PubMed] [Google Scholar]
- 40.Gallo JM, Li S, Guo P, Reed K, Ma J. The effect of P-glycoprotein on paclitaxel brain and brain tumor distribution in mice. Cancer Res. 2003;63:5114–5117. [PubMed] [Google Scholar]
- 41.Fricker G, Miller DS. Modulation of drug transporters at the blood-brain barrier. Pharmacology. 2004;70:169–176. doi: 10.1159/000075545. [DOI] [PubMed] [Google Scholar]
- 42.Kemper EM, van Zandbergen AE, Cleypool C, Mos HA, Boogerd W, Beijnen JH, van Tellingen O. Increased Penetration of Paclitaxel into the Brain by Inhibition of P-Glycoprotein. Clin. Cancer Res. 2003;9:2849–2855. [PubMed] [Google Scholar]
- 43.Fellner S, Bauer B, Miller DS, Schaffrik M, Fankhanel M, Spruss T, Bernhardt G, Graeff C, Farber L, Gschaidmeier H, Buschauer A, Fricker G. Transport of paclitaxel (Taxol) across the blood-brain barrier in vitro and in vivo. J. Clin. Invest. 2002;110:1309–1318. doi: 10.1172/JCI15451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Asperen J, van Tellingen O, Sparreboom A, Schinkel AH, Borst P, Nooijen WJ, Beijnen JH. Enhanced oral bioavailability of paclitaxel in mice treated with the P-glycoprotein blocker SDZ PSC 833. Br. J. Cancer. 1997;76:1181–1183. doi: 10.1038/bjc.1997.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beaulieu E, Demeule M, Ghitescu L, Beliveau R. P-glycoprotein is strongly expressed in the luminal membranes of the endothelium of blood vessels in the brain. Biochem. J. 1997;326(Pt 2):539–544. doi: 10.1042/bj3260539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ojima I, Chen J, Sun L, Borella CP, Wang T, Miller ML, Lin S, Geng X, Kuznetsova L, Qu C, Gallager D, Zhao X, Zanardi I, Xia S, Horwitz SB, Mallen-St Clair J, Guerriero JL, Bar-Sagi D, Veith JM, Pera P, Bernacki RJ. Design, synthesis, and biological evaluation of new-generation taxoids. J. Med. Chem. 2008;51:3203–3221. doi: 10.1021/jm800086e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ojima I, Slater JC, Kuduk SD, Takeuchi CS, Gimi RH, Sun CM, Park YH, Pera P, Veith JM, Bernacki RJ. Syntheses and structure-activity relationships of taxoids derived from 14 beta-hydroxy-10-deacetylbaccatin III. J. Med. Chem. 1997;40:267–278. doi: 10.1021/jm960563e. [DOI] [PubMed] [Google Scholar]
- 48.Ojima I, Slater JC, Michaud E, Kuduk SD, Bounaud PY, Vrignaud P, Bissery MC, Veith JM, Pera P, Bernacki RJ. Syntheses and structure-activity relationships of the second-generation antitumor taxoids: exceptional activity against drug-resistant cancer cells. J. Med. Chem. 1996;39:3889–3896. doi: 10.1021/jm9604080. [DOI] [PubMed] [Google Scholar]
- 49.Ferlini C, Distefano M, Pignatelli F, Lin S, Riva A, Bombardelli E, Mancuso S, Ojima I, Scambia G. Antitumour activity of novel taxanes that act at the same time as cytotoxic agents and P-glycoprotein inhibitors. Br. J. Cancer. 2000;83:1762–1768. doi: 10.1054/bjoc.2000.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vredenburg MR, Ojima I, Veith J, Pera P, Kee K, Cabral F, Sharma A, Kanter P, Greco WR, Bernacki RJ. Effects of orally active taxanes on P-glycoprotein modulation and colon and breast carcinoma drug resistance. J. Natl. Cancer Inst. 2001;93:1234–1245. doi: 10.1093/jnci/93.16.1234. [DOI] [PubMed] [Google Scholar]
- 51.Laccabue D, Tortoreto M, Veneroni S, Perego P, Scanziani E, Zucchetti M, Zaffaroni M, D'Incalci M, Bombardelli E, Zunino F, Pratesi G. A novel taxane active against an orthotopically growing human glioma xenograft. Cancer. 2001;92:3085–3092. doi: 10.1002/1097-0142(20011215)92:12<3085::aid-cncr10150>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 52.Minderman H, Brooks TA, O'Loughlin KL, Ojima I, Bernacki RJ, Baer MR. Broad-spectrum modulation of ATP-binding cassette transport proteins by the taxane derivatives ortataxel (IDN-5109, BAY 59-8862) and tRA96023. Cancer Chemother. Pharmacol. 2004;53:363–369. doi: 10.1007/s00280-003-0745-2. [DOI] [PubMed] [Google Scholar]
- 53.Cisternino S, Bourasset F, Archimbaud Y, Semiond D, Sanderink G, Scherrmann JM. Nonlinear accumulation in the brain of the new taxoid TXD258 following saturation of P-glycoprotein at the blood-brain barrier in mice and rats. Br. J. Pharmacol. 2003;138:1367–1375. doi: 10.1038/sj.bjp.0705150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Metzger-Filho O, Moulin C, de Azambuja E, Ahmad A. Larotaxel: broadening the road with new taxanes. Expert Opin. Investig. Drugs. 2009;18:1183–1189. doi: 10.1517/13543780903119167. [DOI] [PubMed] [Google Scholar]
- 55.Sessa C, Cuvier C, Caldiera S, Bauer J, Van den Bosch S, Monnerat C, Semiond D, Perard D, Lebecq A, Besenval M, Marty M. Phase I clinical and pharmacokinetic studies of the taxoid derivative RPR 109881A administered as a 1-hour or a 3-hour infusion in patients with advanced solid tumors. Ann. Oncol. 2002;13:1140–1150. doi: 10.1093/annonc/mdf174. [DOI] [PubMed] [Google Scholar]
- 56.Cirrito JR, Deane R, Fagan AM, Spinner ML, Parsadanian M, Finn MB, Jiang H, Prior JL, Sagare A, Bales KR, Paul SM, Zlokovic BV, Piwnica-Worms D, Holtzman DM. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-{beta} deposition in an Alzheimer disease mouse model. J. Clin. Invest. 2005;115:3285–3290. doi: 10.1172/JCI25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rice A, Liu Y, Michaelis ML, Himes RH, Georg GI, Audus KL. Chemical modification of paclitaxel (Taxol) reduces P-glycoprotein interactions and increases permeation across the blood-brain barrier in vitro and in situ. J. Med. Chem. 2005;48:832–838. doi: 10.1021/jm040114b. [DOI] [PubMed] [Google Scholar]
- 58.Ballatore C, Hyde E, Deiches RF, Lee VM-Y, Trojanowski JQ, Huryn D, Smith AB., III Paclitaxel C-10 carbamates: potential candidates for the treatment of neurodegenerative tauopathies. Bioorg. Med. Chem. Lett. 2007;17:3642–3646. doi: 10.1016/j.bmcl.2007.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ballatore C, Zhang B, Trojanowski JQ, Lee VM, Smith AB., 3rd. In situ blood-brain barrier permeability of a C-10 paclitaxel carbamate. Bioorg. Med. Chem. Lett. 2008;18:6119–6121. doi: 10.1016/j.bmcl.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Regina A, Demeule M, Che C, Lavallee I, Poirier J, Gabathuler R, Beliveau R, Castaigne JP. Antitumour activity of ANG1005, a conjugate between paclitaxel and the new brain delivery vector Angiopep-2. Br. J. Pharmacol. 2008;155:185–197. doi: 10.1038/bjp.2008.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Demeule M, Regina A, Che C, Poirier J, Nguyen T, Gabathuler R, Castaigne JP, Beliveau R. Identification and design of peptides as a new drug delivery system for the brain. J. Pharmacol. Exp. Ther. 2008;324:1064–1072. doi: 10.1124/jpet.107.131318. [DOI] [PubMed] [Google Scholar]
- 62.Hofle G, Reichenbach H. Anticancer agents from natural products. CRC Press LLC; Boca Raton, FL: 2005. Anticancer agents from natural products; pp. 413–450. [Google Scholar]
- 63.Bollag DM, McQueney PA, Zhu J, Hensens O, Koupal L, Liesch J, Goetz M, Lazarides E, Woods CM. Epothilones, a new class of microtubule-stabilizing agents with a taxol-like mechanism of action. Cancer Res. 1995;55:2325–2333. [PubMed] [Google Scholar]
- 64.Kowalski RJ, Giannakakou P, Hamel E. Activities of the microtubule-stabilizing agents epothilones A and B with purified tubulin and in cells resistant to paclitaxel (Taxol(R)) J. Biol. Chem. 1997;272:2534–2541. doi: 10.1074/jbc.272.4.2534. [DOI] [PubMed] [Google Scholar]
- 65.Giannakakou P, Gussio R, Nogales E, Downing KH, Zaharevitz D, Bollbuck B, Poy G, Sackett D, Nicolaou KC, Fojo T. A common pharmacophore for epothilone and taxanes: molecular basis for drug resistance conferred by tubulin mutations in human cancer cells. Proc. Natl. Acad. Sci. U. S. A. 2000;97:2904–2909. doi: 10.1073/pnas.040546297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.He L, Yang CP, Horwitz SB. Mutations in beta-tubulin map to domains involved in regulation of microtubule stability in epothilone-resistant cell lines. Mol. Cancer Ther. 2001;1:3–10. [PubMed] [Google Scholar]
- 67.Nicolaou KCR,F. Vourloumis, D. Chemical Biology of Epothilones. Angew. Chem. Int. Ed. Engl. 1998;37:2014–2045. doi: 10.1002/(SICI)1521-3773(19980817)37:15<2014::AID-ANIE2014>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 68.Altmann KH. Epothilone B and its analogs - a new family of anticancer agents. Mini Rev. Med. Chem. 2003;3:149–158. doi: 10.2174/1389557033405269. [DOI] [PubMed] [Google Scholar]
- 69.Altmann KH, Pfeiffer B, Arseniyadis S, Pratt BA, Nicolaou KC. The chemistry and biology of epothilones-the wheel keeps turning. ChemMedChem. 2007;2:396–424. doi: 10.1002/cmdc.200600206. [DOI] [PubMed] [Google Scholar]
- 70.Conlin A, Fornier M, Hudis C, Kar S, Kirkpatrick P. Ixabepilone. Nat. Rev. Drug. Discov. 2007;6:953–954. [Google Scholar]
- 71.Michaelis ML, Ansar S, Chen Y, Reiff ER, Seyb KI, Himes RH, Audus KL, Georg GI. {beta}-Amyloid-induced neurodegeneration and protection by structurally diverse microtubule-stabilizing agents. J. Pharmacol. Exp. Ther. 2005;312:659–668. doi: 10.1124/jpet.104.074450. [DOI] [PubMed] [Google Scholar]
- 72.Harris CR, Danishefsky SJ. Complex Target-Oriented Synthesis in the Drug Discovery Process: A Case History in the dEpoB Series. J. Org. Chem. 1999;64:8434–8456. [Google Scholar]
- 73.Lichtner RMR,A, Klar U, Hoffmann J, Buchmann B, Schwede W, Skuballa W. The use of epothilones in the treatment of brain diseases associated with proliferative process. 2003 WO 03/074053 A1. [Google Scholar]
- 74.O'Reilly T, Wartmann M, Brueggen J, Allegrini PR, Floersheimer A, Maira M, McSheehy PM. Pharmacokinetic profile of the microtubule stabilizer patupilone in tumor-bearing rodents and comparison of anti-cancer activity with other MTS in vitro and in vivo. Cancer Chemother Pharmacol. 2008;62:1045–1054. doi: 10.1007/s00280-008-0695-9. [DOI] [PubMed] [Google Scholar]
- 75.Brunden KR, Yao Y, Potuzak JS, Ibarz Ferrer N, Ballatore C, James M, Hogan AML, Trojanowski JQ, Smith AB, III, Lee VM-Y. 2010 Submitted. [Google Scholar]
- 76.Laferriere N, MacRae T, Brown D. Tubulin synthesis and assembly in differentiating neurons. Biochemistry and Cell Biology. 1997;75:103–117. [PubMed] [Google Scholar]
- 77.Black M, Baas P, Humphries S. Dynamics of alpha-tubulin deacetylation in intact neurons. Journal of Neuroscience. 1989;9:358–368. doi: 10.1523/JNEUROSCI.09-01-00358.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan E, Forster C, Yue M, Orne J, Janus C, Mariash A, Kuskowski M, Hyman B, Hutton M, Ashe KH. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–81. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kowalski RJ, Giannakakou P, Gunasekera SP, Longley RE, Day BW, Hamel E. The microtubule-stabilizing agent discodermolide competitively inhibits the binding of paclitaxel (Taxol) to tubulin polymers, enhances tubulin nucleation reactions more potently than paclitaxel, and inhibits the growth of paclitaxel-resistant cells. Mol. Pharmacol. 1997;52:613–622. [PubMed] [Google Scholar]
- 80.Hamel E, Sackett DL, Vourloumis D, Nicolaou KC. The coral-derived natural products eleutherobin and sarcodictyins A and B: effects on the assembly of purified tubulin with and without microtubule-associated proteins and binding at the polymer taxoid site. Biochemistry. 1999;38:5490–5498. doi: 10.1021/bi983023n. [DOI] [PubMed] [Google Scholar]
- 81.Madiraju C, Edler MC, Hamel E, Raccor BS, Balachandran R, Zhu G, Giuliano KA, Vogt A, Shin Y, Fournier JH, Fukui Y, Bruckner AM, Curran DP, Day BW. Tubulin assembly, taxoid site binding, and cellular effects of the microtubule-stabilizing agent dictyostatin. Biochemistry. 2005;44:15053–15063. doi: 10.1021/bi050685l. [DOI] [PubMed] [Google Scholar]
- 82.Ojima I, Chakravarty S, Inoue T, Lin S, He L, Horwitz SB, Kuduk SD, Danishefsky SJ. A common pharmacophore for cytotoxic natural products that stabilize microtubules. Proc. Natl. Acad. Sci. U. S. A. 1999;96:4256–4261. doi: 10.1073/pnas.96.8.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Honore S, Kamath K, Braguer D, Horwitz SB, Wilson L, Briand C, Jordan MA. Synergistic suppression of microtubule dynamics by discodermolide and paclitaxel in non-small cell lung carcinoma cells. Cancer Res. 2004;64:4957–4964. doi: 10.1158/0008-5472.CAN-04-0693. [DOI] [PubMed] [Google Scholar]
- 84.Martello LA, McDaid HM, Regl DL, Yang CP, Meng D, Pettus TR, Kaufman MD, Arimoto H, Danishefsky SJ, Smith AB, III, Horwitz SB. Taxol and discodermolide represent a synergistic drug combination in human carcinoma cell lines. Clin. Cancer Res. 2000;6:1978–1987. [PubMed] [Google Scholar]
- 85.Huang GS, Lopez-Barcons L, Freeze BS, Smith AB, III, Goldberg GL, Horwitz SB, McDaid HM. Potentiation of Taxol Efficacy by Discodermolide in Ovarian Carcinoma Xenograft-Bearing Mice. Clin. Cancer Res. 2006;12:298–304. doi: 10.1158/1078-0432.CCR-05-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Long BH, Carboni JM, Wasserman AJ, Cornell LA, Casazza AM, Jensen PR, Lindel T, Fenical W, Fairchild CR. Eleutherobin, a novel cytotoxic agent that induces tubulin polymerization, is similar to paclitaxel (Taxol (R)) Cancer Res. 1998;58:1111–1115. [PubMed] [Google Scholar]
- 87.Isbrucker RA, Cummins J, Pomponi SA, Longley RE, Wright AE. Tubulin polymerizing activity of dictyostatin-1, a polyketide of marine sponge origin. Biochem. Pharmacol. 2003;66:75–82. doi: 10.1016/s0006-2952(03)00192-8. [DOI] [PubMed] [Google Scholar]
- 88.Edler MC, Buey RM, Gussio R, Marcus AI, Vanderwal CD, Sorensen EJ, Diaz JF, Giannakakou P, Hamel E. Cyclostreptin ( FR182877), an antitumor tubulin-polymerizing agent deficient in enhancing tubulin assembly despite its high affinity for the taxoid site. Biochemistry. 2005;44:11525–11538. doi: 10.1021/bi050660m. [DOI] [PubMed] [Google Scholar]
- 89.Buey RM, Calvo E, Barasoain I, Pineda O, Edler MC, Matesanz R, Cerezo G, Vanderwal CD, Day BW, Sorensen EJ, Lopez JA, Andreu JM, Hamel E, Diaz JF. Cyclostreptin binds covalently to microtubule pores and lumenal taxoid binding sites. Nat. Chem. Biol. 2007;3:117–125. doi: 10.1038/nchembio853. [DOI] [PubMed] [Google Scholar]
- 90.Bai R, Vanderwal CD, Diaz JF, Hamel E. Interaction of a cyclostreptin analogue with the microtubule taxoid site: the covalent reaction rapidly follows binding. J. Nat. Prod. 2008;71:370–374. doi: 10.1021/np800056m. [DOI] [PubMed] [Google Scholar]
- 91.Martello LA, LaMarche MJ, He L, Beauchamp TJ, Smith AB, III, Horwitz SB. The relationship between Taxol and (+)-discodermolide: synthetic analogs and modeling studies. Chem. Biol. 2001;8:843–855. doi: 10.1016/s1074-5521(01)00055-2. [DOI] [PubMed] [Google Scholar]
- 92.Gertsch J, Meier S, Müller M, Altmann K-H. Differential Effects of Natural Product Microtubule Stabilizers on Microtubule Assembly: Single Agent and Combination Studies with Taxol, Epothilone B, and Discodermolide. ChemBioChem. 2009;10:166–175. doi: 10.1002/cbic.200800556. [DOI] [PubMed] [Google Scholar]
- 93.Gaitanos TN, Buey RM, Diaz JF, Northcote PT, Teesdale-Spittle P, Andreu JM, Miller JH. Peloruside A does not bind to the taxoid site on beta-tubulin and retains its activity in multidrug-resistant cell lines. Cancer Res. 2004;64:5063–5067. doi: 10.1158/0008-5472.CAN-04-0771. [DOI] [PubMed] [Google Scholar]
- 94.Huzil JT, Chik JK, Slysz GW, Freedman H, Tuszynski J, Taylor RE, Sackett DL, Schriemer DC. A Unique Mode of Microtubule Stabilization Induced by Peloruside A. J. Mol. Biol. 2008;378:1016–1030. doi: 10.1016/j.jmb.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mooberry SL, Tien G, Hernandez AH, Plubrukarn A, Davidson BS. Laulimalide and isolaulimalide, new paclitaxel-like microtubule-stabilizing agents. Cancer Res. 1999;59:653–660. [PubMed] [Google Scholar]
- 96.Pryor DE, O'Brate A, Bilcer G, Diaz JF, Wang Y, Kabaki M, Jung MK, Andreu JM, Ghosh AK. The Microtubule Stabilizing Agent Laulimalide Does Not Bind in the Taxoid Site, Kills Cells Resistant to Paclitaxel and Epothilones, and May Not Require Its Epoxide Moiety for Activity. Biochemistry. 2002;41:9109–9115. doi: 10.1021/bi020211b. [DOI] [PubMed] [Google Scholar]
- 97.Karjala G, Chan Q, Manzo E, Andersen RJ, Roberge M. Ceratamines, structurally simple microtubule-stabilizing antimitotic agents with unusual cellular effects. Cancer Res. 2005;65:3040–3043. doi: 10.1158/0008-5472.CAN-04-4369. [DOI] [PubMed] [Google Scholar]
- 98.Hamel E, Day BW, Miller JH, Jung MK, Northcote PT, Ghosh AK, Curran DP, Cushman M, Nicolaou KC, Paterson I. Synergistic effects of peloruside A and laulimalide with taxoid site drugs, but not with each other, on tubulin assembly. Mol. Pharmacol. 2006;70:1555–1564. doi: 10.1124/mol.106.027847. [DOI] [PubMed] [Google Scholar]
- 99.Tinley TL, Randall-Hlubek DA, Leal RM, Jackson EM, Cessac JW, Quada JC, Jr., Hemscheidt TK, Mooberry SL. Taccalonolides E and A: Plant-derived Steroids with Microtubule-stabilizing Activity. Cancer Res. 2003;63:3211–3220. [PubMed] [Google Scholar]
- 100.Buey RM, Barasoain I, Jackson E, Meyer A, Giannakakou P, Paterson I, Mooberry S, Andreu JM, DÌaz JF. Microtubule Interactions with Chemically Diverse Stabilizing Agents: Thermodynamics of Binding to the Paclitaxel Site Predicts Cytotoxicity. Chem. Biol. 2005;12:1269–1279. doi: 10.1016/j.chembiol.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 101.Divinski I, Mittelman L, Gozes I. A femtomolar acting octapeptide interacts with tubulin and protects astrocytes against zinc intoxication. J. Biol. Chem. 2004;279:28531–28538. doi: 10.1074/jbc.M403197200. [DOI] [PubMed] [Google Scholar]
- 102.Gozes I, Morimoto BH, Tiong J, Fox A, Sutherland K, Dangoor D, Holser-Cochav M, Vered K, Newton P, Aisen PS. NAP: research and development of a peptide derived from activity-dependent neuroprotective protein (ADNP) CNS Drug Reviews. 2005;11:353–378. doi: 10.1111/j.1527-3458.2005.tb00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vulih-Shultzman I, Pinhasov A, Mandel S, Grigoriadis N, Touloumi O, Pittel Z, Gozes I. Activity-dependent neuroprotective protein snippet NAP reduces tau hyperphosphorylation and enhances learning in a novel transgenic mouse model. J. Pharmacol. Exp. Ther. 2007;323:438–449. doi: 10.1124/jpet.107.129551. [DOI] [PubMed] [Google Scholar]
- 104.Morimoto BH, LannOy I, Fox AW, Gozes I, Stewart A. pharmacokinetics and distribution to brain after intravenous or intranasal administration to rat. Chim. Oggi. 2009;27:16–20. [Google Scholar]
- 105.Xu S, Brunden KR, Trojanowski JQ, Lee VMY. Characterization of tau fibrillization in vitro. Alzheimer's & Dementia: The Journal of the Alzheimer's Association. 2009 doi: 10.1016/j.jalz.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Goedert M, Jakes R, Spillantini MG, Hasegawa M, Smith MJ, Crowther RA. Assembly of microtubule-associated protein tau into Alzheimer-like filaments induced by sulphated glycosaminoglycans. Nature. 1996;383:550–553. doi: 10.1038/383550a0. [DOI] [PubMed] [Google Scholar]
- 107.Chirita CN, Necula M, Kuret J. Anionic micelles and vesicles induce tau fibrillization in vitro. J. Biol. Chem. 2003;278:25644–25650. doi: 10.1074/jbc.M301663200. [DOI] [PubMed] [Google Scholar]
- 108.Crowe A, Ballatore C, Hyde E, Trojanowski JQ, Lee VM-Y. High throughput screening for small molecule inhibitors of heparin-induced tau fibril formation. Biochem. Biophy. Res. Comm. 2007;358:1–6. doi: 10.1016/j.bbrc.2007.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pickhardt M, Gazova Z, von Bergen M, Khlistunova I, Wang Y, Hascher A, Mandelkow EM, Biernat J, Mandelkow E. Anthraquinones inhibit tau aggregation and dissolve Alzheimer's paired helical filaments in vitro and in cells. J. Biol. Chem. 2005;280:3628–3635. doi: 10.1074/jbc.M410984200. [DOI] [PubMed] [Google Scholar]
- 110.Crowe A, Huang W, Ballatore C, Johnson RL, Hogan AM, Huang R, Wichterman J, McCoy J, Huryn D, Auld DS, Smith AB, III, Inglese J, Trojanowski JQ, Austin CP, Brunden KR, Lee VM-Y. Identification of aminothienopyridazine inhibitors of tau assembly by quantitative high-throughput screening. Biochemistry. 2009;48:7732–7745. doi: 10.1021/bi9006435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Groenning M. Binding mode of Thioflavin T and other molecular probes in the context of amyloid fibrils-current status. J. Chem. Biol. 2009 doi: 10.1007/s12154-009-0027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bulic B, Pickhardt M, Schmidt B, Mandelkow EM, Waldmann H, Mandelkow E. Development of Tau Aggregation Inhibitors for Alzheimer's Disease. Angew. Chem. Int. Ed. Engl. 2009;48:2–15. doi: 10.1002/anie.200802621. [DOI] [PubMed] [Google Scholar]
- 113.Brunden KR, Ballatore C, Crowe A, Smith AB, III, Lee VM-Y, Trojanowski JQ. Tau-directed drug discovery for Alzheimer's disease and related tauopathies: A focus on tau assembly inhibitors. Exp. Neurol. 2009 doi: 10.1016/j.expneurol.2009.08.031. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Taniguchi S, Suzuki N, Masuda M, Hisanaga S, Iwatsubo T, Goedert M, Hasegawa M. Inhibition of heparin-induced tau filament formation by phenothiazines, polyphenols, and porphyrins. J. Biol. Chem. 2005;280:7614–23. doi: 10.1074/jbc.M408714200. [DOI] [PubMed] [Google Scholar]
- 115.Chirita C, Necula M, Kuret J. Ligand-dependent inhibition and reversal of tau filament formation. Biochemistry. 2004;43:2879–2887. doi: 10.1021/bi036094h. [DOI] [PubMed] [Google Scholar]
- 116.Khlistunova I, Biernat J, Wang Y, Pickhardt M, von Bergen M, Gazova Z, Mandelkow E, Mandelkow E-M. Inducible Expression of Tau Repeat Domain in Cell Models of Tauopathy: Aggregation is toxic to cells but can be reversed by inhibitor drugs. J. Biol. Chem. 2006;281:1205–1214. doi: 10.1074/jbc.M507753200. [DOI] [PubMed] [Google Scholar]
- 117.Pickhardt M, Larbig G, Khlistunova I, Coksezen A, Meyer B, Mandelkow EM, Schmidt B, Mandelkow E. Phenylthiazolyl-Hydrazide and Its Derivatives Are Potent Inhibitors of tau Aggregation and Toxicity in Vitro and in Cells. Biochemistry. 2007;46:10016–10023. doi: 10.1021/bi700878g. [DOI] [PubMed] [Google Scholar]
- 118.Bulic B, Pickhardt M, Khlistunova I, Biernat J, Mandelkow EM, Mandelkow E, Waldmann H. Rhodanine-based tau aggregation inhibitors in cell models of tauopathy. Angew. Chem. Int. Ed. Engl. 2007;46:9215–9219. doi: 10.1002/anie.200704051. [DOI] [PubMed] [Google Scholar]
- 119.Smith AB, III, Sperry JB, Han Q. Syntheses of (−)-oleocanthal, a natural NSAID found in extra virgin olive oil, the (−)-deacetoxy-oleuropein aglycone, and related analogues. J. Org. Chem. 2007;72:6891–6900. doi: 10.1021/jo071146k. [DOI] [PubMed] [Google Scholar]
- 120.Smith AB, III, Han Q, Breslin PAS, Beauchamp GK. Synthesis and Assignment of Absolute Configuration of (−)-Oleocanthal: A Potent, Naturally Occurring Non-steroidal Anti-inflammatory and Anti-oxidant Agent Derived from Extra Virgin Olive Oils. Org. Lett. 2005;7:5075–5078. doi: 10.1021/ol052106a. [DOI] [PubMed] [Google Scholar]
- 121.Li W, Sperry JB, Crowe A, Trojanowski JQ, Smith AB, III, Lee VMY. Inhibition of tau fibrillization by oleocanthal via reaction with the amino groups of tau. J. Neurochem. 2009;110:1339–1351. doi: 10.1111/j.1471-4159.2009.06224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pickhardt M, Biernat J, Khlistunova I, Wang YP, Gazova Z, Mandelkow EM, Mandelkow E. N-phenylamine derivatives as aggregation inhibitors in cell models of tauopathy. Curr. Alzheimer Res. 2007;4:397–402. doi: 10.2174/156720507781788765. [DOI] [PubMed] [Google Scholar]
- 123.Necula M, Chirita CN, Kuret J. Cyanine dye N744 inhibits tau fibrillization by blocking filament extension: implications for the treatment of tauopathic neurodegenerative diseases. Biochemistry. 2005;44:10227–10237. doi: 10.1021/bi050387o. [DOI] [PubMed] [Google Scholar]
- 124.Congdon EE, Necula M, Blackstone RD, Kuret J. Potency of a tau fibrillization inhibitor is influenced by its aggregation state. Arch. Bichem. Biophys. 2007;465:127–135. doi: 10.1016/j.abb.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chang E, Congdon EE, Honson NS, Duff KE, Kuret J. Structure-activity relationship of cyanine tau aggregation inhibitors. J. Med. Chem. 2009;52:3539–3547. doi: 10.1021/jm900116d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Congdon EE, Figueroa YH, Wang L, Tonuva G, Chang E, Kuret J, Conrad C, Duff KE. Inhibition of tau polymerization with a cyanine dye in two distinct model systems. J. Biol. Chem. 2009;284:20830–20839. doi: 10.1074/jbc.M109.016089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wainwright M, Crossley KB. Methylene Blue - a therapeutic dye for all seasons? J. Chemother. 2002;14:431–443. doi: 10.1179/joc.2002.14.5.431. [DOI] [PubMed] [Google Scholar]
- 128.Peter C, Hongwan D, Küpfer A, Lauterburg BH. Pharmacokinetics and organ distribution of intravenous and oral methylene blue. Eur. J. Clin. Pharmacol. 2000;56:247–250. doi: 10.1007/s002280000124. [DOI] [PubMed] [Google Scholar]
- 129.Wischik CM, Edwards PC, Lai RYK, Roth M, Harrington CR. Selective inhibition of Alzheimer disease-like tau aggregation by phenothiazines. Proc. Natl. Acad. Sci. U. S. A. 1996;93:11213–11218. doi: 10.1073/pnas.93.20.11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ballatore C, Brunden KR, Piscitelli F, James M, Crowe A, Yao M, Hyde E, Trojanowski JQ, Lee VM-Y, Smith AB., III J. Med. Chem. 2010;53:3739–3747. doi: 10.1021/jm100138f. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]