Abstract
Background
Sickle cell disease (SCD) pain is acute or chronic, leads to school absenteeism, impaired health-related quality of life and early mortality. Given that little is known about children with SCD and chronic pain, we 1) described characteristics of these children and 2) evaluated the impact of a multidisciplinary pain management model on health care utilization.
Procedure
A retrospective cohort study of children with SCD evaluated and treated in our institution’s multidisciplinary pain clinic between 1999–2008 was conducted. Referrals occur when children require chronic opioids and/or have frequent pain hospitalizations. Descriptive statistics evaluated patient characteristics and Wilcoxon Signed Rank evaluated change in median number of pain hospitalizations one year before and after referral.
Results
Median age of 19 children identified was 15 years (IQR 11-17); significantly more were female (78.9% vs. 21.1%; p=0.012). At time of referral, all patients reported taking opioids, 68.4% were taking hydroxyurea, half of those not on hydroxyurea started it (n=3), none were chronically transfused and one initiated transfusions upon referral. Majority (89.5%) learned non-pharmacologic pain management techniques. Median number of pain hospitalizations between the year before and after referral significantly decreased [5(IQR 3-6) to 1(IQR 0-4); p=0.006]. To further delineate the pain clinic’s effect, analysis was repeated after removing children initiating hydroxyurea/transfusions upon referral. The significant decrease in hospitalizations persisted [5(IQR 3-6) to 1(IQR 0-4; p=0.022].
Conclusions
A multidisciplinary pain management model appears to have decreased SCD pain hospitalizations. Results of this retrospective study will need to be tested in a prospective randomized trial.
Keywords: sickle cell disease, children, pain management
Introduction
Pain is the leading cause of emergency department visits and hospitalizations for children with SCD [1] leading to school absenteeism and impaired health-related quality of life.[2,3] The frequency of painful episodes increases as children mature from childhood into adolescence[4] and in adults, pain can occur daily and may be consistent with a chronic pain syndrome.[5] Thus, pain in SCD is multifaceted and includes elements of both acute and chronic pain.
Currently, the only medical treatments proven to prevent painful events in SCD are hematopoietic stem cell transplantation (HSCT), chronic blood transfusions and hydroxyurea, however, each of these interventions has limitations. HSCT is curative, but is limited by the need to have an HLA matched sibling donor.[6] There are currently little data on unrelated HSCT or alternative donors for children with SCD. Chronic blood transfusions can lead to iron overload, potential infection, and development of red blood cell alloantibodies.[7] Hydroxyurea is proven to be safe in patients with SCD [8]; however, patients may continue to have pain despite being on hydroxyurea. Due to the above limitations, adjuvant pain management strategies are needed for patients with SCD.
The American Pain Society published guidelines in 1999 regarding the optimal treatment for pain in SCD.[9] The National Institutes of Health (NIH) and National Heart Lung and Blood Institute also published similar guidelines in 2002.[10] These guidelines recommend a multidisciplinary approach to pain management including pharmacological, behavioral, psychological, and physical interventions where all providers work together collaboratively in the management of the patient’s pain.[9,10] These interventions should be combined with patient and family educational needs and a treatment plan should subsequently be formulated. In adults with chronic pain conditions other than SCD, the multidisciplinary pain management model has been shown to improve patients’ pain, decrease health care utilization, and is cost-effective.[11] Data exist proving the effect of psychological interventions on pain management and coping in SCD.[12–14] However, there are a paucity of data proving the effectiveness of a comprehensive multidisciplinary pain management clinic in SCD, where behavioral, medical, and social services are offered to the children and caregivers during a single visit. In addition, the optimal age to introduce this multidisciplinary model has not been well established.
The pain clinic at our institution evaluates and treats patients with a wide variety of chronic pain conditions including headaches, chronic abdominal pain, SCD, and other chronic pain syndromes using the multidisciplinary pain management model described above. Thus, when children at our SCD center are recognized as having recurrent or chronic pain, they are referred to our institution’s pain clinic for evaluation and treatment. However, the effectiveness of this model in children with SCD has never been evaluated.
Given the little known about characteristics and impact of this multidisciplinary pain management model on health care utilization of children with SCD and chronic or recurrent pain, we sought to achieve the following objectives: 1) describe characteristics of children with SCD and chronic or recurrent pain and 2) evaluate the impact of the multidisciplinary pain management model on health care utilization, defined as emergency department visits, hospitalizations, and length of hospital stay. We hypothesized a multidisciplinary pain management model would effectively decrease health care utilization for children with SCD and chronic or recurrent pain.
Methods
We conducted a retrospective longitudinal cohort study of children with SCD ages 2–18 years evaluated and treated in our institution’s pain clinic between 1999–2008. Pain clinic referrals are made when requirements develop for chronic opioids at home and/or frequent pain hospitalizations (3 or more/year) occur despite disease modifying therapy.
The multidisciplinary model used in the pain clinic consists of a pain physician (anesthesiologist with specialization in pediatric pain management), a pediatric psychologist or family and marriage counselor trained in non-pharmacologic pain management techniques, a nurse, and a social worker. During the initial visit, the entire team meets with the patient as a group to complete a medical and psychosocial evaluation. After the initial visit, the team subsequently meets to discuss the patient’s care and a plan of action is formulated. This may consist of medical management of both pain and SCD, psychological interventions such as cognitive behavior therapy for pain management or evaluation and treatment for depression, and social work interventions including initiation of an Individualized Education Program (IEP) or 504 plan. Recommended follow-up care is tailored to the individual patient’s needs and is varied based on the patient. In addition, at the first visit, a formal written pain management action plan is developed for the patient and a copy is given to the patient and family and another copy is kept in the patient’s chart. This action plan includes a stepwise approach for outpatient pain management. Once the child has been evaluated and treated by the pain team as an outpatient, the pain team is usually involved in any subsequent inpatient admissions for painful events. In addition, patients are taught to call the pain team when pain is exacerbated at home and for needed analgesic refills. The pain team involvement was noted in the system-wide accessible sickle dell database so that Emergency Department (ED) providers could directly contact a pain specialist during ED visits. During the duration of the study period, the primary pain management physician and family/marriage therapist were consistent, there were 2 pain psychologists, and the social worker was consistent. Overall, there was limited variability in the composition of the multidisciplinary pain team during the study period.
Baseline demographic data collected included age, gender, genotype, presence of psychiatric diagnoses, pain medications at home, number on hydroxyurea, and asthma diagnosis. Data on sickle cell-related comorbidities were also assessed (i.e., avascular necrosis, acute chest syndrome). Pain medications patients were taking at the end of the study period and compliance with follow-up visits during the study period was also tracked.
Statistical Analysis
Descriptive statistics were performed for all demographic and baseline patient characteristics using appropriate statistical methods. Chi-square was used to evaluate gender differences in the children included in the cohort with the assumption that there would be an equal distribution of males and females. Wilcoxon Signed Ranks Test was used to evaluate the change in health care utilization defined as the change in median number of emergency department visits, hospitalizations, and length of hospital stay one year before compared to one year after the initial visit to the pain clinic. We report proportions and medians and inter-quartile ranges where appropriate. All statistical analyses were conducted with SPSS version 14.0 for Windows (SPSS, Chicago, IL). A p-value of ≤ 0.05 was considered statistically significant.
Results
Study Population
A total of 19 patients were identified during the study period. Table I displays patient characteristics at the time of initial visit to the pain clinic. Our sickle cell center currently serves approximately 300 children with SCD at any given time period. Thus, approximately 6.3% of our total sickle cell population was evaluated and treated in the pain clinic.
Table I.
Patient Characteristics at time of Initial Multidisciplinary Pain Clinic Referral (n=19)
| Variable | N (%) |
|---|---|
| Median age (years) | 15 (IQR 11–17) |
| Gender* | |
| Female | 15 (78.9) |
| Male | 4 (21.1) |
| Genotype | |
| HbSS | 14 (73.7) |
| HbSC | 4 (21.1) |
| HbSG-Philadelphia | 1 (5.2) |
| Hydroxyurea | |
| Yes | 13 (68.4) |
| No | 6 (31.6) |
| Taking Chronic Opioids | 19 (100) |
| Chronic Transfusions (for pain) | 0 |
| Avascular Necrosis | |
| Yes | 5 (26.3) |
| No | 14 (73.7) |
| History of ACS | |
| Yes | 3 (15.8) |
| No | 16 (84.2) |
| Asthma | |
| Yes | 4 (21.1) |
| No | 15 (78.9) |
| Psychologic Comorbidities | |
| Depression | 1 (5.2) |
p=0.012; ACS=Acute Chest Syndrome
Interventions made by Multidisciplinary Pain Management Model
All patients were evaluated by a pain physician and a mental health provider during the initial visit. All patients were prescribed pain medication during this visit. Almost all patients (94.7%; n=18) were prescribed an anti-inflammatory including ibuprofen (61.1%), diclofenac (16.7%), and cyclooxygenase-2 inhibitors (16.7%). Almost half (47.4%; n=9) were given tramadol and all patients were prescribed an opioid. The most common opioids used were morphine (52.6%) and oxycodone (36.8%). Gabapentin was prescribed for one patient. The majority of patients (84.2%; n=16) were given a formal written pain management action plan that consisted of a stepwise approach to managing their pain. Two patients were not given an action plan for unclear reasons and documentation regarding this was missing for 1 patient. One patient (5.3%) had a diagnosis of depression before referral and, after evaluation by the pain psychologist, 2 additional patients (10.5%) were diagnosed with depression and started on antidepressants and psychotherapy. Almost all (89.5%; n=17) were taught non-pharmacologic pain management techniques including cognitive behavioral therapy. We found 36.8% (n=7) of patients had school interventions initiated by the social worker. Of these patients, 57.1% (n=4) had IEPs established and 42.9% (n=3) had 504 plans established. Compliance with follow-up clinic appointments varied among patients. We found 42.2% (n=8) of patients attended >70% of follow-up appointments, 36.8% (n=7) of patients attended 50–70% of appointments and 21.1% (n=4) attended <50% of scheduled follow-up appointments. Of the 13 patients that had hospitalizations for pain after their initial pain clinic visit, the inpatient pain team was consulted for more than half of these admissions in 46.2% of patients. Four patients had no involvement of the pain team during these admissions for reasons that were not clear from the medical record. The SCD physician recommended initiating disease modifying therapy in 4 of the 6 patients not on hydroxyurea at the time of referral (hydroxyurea for 3; chronic red blood cell transfusions for 1).
Pain Hospitalizations Significantly Decreased after Involvement of Pain Clinic
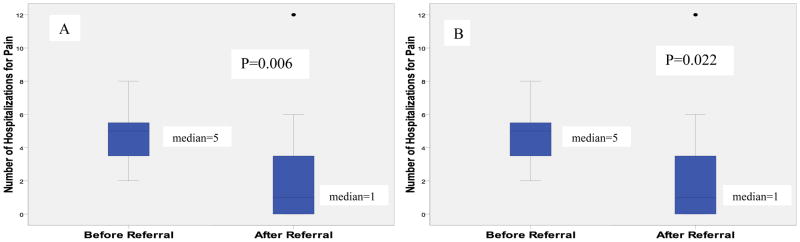
The median number of hospitalizations for pain significantly decreased in the year after the initial visit to the pain clinic compared to the year before for the entire cohort [5 (IQR 3-6) to 1 (IQR 0-4); p=0.006; Wilcoxon Signed Rank] (Figure 1). The total number of all pain hospitalizations in the entire study population decreased by almost 50% after pain clinic referral (n=91 before; n=47 post), demonstrating an overall change in SCD inpatient care. Six patients in the cohort had zero hospitalizations for pain in the year after the initial pain clinic visit and of these 6 patients, 83.3% (n=5) had 100% compliance with their follow-up visits and the remaining patient had 50% compliance with follow-up visits. To further delineate the possible effect of the pain clinic, the analysis was repeated after removing the 4 children started on disease modifying therapy (hydroxyurea or chronic red blood cell transfusions) at the time of the first pain clinic visit. The significant decrease in median hospitalizations persisted [5 (IQR 3-6) to 1 (IQR 0-4; p=0.022; Wilcoxon Signed Rank] and the total number of all pain hospitalizations also decreased by 44.3% after pain clinic referral (n=70 before; n= 39 post). A significant effect on the median number of ED visits [1 (IQR 0-3.25) to 1 (IQR 0-2); p=0.37; Wilcoxon Signed Rank] or mean length of hospital stay [4.6 (SD 2.2) to 5.2 (SD 2.9); p=0.43; Paired t-test] was not seen. At the end of the study period, 68.4% (n=13) no longer had chronic opioids listed on their home medication list. Common pain medications still being used by patients included tramadol, diclofenac, cyclooxygenase-2 inhibitors, and ibuprofen.
Figure 1.
Change in median number of hospitalizations for pain 1 year before and 1 year after initial visit to the multidisciplinary pain clinic. A) All patients in cohort (n=19); B) Analysis excluding patients started on hydroxyurea or chronic transfusions at time of initial pain clinic visit (n=15).
Discussion
Evidence for the effect of multidisciplinary pain management models exists for adults without SCD suffering from chronic pain.[11,15] Despite the American Pain Society and the NIH endorsement of this approach to pain management in SCD, there are a lack of data proving its positive effect in SCD pain management. Our data suggest that this model may decrease pain hospitalizations in children with SCD in the most severe cohort of children, since over two-thirds of the children were already on hydroxyurea when the initial pain clinic referral was made. However, a significant effect on the number of ED visits or the length of hospital stay was not found. The reason for this is unknown and may be due to the fixed small sample size of the cohort. However, we hypothesize that although the multidisciplinary pain management model does not truly modify the underlying disease process and acute pain events may still be severe enough to require acute management with doses of intravenous pain medications, children and parents may be more comfortable attempting to manage subsequent pain at home with oral pain medications knowing they have access to the pain management team as an ongoing resource. In addition, the multidisciplinary pain management team may teach children coping mechanisms that allow them to better deal with their pain at home. Based on these reasons, children and their caregivers may feel more comfortable being discharged from the ED after the receipt of intravenous pain medications. In addition, the presence of a formal written pain management action plan at home may provide children and their caregivers specific home-based pain management strategies to use before they come to the ED or for use when discharged to home. Furthermore, the initial pain clinic visit strongly encourages telephone communication between the patient and the pain clinic to allow for adjustment of pain medications at home. Future directions should be aimed at evaluating the cost-effectiveness of this model due to its ability to decrease hospitalizations, in addition to tracking phone communication between patients and the pain clinic to prove effectiveness of the model from this aspect of pain management.
Although compliance with scheduled follow-up appointments in the pain clinic was variable, there was still an overall decrease in hospitalizations in our cohort. The overall effect of decreased hospitalizations for the entire cohort may be explained by the fact that once a patient is seen in the pain clinic, the pain clinic serves as an ongoing resource for the patient and may continue to facilitate outpatient pain management. However, a trend was seen for better compliance with follow-up appointments leading to a larger decrease in hospitalizations when individual cases were examined. This is evidenced by the fact that all but one patient who had zero hospitalizations in the year after their initial pain clinic visit had 100% compliance with scheduled follow-up visits. The correlation between compliance and the effect on decreased hospitalizations would be important to investigate in a larger prospective study.
The median age of our cohort was 15 years. This is consistent with previously published studies revealing the frequency of painful events and acute care encounters increase as children mature from childhood into adolescence and young adulthood.[4,16] Interestingly, there were a significantly higher proportion of female patients in our study cohort. Albeit a small cohort, previous studies have not shown a gender bias in the frequency of SCD painful events.[4,17] The gender difference seen in our cohort may be due to selection bias. In addition, we do not know whether there was a greater likelihood of males to decline pain clinic intervention, which could contribute to this gender difference. However, prior studies in pain biology and pain conditions other than SCD have found females experience pain differently than males and may have increased pain sensitivity.[18–22] Thus, this gender difference warrants further investigation in a larger cohort of children with SCD.
Only a quarter of our cohort had a diagnosis of avascular necrosis. It has been postulated that much of the chronic pain observed in patients with SCD is from bony changes as is seen in avascular necrosis. The prevalence of avascular necrosis has been estimated to be 27% in children and 41% in adults in studies using screening MRI. [23–25] The fact that the majority of patients in our cohort did not have a diagnosis of avascular necrosis to explain their chronic pain raises the question about other explanations for their chronic pain. Explanations likely include ongoing vascular obstruction from their SCD, but other abnormal neurobiological pain mechanisms such as catastrophizing and peripheral or central sensitization resulting in hypersensitivity, allodynia or elements of neuropathic pain may be contributing to their pain [26,27], especially since over two-thirds of our population continued to have pain despite being prescribed hydroxyurea.
Currently used disease modifying interventions for prevention of pain in SCD including hydroxyurea and chronic red blood cell transfusions, are secondary preventive measures.[28] These medical interventions are implemented after a patient has already suffered significantly from the disease and may have fewer adaptive coping strategies which subsequently lead to higher health care utilization rates.[29] Since our data suggest this multidisciplinary model may be effective in adolescence, perhaps introducing the model earlier in the course of a child’s disease may further improve outcomes and serve as a preventative measure by teaching children coping behaviors for dealing with their pain at an earlier age before maladaptive coping strategies develop.[30,31] However, these data have not yet been validated in a prospective trial and this would be necessary before introducing this model to younger patients earlier in the course of their disease.
Our study is limited by the fact that we had a small, fixed sample size. This may have limited our ability to detect differences in emergency department visits and length of hospital stay. Since this was a retrospective study, there may have been a selection bias since the well patients are not referred to the pain clinic for evaluation and treatment. In addition, the retrospective nature of the study did not allow for comparison to those with similar pain phenotypes that were not referred, or did not follow through with the recommendation to be evaluated and treated by the pain clinic. However, since this was a pre-post analysis, the patient acted as his/her own control. The study included short-term follow-up of patients and patients should be prospectively followed over time to evaluate the sustainability of the effect. It was not known whether males were more likely to decline care in the pain clinic due to perceived stigma or for other reasons, which could bias the cohort toward a female predominance. We did not have data on pain managed at home since data were collected retrospectively, thus we cannot comment on the effect of this model on this important aspect of SCD pain. Finally, important patient reported outcome measurement data such as impact on patients’ functional status and health-related quality of life were not available due to the retrospective nature of the study.
In conclusion, a multidisciplinary pain management model appears to have decreased hospitalizations for pain in children with SCD. In addition, our data suggest children with SCD experiencing chronic or recurrent pain are predominantly female and adolescents. The majority of patients did not have a diagnosis of avascular necrosis to explain their chronic pain, suggesting other neurobiological pain mechanisms may be contributing such as catastrophizing and peripheral or central sensitization resulting in hypersensitivity, allodynia or elements of neuropathic pain. Targeting high risk children earlier in their SCD care using a multidisciplinary pain management model may ultimately improve outcomes for pain in children with SCD.
Acknowledgments
The authors would like to thank M. Scott Brown and Robbie Kattappuram for their assistance with data collection.
Funding: This work was supported in part by a grant from the National Institutes of Health National Heart, Lung, and Blood Institute U54 HL090503 (AB) and K23 HL80092 (JP)
Footnotes
Conflict of Interest Statement
None for any of the authors.
References
- 1.Hoffmann RG. Hematology: Basic Principles and Practice. Philadelphia: Elsevier Churchill Livingstone; 2005. [Google Scholar]
- 2.Brandow AM, Brousseau DC, Pajewski NM, et al. Vaso-occlusive painful events in sickle cell disease: impact on child well-being. Pediatric Blood & Cancer. 2010;54(1):92–97. doi: 10.1002/pbc.22222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandow AM, Brousseau DC, Panepinto JA, et al. Postdischarge pain, functional limitations and impact on caregivers of children with sickle cell disease treated for painful events. British Journal of Haematology. 2009;144(5):782–788. doi: 10.1111/j.1365-2141.2008.07512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panepinto JA, Brousseau DC, Hillery CA, et al. Variation in hospitalizations and hospital length of stay in children with vaso-occlusive crises in sickle cell disease. Pediatr Blood Cancer. 2005;44(2):182–186. doi: 10.1002/pbc.20180. [DOI] [PubMed] [Google Scholar]
- 5.Smith WR, Penberthy LT, Bovbjerg VE, et al. Daily assessment of pain in adults with sickle cell disease. Ann Intern Med. 2008;148(2):94–101. doi: 10.7326/0003-4819-148-2-200801150-00004. [DOI] [PubMed] [Google Scholar]
- 6.Panepinto JA, Walters MC, Carreras J, et al. Matched-related donor transplantation for sickle cell disease: report from the Center for International Blood and Transplant Research. Br J Haematol. 2007;137(5):479–485. doi: 10.1111/j.1365-2141.2007.06592.x. [DOI] [PubMed] [Google Scholar]
- 7.Castellino SM, Combs MR, Zimmerman SA, et al. Erythrocyte autoantibodies in paediatric patients with sickle cell disease receiving transfusion therapy: frequency, characteristics and significance. Br J Haematol. 1999;104(1):189–194. doi: 10.1046/j.1365-2141.1999.01127.x. [DOI] [PubMed] [Google Scholar]
- 8.Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N Engl J Med. 1995;332(20):1317–1322. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- 9.Benjamin LJDC, Jacox AK, Odesina V, Phoenix D, Shapiro B, Strafford M, Treadwell M. Guideline for the management of acute and chronic pain in sickle cell disease [American Pain Society clinical practice guideline series No 1] Glenview, IL: 1999. Report. [Google Scholar]
- 10.Volume Pub No.02=2117. NIH; 2002. The Management of Sickle Cell Disease; pp. 161–165. [Google Scholar]
- 11.Flor H, Fydrich T, Turk DC. Efficacy of multidisciplinary pain treatment centers: a meta-analytic review. Pain. 1992;49(2):221–230. doi: 10.1016/0304-3959(92)90145-2. [DOI] [PubMed] [Google Scholar]
- 12.Gil KM, Anthony KK, Carson JW, et al. Daily coping practice predicts treatment effects in children with sickle cell disease. J Pediatr Psychol. 2001;26(3):163–173. doi: 10.1093/jpepsy/26.3.163. [DOI] [PubMed] [Google Scholar]
- 13.Gil KM, Carson JW, Sedway JA, et al. Follow-up of coping skills training in adults with sickle cell disease: analysis of daily pain and coping practice diaries. Health Psychol. 2000;19(1):85–90. doi: 10.1037//0278-6133.19.1.85. [DOI] [PubMed] [Google Scholar]
- 14.Chen E, Cole SW, Kato PM. A review of empirically supported psychosocial interventions for pain and adherence outcomes in sickle cell disease. J Pediatr Psychol. 2004;29(3):197–209. doi: 10.1093/jpepsy/jsh021. [DOI] [PubMed] [Google Scholar]
- 15.Cipher DJ, Fernandez E, Clifford AC. Cost-Effectiveness and Health Care Utilization in a Multidisciplinary Pain Center: Comparison of Three Treatment Groups. Journal of Clinical Psychology in Medical Settings. 2001;8(4):237–244. [Google Scholar]
- 16.Brousseau DC, Owens PL, Mosso AL, et al. Acute care utilization and rehospitalizations for sickle cell disease. JAMA. 303(13):1288–1294. doi: 10.1001/jama.2010.378. [DOI] [PubMed] [Google Scholar]
- 17.McClish DK, Levenson JL, Penberthy LT, et al. Gender differences in pain and healthcare utilization for adult sickle cell patients: The PiSCES Project. J Womens Health (Larchmt) 2006;15(2):146–154. doi: 10.1089/jwh.2006.15.146. [DOI] [PubMed] [Google Scholar]
- 18.Paller CJ, Campbell CM, Edwards RR, et al. Sex-based differences in pain perception and treatment. Pain Med. 2009;10(2):289–299. doi: 10.1111/j.1526-4637.2008.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fillingim RB, King CD, Ribeiro-Dasilva MC, et al. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10(5):447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paulson PE, Minoshima S, Morrow TJ, et al. Gender differences in pain perception and patterns of cerebral activation during noxious heat stimulation in humans. Pain. 1998;76(1–2):223–229. doi: 10.1016/s0304-3959(98)00048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang L, Heitkemper MM. Gender differences in irritable bowel syndrome. Gastroenterology. 2002;123(5):1686–1701. doi: 10.1053/gast.2002.36603. [DOI] [PubMed] [Google Scholar]
- 22.Greenspan JD, Craft RM, LeResche L, et al. Studying sex and gender differences in pain and analgesia: a consensus report. Pain. 2007;132 (Suppl 1):S26–45. doi: 10.1016/j.pain.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Almeida A, Roberts I. Bone involvement in sickle cell disease. Br J Haematol. 2005;129(4):482–490. doi: 10.1111/j.1365-2141.2005.05476.x. [DOI] [PubMed] [Google Scholar]
- 24.Ware HE, Brooks AP, Toye R, et al. Sickle cell disease and silent avascular necrosis of the hip. J Bone Joint Surg Br. 1991;73(6):947–949. doi: 10.1302/0301-620X.73B6.1955442. [DOI] [PubMed] [Google Scholar]
- 25.Adekile AD, Gupta R, Yacoub F, et al. Avascular necrosis of the hip in children with sickle cell disease and high Hb F: magnetic resonance imaging findings and influence of alpha-thalassemia trait. Acta Haematol. 2001;105(1):27–31. doi: 10.1159/000046529. [DOI] [PubMed] [Google Scholar]
- 26.Schmelzle-Lubiecki BM, Campbell KA, Howard RH, et al. Long-term consequences of early infant injury and trauma upon somatosensory processing. Eur J Pain. 2007;11(7):799–809. doi: 10.1016/j.ejpain.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 27.Curatolo M, Petersen-Felix S, Arendt-Nielsen L, et al. Central hypersensitivity in chronic pain after whiplash injury. Clin J Pain. 2001;17(4):306–315. doi: 10.1097/00002508-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 28.U.S. Preventive Services Task Force. Guide to clinical preventive services: report of the US Preventive Services Task Force. 2. Baltimore, MD: 1996. Report. [Google Scholar]
- 29.Gil KM, Williams DA, Thompson RJ, Jr, et al. Sickle cell disease in children and adolescents: the relation of child and parent pain coping strategies to adjustment. J Pediatr Psychol. 1991;16(5):643–663. doi: 10.1093/jpepsy/16.5.643. [DOI] [PubMed] [Google Scholar]
- 30.Gil KM, Wilson JJ, Edens JL. The stability of pain coping strategies in young children adolescents, and adults with sickle cell disease over an 18-month period. Clin J Pain. 1997;13(2):110–115. doi: 10.1097/00002508-199706000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Gil KM, Edens JL, Wilson JJ, et al. Coping strategies and laboratory pain in children with sickle cell disease. Ann Behav Med. 1997;19(1):22–29. doi: 10.1007/BF02883423. [DOI] [PubMed] [Google Scholar]